- 1Department of Urology, Taizhou Central Hospital (Taizhou University Hospital), Taizhou, Zhejiang, China
- 2Department of Nephrology, Jiaxing Hospital of Traditional Chinese Medicine, Jiaxing, Zhejiang, China
- 3Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 4Department of Urology, The First Affiliated Hospital of Nanchang University, Nanchang, China
Background: At present, androgen deprivation therapy (ADT) is still the standard regimen for patients with metastatic and locally advanced prostate cancer (PCa). The level of androgen receptor splice variant-7 (AR-V7) in men with castration-resistant prostate cancer (CRPC) has been reported to be elevated compared with that in patients diagnosed with hormone-sensitive prostate cancer (HSPC).
Aim: Herein, we performed a systematic review and cumulative analysis to evaluate whether the expression of AR-V7 was significantly higher in patients with CRPC than in HSPC patients.
Methods: The commonly used databases were searched to identify the potential studies reporting the level of AR-V7 in CRPC and HSPC patients. The association between CRPC and the positive expression of AR-V7 was pooled by using the relative risk (RR) with the corresponding 95% confidence intervals (CIs) under a random-effects model. For detecting the potential bias and the heterogeneity of the included studies, sensitivity analysis and subgroup analysis were performed. Publication bias was assessed Egger’s and Begg’s tests. This study was registered on PROSPERO (ID: CRD42022297014).
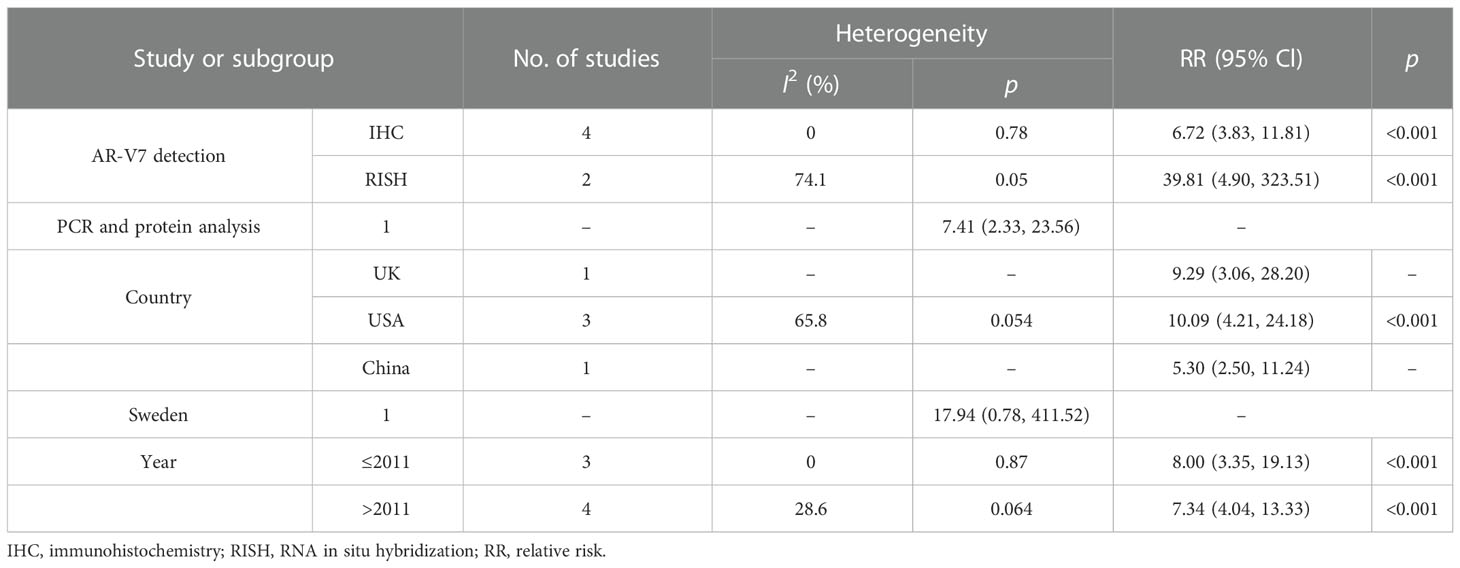
Results: This cumulative analysis included 672 participants from seven clinical trials. The study group contained 354 CRPC patients, while the other group contained 318 HSPC patients. Pooled results from the seven eligible studies showed that the expression of positive AR-V7 was significantly higher in men with CRPC compared to those with HSPC (RR = 7.55, 95% CI: 4.61–12.35, p < 0.001). In the sensitivity analysis, the combined RRs did not change substantially, ranging from 6.85 (95% CI: 4.16–11.27, p < 0.001) to 9.84 (95% CI: 5.13–18.87, p < 0.001). In the subgroup analysis, a stronger association was detected in RNA in situ hybridization (RISH) measurement in American patients, and those studies were published before 2011 (all p < 0.001). There was no significant publication bias identified in our study.
Conclusion: Evidence from the seven eligible studies demonstrated that patients with CRPC had a significantly elevated positive expression of AR-V7. More investigations are still warranted to clarify the association between CRPC and AR-V7 testing.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022297014.
Introduction
According to the data from Cancer Statistics 2022, prostate cancer (PCa) is the most frequently diagnosed male cancer in Western countries (1, 2). Moreover, PCa is one of the leading causes of cancer mortality in developed countries (1, 2). The growth and differentiation of normal prostate cells depend on androgens for the activation of androgen receptors (ARs) (3). Also, androgens play an essential role during all phases of the growth of PCa cells (4). AR signaling is the foundation for the proliferation and survival of PCa cells. A human AR gene can be found on chromosome Xq11-12. It is composed of eight exons encoding a 110-kDa protein. Structurally, the human AR protein is composed of an N-terminal transactivation domain, a hinge region, a central DNA-binding domain, and a C-terminal ligand-binding domain (5). The binding of androgen to the AR ligand-binding domain (LBD) allows the ligand-bound receptor to enter the nucleus and regulate androgen-responsive genes in the nucleus (6). At present, androgen deprivation therapy (ADT) is still the mainstay therapy for metastatic and advanced PCa. To a great extent, men with advanced PCa may initially respond to ADT, termed hormone-sensitive prostate cancer (HSPC). Unfortunately, the majority of patients may experience progression to castration-resistant prostate cancer (CRPC) within a median time frame of 24 to 36 months, although the levels of androgens continue to be low (7).
The current evidence suggests that CRPC may be not independent of the effect of androgen, but AR signaling continues to be essential (8). The researchers found that CRPC could express not only AR but also the androgen-responsive genes. The AR signaling pathway is still functional when the androgen is depleted (9). The AR axis still plays a role in CRPC and is accountable for the progression of the disease (10), and a new generation of AR-directed agents have recently emerged, i.e., androgen biosynthesis inhibitor “abiraterone” (11) and the AR antagonist “enzalutamide” (12). The mechanisms of ablation-resistant AR-mediated signaling pathways have not yet been fully elucidated. The mechanisms by which AR are activated in CRPC have been hypothesized to be different, including mutation and augmentation of AR gene (13, 14). AR may turn out to be more susceptible to stimulation by androgens or other ligands due to intratumoral synthesis of androgens, as well as epigenetic and genetic alterations (15–17). Moreover, the androgen receptor splice variants (AR-Vs) are frequently expressed in CRPC (18, 19). To date, more than 20 AR-Vs were identified, and AR-V7 (also named AR3) is one of the most clinically significant variants (20).
AR-V7 has been identified as one of the leading splice variants in both localized and advanced PCa (21). AR-V7 is deficient with the ligand-binding domain (androgen-binding site) but retains the transactivating N-terminal domain. Since AR-V7 serves as an important transcription factor, it constitutively activates and promotes the activation of its target genes (22). Several clinical trials have reported that the AR-V7 level was significantly greater in CRPC when compared with HSPC (23–25). According to these studies, a trend toward a higher level of AR-V7 was observed in patients with CRPC.
Since a number of studies have identified the potential relationship between CRPC and AR-V7, it is clinically meaningful to summarize the evidence on this issue by conducting a meta-analysis. In this study, we performed a cumulative study to summarize and analyze the evidence on the association between CRPC and the AR-V7 expression.
Methods
The current cumulative analysis was conducted according to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (26), which was registered on the PROSPERO (ID: CRD42022297014).
Search strategy
The Medline, Cochrane Library, and Embase were searched for systematic literature reviews. The time frame for searching the eligible studies was up to April 2022. Searches were conducted by using the subject headings and keywords. The following search terms were used: ((((((“Prostatic Neoplasms”[Mesh]) OR (Prostate Cancer)) OR (Prostatic Cancer)) AND ((((AR-V7) OR AR3) OR receptor splicing variant 7) OR androgen receptor 3))) AND castration-resistant prostate cancer). Moreover, we further reviewed the reference lists of the relevant articles to detect more eligible studies. Participants and the language of the search were restricted to American patients and English, respectively.
Quantification of AR-V7
Clinical trials in which AR-V7 was investigated by any of the existing instruments were considered to be eligible. These included immunohistochemical (IHC) staining analysis, RNA in situ hybridization (ISH), PCR, and protein analysis.
Study selection
Study eligibility was fully determined by the PICOS criteria, namely, the patient population, intervention or exposure, comparison, and outcome (PICOS) study design. The inclusion criteria included the following: 1) participants: men with prostate cancer PCa. 2) Interventions: CRPC. 3) Comparisons: HSPC. 4) Outcomes: the positive expression of AR. 5) Study design: any study designs. Furthermore, additional studies included in this review were supposed to provide the relative risk (RR) estimates with the corresponding 95% confidence intervals (CIs). A list of exclusion criteria was provided, as follows: 1) no control data; 2) updated or duplicated data; 3) review articles; 4) meeting abstract, commentaries, editorials, congress reports, letters, or case reports; and 5) animal or in vitro experiments.
Quality assessment and data extraction
According to the predetermined selection criteria, two authors independently extracted the data. Data were obtained from the included studies, as follows: names of the first author, study design, publication year, study areas, the sample sizes of the study group and the control group, methods of AR-V7 detection, and the effect measures (HR, RR, or OR) with their 95% CI. The quality of cross-sectional studies was assessed by using the quality methodology checklist (27). The Newcastle–Ottawa Scale was applied to evaluate the study quality of the case–control/cohort studies (28).
Statistical analyses
The strength of the association between CRPC and AR expression was assessed by the pooled RRs and 95% CIs. Results with p-values <0.05 were defined as statistically significant. Heterogeneity across studies was determined by using the I2 statistic and Cochran’s Q statistic (29). Fixed-effect models were used when there was no significant heterogeneity (I2 < 50%, p > 0.10). Otherwise, a random-effects model was applied. Moreover, an analysis of sensitivity was conducted by omitting one study at one time to assess how it affected the overall risk estimate. The origins of heterogeneity were further examined by subgroup analyses. Publication biases were determined by using Begg’s and Egger’s tests (30, 31). p > 0.05 indicated no publication bias, while p < 0.05 was judged to be a statistical publication bias. All the analyses presented within the present study were conducted the Stata 12.0 software (Stata Corp LP, College Station, TX, USA).
Results
Results from the literature search
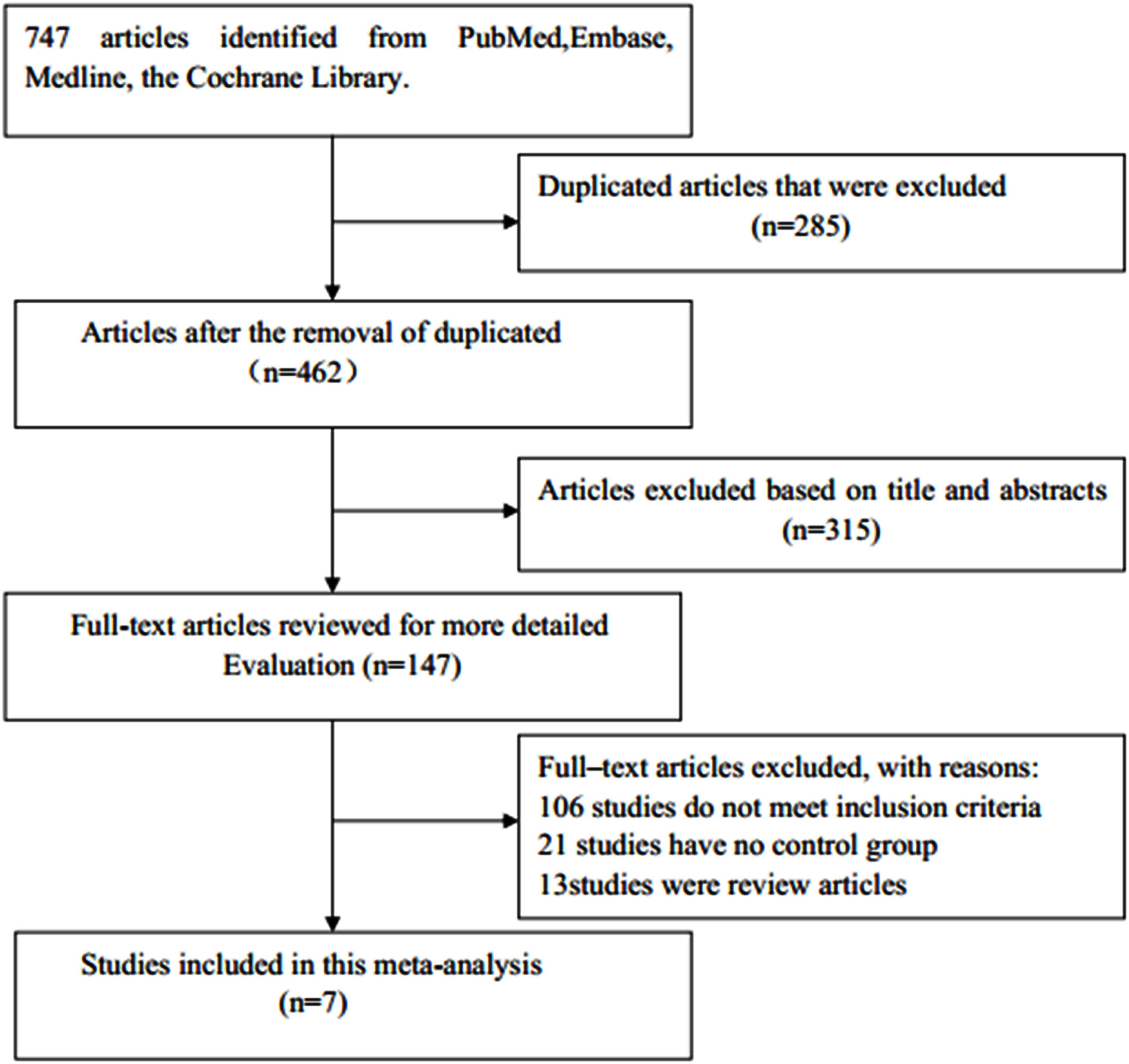
A diagram of the study selection process is shown in Figure 1. A total number of 747 articles were identified in the initial search, of which 285 duplicates were eliminated. Of the remaining articles, after the titles and abstracts were read, 315 articles were excluded. A total of 147 potentially relevant studies remained for further review. Among them, 106 studies were eliminated for not meeting the inclusion criteria, 21 for having no control group, and 13 for being reviews. Finally, seven observational studies were included in this meta-analysis (23–25, 32–35).
Study characteristics
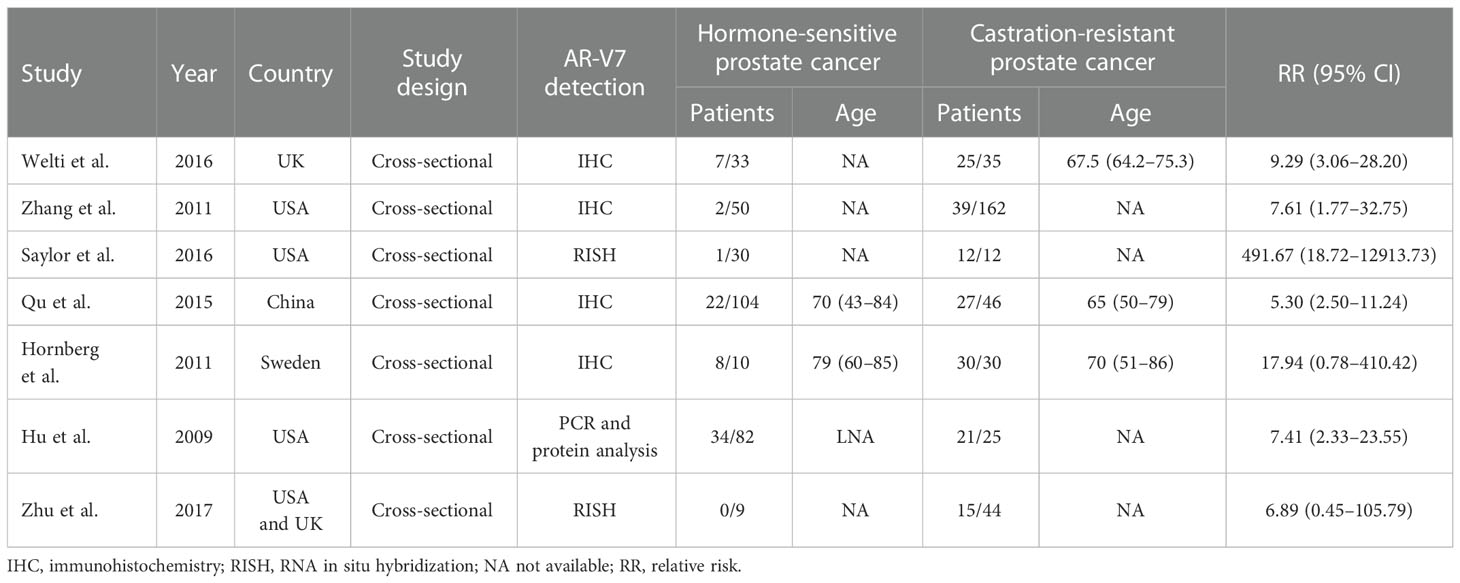
The characteristics of eligible publications are listed in Table 1. The study design of all included studies was cross-sectional. All of the eligible publications were published from 2009 to 2017. Among the seven included studies, a total of 672 participants were enrolled, 354 of whom were CRPC patients, while the remaining 318 participants were HSPC patients (the sample sizes ranged from 9 to 162). The assessment of AR-V7 expression was inconsistent among studies.
Study quality
Supplementary Table 1 summarizes the quality assessment results for the cross-sectional studies.
Synthesis of results
No significant statistical heterogeneity was detected among all the eligible studies (I2 = 20.6%, p = 0.272); thus, the fixed-effects model was conducted to pool the data. As displayed in Figure 2, the combined results revealed that patients with CRPC had a significantly higher expression of AR-V7 than the individuals with HSPC (RR = 7.55, 95% CI: 4.61–12.35, p < 0.001), which showed that CRPC was strongly correlated with an increased positive expression of AR-V7.
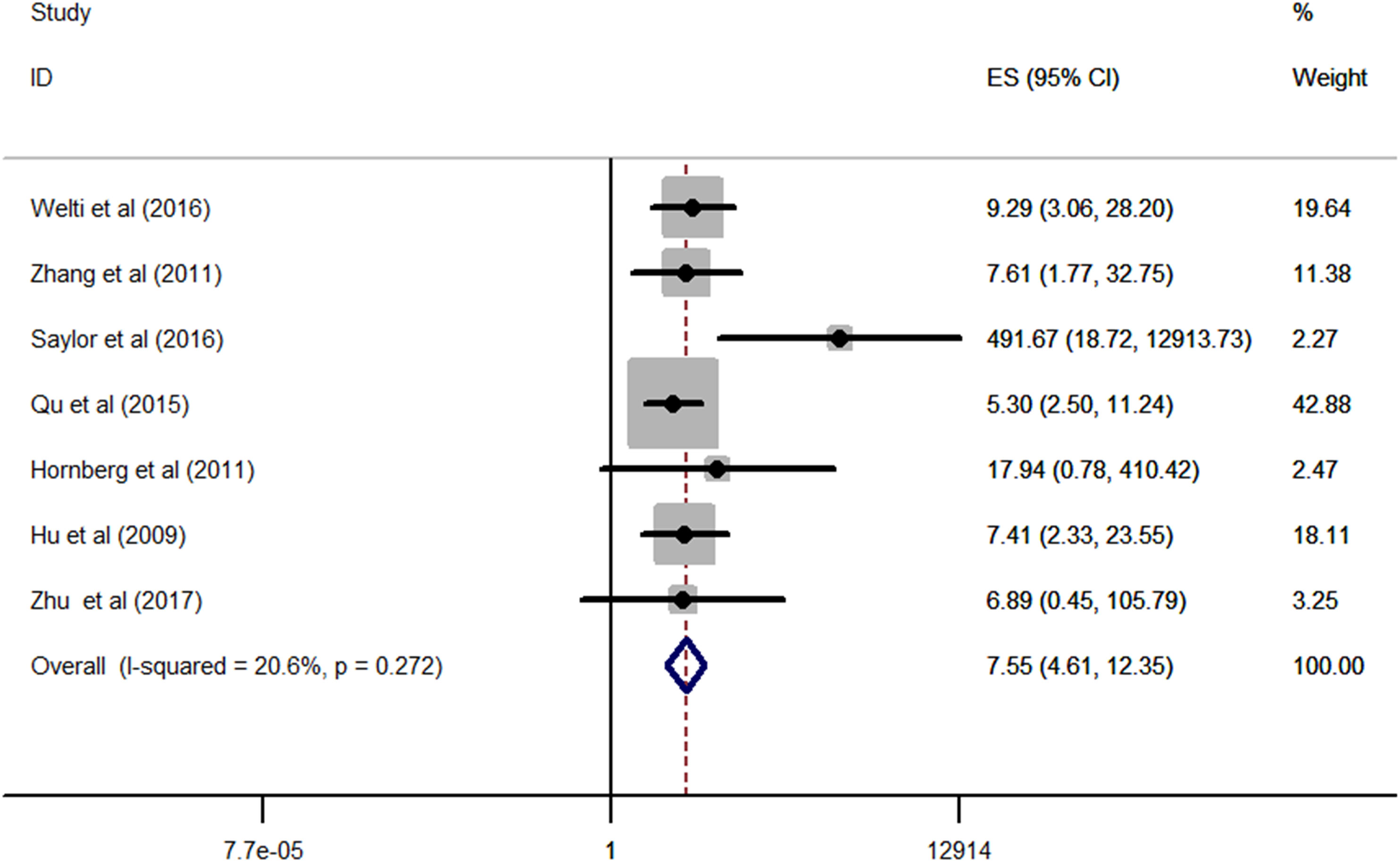
Figure 2 Forest plots of meta-analysis of the included studies on the association between CRPC and positive expression of AR-V7. CRPC, castration-resistant prostate cancer.
Sensitivity analysis
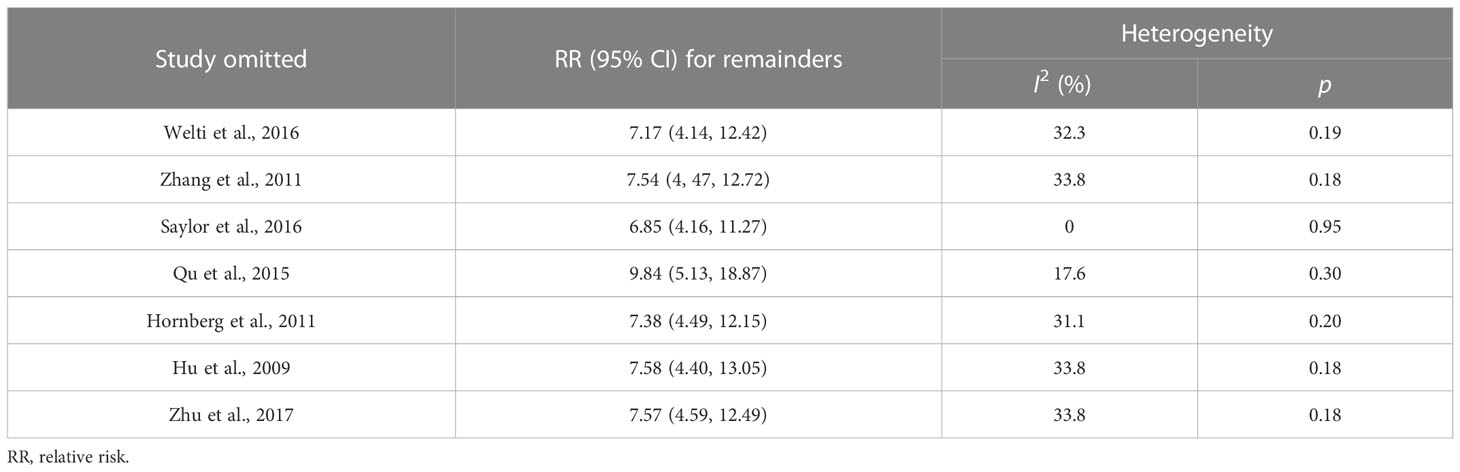
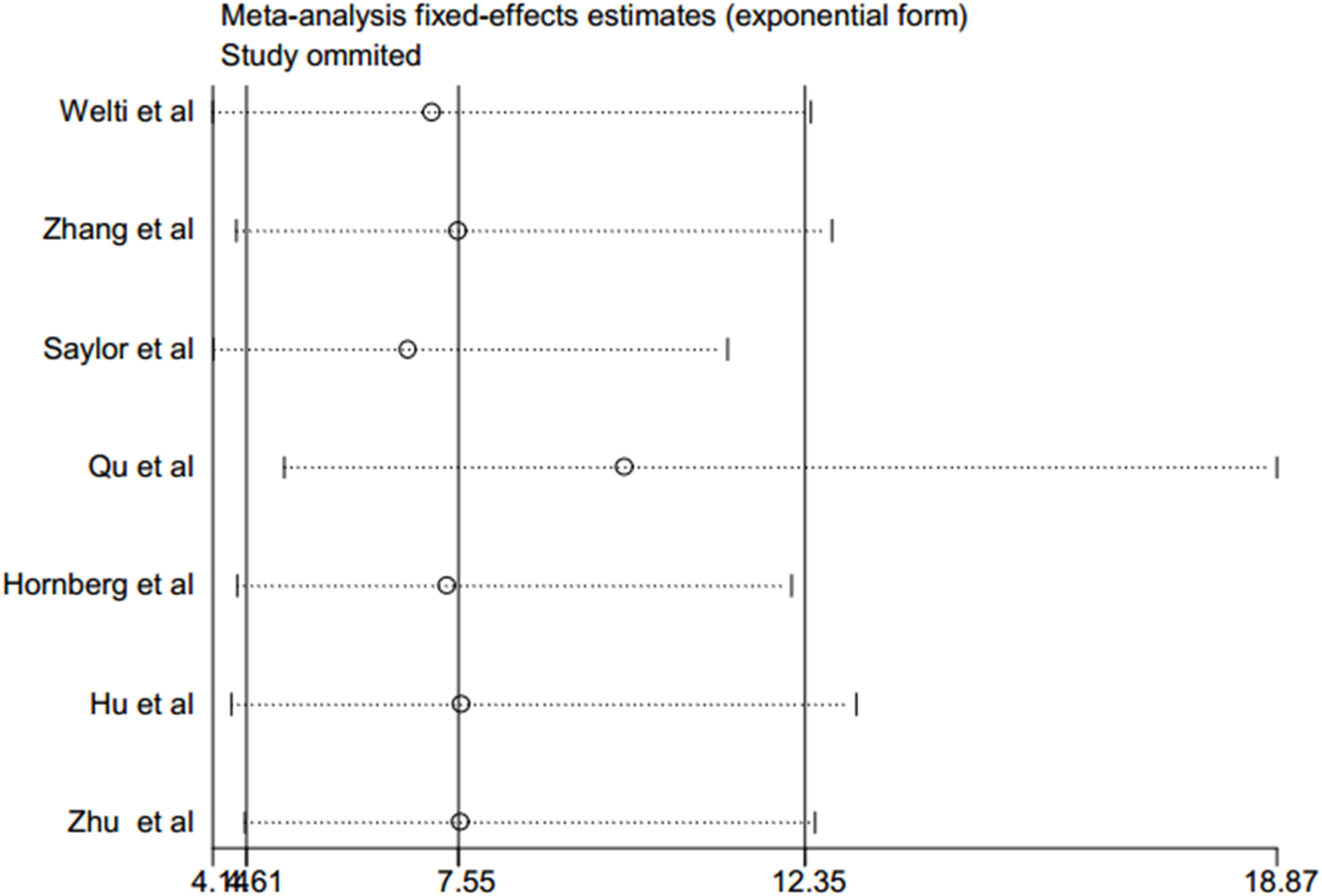
A sensitivity analysis of this meta-analysis was conducted to evaluate its reliability with regard to the association between CRPC and positive expression of AR-V7. Each of the individual studies was excluded in turn to recalculate the synthesized RR. The overall pooled return on investment did not change substantially, with a range from 6.85 (95% CI: 4.16–11.27, p < 0.001) to 9.84 (95% CI: 5.13–18.87, p < 0.001) after eliminating any one of the included studies (Table 2 and Figure 3). These results suggested that no single study dominated the combined RR, which strengthened the evidence of this study.
Subgroup analyses
To further obtain the potential relationship between CRPC and the positive expression of AR-V7, subgroup analyses were performed according to the methods of AR-V7 detection, the geographical region, and the publication year (Table 3). In the subgroup analysis according to the methods of AR-V7 detection, a stronger association was detected in the RNA in situ hybridization (RISH) group (RR = 39.81, 95% CI: 4.90–323.51, p < 0.001) compared with the IHC group (RR = 6.72, 95% CI: 3.83–11.81, p < 0.001) and PCR and protein analysis group (RR = 7.41, 95% CI: 2.33–23.56, p < 0.001). Moreover, in the subgroup analysis according to the geographical region, a statistically significant association between CRPC and positive expression of AR-V7 could be observed in the UK, the USA, and China, with RR (95% CIs) of 9.29 (3.06–28.20, p < 0.001), 10.09 (4.21–24.18, p < 0.001), and 5.30 (2.50–11.24, p < 0.001), respectively. However, a similar association was not identified in Sweden (RR = 17.94, 95% CI: 0.78–411.52, p > 0.05). Finally, when further stratified by publication years 2009–2011 and 2012–2017, the RR of CRPC associated with positive AR-V7 expression was 8.00 (95% CI: 3.35–19.13, p < 0.001) and 7.34 (95% CI: 4.04–13.33, p < 0.001), respectively.
Publication bias
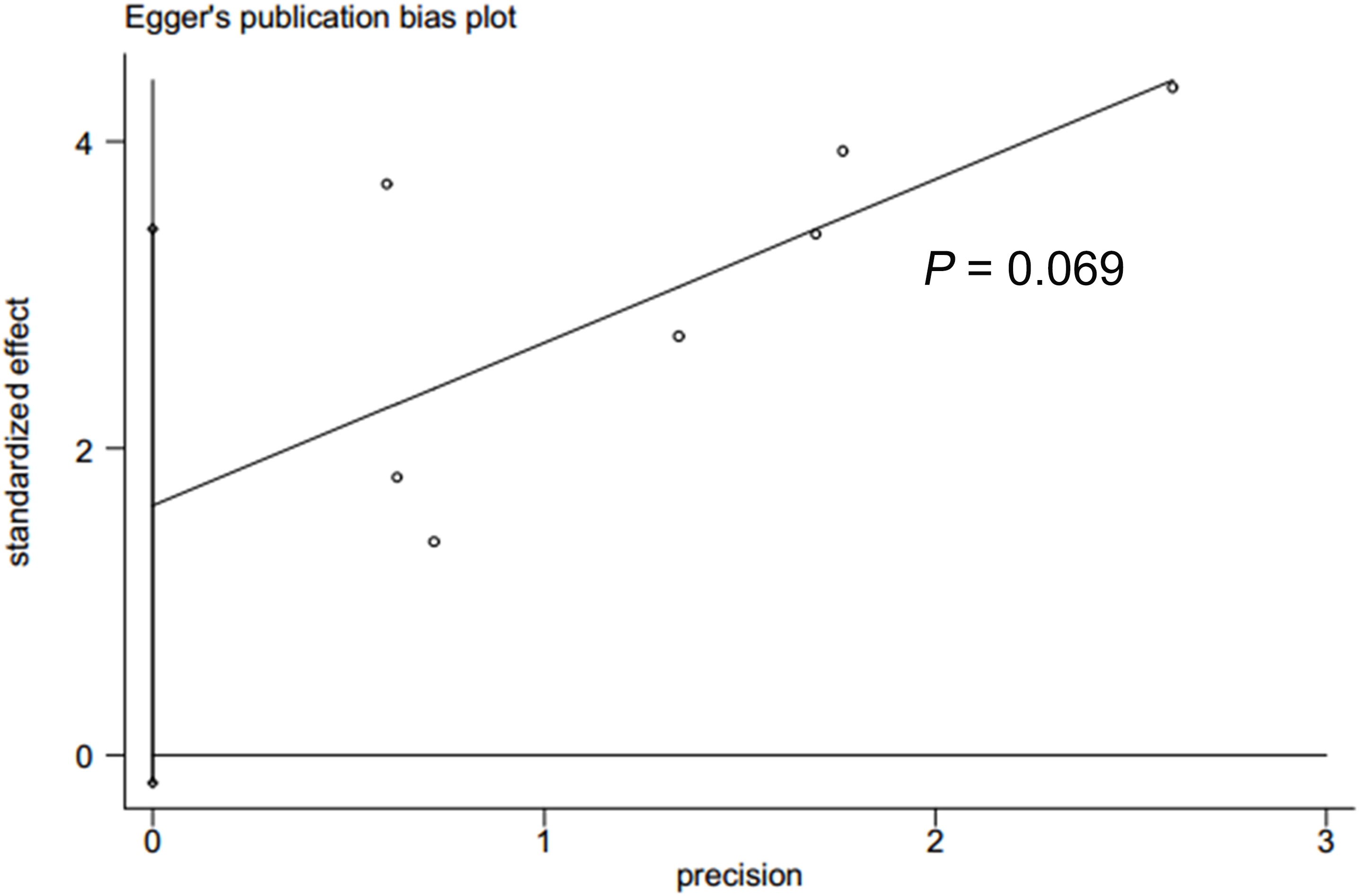
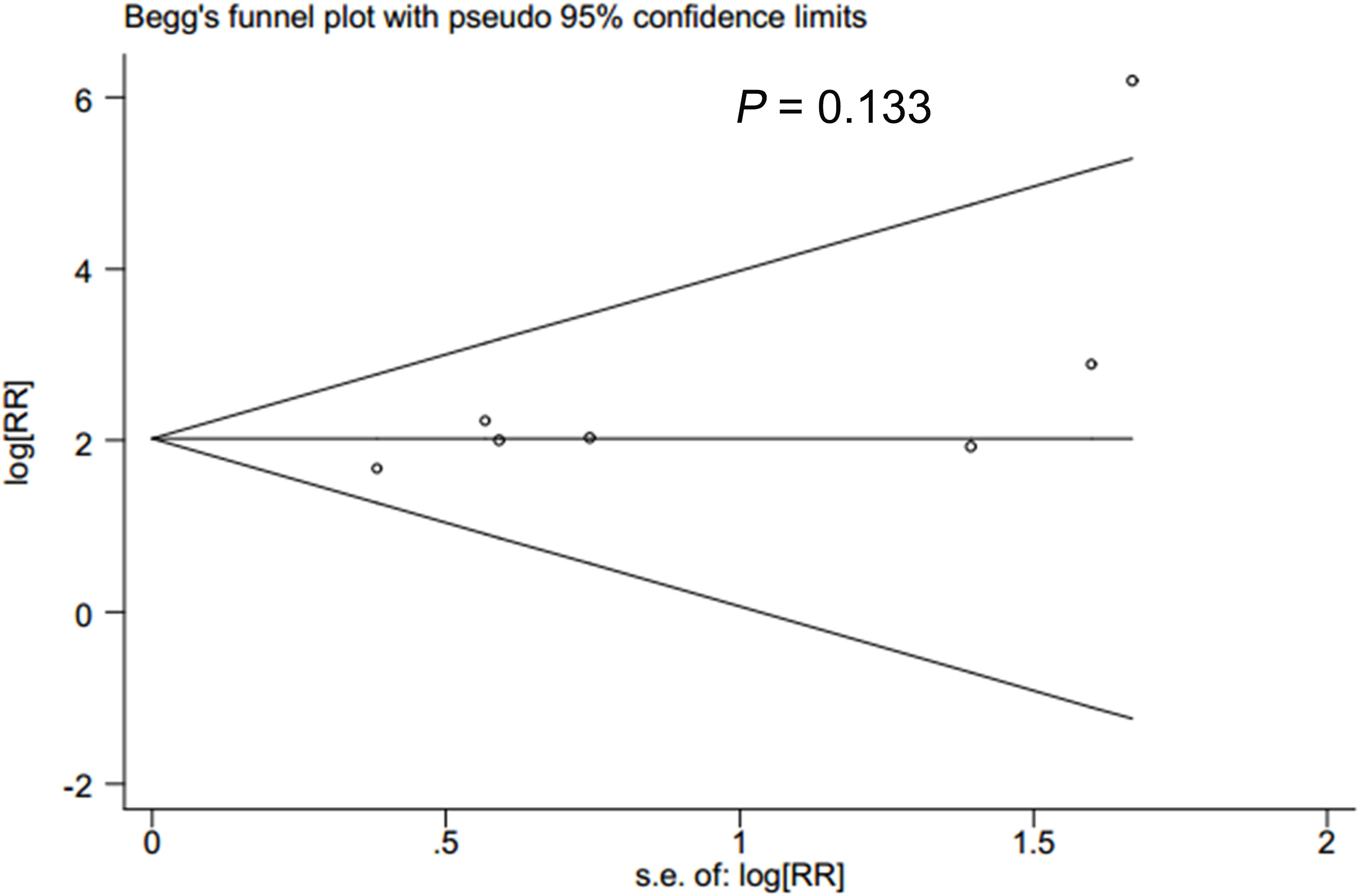
In accordance with Begg’s rank correlation analysis and Egger’s linear regression analysis, no publication bias was observed in the studies reviewed (Begg’s, p > |z| = 0.133; Egger, p > |t| = 0.069) (Figures 4, 5).
Discussion
Recently, numerous studies (36, 37) had speculated the relationship between CRPC and the positive expression of AR-V7. In this study, we summarized the evidence of the association between CRPC and positive AR-V7 expression. Based on data from the seven included studies, the present study suggested that CRPC patients have a significantly increased positive expression of AR-V7 than the individuals with HSPC. The results derived from this study were consistent with several previous clinical studies (23–25), which demonstrated that the expression of AR-V7 protein was more frequently identified in men with CRPC compared to those with HSPC. Furthermore, we also examined the impacts of potential confounders on our findings by performing the subgroup analyses. A stronger association was detected in RISH measurement in American patients, and those studies were published before 2011 (all p < 0.001). Based on the sensitivity analyses, the positive association between CRPC and AR-V7 remained significant in nearly all of the included studies.
A majority of tumors eventually progress to CRPC after a period of an initial response to the ADT regimen (38). The underlying mechanism for the development and progression of CRPC is the expression of AR-Vs, particularly AR-V7, which is a popular area of related research. In ligand-free forms of AR-V7, ligand-binding domains are absent, but transcriptional element binding domains are preserved, facilitating intracellular AR signaling even in the absence of androgens or antiandrogens (39). It is capable of translocating into nuclei and binding AR-responsive elements without ligand interaction and regulating gene transcription (32).
Accumulating evidence has demonstrated that there is a correlation between the level of AR-V7 and resistance to both abiraterone and enzalutamide, as well as poor survival in CRPC (40). In a previous study developed by Seitz et al. (41), 85 CRPC patients were under abiraterone (n = 56) or enzalutamide (n = 29) treatments. High AR-V7 expression levels were associated with shorter prostate-specific antigen (PSA)–progression-free survival (PSA-PFS) (median, 2.4 vs. 3.7 months; p < 0.001), shorter clinical progression-free survival (median, 2.7 vs. 5.5 months; p < 0.001), and shorter overall survival (median, 4.0 vs. 13.9 months; p < 0.001). Moreover, Todenhöfer et al. (42) found that AR-V7 transcripts in peripheral blood were significantly associated with a shorter median PSA-PFS (0.7 vs. 4.0 months, p < 0.001) and median overall survival (5.5 vs. 22.1 months, p < 0.001) in CRPC patients treated with abiraterone. This association was confirmed in another clinical study that demonstrated that patients with positive expression of AR-V7 had a poor prognosis than those with negative AR-V7 expression in CRPC treatment with either enzalutamide or abiraterone (43).
Preclinical studies have shown that expression of AR-V7 protein might be a treatment-specific biomarker in CRPC (44). AR-V7-positive patients would benefit from taxanes more than those managed with androgen receptor signaling inhibitors (ARSIs). Antonarakis et al. (45) reported that PSA responses were higher in AR-V7-positive CRPC patients. Moreover, PSA-PFS and clinical and/or radiographic PFS were significantly longer in taxane-treated than those under enzalutamide or abiraterone treatments. However, the outcomes turned out to be non-significant different when the therapy type was changed to AR-V7-negative CRPC. Similar to their results, Scher et al. (46) observed that circulating tumor cell expression of AR-V7 protein in CRPC patients was correlated with superior survival on taxane therapy over ARSI-directed therapy. This evidence suggested the presence of AR-V7 was associated with a better clinical outcome for patients treated with taxanes when compared to those under enzalutamide or abiraterone therapies. Based on the above studies, detecting the AR-V7 expression might be useful to serve as a therapeutic biomarker in patients with CRPC.
In this study, the AR-V7 expression in CRPC patients was found to be significantly ascending as compared with that of patients with HSPC. Moreover, on the basis of the findings in previous studies, the AR-V7 expression might be correlated to resistance to abiraterone and enzalutamide treatment but not taxane chemotherapy. Thus, CRPC patients with positive expression of AR-V7 are recommended to be treated with taxane rather than ARS inhibitors. Accordingly, the AR-V7 status should be evaluated when managing patients with CRPC in clinical practice.
It was speculated that the possible mechanism of progression from HSPC (also known as castration-sensitive prostate cancer (CSPC)) to CRPC is the existence of AR splice variants. AR-V1 was the most common AR-V in hormone-naïve PCa, while AR-V7 was the most common during ADT and in the CRPC stage (47). However, several other variants also have been found to play roles in this action. AR-Vs can be categorized into the following four groups depending on their nuclear localization ability: ligand stimulated in a similar manner to canonical full-length AR (AR-FL) (i.e., AR-23), constitutively active (i.e., AR-V3, 4, 7, and 12), conditionally active (i.e., AR45, AR-V1, and 9), and inactive (i.e., AR-V13, 14, and AR8) (48, 49). For example, in addition to AR-V7, AR-V3, AR-V7, and AR-V9 were also found to be co-expressed in the metastases of CRPC (50). Interestingly, the mRNA of expression of these variants (including AR-V7) was also detected in benign prostatic hyperplasia (BPH) and hormone-naïve primary tumors with lower abundance and frequency (51). De Laere et al. assessed 30 circulating tumor cell (CTC) samples from 26 metastatic CRPC (mCRPC) patients, and 15/26 (57.7%) patients were AR-V-positive with AR-V7 being the most frequently detected variant (12/15, 80%), followed by AR-V3 (11/15, 73%), AR45 (10/15, 67%), AR-V9 (6/15, 40%), AR-V1 (5/15, 30%), AR-V2 (3/15, 20%), and AR-V5 (3/15, 20%) (52). However, a subsequent study (53) developed by To et al. showed that AR-V7 or AR-V9 expression does not predict outcomes in mCRPC patients receiving abiraterone or enzalutamide by conducting a whole blood assay. Nevertheless, based on outcomes from the majority of the previous relevant studies and the meta-analysis of our, AR-V7 was found to be associated with the development and progression of CRPC. At present, most investigators are focusing on AR-V7, but the clinical importance of the detection of other AR-Vs in CRPC is largely unknown, and it deserves further research. According to the above evidence, the specific role of AR-V7 in CRPC is still controversial among different studies. In addition to AR-V7, the expression of several other variants should also be evaluated in both blood and tissues when making a decision on CRPC patients.
In addition to distinct demographic characteristics (i.e., race, sample size, and age), different disease states, and the diverse treatments of CRPC or CSPC, it should be known that various detection methods and measurements for assessing the AR-V7 might also play a role in the outcomes among different studies. For example, Li and colleagues (54) showed that 21% (64/310) of the patients with metastatic CSPC had positive AR-V7 immunohistochemical staining on diagnostic biopsies using clone EPR15656 from Abcam. However, a more recent study (4) reported by Sowalsky et al. demonstrated that AR-V7 mRNA and protein abundance was low in CSPC prior to treatment using robustly validated assays applying both IHC and RNA sequencing-based approaches. The teams previously showed that AR-V7 protein was rarely expressed (<1%) in primary PC but is frequently detected (75% of cases) following androgen deprivation therapy (55). The authors, as have others, also demonstrated that AR-V7 mRNA was commonly identified in benign prostate and primary prostate cancer tissues (4, 51). At present, the majority of the studies were conducted in the metastatic CRPC setting. Our meta-analysis has confirmed the positive relationship between a high level of AR-V7 and the risk of CRPC. However, the clinical importance of AR-V7 detection in HSPC is rarely reported. In HSPC/CSPC research, Li et al. and Sowalsky et al. presented conflicting results about the expression of AR-V7 in CSPC. The following factors may cause this inconsistency: 1) the participants: Li et al. investigated Chinese metastatic HSPC (mHSPC) patients receiving ADT, while Sowalsky et al. investigated US and UK HSPC patients with high-risk localized prostate cancer treated with ADT plus enzalutamide prior to surgery (that is, non-metastatic). 2) The antibody: the AR-V7 antibody used in their studies was different (Li used clone EPR15656 from Abcam; Sowalsky used clone RM7 from RevMAb and Abcam clone EPR15656). 3) The assessment methods for IHC: diverse dilution rate (data were not available) and the cutoff were used for defining “positive or negative” samples. Thus, in the future, evidence for the potential link between AR-V7 and CSPC development may be enhanced if the detection methods are under the same condition. In the CRPC setting, the positive association between AR-V7 and CRPC seems to be a little controversial.
The relationship between AR-V7 and CRPC has been extensively investigated, but whether AR-V7 itself or the ratio of full-length (FL) AR and AR-V7 plays a key role in CRPC remains to be resolved. It is common for patients with CRPC to co-express the full-length receptor and spliced variant AR-V7 (56). After hormone-sensitive PCa progression into CRPC, the expression of full-length AR and AR-V7 increases. AR splice variants exert activating effects in DNA repair genes similar to full-length androgen receptors. However, even in the absence of AR-FL, AR splice variants can provide the necessary transcriptional support for DNA repair genes. AR-Vs have mRNA sequences that are structurally different from the canonical full-length AR. The ARV-7 preferentially leads to the expression of cell cycle regulatory genes, while the full-length AR represses that program and favors instead genes related to metabolism, differentiation, and macromolecular synthesis. Blocking AR-FL using antiandrogens has been shown to retain AR-V activity (47). It was reported that the antibody used for the detection of AR-V7 has high specificity with no cross-reaction to full-length AR (57). In the study of Li et al., mHSPC patients were AR-FL-positive, and 64 (21%) were AR-V7-positive (54).
However, it was reported that AR-V7 could activate target genes irrespective of AR-FL, leading to the development and growth of prostate cancer under low androgen levels (58). AR-V7 are already located at a high fraction in the nuclei of primary PCA cells, while AR-FL remains cytoplasmatic in the absence of activating ligands (59). AR-FL and many AR-Vs may share common chromatin binding sites, genomic binding sites, and transcriptional programs specific to AR-V7. Although AR-V7 co-exists with AR-FL, genomic functions mediated by AR-V7 do not require the presence of AR-FL. AR-V7 expression was found to be lower than AR-FL in CRPC clinical samples (33). AR-V7 is negatively regulated by androgen signaling mediated by AR-FL (60). Detection of AR-V7 indicates the resistance of CRPC to AR signaling inhibitors (43). It was reported that dihydrotestosterone treatment results in a greater decrease in the AR-V7 expression than AR-FL, indicating a mechanism that preferentially reduces the expression of AR-V7 (58). It was also reported that AR-V7 and the AR-V7/AR-FL ratio increase as the disease progresses from CSPC to CRPC (18). Moreover, high nuclear AR-V7 expression and high nuclear AR-V7/AR-FL ratio were associated with a shorter biochemical recurrence-free survival of PCa (59). Among the CRPC patients before their treatment with abiraterone or enzalutamide, positive AR-V7 detection, but not higher AR-FL was significantly associated with shorter PSA-PFS (35). Based on this evidence, both AR-V7 itself and the AR-V7/AR-FL ratio play important roles in CRPC development, but AR-V7 plays a central functional role in this action. However, studies on the relationship between the AR−V7/AR−FL ratio and CRPC are insufficient; whether AR-V7/AR-FL forms heterodimers under low androgen conditions needs further investigation.
Our study is a high-quality cumulative analysis that was conducted following the PRISMA statement. However, some non-negligible limitations should be acknowledged when interpreting the outcomes derived from this study. First, heterogeneity was unavoidable among different studies, even though the potential causes of heterogeneity were identified by sensitivity analysis and subgroup analyses. The effects of small sample studies might be problematic in these meta-analyses, which resulted in the exaggeration of the summary estimates. Second, the various methods of assessment for AR-V7 among studies might also be one of the limitations of this study. Different kinds of assessments for AR-V7 may have a huge impact on the pooled analysis. Therefore, diverse measurements for detecting the AR-V7 expression might be another source of heterogeneity.
Conclusion
Our study demonstrated that the positive expression of AR-V7 was significantly higher in patients with CRPC than that in those with HSPC. To improve clinical prognostic, the level of AR-V7 should be evaluated when clinicians determined the preferred treatments for CRPC. However, the present evidence was based on retrospective clinical trials with limited included studies. Therefore, further high-quality, large sample size and multicenter cohort studies are still warranted to validate our findings. At present, the predictive effects of the AR-V7 expression in CSPC/HSPC are controversial among studies, and this needs to be addressed in the near future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
ShaZ: project development and manuscript writing. JL and MS: data collection. XL: data analysis. LZ and ShiZ: manuscript editing. All authors contributed to the article and approved the submitted version.
Funding
The present study was supported by the Natural Science Foundation of Zhejiang Province (No. LQ22H040009), the Health Science and Technology Program (No. 2022RC297), the National Natural Science Foundation of China (No. 82060007).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1053111/full#supplementary-material
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Bogemann M, Shore ND, Smith MR, Tammela T, Ulys A, Vjaters E, et al. Efficacy and safety of darolutamide in patients with nonmetastatic castration-resistant prostate cancer stratified by prostate-specific antigen doubling time: Planned subgroup analysis of the phase 3 ARAMIS trial. Eur Urol (2022) S0302-2838(22):02532–5. doi: 10.1016/j.eururo.2022.07.018
3. Jamroze A, Chatta G, Tang DG. Androgen receptor (AR) heterogeneity in prostate cancer and therapy resistance. Cancer Lett (2021) 518:1–9. doi: 10.1016/j.canlet.2021.06.006
4. Sowalsky AG, Figueiredo I, Lis RT, Coleman I, Gurel B, Bogdan D, et al. Assessment of androgen receptor splice variant-7 as a biomarker of clinical response in castration-sensitive prostate cancer. Clin Cancer Res (2022) 28(16):3509–25. doi: 10.1158/1078-0432.CCR-22-0851
5. El KS, Dubois V, van Royen ME, Houtsmuller AB, Pavlova E, Atanassova N, et al. The androgen receptor depends on ligand-binding domain dimerization for transcriptional activation. EMBO Rep (2021) 22(12):e52764. doi: 10.15252/embr.202152764
6. Ozturan D, Morova T, Lack NA. Androgen receptor-mediated transcription in prostate cancer. Cells (2022)11(5):898. doi: 10.3390/cells11050898
7. Lin E, Garmo H, Van Hemelrijck M, Zethelius B, Stattin P, Hagstrom E, et al. Association of gonadotropin-releasing hormone agonists for prostate cancer with cardiovascular disease risk and hypertension in men with diabetes. JAMA Netw Open (2022) 5(8):e2225600. doi: 10.1001/jamanetworkopen.2022.25600
8. Saad F, de Bono J, Barthelemy P, Dorff T, Mehra N, Scagliotti G, et al. Patient-reported outcomes in men with metastatic castration-resistant prostate cancer harboring DNA damage response alterations treated with talazoparib: Results from TALAPRO-1. Eur Urol (2022) S0302-2838(22):02407–1. doi: 10.1016/j.eururo.2022.05.030
9. Zhou J, Wang Y, Wu D, Wang S, Chen Z, Xiang S, et al. Orphan nuclear receptors as regulators of intratumoral androgen biosynthesis in castration-resistant prostate cancer. Oncogene (2021) 40(15):2625–34. doi: 10.1038/s41388-021-01737-1
10. Coutinho I, Day TK, Tilley WD, Selth LA. Androgen receptor signaling in castration-resistant prostate cancer: A lesson in persistence. Endocr Relat Cancer (2016) 23(12):T179–97. doi: 10.1530/ERC-16-0422
11. Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol (2008) 26(28):4563–71. doi: 10.1200/JCO.2007.15.9749
12. Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: A phase 1-2 study. Lancet (2010) 375(9724):1437–46. doi: 10.1016/S0140-6736(10)60172-9
13. Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res (2001) 61(9):3550–5.
14. Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med (1995) 332(21):1393–8. doi: 10.1056/NEJM199505253322101
15. Gregory CW, Fei X, Ponguta LA, He B, Bill HM, French FS, et al. Epidermal growth factor increases coactivation of the androgen receptor in recurrent prostate cancer. J Biol Chem (2004) 279(8):7119–30. doi: 10.1074/jbc.M307649200
16. Guo Z, Dai B, Jiang T, Xu K, Xie Y, Kim O, et al. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell (2006) 10(4):309–19. doi: 10.1016/j.ccr.2006.08.021
17. Lopez J, Anazco-Guenkova AM, Monteagudo-Garcia O, Blanco S. Epigenetic and epitranscriptomic control in prostate cancer. Genes (Basel) (2022) 13(2):378. doi: 10.3390/genes13020378
18. Kanayama M, Lu C, Luo J, Antonarakis ES. AR splicing variants and resistance to AR targeting agents. Cancers (Basel) (2021) 13(11):2563. doi: 10.3390/cancers13112563
19. Wach S, Taubert H, Cronauer M. Role of androgen receptor splice variants, their clinical relevance and treatment options. World J Urol (2020) 38(3):647–56. doi: 10.1007/s00345-018-02619-0
20. Chen Y, Lan T. Molecular origin, expression regulation, and biological function of androgen receptor splicing variant 7 in prostate cancer. Urol Int (2021) 105(5-6):337–53. doi: 10.1159/000510124
21. Thomas R, Jerome JM, Dang TD, Souto EP, Mallam JN, Rowley DR. Androgen receptor variant-7 regulation by tenascin-c induced src activation. Cell Commun Signal (2022) 20(1):119. doi: 10.1186/s12964-022-00925-0
22. Zhang T, Karsh LI, Nissenblatt MJ, Canfield SE. Androgen receptor splice variant, AR-V7, as a biomarker of resistance to androgen axis-targeted therapies in advanced prostate cancer. Clin Genitourin Cancer (2020) 18(1):1–10. doi: 10.1016/j.clgc.2019.09.015
23. Welti J, Rodrigues DN, Sharp A, Sun S, Lorente D, Riisnaes R, et al. Analytical validation and clinical qualification of a new immunohistochemical assay for androgen receptor splice variant-7 protein expression in metastatic castration-resistant prostate cancer. Eur Urol (2016) 70(4):599–608. doi: 10.1016/j.eururo.2016.03.049
24. Zhang X, Morrissey C, Sun S, Ketchandji M, Nelson PS, True LD, et al. Androgen receptor variants occur frequently in castration resistant prostate cancer metastases. PloS One (2011) 6(11):e27970. doi: 10.1371/journal.pone.0027970
25. Saylor PJ, Lee RJ, Arora KS, Deshpande V, Hu R, Olivier K, et al. In situ hybridization for androgen receptor splice variant AR-V7 as a prognostic biomarker for metastatic castration-sensitive prostate cancer. Clin Cancer Res (2017) 23(2):363–9. doi: 10.1158/1078-0432.CCR-16-0237
26. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
27. Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: A tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol (2003) 3:25. doi: 10.1186/1471-2288-3-25
28. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
29. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
30. Shahjouei S, Li J, Koza E, Abedi V, Sadr AV, Chen Q, et al. Risk of subsequent stroke among patients receiving outpatient vs inpatient care for transient ischemic attack: A systematic review and meta-analysis. JAMA Netw Open (2022) 5(1):e2136644. doi: 10.1001/jamanetworkopen.2021.36644
31. Lopes RB, Bernal-Cordoba C, Fausak ED, Silva-Del-Rio N. Effect of prebiotics on growth and health of dairy calves: A protocol for a systematic review and meta-analysis. PloS One (2021) 16(6):e0253379. doi: 10.1371/journal.pone.0253379
32. Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res (2009) 69(1):16–22. doi: 10.1158/0008-5472.CAN-08-2764
33. Qu Y, Dai B, Ye D, Kong Y, Chang K, Jia Z, et al. Constitutively active AR-V7 plays an essential role in the development and progression of castration-resistant prostate cancer. Sci Rep (2015) 5:7654. doi: 10.1038/srep07654
34. Hornberg E, Ylitalo EB, Crnalic S, Antti H, Stattin P, Widmark A, et al. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PloS One (2011) 6(4):e19059. doi: 10.1371/journal.pone.0019059
35. Zhu Y, Sharp A, Anderson CM, Silberstein JL, Taylor M, Lu C, et al. Novel junction-specific and quantifiable In situ detection of AR-V7 and its clinical correlates in metastatic castration-resistant prostate cancer. Eur Urol (2018) 73(5):727–35. doi: 10.1016/j.eururo.2017.08.009
36. Ashizawa T, Nagata M, Nakamura S, Hirano H, Nagaya N, Lu Y, et al. Efficacy of cabazitaxel and androgen splicing variant-7 status in circulating tumor cells in Asian patients with metastatic castration-resistant prostate cancer. Sci Rep (2022) 12(1):18016. doi: 10.1038/s41598-022-22854-1
37. Sugiura M, Sato H, Okabe A, Fukuyo M, Mano Y, Shinohara KI, et al. Identification of AR-V7 downstream genes commonly targeted by AR/AR-V7 and specifically targeted by AR-V7 in castration resistant prostate cancer. Transl Oncol (2021) 14(1):100915. doi: 10.1016/j.tranon.2020.100915
38. Laudato S, Aparicio A, Giancotti FG. Clonal evolution and epithelial plasticity in the emergence of AR-independent prostate carcinoma. Trends Cancer (2019) 5(7):440–55. doi: 10.1016/j.trecan.2019.05.008
39. Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res (2008) 68(13):5469–77. doi: 10.1158/0008-5472.CAN-08-0594
40. Di Lorenzo G, Zappavigna S, Crocetto F, Giuliano M, Ribera D, Morra R, et al. Assessment of total, PTEN(-), and AR-V7(+) circulating tumor cell count by flow cytometry in patients with metastatic castration-resistant prostate cancer receiving enzalutamide. Clin Genitourin Cancer (2021) 19(5):e286–98. doi: 10.1016/j.clgc.2021.03.021
41. Seitz AK, Thoene S, Bietenbeck A, Nawroth R, Tauber R, Thalgott M, et al. AR-V7 in peripheral whole blood of patients with castration-resistant prostate cancer: Association with treatment-specific outcome under abiraterone and enzalutamide. Eur Urol (2017) 72(5):828–34. doi: 10.1016/j.eururo.2017.07.024
42. Todenhofer T, Azad A, Stewart C, Gao J, Eigl BJ, Gleave ME, et al. AR-V7 transcripts in whole blood RNA of patients with metastatic castration resistant prostate cancer correlate with response to abiraterone acetate. J Urol (2017) 197(1):135–42. doi: 10.1016/j.juro.2016.06.094
43. Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med (2014) 371(11):1028–38. doi: 10.1056/NEJMoa1315815
44. Stone L. Prostate cancer: AR-V7 status in CTCs is a treatment-specific biomarker. Nat Rev Urol (2016) 13(8):433. doi: 10.1038/nrurol.2016.113
45. Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Nakazawa M, et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol (2015) 1(5):582–91. doi: 10.1001/jamaoncol.2015.1341
46. Scher HI, Lu D, Schreiber NA, Louw J, Graf RP, Vargas HA, et al. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol (2016) 2(11):1441–9. doi: 10.1001/jamaoncol.2016.1828
47. Tolkach Y, Kremer A, Lotz G, Schmid M, Mayr T, Forster S, et al. Androgen receptor splice variants contribute to the upregulation of DNA repair in prostate cancer. Cancers (Basel) (2022) 14(18):4441. doi: 10.3390/cancers14184441
48. Lu C, Luo J. Decoding the androgen receptor splice variants. Transl Androl Urol (2013) 2(3):178–86. doi: 10.3978/j.issn.2223-4683.2013.09.08
49. Lu C, Brown LC, Antonarakis ES, Armstrong AJ, Luo J. Androgen receptor variant-driven prostate cancer II: Advances in laboratory investigations. Prostate Cancer Prostatic Dis (2020) 23(3):381–97. doi: 10.1038/s41391-020-0217-3
50. Kallio H, Hieta R, Latonen L, Brofeldt A, Annala M, Kivinummi K, et al. Constitutively active androgen receptor splice variants AR-V3, AR-V7 and AR-V9 are co-expressed in castration-resistant prostate cancer metastases. Br J Cancer (2018) 119(3):347–56. doi: 10.1038/s41416-018-0172-0
51. He MX, Cuoco MS, Crowdis J, Bosma-Moody A, Zhang Z, Bi K, et al. Transcriptional mediators of treatment resistance in lethal prostate cancer. Nat Med (2021) 27(3):426–33. doi: 10.1038/s41591-021-01244-6
52. De Laere B, van Dam PJ, Whitington T, Mayrhofer M, Diaz EH, Van den Eynden G, et al. Comprehensive profiling of the androgen receptor in liquid biopsies from castration-resistant prostate cancer reveals novel intra-AR structural variation and splice variant expression patterns. Eur Urol (2017) 72(2):192–200. doi: 10.1016/j.eururo.2017.01.011
53. To SQ, Kwan EM, Fettke HC, Mant A, Docanto MM, Martelotto L, et al. Expression of androgen receptor splice variant 7 or 9 in whole blood does not predict response to androgen-axis-targeting agents in metastatic castration-resistant prostate cancer. Eur Urol (2018) 73(6):818–21. doi: 10.1016/j.eururo.2018.01.007
54. Li H, Zhang Y, Li D, Ma X, Xu K, Ding B, et al. Androgen receptor splice variant 7 predicts shorter response in patients with metastatic hormone-sensitive prostate cancer receiving androgen deprivation therapy. Eur Urol (2021) 79(6):879–86. doi: 10.1016/j.eururo.2021.01.037
55. Sharp A, Coleman I, Yuan W, Sprenger C, Dolling D, Rodrigues DN, et al. Androgen receptor splice variant-7 expression emerges with castration resistance in prostate cancer. J Clin Invest (2019) 129(1):192–208. doi: 10.1172/JCI122819
56. Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer (2015) 15(12):701–11. doi: 10.1038/nrc4016
57. Li H, Wang Z, Xiao W, Yan L, Guan W, Hu Z, et al. Androgen-receptor splice variant-7-positive prostate cancer: A novel molecular subtype with markedly worse androgen-deprivation therapy outcomes in newly diagnosed patients. Mod Pathol (2018) 31(1):198–208. doi: 10.1038/modpathol.2017.74
58. Yu Z, Chen S, Sowalsky AG, Voznesensky OS, Mostaghel EA, Nelson PS, et al. Rapid induction of androgen receptor splice variants by androgen deprivation in prostate cancer. Clin Cancer Res (2014) 20(6):1590–600. doi: 10.1158/1078-0432.CCR-13-1863
59. Chen X, Bernemann C, Tolkach Y, Heller M, Nientiedt C, Falkenstein M, et al. Overexpression of nuclear AR-V7 protein in primary prostate cancer is an independent negative prognostic marker in men with high-risk disease receiving adjuvant therapy. Urol Oncol (2018) 36(4):161.e19–161.e30. doi: 10.1016/j.urolonc.2017.11.001
60. Hu R, Lu C, Mostaghel EA, Yegnasubramanian S, Gurel M, Tannahill C, et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res (2012) 72(14):3457–62. doi: 10.1158/0008-5472.CAN-11-3892
Keywords: androgen receptor variant-7, castration-resistant prostate cancer, systematic review, cumulative analysis, expression
Citation: Zhao S, Liao J, Zhang S, Shen M, Li X and Zhou L (2023) The positive relationship between androgen receptor splice variant-7 expression and the risk of castration-resistant prostate cancer: A cumulative analysis. Front. Oncol. 13:1053111. doi: 10.3389/fonc.2023.1053111
Received: 25 September 2022; Accepted: 09 January 2023;
Published: 14 February 2023.
Edited by:
Yafeng Ma, Ingham Institute of Applied Medical Research, AustraliaReviewed by:
Di Gu, First Affiliated Hospital of Guangzhou Medical University, ChinaNagalakshmi Nadiminty, University of Toledo, United States
Copyright © 2023 Zhao, Liao, Zhang, Shen, Li and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Libo Zhou, zhoulibo37@163.com
†These authors share first authorship
‡ORCID: Libo Zhou, orcid.org/0000-0003-0107-3079
 Shankun Zhao
Shankun Zhao Jian Liao2†
Jian Liao2†