- Department of Outpatient Chemotherapy, Harbin Medical University Cancer Hospital, Harbin, China
Background: This study aims to comprehensively summarize the colorectal survival rate in China. Method: In PubMed and Web of Science, keywords such as “colorectal cancer”, “survival” and “China” were used to search literatures in the past 10 years. Random effect models were selected to summarize 1-year, 3-year, and 5-year survival rates, and meta-regression and subgroup analyses were performed on the included studies.
Results: A total of 16 retrospective and prospective studies providing survival rates for colorectal cancer in China were included. The 1-year, 3-year, and 5-year survival rates of colorectal cancer in China were 0.79, 0.72 and 0.62, respectively. In the included studies, the 5-year survival rates of stage I (5474 cases), stage II (9215 cases), stage III (8048 cases), and stage IV (4199 cases) colorectal cancer patients were 0.85, 0.81, 0.57 and 0.30, respectively. Among them, the 5-year survival rates of colorectal cancer were 0.82, 0.76, 0.71, 0.67, 0.66, 0.65 and 0.63 in Tianjin, Beijing, Guangdong, Shandong, Liaoning, Zhejiang and Shanghai, respectively.
Conclusion: The 5-year survival rate in China is close to that of most European countries, but still lower than Japan and South Korea, and the gap is gradually narrowing. Region, stage, differentiation, pathological type, and surgical approach can affect 5-year survival in colorectal cancer.
Systematic review registration: https://www.crd.york.ac.uk/prospero/ identifier, CRD42022357789.
1. Introduction
Colorectal cancer (CRC) is the third most common cancer in the world. In 2020, there were 1.9 million new cases of CRC and 93,5000 related deaths (1) Currently, China is undergoing cancer transition with an increasing burden of gastrointestinal cancer. The incidences of CRC increased rapidly (2). In recent years, the economic burden associated with CRC has been rising. Studies have shown that CRC-related healthcare spending is growing rapidly, with overall direct healthcare expenditure per CRC patient in China exceeding GDP per capita in the same year (3). The proportion of rectal cancer in China decreased from 71.2% in the 1980s to 66.7% in the 1990s, while the proportion of colon cancer increased from 10.9% to 15.2% during the same period. In China, the incidence of left and right CRC is basically the same. Among all CRC patients, 49.2% are observed on the right side and 49.4% are observed on the left side. Adenocarcinoma remains the most common type of CRC (4).CRC-related genetic syndromes, such as Lynch syndrome and familial adenomatous polyposis are responsible for 5%−10% of all CRC cases (5). A retrospective study from China showed that Lynch syndrome is an autosomal dominant hereditary Disease, representing 4% of all CRC cases (6).
Survival rate is one of the most critical indicators to measure the therapeutic effect and prognosis of a certain disease. Many social factors will affect the survival of colorectal cancer patients, and the disease itself, such as stage, differentiation, pathological type, tumor site, inflammatory factor, age and gender, will also affect survival (7). The 5-year survival rate of patients with stage I colon cancer was as high as 96.6%, while the 5-year survival rate of patients with stage IV colon cancer was only 34.3% (8). Research has reported that younger patients have a worse prognosis than older patients (9). A single-institution retrospective study showed better survival after radical resection of left colon cancer than right colon cancer, with a significant difference in 5-year overall survival between right and left colon cancer (82.1% vs. 88.7%, P < 0.05) (10). In the past few decades, the survival rate of CRC patients has improved significantly with the improvement of diagnostic technology and treatment, but there are still significant regional differences in the survival rate of CRC patients across the country.
There are many researches on the survival rate of CRC in China, providing valuable experience for the treatment and prognosis by understanding the survival rate of colorectal cancer in different regions and at different times. The purpose of this study is to analyze and summarize the survival rate of CRC in China.
2. Materials and methods
2.1. Study design
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA). By applying the Problem/Population, Intervention, Comparison, and Outcome (PICO) framework, the patients involved in our meta-analysis were colorectal cancer patients. We do not have a specific definition of “Intervention”. Articles that provided survival were included. In all included studies, the “Comparison” element of the PICO framework was not involved because we performed a pooling of single-group rates. The primary outcomes were 1-year, 3-year, and 5-year survival rates.
2.2. Search strategy
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statements checklist. A comprehensive, computerized literature search was conducted in PubMed and Web of Science for relevant studies between January 2011 and December 2021. The keywords and MeSH terms we used for retrieval were: (colorectal) or (colon) or (rectum) and (Neoplasms) or (tumor) or (cancer) or (Malignancy) or (carcinoma) and (survival) or (survival rate) or (survival analysis) or (prognosis) and (China). The search strategy was repeatedly performed until no new relevant articles were found. In addition, we reviewed references in the retrieved articles to search for additional relevant studies. All articles were evaluated by two authors based on the eligibility criteria we designed.
2.3. Selection of researches
First, we checked titles and abstracts of articles that were searched by using keywords to exclude irrelevant articles. Then the retrieved literatures were imported into “EndNote X9” software to exclude the duplicated ones before being screened by two reviewers independently. The exclusion criteria for our studies were as follows. (1) Studies were published in the year before 2011. (2) Articles that do not accurately provide complete survival information. (3) Studies with a sample size of less than 500 patients. (4) Article type is meta-analysis or review. (5) The language of the article is non-English.
2.4. Quality evaluation and data extraction
We evaluated comparative studies using the Newcastle-Ottawa Quality Assessment Scale for Cohort Studies. For the methodological quality evaluation of randomized controlled trials, we took reference to the Cochrane Handbook for Systematic Reviews of Interventions. Data were extracted independently by two researchers and any discrepancies in the data were settled by consensus. If necessary, a third researcher was expected to participate in the discussion and make a decision.
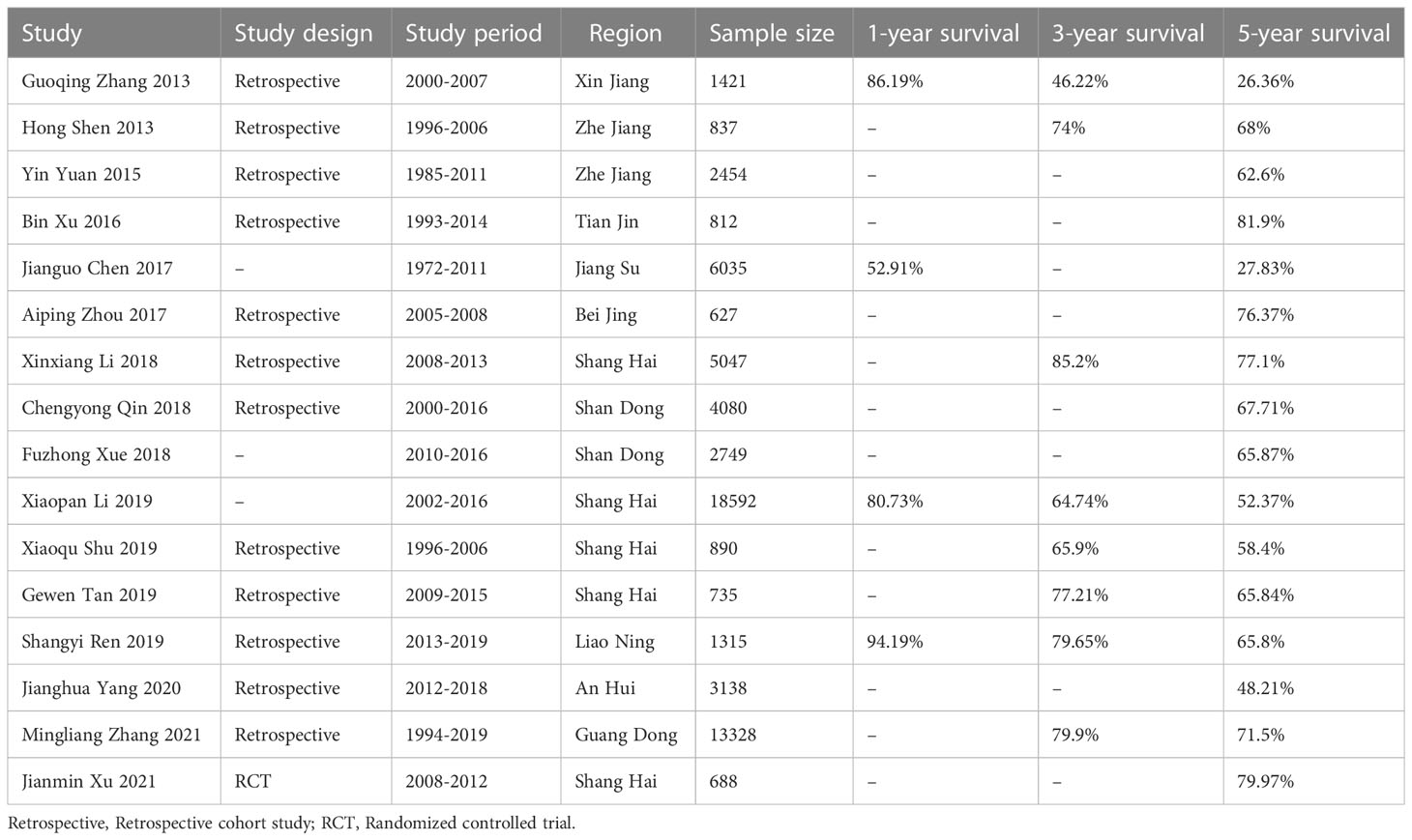
Data were extracted independently by two researchers using pre-designed standard forms, including corresponding author, study design, publication year, research year, region of patients, number of patients, age, sex, tumor site, tumor stage, differentiation, pathological type, surgical approach and 1-, 3- and 5-year survival rates. All data were extracted directly from the original text or calculated from the data known in the original text.
2.5. Data analysis
Firstly, we generated combined 1-, 3- and 5-year survival rates. Second, we performed subgroup analysis and meta-regression analysis when heterogeneity existed between the studies. Subgroup analysis was performed based on the factors of study region, stage, differentiation, pathological type, surgical approach, age and gender. The 5-year survival rates of different subgroups were calculated. The factors of sample size, publication year, research year and study region were included into the meta-regression model for meta-regression analysis. Sensitivity analysis was performed to investigate the risk of publication bias for individual studies. Finally, Egger bias test and funnel plot were used to evaluate the risk of publication bias in the included researches. We used Stata13 for meta-analysis and Graphpad for mapping.
3. Results
3.1. Included researches and their characteristics
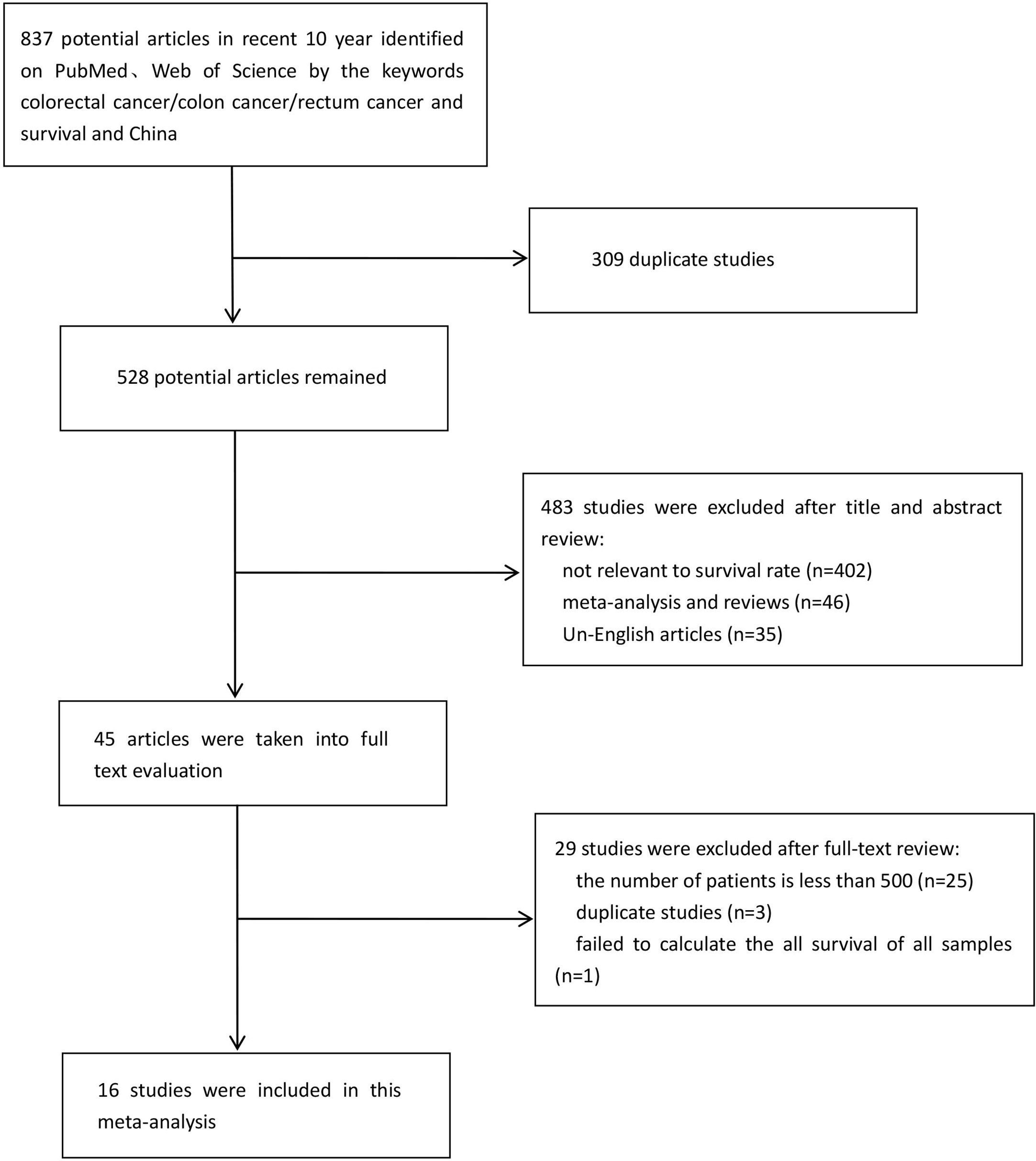
Sixteen researches were eventually included in the meta-analysis (11–26). The combined search identified 837 literatures published, of which 309 duplicates were excluded. 483 were rejected based on the title and abstract evaluation (402 articles did not mention survival rate, 46 articles were meta-analyses and reviews, and 35 articles were non-English), and the remaining 45 articles underwent full-text evaluation. In order to minimize publication bias and make the included researches more representative, we excluded 25 researches with the number of cases fewer than 500. Three researches (13, 27, 28) were proven to be based on the same patient population, so only the one with the most comprehensive information was included (13). There were two researches (26, 29) with the same situation, and we excluded the one with less comprehensive information (29). One epidemiological research was excluded because it was unable to calculate overall survival rate of CRC patients. A flow diagram of the literature selection process used in this study was shown in Figure 1. The 16 researches with 62,748 patients, including 4 from Shanghai, 2 each from Zhejiang and Shandong provinces, and 1 each from Guangdong, Anhui and Jiangsu provinces, Tianjin, Beijing and Xinjiang. Table 1 summarizes the features of the included researches.
3.2. Survival rate
The median follow-up of the 16 researches was 19.76 to 130 months. Four researches mentioned 1-year survival rate of 27363 patients; eight researches included information on 3-year survival rate of 42,165 patients; sixteen researches provided enough data to calculate 5-year survival rates. The 1-, 3- and 5-year survival rates of Chinese CRC patients summarized by the random-effect model were 0.79 (95%CI: 0.63-0.94), 0.72 (95%CI: 0.64-0.79) and 0.62 (95%CI: 0.54-0.70) respectively (Figure 2).
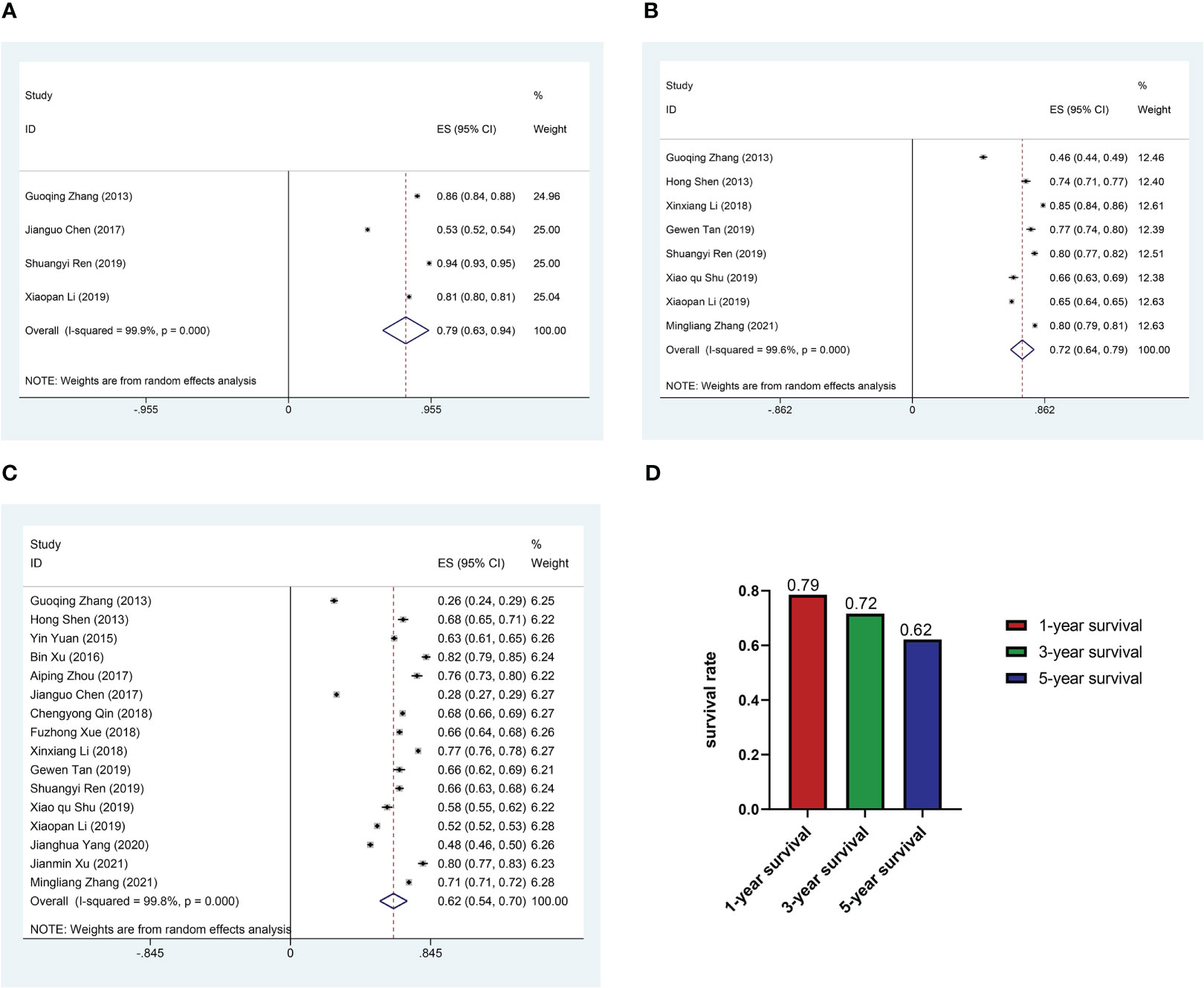
Figure 2 Pooled survival rate. (A) Forest plot of pooled 1-year survival rate in patients with colorectal cancer. (B) Forest plot of pooled 3-year survival rate in patients with colorectal cancer. (C) Forest plot of pooled 5-year survival rate in patients with colorectal cancer. (D) Histogram of pooled 1, 3, 5-year survival rate in patients with colorectal cancer.
3.3. Subgroup analysis
In order to reduce heterogeneity and more deeply compare the impact of patient characteristics on survival rate, we performed the subgroup analysis. The results showed significant differences in 5-year survival rates among CRC patients in different regions. Based on regional subgroup analysis, Tianjin had the highest 5-year survival rate (0.82), followed by Beijing (0.76), Guangdong (0.71), Shandong (0.67), Liaoning (0.66), Zhejiang (0.65), Shanghai (0.63), and Xinjiang had the lowest 5-year survival rate (0.26, 95%CI:0.24-0.29) (Figure 3). The subgroup analysis of different stages of the cancer showed that the 5-year survival rate at stage I was 0.85 (95%CI:0.80-0.90), at stage II was 0.81 (95%CI:0.78-0.85), at stage III was 0.57 (95%CI:0.49-0.65), and at stage IV was only 0.30 (95%CI:0.15-0.46) (Figure 4A). Four researches directly compared 5-year survival rates among patients with different degrees of differentiation, with 5-year survival rates of 0.77 (95%CI: 0.70-0.85) and 0.72 (95%CI: 0.68 -0.77) in the highly and moderately differentiated subgroups respectively, and 0.57 (95%CI: 0.49 - 0.65) in the poorly differentiated subgroup (Figure 4B). In addition, according to the subgroup analysis based on different pathological types of the tumor, the 5-year survival rate was 0.68 (95%CI:0.63-0.73) in the adenocarcinoma subgroup and 0.55 (95%CI: 0.52-0.59) in the mucinous adenocarcinoma subgroup (Figure 4C). The 5-year survival rate of radical surgery patients in our study was 0.73(95%CI:0.71-0.76), while the 5-year survival rate of palliative surgery patients was only 0.15(95%CI:0.09-0.22) (Figure 4D). The histogram summarizes the 5-year survival rate of patients in subgroups with different tumor stage, differentiation, pathological type, surgical approach (Figure 4E). There were no significant differences in subgroup analysis based on age, sex, and tumor site, as shown in Supplementary Figure 1 and Supplementary Figure 2.
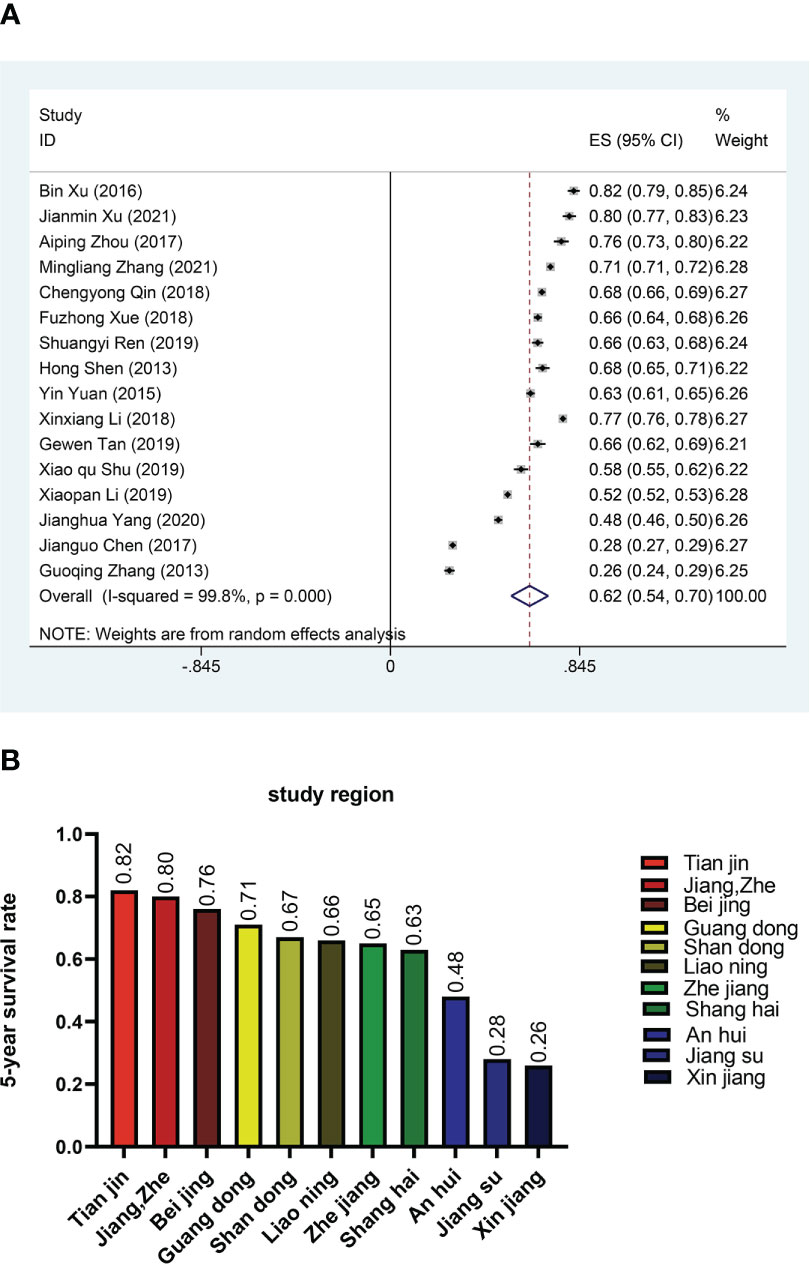
Figure 3 Survival rates in different regions (A) Forest plot of survival rates in different regions. (B) Histogram of survival rates in different regions.
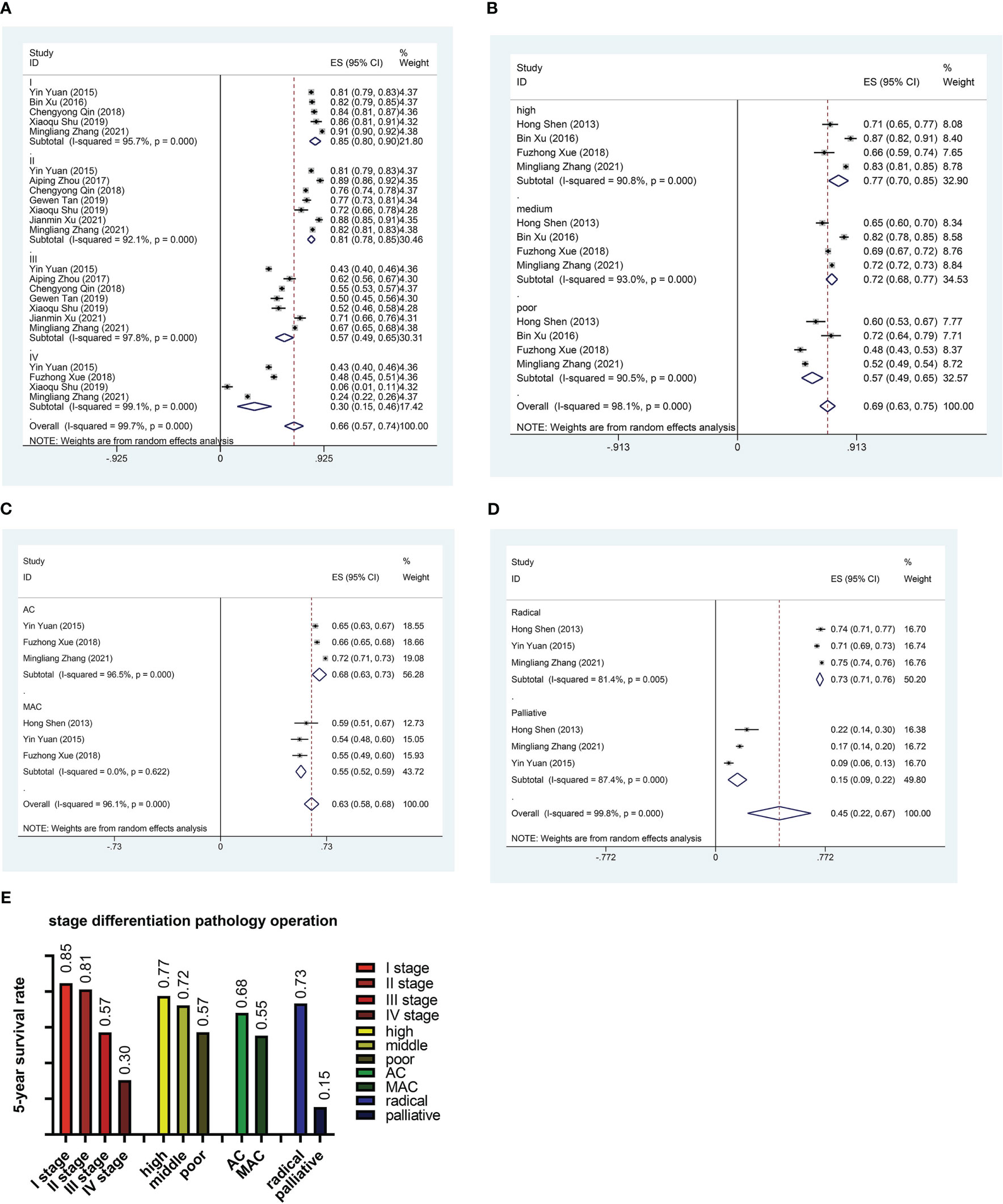
Figure 4 Subgroup analysis. (A) Forest plot of subgroup analysis based on stage. (B) Forest plot of subgroup analysis based on differentiation. (C) Forest plot of subgroup analysis based on pathological type. (D) Forest plot of subgroup analysis based on surgical method. (E) Histogram of subgroup analysis based on stage, differentiation, pathological type and surgical method. AC, Adenocarcinoma; MAC, Mucinous adenocarcinoma.
3.4. Meta-regression analysis
We performed the meta-regression analysis to explore the potential causes of heterogeneity, fitting factors of sample size, publication year, research year and study region into a univariate model. The results showed that sample size and study region were the main causes for heterogeneity, with P values of 0.001 and 0.000 respectively. Meta-regression analysis based on publication year and hospitalization year of the patients found no significant heterogeneity, with P values of 0.075 and 0.437 (Table S1, Supplementary Figure 4).
3.5. Publication bias and evaluation of reference quality
In the evaluation of publication bias in the included studies, we found asymmetry in funnel plot (Supplementary Figure 4), however, it was proven in the more sensitive Egger test that publication bias of the included researches did not exist (P=0.508) (Figure 5A). Sensitivity analysis showed that there was rather significant heterogeneity in the researches from Guoqing Zhang’s research group and Jianguo Chen’s research group (11, 15) (Figure 5B). We believe that heterogeneity may be due to differences in regions and publication years of the study. The economic development, medical level, lifestyle and dietary habits differs from regions, and might affect survival rates in CRC patients. As for Chen’s study, the diagnosis year for the included patients is 1993-2007, which may lead to publication bias. After experiencing regional economic development, improvement of comprehensive treatment options, and changes in healthcare and services, CRC survival rates vary from different years. However, this article can truly reflect the prognosis of CRC patients in China over the past 50 years. We then assessed the methodological quality of all included researches. In one randomized controlled trial, Cochrane Handbook for Systematic Reviews of Interventions alone was used to evaluate its methodological quality, and the risk was evaluated as low for all parts. One epidemiological research (15) provided effective survival rates for the meta-analysis, but there was not enough information for the evaluation of methodological quality, so the rate was rather low (4 stars), while the quality of rest of the researches were all equal to or higher than 5 stars (Table S1).
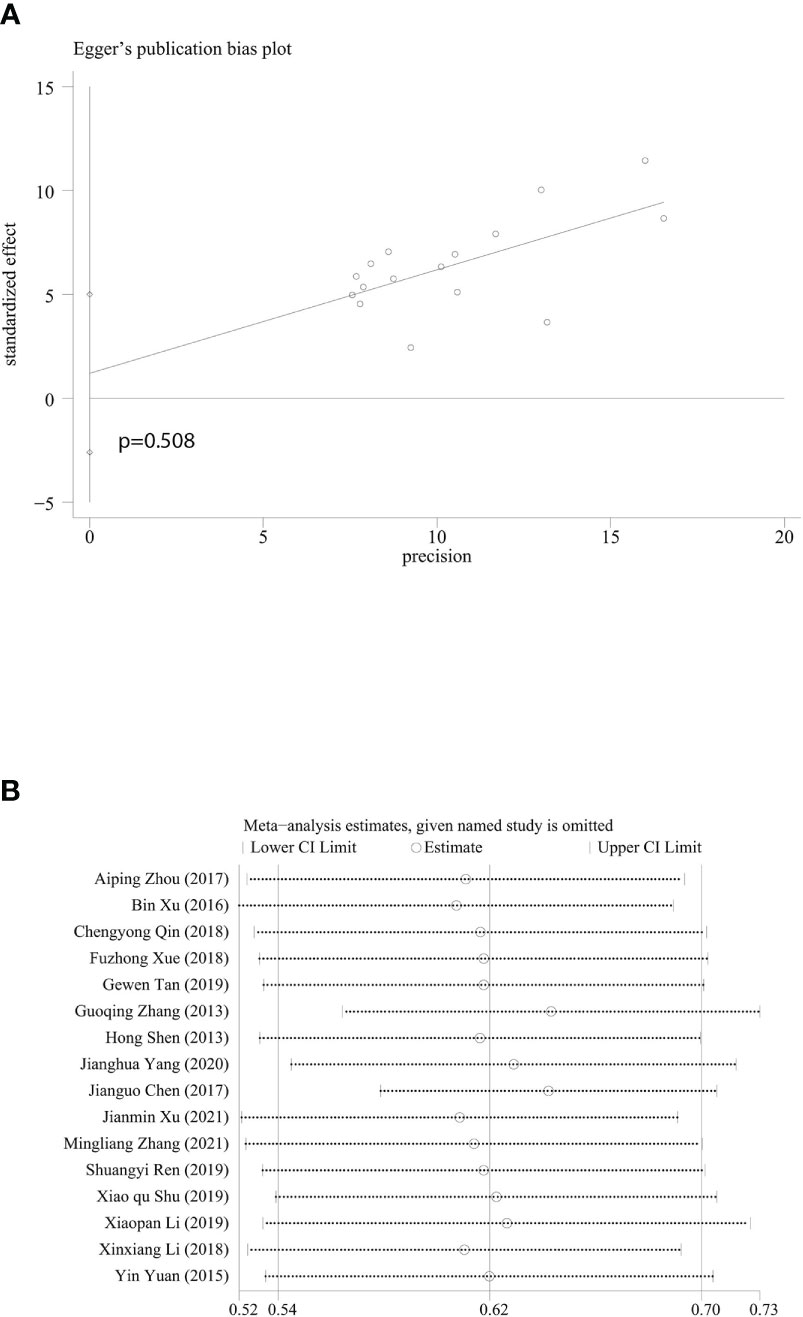
Figure 5 Egger’s publication bias plot and sensitivity analysis. (A) Egger’s publication bias plot based on 5-year survival rate. (B) Sensitivity analysis based on 5-year survival rate. Every horizontal line representing combined 5-year survival and the range of 95% CI after omitting study of the included studies one by one.
4. Discussion
The 1-, 3- and 5-year survival rates of patients with CRC in China were obtained in this meta-analysis. Among the included studies, the pooled 1-year and 3-year survival rates were 0.79 (95%CI: 0.63-0.94) and 0.72 (95%CI: 0.64-0.79), respectively. According to a population-based data analysis, the 1-year survival rates of patients with CRC in Australia, Canada, Norway, Denmark, and the United Kingdom were 0.849, 0.835, 0.824, 0.777, and 0.747, respectively (30). The 1-year survival rates in these regions were basically consistent with our findings. The pooled 5-year survival rate of the 16 included studies was 0.62 (95% CI: 0.54-0.70). A study published in 2019 reported 5-year survival rates for CRC patients in Australia, Canada, Denmark, Ireland, New Zealand, Norway and the United Kingdom. The highest survival rate was 0.708 (95%CI: 0.70-0.715) in Australia, and the lowest was 0.589 (95%CI: 0.586-0.593) in The UK (31). The 5-year survival rate of CRC in China was close to most European countries. Comparatively, Japan, an Asian country, has a 5-year survival rate of 0.73 for CRC, 0.628 for South Korea; 0.61 for Iran; 0.582 for Jordan and 0.342 for Malaysia (32–36). The 5-year survival rate in China was still lower than Japan and South Korea, but the gap was gradually narrowing.
Many factors can affect the prognosis of CRC patients, such as economic status, cancer stage, histological type, tumor location, and age at diagnosis (37, 38). The incidence and mortality of CRC in regions with high Human Development Index (HDI) in the world are at least twice as high as those in regions with low HDI (39). We found that region had an effect on 5-year survival. CRC is a multifactorial disease caused by lifestyle, genetic and environmental factors (40–42). China has a wide geographical area, and different regions have different lifestyles and dietary habits, which lead to different survival rates of CRC in different regions. A study from Malaysian also confirmed wide regional differences in CRC survival (43). In our research, Tianjin had the highest 5-year survival rate (0.82, 95%CI: 0.79-0.85), and Xinjiang had the lowest 5-year survival rate (0.26, 95%CI:0.24-0.29). Due to the small number of included studies, only one study mentioned the 5-year survival rate in Xinjiang (0.26), so it cannot comprehensively represent the 5-year survival rate of patients with CRC in the entire Xinjiang region. The high 5-year survival rate in Tianjin was also due to the fact that the study targeted at stage I CRC patients. For CRC, screening is conducive to early diagnosis and can improve the survival rate of CRC patients. Thus, CRC screening has been recommended in clinical practice guidelines in many countries (44, 45). The implementation of the National Danish Colorectal Cancer Screening Programme was considered a success and the programme was hopefully in the process of reducing colorectal cancer morbidity and mortality in Denmark (46). Policies vary from region to region, and some cities have cancer screening programs in place. For example, in Shanghai, as early as 2013, the CRC screening program was incorporated into community medical services, which greatly improved the 5-year survival rate of patients with colorectal cancer in Shanghai (20).
The pathological stage of tumor at the initial diagnosis is the most important factor in determining the behavior and prognosis for CRC, and mortality rises with tumor stage (47, 48). In our study, the pooled 5-year survival rate at stage I was 0.85, 0.81 at stage II, 0.57 at stage III, and 0.30 at stage IV. It is difficult for patients at stage III and IV to achieve radical cure of the disease and reduce the survival rate. Due to the deeper tumor infiltration, the cancer cells involve surrounding tissues, organs and regional lymph nodes. Rajaa Chatila’s study also confirmed that stage was a major determinant of prognosis in patients with CRC. After adjusting for age and gender in his study, there was a highly significant difference between stage IV patients and stage I patients (HR = 8.81, 95% CI: 3.20-24.22, p = 0.000) (49). Incorporating the surgical method (radical or palliative) into the nomogram model can visually display that the surgical method was an independent prognostic factor affecting the overall survival rate of CRC patients (50). Therefore, the 5-year survival rate of radical surgery patients in our study was 0.73, while the 5-year survival rate of palliative surgery patients was only 0.15. In the subgroup analysis, the 5-year survival rate of the well-differentiated subgroup was 0.77, the 5-year survival rate of the moderately differentiated subgroup was 0.72, and the 5-year survival rate of the poorly differentiated subgroup was 0.57. The existence of prognostic differences between mucinous and non-mucinous colorectal carcinoma, mucinous differentiation results in increased hazard of death (51). In our results, the 5-year survival rates for adenocarcinoma and mucinous adenocarcinoma were 0.68 and 0.55, respectively. Mucinous adenocarcinoma showed a lower 5-year survival rate. The reason may be that mucinous adenocarcinoma, a pathological type, has different characteristics from adenocarcinoma, including younger patients, an advanced stage at diagnosis, and more prone to metastasis (52).
The results of subgroup analysis showed that there was no significant difference in the 5-year survival rate with different age, gender, and tumor site. The 5-year survival rates of patients <60 years and ≥60 years were 0.70 and 0.67, while 0.67 and 0.70 in male and female, respectively. Primary tumor site affects prognosis in patients with CRC. Although there have been studies reporting that right-sided colon cancer has worse overall survival compared to left-sided colon cancer, in our study, there was no significant difference between the two (0.74 vs. 0.71) (53–57). The 5-year survival rates of colon and rectal cancer subgroups were almost equal (0.70 vs. 0.69). It may be related to the fact that we included too few studies, with only 6 studies summarizing 5-year survival in patients with colon/rectal cancer and only 3 studies comparing 5-year survival in the left colon versus the right colon.
One of the limitations of this study is that although we included up to 62748 patients, the number of studies included was small. In order to minimize publication bias and make the included researches more representative, we chose to include researches from large study centers. We determined a threshold of 500 cases based on the actual number of patients in the articles. We hope that this threshold can reduce bias. Many studies did not mention 1-year and 3-year survival, resulting in only 4 studies summarizing 1-year survival and 8 studies summarizing 3-year survival. The information about the survival rate in many studies was not comprehensive. We obtained the survival rate by calculation, so there may be minor deviations. Lynch syndrome is a common CRC-related genetic syndrome. Unfortunately, available research data could not support comparisons of survival rates for genetic and non-hereditary colorectal cancers. The vast majority of Chinese cities have not published studies on CRC survival rates, so it was impossible to summarize the survival rates in various regions of China. Moreover, there were too few related studies in some areas, which is prone to the phenomenon of generalization like the 5-year survival rate in Xinjiang. Summarized information on survival rates of CRC patients in China is lacking. Our study complements the 5-year survival rate information for CRC in different regions and different clinicopathological features in China.
5. Conclusions
The 5-year survival rate in China is close to that of most European countries, but still lower than Japan and South Korea, and the gap is gradually narrowing. Region, stage, differentiation, pathological type, and surgical approach can affect 5-year survival in colorectal cancer.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
HL and JL contributed to the research concept and design. RW and XW retrieved and filtered articles, and XP and BX extracted data. RW and JL analyzed the data. RW, XW, JS, and ST explained the data. RW and JL drafted manuscript. HL and JL contributed to critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Natural Science Foundation of China (grant nos. U20A20376 and 61972116), Beijing Award Foundation (YXJL-2020-0818-0478), Wu Jieping Medical Foundation (320.6750.2020-19-20), Heilongjiang Province Postdoctoral Science Foundation (LBHZ21189), Harbin Medical University Innovative Science Research Funded Project (grant no.2022-KYYWF-0289) and China Postdoctoral Science Foundation (grant no.2022MD713747).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1033154/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel LR, Torre AL, Ahmedin DVM. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2020) 70(4):313. doi: 10.3322/caac.21492
2. Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) (2021) 134(7):783–91. doi: 10.1097/CM9.0000000000001474
3. Huang HY, Shi JF, Guo LW, Bai YN, Liao XZ, Liu GX, et al. Expenditure and financial burden for the diagnosis and treatment of colorectal cancer in China: A hospital-based, multicenter, cross-sectional survey. Cancer Commun (2017) 36(1):1–15. doi: 10.1186/s40880-017-0209-4
4. Yang Y, Han Z, Li X, Huang A, Shi J, Gu J, et al. Epidemiology and risk factors of colorectal cancer in China. Chin J Cancer Res (2020) 32(6):729. doi: 10.21147/j.issn.1000-9604.2020.06.06
5. Monahan KJ, Bradshaw N, Dolwani S, Desouza B, Dunlop MG, East JE, et al. Guidelines for the management of hereditary colorectal cancer from the British society of gastroenterology (BSG)/Association of coloproctology of great Britain and Ireland (ACPGBI)/United kingdom cancer genetics group (UKCGG). Gut (2020) 69(3):411–44. doi: 10.1136/gutjnl-2019-319915
6. Lee J, Xiao YY, Sun YY, Balderacchi J, Clark B, Desani J, et al. Prevalence and characteristics of hereditary non-polyposis colorectal cancer (HNPCC) syndrome in immigrant Asian colorectal cancer patients. BMC Cancer (2017) 17(1):1–9. doi: 10.1186/s12885-017-3799-y
7. Jin LJ, Chen WB, Zhang XY, Bai J, Zhao HC, Wang ZY. Analysis of factors potentially predicting prognosis of colorectal cancer. World J Gastrointest Oncol (2019) 11(12):1206. doi: 10.4251/wjgo.v11.i12.1206
8. Pang X, Xu B, Lian J, Wang R, Wang X, Shao J, et al. Real-world survival of colon cancer after radical surgery: A single-institutional retrospective analysis. Front Oncol (2022) 12. doi: 10.1097/MD.0000000000021304
9. Zhang Y, Wang Y, Liu X, Chen B, Zhuang J, Li S, et al. Worse prognosis in young patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy: A comparative study. Medicine (2020) 99(35):e21304. doi: 10.1097/MD.0000000000021304
10. Lim DR, Kuk JK, Kim T, Shin EJ. Comparison of oncological outcomes of right-sided colon cancer versus left-sided colon cancer after curative resection: which side is better outcome? Medicine (2017) 96(42):e8241. doi: 10.1097/MD.0000000000008241
11. Yusup A, Wang HJ, Rahmutula A, Sayim P, Zhao ZL, Zhang GQ. Clinical features and prognosis in colorectal cancer patients with different ethnicities in Northwest China. World J Gastroenterol: WJG (2013) 19(41):7183–8. doi: 10.3748/wjg.v19.i41.7183
12. Yuan Y, Li MD, Hu HG, Dong CX, Chen JQ, Li XF, et al. Prognostic and survival analysis of 837 Chinese colorectal cancer patients. World J Gastroenterol: WJG (2013) 19(17):2650–9. doi: 10.3748/wjg.v19.i17.2650
13. Tan Y, Fu J, Li X, Yang J, Jiang M, Ding K, et al. A minor (< 50%) signet-ring cell component associated with poor prognosis in colorectal cancer patients: a 26-year retrospective study in China. PLos One (2015) 10(3):e0121944. doi: 10.1371/journal.pone.0121944
14. Xu B, Yu L, Zhao LZ, Ma DW. Prognostic factors in the patients with T2N0M0 colorectal cancer. World J Surg Oncol (2016) 14(1):1–6. doi: 10.1186/s12957-016-0826-4
15. Chen JG, Zhu J, Zhang YH, Zhang YX, Yao DF, Chen YS, et al. Cancer survival in qidong between 1972 and 2011: A population−based analysis. Mol Clin Oncol (2017) 6(6):944–54. doi: 10.3892/mco.2017.1234
16. Qin Q, Yang L, Sun YK, Ying JM, Song Y, Zhang W, et al. Comparison of 627 patients with right-and left-sided colon cancer in China: Differences in clinicopathology, recurrence, and survival. Chronic Dis Transl Med (2017) 3(01):51–9. doi: 10.1016/j.cdtm.2017.02.004
17. Shen L, Mo M, Jia L, Jia H, Li Q, Liang L, et al. Poorer prognosis in young female patients with non-metastatic colorectal cancer: a hospital-based analysis of 5,047 patients in China. Cancer Manag Res (2018) 10:653–61. doi: 10.2147/CMAR.S159901
18. Li X, Zhao Q, An B, Qi J, Wang W, Zhang D, et al. Prognostic and predictive value of the macroscopic growth pattern in patients undergoing curative resection of colorectal cancer: a single-institution retrospective cohort study of 4,080 Chinese patients. Cancer Manag Res (2018) 10:1875–87. doi: 10.2147/CMAR.S165279
19. Li J, Gu J, Ma X, Li X, Liu X, Kang F, et al. Development and validation of a nomogram for predicting survival in Chinese han patients with resected colorectal cancer. J Surg Oncol (2018) 118(6):1034–41. doi: 10.1002/jso.25213
20. Li X, Zhou Y, Luo Z, Gu YA, Chen Y, Yang C, et al. The impact of screening on the survival of colorectal cancer in shanghai, China: A population based study. BMC Public Health (2019) 19(1):1–9. doi: 10.1186/s12889-019-7318-8
21. Cui Y, Wen W, Zheng T, Li H, Gao YT, Cai H, et al. Use of antihypertensive medications and survival rates for breast, colorectal, lung, or stomach cancer. Am J Epidemiol (2019) 188(8):1512–28. doi: 10.1093/aje/kwz106
22. You W, Sheng N, Yan L, Chen H, Gong J, He Z, et al. The difference in prognosis of stage II and III colorectal cancer based on preoperative serum tumor markers. J Cancer (2019) 10(16):3757–66. doi: 10.7150/jca.31660
23. Feng Z, Shi X, Zhang Q, Zhang X, Li X, Chen Z, et al. Analysis of clinicopathological features and prognosis of 1315 cases in colorectal cancer located at different anatomical subsites. Pathol Res Pract (2019) 215(10):152560. doi: 10.1016/j.prp.2019.152560
24. Wang Z, Du Z, Liu Y, Wang W, Liang M, Zhang A, et al. Comparison of the clinicopathological features and prognoses of patients with schistosomal and nonschistosomal colorectal cancer. Oncol Lett (2020) 19(3):2375–83. doi: 10.3892/ol.2020.11331
25. Zhu D, Xia J, Gu Y, Lin J, Ding K, Zhou B, et al. Preoperative hepatic and regional arterial chemotherapy in patients who underwent curative colorectal cancer resection: A prospective, multi-center, randomized controlled trial. Ann Surg (2021) 273(6):1066–75. doi: 10.1097/SLA.0000000000004558
26. Fang L, Yang Z, Zhang M, Meng M, Feng J, Chen C. Clinical characteristics and survival analysis of colorectal cancer in China: A retrospective cohort study with 13,328 patients from southern China. Gastroenterol Rep (2021) 9(6):571–82. doi: 10.1093/gastro/goab048
27. Fu J, Yang J, Tan Y, Jiang M, Wen F, Huang Y, et al. Young patients (≤ 35years old) with colorectal cancer have worse outcomes due to more advanced disease: a 30-year retrospective review. Medicine (2014) 93(23):e135. doi: 10.1097/MD.0000000000000135
28. Fu JF, Huang YQ, Yang J, Yi CH, Chen HL, Zheng S. Clinical characteristics and prognosis of young patients with colorectal cancer in Eastern China. World J Gastroenterol: WJG (2013) 19(44):8078–84. doi: 10.3748/wjg.v19.i44.8078
29. Li J, Xie Y, Huang Z, Shen D, Zhuang Z, Zhu M, et al. Current treatment and surveillance modalities are not sufficient for advanced stage III colon cancer: Result from a multicenter cohort analysis. Cancer Med (2021) 10(24):8924–33. doi: 10.1002/cam4.4417
30. Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the international cancer benchmarking partnership): an analysis of population-based cancer registry data. Lancet (2011) 377(9760):127–38. doi: 10.1016/S0140-6736(10)62231-3
31. Arnold M, Rutherford MJ, Bardot A, Ferlay J, Andersson TM, Myklebust TÅ, et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): A population-based study. Lancet Oncol (2019) 20(11):1493–505. doi: 10.1016/S1470-2045(19)30456-5
32. Tamakoshi A, Nakamura K, Ukawa S, Okada E, Hirata M, Nagai A, et al. Characteristics and prognosis of Japanese colorectal cancer patients: The BioBank Japan project. J Epidemiol (2017) 27(Supplement_III):S36–42. doi: 10.1016/j.je.2016.12.004
33. Bong JW, Lee JA, Ju Y, Seo J, Kang SH, Lee SI, et al. Treatment outcomes of patients with involved resection margin after rectal cancer surgery: A nationwide population-based cohort study in south Korea. Asia Pac J Clin Oncol (2022) 18(4):378–87. doi: 10.1111/ajco.13608
34. Moghimi-Dehkordi B, Safaee A, Zali MR. Prognostic factors in 1,138 Iranian colorectal cancer patients. Int J Colorectal Dis (2008) 23(7):683–8. doi: 10.1007/s00384-008-0463-7
35. Sharkas GF, Arqoub KH, Khader YS, Tarawneh MR, Nimri OF, Al-Zaghal MJ, et al. Colorectal cancer in Jordan: Survival rate and its related factors. J Oncol (2017) 2017. doi: 10.1155/2017/3180762
36. Ghazali AK, Musa KI, Naing NN, Mahmood Z. Prognostic factors in patients with colorectal cancer at hospital universiti sains Malaysia. Asian J Surg (2010) 33(3):127–33. doi: 10.1016/S1015-9584(10)60022-X
37. Rasouli MA, Moradi G, Roshani D, Nikkhoo B, Ghaderi E, Ghaytasi B. Prognostic factors and survival of colorectal cancer in Kurdistan province, Iran: A population-based study (2009–2014). Medicine (2017) 96(6):e5941. doi: 10.1097/MD.0000000000005941
38. Leong E, Ong SK, Madli F, Tan A, Lai D, Basir N, et al. Survival rates and associated factors of colorectal cancer patients in Brunei darussalam. Asian Pac J Cancer Prev: APJCP (2020) 21(1):259–65. doi: 10.31557/APJCP.2020.21.1.259
39. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut (2017) 66(4):683–91. doi: 10.1136/gutjnl-2015-310912
40. Aran V, Victorino AP, Thuler LC, Ferreira CG. Colorectal cancer: Epidemiology, disease mechanisms and interventions to reduce onset and mortality. Clin Colorectal Cancer (2016) 15(3):195–203. doi: 10.1016/j.clcc.2016.02.008
41. Chong DQ, Banbury BL, Phipps AI, Hua X, Kocarnik J, Peters U, et al. Association of family history and survival in patients with colorectal cancer: A pooled analysis of eight epidemiologic studies. Cancer Med (2018) 7(5):2192–9. doi: 10.1002/cam4.1470
42. Schoen RE, Razzak A, Kelly JY, Berndt SI, Firl K, Riley TL, et al. Incidence and mortality of colorectal cancer in individuals with a family history of colorectal cancer. Gastroenterology (2015) 149(6):1438–45. doi: 10.1053/j.gastro.2015.07.055
43. Ghazali AK, Keegan T, Taylor BM. Spatial variation of survival for colorectal cancer in Malaysia. Int J Environ Res Public Health (2021) 18(3):1052. doi: 10.3390/ijerph18031052
44. Rehm CD, Peñalvo JL, Afshin A, Mozaffarian D. Dietary intake among US adults, 1999-2012. Jama (2016) 315(23):2542–53. doi: 10.1001/jama.2016.7491
45. Pesola F, Eloranta S, Martling A, Saraste D, Smedby KE. Family history of colorectal cancer and survival: A Swedish population-based study. J Intern Med (2020) 287(6):723–33. doi: 10.1111/joim.13036
46. Njor SH, Friis-Hansen L, Andersen B, Søndergaard B, Linnemann D, Jørgensen JCR, et al. Three years of colorectal cancer screening in Denmark. Cancer Epidemiol (2018) 57:39–44. doi: 10.1016/j.canep.2018.09.003
47. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin (2017) 67(2):93–9. doi: 10.3322/caac.21388
48. González LV, de Miguel Ibáñez R, Sotos FE. Colorectal cancer prevalence and survival in cuenca (Spain). J Gastrointest Cancer (2022) 2022:1–10. doi: 10.1007/s12029-021-00784-x
49. Chatila R, Mansour J, Mugharbil A, Nsouli G, O’Son L, Sayad E, et al. Epidemiology and survival of colorectal cancer in Lebanon: A Sub-national retrospective analysis. Cancer Control (2021) 28:10732748211041221. doi: 10.1177/10732748211041221
50. Lv J, Guo P. A nomogram model for predicting prognosis of obstructive colorectal cancer. World J Surg Oncol (2021) 19(1):1–11. doi: 10.1186/s12957-021-02445-6
51. Verhulst J, Ferdinande L, Demetter P, Ceelen W. Mucinous subtype as prognostic factor in colorectal cancer: A systematic review and meta-analysis. J Clin Pathol (2012) 65(5):381–8. doi: 10.1136/jclinpath-2011-200340
52. Wu X, Lin H, Li S. Prognoses of different pathological subtypes of colorectal cancer at different stages: A population-based retrospective cohort study. BMC Gastroenterol (2019) 19(1):1–8. doi: 10.1186/s12876-019-1083-0
53. Ge Y, Lei S, Cai B, Gao X, Wang G, Wang L, et al. Incidence and prognosis of pulmonary metastasis in colorectal cancer: A population-based study. Int J Colorectal Dis (2020) 35(2):223–32. doi: 10.1007/s00384-019-03434-8
54. Shida D, Inoue M, Tanabe T, Moritani K, Tsukamoto S, Yamauchi S, et al. Prognostic impact of primary tumor location in stage III colorectal cancer-right-sided colon versus left-sided colon versus rectum: A nationwide multicenter retrospective study. J Gastroenterol (2020) 55(10):958–68. doi: 10.1007/s00535-020-01706-7
55. Zheng C, Jiang F, Lin H, Li S. Clinical characteristics and prognosis of different primary tumor location in colorectal cancer: A population-based cohort study. Clin Transl Oncol (2019) 21(11):1524–31. doi: 10.1007/s12094-019-02083-1
56. Lee L, Erkan A, Alhassan N, Kelly JJ, Nassif GJ, Albert MR, et al. Lower survival after right-sided versus left-sided colon cancers: Is an extended lymphadenectomy the answer? Surg Oncol (2018) 27(3):449–55. doi: 10.1016/j.suronc.2018.05.031
Keywords: colorectal cancer, overall survival, meta-analysis, China, epidemiology
Citation: Wang R, Lian J, Wang X, Pang X, Xu B, Tang S, Shao J and Lu H (2023) Survival rate of colorectal cancer in China: A systematic review and meta-analysis. Front. Oncol. 13:1033154. doi: 10.3389/fonc.2023.1033154
Received: 31 August 2022; Accepted: 20 February 2023;
Published: 03 March 2023.
Edited by:
Jaques Waisberg, Faculdade de Medicina do ABC, BrazilReviewed by:
Demetrius Germini, Hospital do Servidor Público Estadual, BrazilRogerio Palma, Faculdade de Medicina do ABC, Brazil
Copyright © 2023 Wang, Lian, Wang, Pang, Xu, Tang, Shao and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haibo Lu, luhaibo@hrbmu.edu.cn
†These authors have contributed equally to this work
 Ren Wang
Ren Wang Jie Lian
Jie Lian Xin Wang
Xin Wang Xiangyi Pang
Xiangyi Pang Benjie Xu
Benjie Xu Shuli Tang
Shuli Tang Jiayue Shao
Jiayue Shao Haibo Lu
Haibo Lu