- 1Department of Pharmacy, University of Naples Federico II, Naples, Italy
- 2Department of Pharmacognosy and Biotechnology, School of Pharmacy, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 3Department of Experimental Oncology, National Cancer Institute -IRCCS “G.Pascale” Foundation, Naples, Italy
- 4Department of Pharmacy, University of Pisa, Pisa, Italy
Melanoma is the most common form of skin cancer. Given its high mortality, the interest in the search of preventive measures, such as dietary factors, is growing significantly. In this study we tested, in vitro and in vivo, the potential anti-cancer effect of the acetyl deacylasadisulfide (ADA), a vinyl disulfide compound, isolated and purified from asafoetida a foul-smelling oleo gum-resin of dietary and medicinal relevance. ADA markedly suppressed proliferation of human melanoma cell lines by inducing apoptosis. Moreover, treatment of melanoma cells with ADA reduced nuclear translocation and activation of NF-κB, decreased the expression of the anti-apoptotic proteins c-FLIP, XIAP, and Bcl-2 and inhibited the phosphorylation and activation of both AKT and ERK proteins, two of the most frequently deregulated pathways in melanoma. Finally, the results obtained in vitro were substantiated by the findings that ADA significantly and dose-dependently reduced lung metastatic foci formation in C57BL/6 mice. In conclusion, our findings suggest that ADA significantly inhibits melanoma progression in vivo and could represent an important lead compound for the development of new anti-metastatic agents.
Introduction
Hydrogen sulfide (H2S) is a gaseous signaling molecule that plays important roles in a variety of biological functions, in health and disease (Szabo, 2007; Kimura, 2011; Whiteman et al., 2011; Wang, 2012). The physiological production of H2S is mainly deputed to the activity of three enzymes: Cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST). Recently endogenous generated H2S has been involved in the regulation of cancer biological processes and, according to the cancer type, different roles can be ascribed to the molecule as recently reviewed (Szabo, 2016). In colorectal cancer, ovarian cancer, and breast cancer an increase in the expression of CBS in cancer cells has been associated to the promotion of cell proliferation and cellular bioenergetics (Hellmich and Szabo, 2015). In human melanoma, H2S generated by over-expressed CSE appears to be involved in the progression of the disease (Panza et al., 2015). While inhibition of H2S biosynthesis produces anticancer effects, many reports show that H2S donors, of either natural or chemical origin, exert anticancer actions in vitro and in vivo (Hellmich et al., 2015; Panza et al., 2015). Thus, it has been hypothesized that low (endogenous) levels of H2S tend to promote, while higher levels released from donors (exogenous), tend to inhibit cancer cell proliferation (Hellmich et al., 2015).
Ferula assa-foetida is the main source of asafoetida, an oleo-gum resin obtained by incision of the roots of various plants from the genus Ferula (family Umbelliferae) native to Central Asia, Afghanistan and Iran (Mahendra and Bisht, 2012). Asafoetida chiefly subsume resin (40–65%), gum (20–25%), and volatile oil (3–17%) the latter consisting of disulfides as its major components that are responsible for the characteristic odor of asafoetida (Iranshahy and Iranshahi, 2011). Asafoetida is a popular ingredient in the Indian cuisine and it is also used in traditional medicine for treating many human diseases such as asthma, gastro-intestinal disorders, influenza, and more recently, a cancer chemopreventive role for asafoetida has been described (Kim et al., 2011; Kiani et al., 2015; Oh et al., 2015). Different mechanisms seem to impact on this activity such as radical scavenging activity of sulfur-containing compounds even if the exact mechanism through which asafoetida behaves as ant-tumor agent has yet to be elucidated. In the present study, we have isolated and purified from asafoetida a new H2S-donor, ADA, studied and clarified its mechanism of action in vitro and demonstrated its anti-tumoral activity in vivo.
Methods
Plant Material and Isolation of Vinyl Disulfides
The latex of F. asafoetida was collected by incision of the root collar from plants growing in Jandagh (Isfahan, Iran) at an altitude of 1500 m above sea level. The plant material was identified by Dr. Mohammad-Reza Kanani, Department of Biology, Medicinal Plants and Drugs Research Institute, Shahid Beheshti University, Tehran, Iran, where a voucher specimen is kept (School of Pharmacy, Isfahan, No. 5729). The latex (100 g) was dried and extracted with acetone (2 × 1 L) for 2 days with continuous shaking to afford a gummy residue (30 g), that was fractionated by gravity column chromatography on silica gel and further purified by HPLC to obtain pure compounds as previously described (Shokoohinia et al., 2013).
Reagents and Cell Culture
Normal human epidermal melanocytes (NHEM) were purchased from Lonza (Walkersville, MD, USA) and were grown in Melanocyte growth medium 2 (Lonza). The melanoma cells lines B16/F10, Sk-Mel-5, and Sk-Mel-28 were purchased from IRCCS AOU San Martino—IST (Genova, Italy), A375 from Sigma-Aldrich (Milan, Italy), and WM3060 and WM983A were purchased from Rockland (Limerick, Ireland). B16/F10, Sk-Mel-5, Sk-Mel-28, and A375 were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, 2 mmol/L L-glutamine, 100 μmol/L non-essential amino acids, penicillin (100 U/mL), streptomycin (100 μg/mL), and 1 mmol/L sodium pyruvate (all from Sigma-Aldrich, Milan, Italy). WM3060 and WM983A were cultured in Tumor Specialized Media (1:5 Leibovitz's—MCDB153), containing 2% Inactivated FBS and 1.68 mM CaCl2. Cells were grown at 37°C in a humidified incubator under 5% CO2. The cell line PES 43 was isolated from a lung metastases of a patient from the National Cancer Institute, G. Pascale Foundation (Scala et al., 2006) and cultured in Iscove's modified Dulbecco's medium (Cambrex Bioscience, Verviers, Belgium) supplemented with heat-inactivated 10% fetal bovine serum, penicillin, and streptomycin (100 units/mL each). ADA was diluted in DMSO to produce a stock solution of 10 mM for in vitro experiments. All cell lines used in this study were characterized by the cell bank were they were purchased.
Proliferation Assay
Cell proliferation was measured by the 3-[4,5-dimethyltiazol-2yl]-2,5 diphenyl tetrazolium bromide (MTT) assay. The melanoma cells (A375, SK-Mel-5, SK-Mel-28, PES43, B16/F10, WM3060, and WM983A) and the NHEM cells were seeded on 96-well plates (1 × 104 cells/well) and treated with ADA or with the other compounds: Propionyl deacylasadisulfide (PDA); methoxylatifolone (MEF); foetisulfide A (FSA); arachyl deacylasadisulfide (ARDA); deacylasadisulfide (DA)]; (10-30-100 μM) for 24–48–72 h before adding 25 μl of MTT (Sigma, Milan, Italy) (5 mg/ml in saline). Cells were incubated for additional 3 h at 37°C. Thereafter, cells were lysed and dark blue crystals were solubilized with a solution containing 50% (vol/vol) N,N-dimethyl formamide, 20% (wt/vol) sodium dodecylsulfate with an adjusted pH of 4.5. The OD of each well was measured with a microplate spectrophotometer (TitertekMultiskan MCC/340) equipped with a 620-nm filter.
Amperometric Measurement of Hydrogen Sulfide Realease from ADA
The characterization of the potential H2S-generating properties of the tested compound ADA has been carried out by an amperometric approach, through the Apollo-4000 free radical analyzer (WPI) detector and H2S-selective mini-electrodes. The experiments have been carried out at room temperature (20°C). Following the manufacturer's instructions, a “PBS buffer 10x” was prepared (NaH2PO4·H2O 1.28 g, Na2HPO4.12H2O 5.97 g, NaCl 43.88 g in 500 ml H2O) and stocked at 4°C. Immediately before the experiments, the “PBS buffer 10x” was diluted using distilled water (1:10) to obtain the assay buffer and the pH adjusted to 7.4. The H2S-selective mini-electrode was equilibrated in 10 ml of the assay buffer, until the recovery of a stable baseline. Then, 100 μl of a DMSO solution of ADA was added (the final concentration of the tested compound was 100 μM; the final concentration of DMSO in the assay buffer was 1%). The eventual generation of H2S was observed for 20 min. Preliminary experiments demonstrated that DMSO 1% did not produce any interference on the amperometric recording. When required by the experimental protocol, the nucleophil L-cysteine (4 mM) was added 10 min before the addition of ADA. L-Cysteine did not produce any amperometric response. The correct relationship between the amperometric currents (recorded in pA) and the corresponding concentrations of H2S was previously determined by suitable calibration curves, which were obtained by the use of increasing concentrations of NaHS (1, 3, 5, and 10 μM) at pH 4.0.
Flow Cytometry
Apoptosis was detected with an Annexin V-FITC kit purchased from BD Pharmingen (San Diego, CA, USA) according to the manufacturer's instructions. PES 43 cells were seeded in 35 mm culture dishes and allowed to attach overnight. The cells were treated with ADA (100 μM) for 24–48–72 h, collected, and washed twice with PBS. Samples were then taken to determine baseline and drug-induced apoptosis by Annexin V-FITC/Propidium Iodide (PI) (Beckman Coulter; Brea, CA) double staining or PI staining and flow cytometry analysis using a FACSCanto II 6-color flow cytometer (Becton Biosciences, San Jose, CA), as described previously (Ianaro et al., 2009). To detect early and late apoptosis, both adherent and floating cells were harvested together and resuspended in annexin V binding buffer (10 mM HEPES/NaOH pH 7.4, 140 mM NaCl, 2.5 mM CaCl2) at a concentration of 106 cells/mL. Subsequently, 5 μL of FITC-conjugated Annexin V and 5 μL of PI were added to 100 μL of the cell suspension (105 cells). The cells were incubated for 15 min at room temperature in the dark. Finally, 400 μL of annexin V binding buffer was added to each tube. A minimum of 50000 events for each sample were collected and data were analyzed using FacsDiva software (Becton Biosciences).
Preparation of Cellular Extracts and Western Blot Analysis
PES 43 and A375 cells were treated with ADA 100 μM for 15–30–60'or for 3–6–24–48 h. Whole-cell or nuclear extracts were prepared as previously described (Ialenti et al., 2005; Panza et al., 2016). The protein concentration was measured by the Bradford method (Bio-Rad, Milan, Italy). Equal amounts of protein (40 μg/sample) from whole or nuclear cell extracts were separated by sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto a nitrocellulose membranes (Trans-Blot Turbo Transfer Starter System, Biorad). The membranes were blocked for 2 h in 5% low-fat milk in PBS with 0.1% Tween 20 (PBST) at room temperature. Then the filters were incubated with the following primary antibodies: IκBα (sc-1643 Cruz Biotechnology, Santa Cruz, CA; diluted 1:200); Bcl-2 (2876, Cell Signaling, USA; diluted 1:1000), caspase 3 (9662, Cell Signaling, USA; diluted 1:1000), PARP (9542, Cell Signaling, USA; diluted 1:1000), p44/42 MAPK (Erk1/2) (9102, Cell Signaling, USA; diluted 1:1000), Phospho-p44/42 Erk MAPK (Erk1/2, Thr202/Tyr204) XP (4370, Cell Signaling, USA; diluted 1:2000), AKT (9272, Cell Signaling, USA; diluted 1:1000), Phospho-AKT (Ser473) XP (4060, Cell Signaling, USA; diluted 1:2000), c-FLIP (06-864, Millipore; diluted 1 μg/ml); XIAP (R&D System, Minneapolis; 1 μg/ml); NF-κB p65 (F-6): (sc-8008 Santa Cruz Biotechnology, Santa Cruz, CA; diluted 1:200); β-actin (Santa Cruz Biotechnology, Santa Cruz, CA; diluted 1:1000), GAPDH (2118, Cell Signaling, USA; diluted 1:1000) overnight at 4°C. The membranes were washed 3 times with PBST and then incubated with horseradish peroxidase-conjugated antibodies (Santa Cruz Biotechnology, Santa Cruz, CA; diluted 1:2000) for 2 h at room temperature. The immune complexes were visualized by the ECL chemiluminescence method and acquired by the Image Quant 400 system (GE Healthcare).
Invasion Assay
The assay was performed using chambers with polycarbonate filters with 8-μm nominal pore size (Millipore, USA) coated on the upper side with Matrigel (Becton Dickinson Labware, USA) as previously described (Ivanov et al., 2003). Briefly, the chambers were placed into a 24-well plate and melanoma cells (2.5 × 105/mL) were plated in the upper chamber, with or without ADA (10–30 μM), in serum-free DMEM. After the incubation period (16 h), the filter was removed, and non-migrant cells on the upper side of the filter were detached with the use of a cotton swab. Filters were fixed with 4% formaldehyde for 15 min, and cells located in the lower filter were stained with 0.1% crystal violet for 20 min and then washed with PBS. The filters were examined microscopically and cellular invasion was determined by counting the number of stained cells on each filter in at least 4–5 randomly selected fields. Resultant data are presented as a mean of invaded cells ± SEM /microscopic field of three independent experiments.
Animals
The experimental procedures, according to Italian (DL 26/2014) and European (n. 63/2010/UE) regulations on the protection of animals used for experimental and other scientific purposes, were approved by the Italian Ministry. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). Mice were observed daily and humanely euthanized by CO2 inhalation if a solitary subcutaneous tumor exceeded 1.5 cm in diameter or mice showed signs referable to metastatic cancer. All efforts were made to minimize suffering. Female C57BL/6 mice (18−20 g) were purchased from Charles River Laboratories, Inc. Mice were housed at the Animal Research Facility of the Department of Pharmacy of the University of Naples Federico II.
Tumor Metastasis Assay
B16/F10 (5 × 105) murine melanoma cells were collected in PBS and injected via tail vein into syngeneic C57BL/6J mice. The mice were equally randomized into three groups (8 mice/group): 0.9% normal saline control group, ADA 5 mg·kg−1 group, ADA 50 mg·kg−1 group. Starting on the 1st day after tumor cell inoculation, test compounds were given orally every day for 14 days. Then the mice were weighed and sacrificed. The lungs were removed and washed with PBS. The percentage of metastatic area was calculated using ImageJ software (ImageJ).
Data Analysis
Data are expressed as mean ± SEM of n experiments. Data were analyzed and presented using GRAPHPAD PRISM software (GraphPad). Significance was determined using Student's 2-tailed t-test. Results were considered significant at P-values ≤ 0.05 and are labeled with a single asterisk. In addition, P-values ≤ 0.01 and 0.001 are designated with double and triple asterisks, respectively.
Results
ADA Suppresses the Proliferation of Human Melanoma Cells in Vitro
In preliminary experiments, the disulfide compounds obtained from asafoetida were evaluated for their anti-proliferative activity on human melanoma cells A375 (Figure 1). After an initial screening, we found that all the sulfur-containing compounds ADA, PDA, FSA, ARDA, and DA inhibited the proliferation of melanoma cells although with different potency (Figure 1). On the other hand, the structurally unrelated MEF was practically inactive. Therefore, the disulfide group is essential for the anti-proliferative effect. Among the active compounds ADA and PDA showed similar activity on the human melanoma cell line A375 (IC50 51.4 and 54.6 μM, respectively). The selection of ADA for further investigation was based on the relative abundance of this metabolite in the plant, the higher availability for pharmacological evaluation as well as the presence of a clear concentration-related effect as compared to PDA.
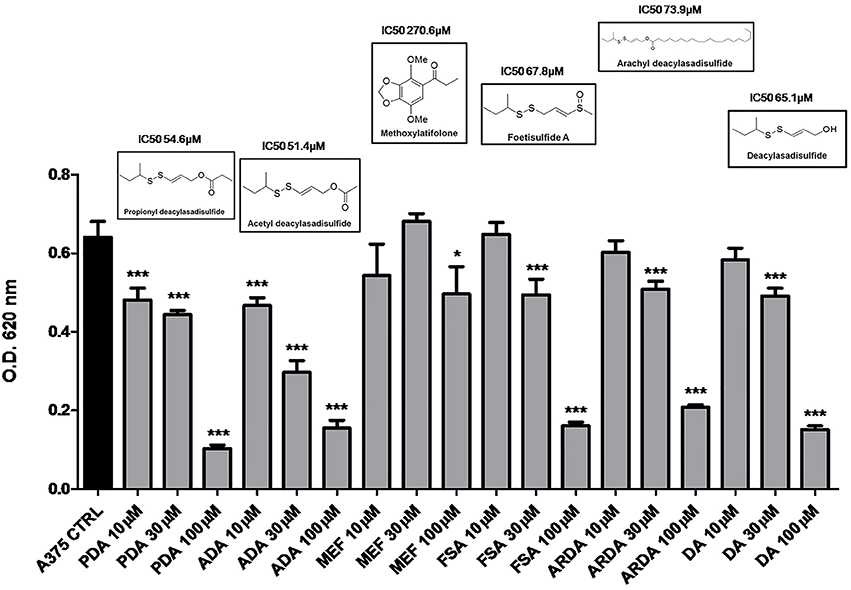
Figure 1. Disulfide compounds from asafetida inhibit the proliferation of human melanoma cells A375. A375 cells were treated with increasing concentration (10–30–100 μM) of several disulfide compounds (propionyl deacylasadisulfide, PDA; acetyl deacylasadisulfide, ADA; foetisulfide A, FSA; arachyl deacylasadisulfide, ARDA; deacylasadisulfide, DA) and one no sulfur-containing compound (methoxylatifolone, MEF). Growth inhibition was measured at 72 h using the MTT assay and is expressed as OD values. All the compounds tested, with the only exception of the MEF, inhibited the proliferation of melanoma cells although with different potency. Experiments were run in triplicate, each performed in quadruplicate (*P < 0.05; ***P < 0.001 vs. control [CTRL]).
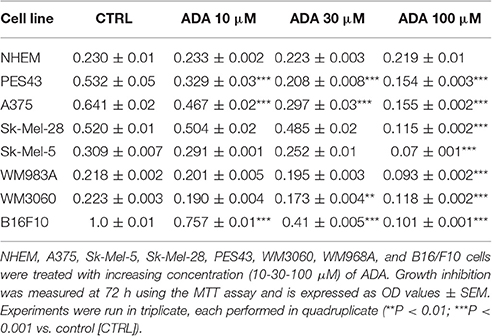
ADA also inhibited the proliferation of all the other human melanoma cell lines employed in this study, namely SK-Mel-5, SK-Mel-28, WM983A, and PES43 cell lines, all carrying the BRAFv600E mutation and the WM3060 cell line wild type for BRAF, without affecting NHEM proliferation (Table 1). A marked inhibition of cell proliferation was observed also in the murine cell line B16/F10 after incubation with increasing concentration of ADA (Table 1). Among the human cell lines employed the high metastatic PES43 and A375 were the most sensitive to the anti-proliferative activity of ADA. In fact, as shown in Table 1 increasing concentration of ADA (10-30-100 μM) inhibited PES43 proliferation at 72 h by 38, 61, and 71%, respectively (P < 0.001, n = 5) and A375 cell proliferation by 28, 54, and 76% respectively (P < 0.001, n = 5). Thus, these cell lines were selected for the molecular studies.
ADA Induces Apoptosis and Activation of Caspase-3 in Human Melanoma Cells
To verify if the anti-proliferative effect of ADA was related to its ability to induce apoptosis of cancer cells, the cytofluorimetric assay, with Annexin V/PI dual staining, was carried out on melanoma cells PES43 and A375. This dual staining distinguishes between unaffected (unlabeled; quadrant 3, Q3), early apoptotic (Annexin V positive; quadrant 4, Q4), late apoptotic (Annexin V positive, PI positive; quadrant 2, Q2), and necrotic (PI positive; quadrant 1, Q1) cells. Treatment of PES 43 and A375 cells for 24–48–72 h with ADA (100 μM) resulted in a significant and time–dependent induction of apoptosis (Figures 2A,B,D,E). The pro-apoptotic effect of ADA was confirmed by a time-dependent cleavage of caspase 3, the main effector caspase, and of its substrate poly (adenosine diphosphate-ribose) polymerase (PARP) (Figures 2C,F).
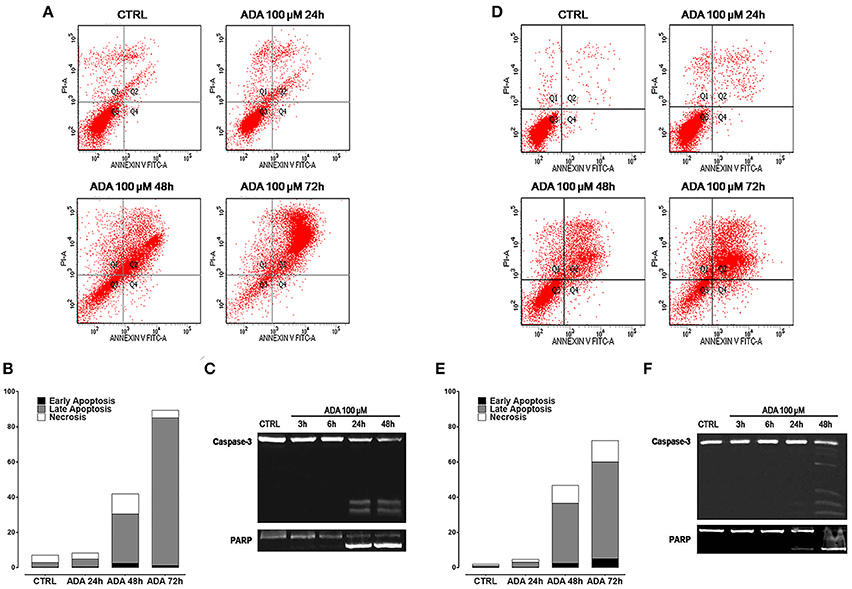
Figure 2. ADA induces apoptosis in metastatic melanoma cells PES43 and A375. PES43 and A375 cells were treated with ADA (100 μM) at different time points and apoptosis was determined by flow cytometry analysis. Apoptosis was determined by annexin V/propidium iodide (PI) staining, which detects the externalization of phosphatidylserine (PS). This dual staining distinguishes between unaffected cells (unlabeled; quadrant 3, Q3), early apoptotic cells (annexin V positive; quadrant 4, Q4), late apoptotic cells (annexin V positive, PI positive; quadrant 2, Q2), and necrotic (PI positive; quadrant 1, Q1). Treatment of PES43 cells (A) or A375 cells (D) for 24–48–72 h with ADA (100 μM) resulted in a time-dependent induction of apoptosis. (B,E) Quantitative analysis of apoptosis at various time points showing that at 72 h about 80% of PES43 cells (B) or A375 cells (E) treated with ADA exhibit markers of late apoptosis. Experiments (n = 5) were run in triplicate. (C,F) Western blot analysis of caspase 3 and PARP in PES43 (C) or A375 cells (F) whole-cell lysates. PES43 or A375 cells were incubated with ADA 100 μM at different time points and at 24 and 48 h cleavage of caspase 3 and of its substrate PARP was observed.
ADA Inhibits NF-κB Activation and Down-Regulates NF-κB Dependent Anti-Apoptotic Genes
It has been reported that in melanoma constitutive activation of NF-κB confers tumor survival capacity and apoptosis avoidance (Ueda and Richmond, 2006). In order to analyze if the inhibition of apoptosis induced by ADA was involving the NF-κB pathway, PES 43 and A375 cells were treated with ADA 100 μM for 15, 30, and 60 min and western blot analysis was carried out on cellular extracts. As shown in Figures 3A,E an inhibition of IκBα degradation at the earliest time points following ADA incubation was observed. This effect was abided by the inhibition of NF-κB nuclear translocation as demonstrated by a reduction in band intensity of the subunit p65 (Figures 3B,F). Finally, western blot analysis showed that ADA markedly decreased the expression of three anti-apoptotic proteins whose expression is modulated by the transcriptional activity of NF-κB, (XIAP, c-FLIP, and Bcl-2) confirming NF-κB involvement (Figures 3C,G).
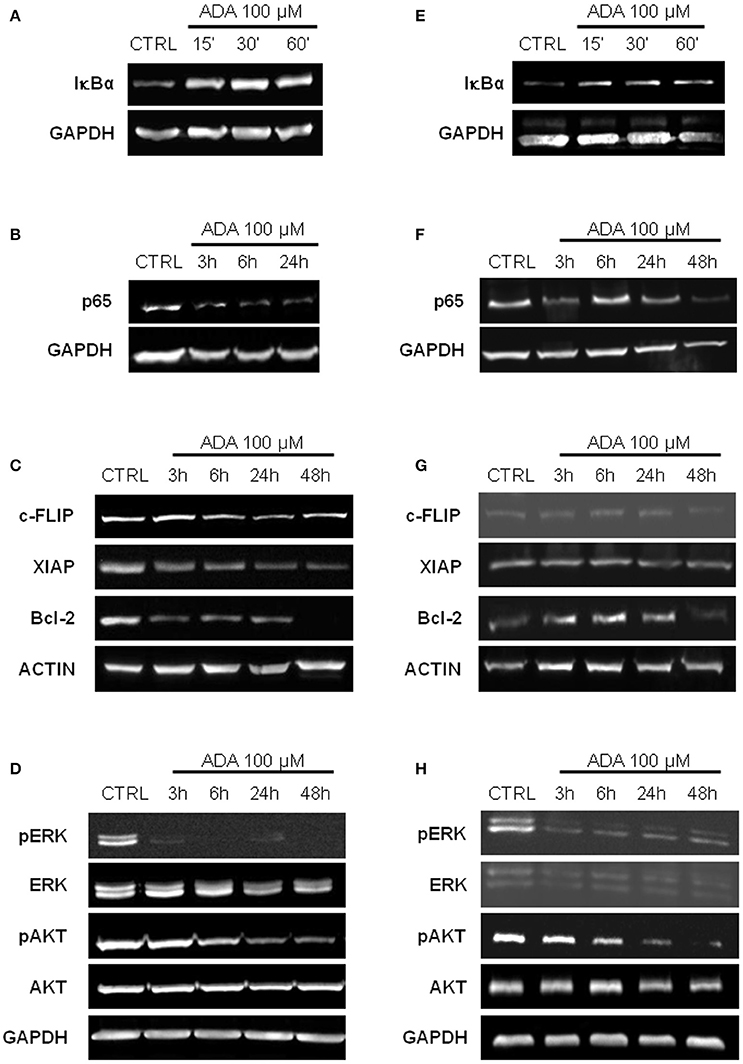
Figure 3. ADA inhibits NF-κB activation and down-regulates MAPK/ERK and PI3K/AKT pathways in metastatic melanoma cells. Western blot analysis carried out on the whole-cell extracts obtained from PES43 (A) and A375 (E) cells treated with ADA 100 μM shows an inhibition of IκBα degradation at 30 and 60 min. Nuclear extracts from control and ADA-treated PES43 (B) and A375 (F) cells collected at 3–6–24–48 h were analyzed by Western blot for NF-κB activation as p65 nuclear translocation. Both cell lines displayed a constitutively high expression of p65 into the nucleus that was reduced by ADA. Western blot analysis of c-FLIP, XIAP, and Bcl-2 carried out on PES43 (C) and A375 (G) cells treated with ADA 100 μM for 3–6–24–48 h. ADA decreased the expression of all the anti-apoptotic genes analyzed in both cell lines. Actin and GAPDH were detected as loading control. Experiments (n = 5) were run in triplicate. Western blot analysis of phospho- and total Akt and phospho- and total ERK in PES43 (D) and A375 (H) cells treated with ADA (100 μM) for 3–6–24–48 h. Both phospho-AKT (p-AKT) and phospho-ERK (p-ERK) band intensity was time-dependently reduced following treatment with ADA (100 μM). GAPDH was detected as a loading control. Experiments (n = 5) were run in triplicate.
Effect of ADA on MAPK/ERK and PI3/AKT Pathways
The Mitogen-Activated Protein Kinase (MAPK)/ERK and the Phosphoinositide 3-Kinase (PI3K)/AKT pathways are two of the most frequently deregulated pathways in melanoma (Hodis et al., 2012). They play an important role in melanoma development and progression and are involved in the mechanism of resistance to targeted therapy (Flaherty et al., 2012). As shown in Figures 3D,H, both phospho-AKT (p-AKT) and phospho-ERK (p-ERK) band intensity was time-dependently reduced following treatment with ADA (100 μM).
Effect of ADA on Cells Invasion
To determine whether ADA affected the invasive ability of the metastatic melanoma cells PES 43 and A375, we performed cell invasion assays using a transwell system. As shown in Figures 4A,B, ADA at 10 and 30 μM significantly inhibited the invasiveness of PES43 cell line by 33 and 63%, respectively (P < 0.01; P < 0.001 vs. control; n = 5). ADA (10–30 μM) produced a comparable effect on A375 cell line (Figures 4C,D) inhibiting the invasiveness by 45 and 67% respectively (P < 0.001 vs. control; n = 5).
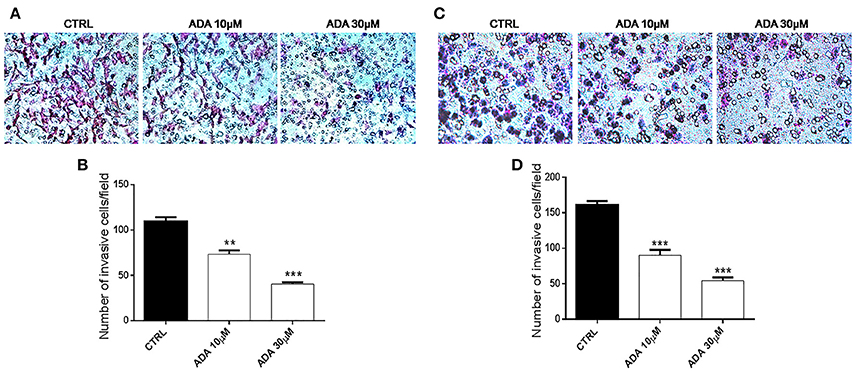
Figure 4. ADA inhibits the invasion of metastatic melanoma cells. ADA (10–30 μM) suppresses PES43 and A375 cell invasion as measured by a transwell cell invasion assay. Representative field of invasive cells PES43 (A) and A375 (C) on the membrane Average number of invasive cells PES43 (B) and A375 (D) from triplicate measurements. (**P < 0.01; ***P < 0.001 vs. control [CTRL]).
ADA Inhibits Metastatic Melanoma In vivo in Mice
To properly investigate on the role of ADA on melanoma progression, we exploited a murine model of metastatic melanoma induced following the injection of the murine melanoma cells B16/F-10 into the tail vein of C57BL/6J mice. Groups of mice were treated with different doses of ADA (5 and 50 mg·kg−1, p.o.) while control group received the vehicle only. Fourteen days after tumor implant, lungs were removed and the percentage of metastatic affected area was calculated by using the software Image J. As shown in Figures 5A,B, ADA (5–50 mg·kg−1) caused a dose-dependent reduction of lung metastases. The higher dose tested e.g., 50 mg·kg−1 reduced the total area of about 96% (2.74% vs. control 68.1%).
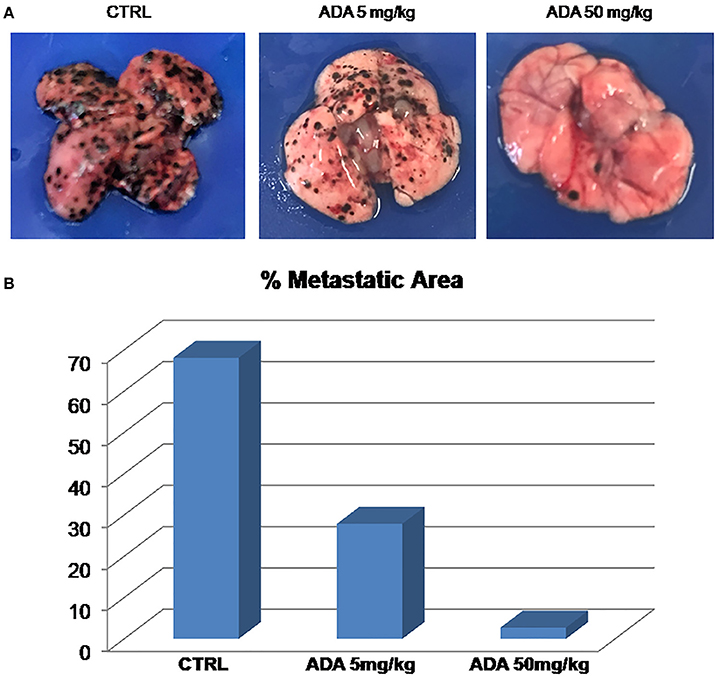
Figure 5. ADA inhibits B16 melanoma metastasis in vivo. B16-F10 mouse melanoma cells were injected through the lateral tail vein of C57BL/6J mice (n = 8 per group). ADA (5 or 50 mg·kg−1) was given orally to mice; control mice received vehicle only. ADA 5 and 50 mg·kg−1 reduced the metastatic area by 60 and 96% respectively. (A) Representative macroscopic pictures of mouse lungs, 14 days after inoculation. (B) Histogram with the percentage of metastatic affected area.
ADA Releases Hydrogen Sulfide
The incubation of ADA 100 μM in aqueous solution in presence of the nucleophil L-cysteine led to the generation of a time-related increase in H2S concentration. The rise of the H2S production reached a steady-state after about 10 min and the highest concentration of H2S, recorded after 20 min was 5.05 ± 0.04 μM (Figure 6).
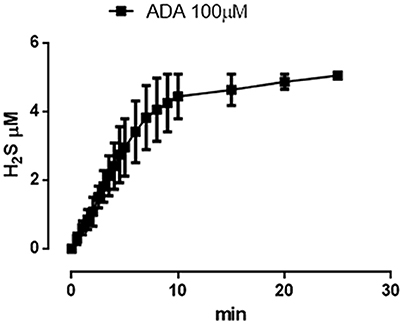
Figure 6. ADA releases hydrogen sulfide. Curve describes the increase of H2S concentration, with respect to time, following the incubation of ADA (100 μM) in the assay buffer in the presence of the nucleophile L-cysteine. H2S was recorded by amperometry; the vertical bars indicate the SEM.
Discussion
In the present study, we describe for the first time, the anti-cancer properties of ADA, a naturally occurring H2S donor, isolated and purified from Ferula assa-foetida L. Sulfur compounds contained within vegetables may be chemically or enzymatically transformed in the human body with subsequent formation of H2S (Jacob et al., 2008) and their consumption has been associated to chemopreventive effects (Krasilnikov et al., 2003). In particular, the garlic-derived organic polysulfide DATS has been shown to exhibit anticancer activity (Wang et al., 2012). Furthermore, epidemiological studies have shown that people assuming a diet rich in cruciferous vegetables (i.e., broccoli and cabbage) have a minor incidence of breast, lung, prostate, colon, and bladder cancer (Spitz et al., 2000; Ambrosone et al., 2004; Tang et al., 2008; Traka et al., 2008). This activity has been mainly ascribed to the isothiocyanate component abundantly present in these vegetables. Phytochemicals rich in sulfur, in particular diet-derived compounds, have therefore been proposed and applied in clinical trials as cancer chemopreventive/chemotherapeutic agents. These results along with studies performed using inhibitors of the enzymes responsible of H2S production have led to hypothesize a role for this metabolic pathway in cancer as recently reviewed (Szabo, 2016). We have previously established that sulfurated compounds can inhibit human melanoma cell proliferation (Panza et al., 2015). Here we describe the anti-tumoral activity of a newly identified natural H2S-donor from Ferula assa-foetida L. Interestingly, among all the compounds screened only the non-sulfur containing methoxylatifolone resulted inactive. This evidence further support our working hypothesis that the presence of sulfur is required in order to display anti-proliferative activity in melanoma. Indeed, all the sulfur containing compounds isolated were active on A375 melanoma cells and, among these, ADA and PDA showed the highest potency. ADA was selected to perform the study for the following reasons: (i) it displayed a clear concentration-dependent effect, as opposite to PDA that appeared to have a threshold concentration; (ii) it was the most abundant vinyl disulfide present in Ferula assa-foetida. The latter aspect has to be outlined since the abundance of a purified natural compound makes it possible to perform a more in deep and accurate pharmacological evaluation of its biological activities. Thus, we further investigated on ADA activity by using a panel of melanoma cell lines. Among the human cells tested, the metastatic cell lines PES 43 and A375 resulted the most sensible to the anti-proliferative activity of ADA and thus were selected for the mechanistic studies. Since in many cases the anti-proliferative effect of H2S-releasing molecules is related to their ability to activate the apoptotic machinery we investigated on this biochemical event performing flow citometry and western blot analysis on both cell lines. The results confirmed that ADA anti-proliferative effect relies on induction of apoptosis. This was confirmed by the cytofluorimetric assay with Annexin V/PI dual staining and by the time-dependent cleavage of caspase 3 and of its substrate PARP. In order to better understand the mechanism(s) underlying the apoptotic effect we investigated on the downstream signaling, looking at a possible involvement of NF-κB. Indeed, this pleiotropic transcription factor plays important roles in controlling cell proliferation, apoptosis, and oncogenesis. NF-κB activation has been also shown to promote melanoma tumor progression (Richmond, 2002; Ivanov et al., 2003). In fact, in late melanoma stage, NF-κB is up-regulated and inhibits cell apoptosis by enhancing the expression of several anti-apoptotic genes such as XIAP (Deveraux et al., 1998), c-FLIP (Micheau et al., 2001), and BclxL genes (Ravi et al., 2001). In most non-transformed cell types, NF-κB complexes bound to an inhibitor, IκB, are largely cytoplasmic. On activation, the IκB proteins become phosphorylated, ubiquitinated, and subsequently degraded. Freed NF-κB accumulates in the nucleus, where it enhances the transcription of specific genes. We demonstrated, by western blot analysis, that ADA inhibited both the phosphorylation and degradation of IkBα thereby preventing the nuclear translocation of the p65 subunit containing the transcriptional activation domain. The involvement of NF-κB was further supported by the finding that the expression of the anti-apoptotic genes c-FLIP, XIAP and Bcl-2 was also reduced. Indeed, all these three genes are transcriptionally regulated by NF-κB. As well as for NF-κB also the serine/threonine kinase Akt is constitutively activated in human melanomas (Madhunapantula et al., 2011). Although it may not be essential for initiation of melanoma, Akt activation facilitates melanoma progression by enhancing cell survival through up-regulation of NF-κB (Dhawan et al., 2002). Our results clearly show that ADA down-regulates Akt activity by reducing the phosphorylation on Ser473. Therefore, ADA inhibits the constitutive activated PI3K/Akt pathway and its downstream target NF-κB in melanoma cells.
Another key player involved in melanoma progression and resistance to current therapies is the Ras/Raf/MAPK pathway that is strictly linked to Akt pathway (Dhillon et al., 2007; Sullivan and Flaherty, 2013). In fact, several new drugs interfering with these two pathways are in advanced clinical phase for the treatment of resistant metastatic melanoma. Our results demonstrated an inhibitory effect of ADA also on the Ras/Raf/MAPK pathway. In fact, following incubation of melanoma cells with ADA, we observed a reduction of the phosphorylation and activation of ERK1/2. The dual inhibitory effect exhibited by ADA on both activating pathways is in line with the therapeutic strategy that are currently clinically explored in melanoma. It is well-known that mutant B-RAF may indirectly activate NF-κB through constitutive activation of the downstream effector protein ERK1/2 and the up-regulation of inflammatory cytokines leading to increases in survival, proliferation and invasiveness (Norris and Baldwin, 1999). In particular the increased ability of cancer cells to invade adjacent tissues is one of the critical steps leading to metastasis (Chaffer and Weinberg, 2011). This process is poorly understood even if it is the major cause of cancer-related mortality. Therefore, many investigators are trying to find strategies to suppress tumor growth as well as tumor metastasis. We addressed this particular issue by performing in vitro and in vivo experiments. Firstly, we demonstrated that ADA inhibits melanoma cells invasive capability in vitro by almost 70% at the higher dose used. All these in vitro evidence clearly indicated that ADA, by interfering with more than one mechanism involved in melanoma progression and spreading, has the potential to act also in vivo. In order to address this issue we used a model of pulmonary metastasis in mice that mimic the common and virulent phenomena of human metastasis and is widely used for the pre-clinical evaluation of drugs (Overwijk and Restifo, 2001). The injection of ADA to mice significantly suppressed (about 96% as compared to untreated mice) metastatic tumor growth in the lung suggesting that ADA reduced migration and growth of melanoma cancer cells in lung tissue. Of particular interest is the fact that the anti-tumor effect displayed by ADA is more sustained of that displayed by the well-characterized H2S-releasing molecule DATS (Panza et al., 2015).
It is necessary to point out that B16-F10, as any tumor cells, once injected intravenously migrate into the lungs. Therefore, the term pulmonary metastasis is widely used even though every resulting pulmonary nodule is technically an independent “primary” tumor rather than a true metastasis (Overwijk and Restifo, 2001). Nevertheless, within the limit of this preclinical model, ADA also in vivo displayed a significant and marked anticancer activity.
A recent investigation has reported a detailed picture of the sulfur-containing compounds of asafoetida. These mixed S-alkyl-S-alkenyldisulfides are able to release H2S due to their electrophilic nature and tendency to react with different nucleophilic (Nu:) agents (Shokoohinia et al., 2013).
In conclusion, we have established that ADA, a sulfur-containing compound of asafetida, not only suppresses melanoma cell growth and tumor cell migration in vitro but it is also active in vivo. Metastasis in cancer patients is associated with poor prognosis and death. Therefore, the finding of new strategies to suppress tumor growth and tumor metastasis is an unmet clinical need. The results of our study point toward this aim indicating ADA as a lead compound for the development of a new class of drugs active in metastatic melanoma based on the presence of labile sulfur in their structure.
Author Contributions
PD and EP were responsible for acquisition, analysis and interpretation of data, and redaction of the manuscript. CA, GE, RC, and VC carried out acquisition, analysis, and interpretation of data. OT, YS and GP were responsible for the critical reading of the manuscript. GC was responsible for interpretation of data and critical reading of the manuscript. AI performed conception and design, analysis and interpretation of data, and redaction of the manuscript. All authors read and approved the final manuscript.
Funding
Funding of this research was provided by the Italian Government grants (PRIN 2012 no: 2012WBSSY4_005).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
H2S, hydrogen sulfide; ADA, acetyl deacylasadisulfide; PDA, propionyl deacylasadisulfide; FSA, foetisulfide A; ARDA, arachyl deacylasadisulfide; DA, deacylasadisulfide; MEF, methoxylatifolone.
References
Ambrosone, C. B., McCann, S. E., Freudenheim, J. L., Marshall, J. R., Zhang, Y., and Shields, P. G. (2004). Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J. Nutr. 134, 1134–1138.
Chaffer, C. L., and Weinberg, R. A. (2011). A perspective on cancer cell metastasis. Science 331, 1559–1564. doi: 10.1126/science.1203543
Deveraux, Q. L., Roy, N., Stennicke, H. R., Van Arsdale, T., Zhou, Q., Srinivasula, S. M., et al. (1998). IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 17, 2215–2223. doi: 10.1093/emboj/17.8.2215
Dhawan, P., Singh, A. B., Ellis, D. L., and Richmond, A. (2002). Constitutive activation of Akt/protein kinase B in melanoma leads to up-regulation of nuclear factor-kappaB and tumor progression. Cancer Res. 62, 7335–7342.
Dhillon, A. S., Hagan, S., Rath, O., and Kolch, W. (2007). MAP kinase signalling pathways in cancer. Oncogene 26, 3279–3290. doi: 10.1038/sj.onc.1210421
Flaherty, K. T., Infante, J. R., Daud, A., Gonzalez, R., Kefford, R. F., Sosman, J., et al. (2012). Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Engl. J. Med. 367, 1694–1703. doi: 10.1056/Nejmoa1210093
Hellmich, M. R., Coletta, C., Chao, C., and Szabo, C. (2015). The therapeutic potential of cystathionine beta-synthetase/hydrogen sulfide inhibition in cancer. Antioxid. Redox Signal. 22, 424–448. doi: 10.1089/ars.2014.5933
Hellmich, M. R., and Szabo, C. (2015). Hydrogen sulfide and cancer. Handb. Exp. Pharmacol. 230, 233–241. doi: 10.1007/978-3-319-18144-8_12
Hodis, E., Watson, I. R., Kryukov, G. V, Arold, S. T., Imielinski, M., Theurillat, J. P., et al. (2012). A landscape of driver mutations in melanoma. Cell 150, 251–263. doi: 10.1016/j.cell.2012.06.024
Ialenti, A., Di Meglio, P., D'Acquisto, F., Pisano, B., Maffia, P., Grassia, G., et al. (2005). Inhibition of cyclooxygenase-2 gene expression by the heat shock response in J774 murine macrophages. Eur. J. Pharmacol. 509, 89–96. doi: 10.1016/j.ejphar.2004.10.052
Ianaro, A., Tersigni, M., Belardo, G., Di Martino, S., Napolitano, M., Palmieri, G., et al. (2009). NEMO-binding domain peptide inhibits proliferation of human melanoma cells. Cancer Lett. 274, 331–336. doi: 10.1016/j.canlet.2008.09.038
Iranshahy, M., and Iranshahi, M. (2011). Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin)-a review. J. Ethnopharmacol. 134, 1–10. doi: 10.1016/j.jep.2010.11.067
Ivanov, V. N., Bhoumik, A., and Ronai, Z. (2003). Death receptors and melanoma resistance to apoptosis. Oncogene 22, 3152–3161. doi: 10.1038/sj.onc.1206456
Jacob, C., Anwar, A., and Burkholz, T. (2008). Perspective on recent developments on sulfur-containing agents and hydrogen sulfide signaling. Planta Med. 74, 1580–1592. doi: 10.1055/s-0028-1088299
Kiani, A., Almasi, K., Shokoohinia, Y., Sadrjavadi, K., Nowroozi, A., and Shahlaei, M. (2015). Combined spectroscopy and molecular modeling studies on the binding of galbanic acid and MMP9. Int. J. Biol. Macromol. 81, 308–315. doi: 10.1016/j.ijbiomac.2015.08.005
Kilkenny, C., Browne, W., Cuthill, I. C., Emerson, M., and Altman, D. G. (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br. J. Pharmacol. 160, 1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x
Kim, K. H., Lee, H. J., Jeong, S. J., Lee, H. J., Lee, E. O., Kim, H. S., et al. (2011). Galbanic acid isolated from Ferula assafoetida exerts in vivo anti-tumor activity in association with anti-angiogenesis and anti-proliferation. Pharm. Res. 28, 597–609. doi: 10.1007/s11095-010-0311-7
Kimura, H. (2011). Hydrogen sulfide: its production, release and functions. Amino Acids 41, 113–121. doi: 10.1007/s00726-010-0510-x
Krasilnikov, M., Ivanov, V. N., Dong, J., and Ronai, Z. (2003). ERK and PI3K negatively regulate STAT-transcriptional activities in human melanoma cells: implications towards sensitization to apoptosis. Oncogene 22, 4092–4101. doi: 10.1038/sj.onc.1206598
Madhunapantula, S. V, Mosca, P. J., and Robertson, G. P. (2011). The Akt signaling pathway: an emerging therapeutic target in malignant melanoma. Cancer Biol. Ther. 12, 1032–1049. doi: 10.4161/cbt.12.12.18442
Mahendra, P., and Bisht, S. (2012). Ferula asafoetida: traditional uses and pharmacological activity. Pharmacogn. Rev. 6, 141–146. doi: 10.4103/0973-7847.99948
McGrath, J. C., Drummond, G. B., McLachlan, E. M., Kilkenny, C., and Wainwright, C. L. (2010). Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br. J. Pharmacol. 160, 1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x
Micheau, O., Lens, S., Gaide, O., Alevizopoulos, K., and Tschopp, J. (2001). NF-κB signals induce the expression of c-FLIP. Mol. Cell. Biol. 21, 5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001
Norris, J. L., and Baldwin, A. S. Jr. (1999). Oncogenic Ras enhances NF-kappaB transcriptional activity through Raf-dependent and Raf-independent mitogen-activated protein kinase signaling pathways. J. Biol. Chem. 274, 13841–13846.
Oh, B. S., Shin, E. A., Jung, J. H., Jung, D. B., Kim, B., Shim, B. S., et al. (2015). Apoptotic effect of galbanic acid via activation of caspases and inhibition of Mcl-1 in H460 non-small lung carcinoma cells. Phytother. Res. 29, 844–849. doi: 10.1002/ptr.5320
Overwijk, W. W., and Restifo, N. P. (2001). B16 as a mouse model for human melanoma. Curr. Protoc. Immunol. Chapter 20, Unit 20, 21. doi: 10.1002/0471142735.im2001s39
Panza, E., De Cicco, P., Armogida, C., Scognamiglio, G., Gigantino, V., Botti, G., et al. (2015). Role of the cystathionine gamma lyase/hydrogen sulfide pathway in human melanoma progression. Pigment Cell Melanoma Res. 28, 61–72. doi: 10.1111/pcmr.12312
Panza, E., De Cicco, P., Ercolano, G., Armogida, C., Scognamiglio, G., Anniciello, A. M., et al. (2016). Differential expression of cyclooxygenase-2 in metastatic melanoma affects progression free survival. Oncotarget 7, 57077–57085. doi: 10.18632/oncotarget.10976
Ravi, R., Bedi, G. C., Engstrom, L. W., Zeng, Q., Mookerjee, B., Gelinas, C., et al. (2001). Regulation of death receptor expression and TRAIL/Apo2L-induced apoptosis by NF-kappaB. Nat. Cell Biol. 3, 409–416. doi: 10.1038/35070096
Richmond, A. (2002). Nf-kappa B, chemokine gene transcription and tumour growth. Nat. Rev. Immunol. 2, 664–674. doi: 10.1038/nri887
Scala, S., Giuliano, P., Ascierto, P. A., Ierano, C., Franco, R., Napolitano, M., et al. (2006). Human melanoma metastases express functional CXCR4. Clin. Cancer Res. 12, 2427–2433. doi: 10.1158/1078-0432.CCR-05-1940
Shokoohinia, Y., Chianese, G., Appendino, G., Di Marzo, V., De Petrocellis, L., Ghannadi, A., et al. (2013). Some like it pungent and vile. TRPA1 as a molecular target for the malodorous vinyl disulfides from asafoetida. Fitoterapia 90, 247–251. doi: 10.1016/j.fitote.2013.08.001
Spitz, M. R., Duphorne, C. M., Detry, M. A., Pillow, P. C., Amos, C. I, Lei, L., et al. (2000). Dietary intake of isothiocyanates: evidence of a joint effect with glutathione S-transferase polymorphisms in lung cancer risk. Cancer Epidemiol. Biomarkers Prev. 9, 1017–1020.
Sullivan, R. J., and Flaherty, K. T. (2013). Resistance to BRAF-targeted therapy in melanoma. Eur. J. Cancer 49, 1297–1304. doi: 10.1016/j.ejca.2012.11.019
Szabo, C. (2007). Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 6, 917–935. doi: 10.1038/nrd2425
Szabo, C. (2016). Gasotransmitters in cancer: from pathophysiology to experimental therapy. Nat. Rev. Drug Discov. 15, 185–203. doi: 10.1038/nrd.2015.1
Tang, L., Zirpoli, G. R., Guru, K., Moysich, K. B., Zhang, Y., Ambrosone, C. B., et al. (2008). Consumption of raw cruciferous vegetables is inversely associated with bladder cancer risk. Cancer Epidemiol. Biomarkers Prev. 17, 938–944. doi: 10.1158/1055-9965.EPI-07-2502
Traka, M., Gasper, A. V, Melchini, A., Bacon, J. R., Needs, P. W., Frost, V., et al. (2008). Broccoli consumption interacts with GSTM1 to perturb oncogenic signalling pathways in the prostate. PLoS ONE 3:e2568. doi: 10.1371/journal.pone.0002568
Ueda, Y., and Richmond, A. (2006). NF-kappaB activation in melanoma. Pigment Cell Res. 19, 112–124. doi: 10.1111/j.1600-0749.2006.00304.x
Wang, H. C., Pao, J., Lin, S. Y., and Sheen, L. Y. (2012). Molecular mechanisms of garlic-derived allyl sulfides in the inhibition of skin cancer progression. Ann. N. Y. Acad. Sci. 1271, 44–52. doi: 10.1111/j.1749-6632.2012.06743.x
Wang, R. (2012). Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev. 92, 791–896. doi: 10.1152/physrev.00017.2011
Keywords: melanoma, skin cancer, hydrogen sulfide, apoptosis, metastasis
Citation: De Cicco P, Panza E, Armogida C, Ercolano G, Taglialatela-Scafati O, Shokoohinia Y, Camerlingo R, Pirozzi G, Calderone V, Cirino G and Ianaro A (2017) The Hydrogen Sulfide Releasing Molecule Acetyl Deacylasadisulfide Inhibits Metastatic Melanoma. Front. Pharmacol. 8:65. doi: 10.3389/fphar.2017.00065
Received: 24 November 2016; Accepted: 31 January 2017;
Published: 27 February 2017.
Edited by:
Salvatore Cuzzocrea, University of Messina, ItalyReviewed by:
Bashir M. Rezk, Southern University at New Orleans, USAMarcelo Nicolas Muscara, University of Sao Paulo, Brazil
Copyright © 2017 De Cicco, Panza, Armogida, Ercolano, Taglialatela-Scafati, Shokoohinia, Camerlingo, Pirozzi, Calderone, Cirino and Ianaro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Cirino, cirino@unina.it
Angela Ianaro, ianaro@unina.it
†These authors have contributed equally to this work.
 Paola De Cicco
Paola De Cicco Elisabetta Panza
Elisabetta Panza Chiara Armogida1
Chiara Armogida1 Giuseppe Ercolano
Giuseppe Ercolano Vincenzo Calderone
Vincenzo Calderone Giuseppe Cirino
Giuseppe Cirino Angela Ianaro
Angela Ianaro