- 1Department of Pharmacy, University of Malakand, Chakdara, Pakistan
- 2Department of Chemistry, COMSATS University Islamabad, Abbottabad, Pakistan
- 3Department of Biotechnology and Genetic Engineering, Kohat University of Science and Technology, Kohat, Pakistan
Isodon rugosus Wall. ex. Benth is an important species and is used in folk medicine for different types of pains such as abdominal pain, earache, toothache, gastric, and generalized body pain. Recently, we also have reported the antinociceptive potential of chloroform fraction of I. rugosus. In this research, we have investigated the antinociceptive, antioxidant and anti-cholinesterase potentials of essential oils from I. rugosus (Ir.EO), and have determined a possible mechanism of anti-nociception. The Ir.EO was subjected to gas chromatography-mass spectroscopy analysis to find out its chemical constituents. The Ir.EO was assayed for analgesic potential following acetic acid induced writhing, formalin test and hot plate method in animal models. The antioxidant activity was conducted against DPPH and ABTS free radicals following spectroscopic analysis. The cholinesterase inhibitory assays were performed using Ellman's assay. The GC-MS analysis of Ir.EO revealed the identification of 141 compounds. Ir.EO demonstrated strong antinociceptive potential in all three in-vivo models. With the use of nalaxone, it was confirmed that the essential oil was acting on the central pathway of nociception. The Ir.EO also exhibited strong free radicals scavenging potential, exhibiting IC50 values of 338 and 118 μg/ml for DPPH and ABTS free radicals respectively. In AChE and BChE inhibitory assays, the observed IC50 values were 93.56 and 284.19 μg/ml respectively. The encouraging antinociceptive, antioxidant and anticholinesterase results revealed that Ir.EO is a rich source of bioactive compounds as obvious from the GC-MS results.
Introduction
Globally, a large number of medicines are available for the treatment of pain and associated disorders. Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used for the management of pain and inflammation due to their strong efficacy (Zarin et al., 2005). However, their use is associated with severe side effects. Alternatively, the drugs from natural origins are considered to be relatively safe and are associated with fewer unwanted effects. Natural products, especially the plants play a vital role in the discovery of new chemical entities with potential therapeutic values (Rates, 2001; Ayaz et al., 2017a). The traditional use of plants is therefore a logical strategy to find out natural therapeutic agents for different ailments like pain and inflammation (Gupta et al., 2006). Despite the development of therapeutic agents for pain, there is still a demand to search out novel agents which could treat pain and related disorders more efficiently (Calixto et al., 2000).
The reactive oxygen species (ROS) are produced within the body as a result of redox processes and aerobic respiration. These ROS invade lipids, proteins, enzymes, DNA and RNA and ultimately damage the cells. These biomolecules play a vital role in stimulation, propagation and maintenance of inflammatory processes, as well as pain and neurodegenerative disorders (Zhu et al., 2004). These unwanted effects can be reduced by the use of antioxidants which either reduce the production of ROS or diminish them before reaction (Khalil et al., 1999; Cuzzocrea et al., 2001). In this regard, the essential oils isolated from herbal sources may be considered for the management of pain, inflammation, and free radicals scavenging. Several plants have been reported with strong antioxidant potentials against free radicals (Ahmad et al., 2015; Ayaz et al., 2015).
Alzheimer's disease (AD) is a common neurodegenerative disorder characterized by cognitive hypo-function, behavioral turbulence and difficulties in life activities (Ali et al., 2017; Ayaz et al., 2017b). AD is believed to be the major cause of dementia in elder population (Ullah et al., 2016). According to the statistics, 27 million people are affecting globally from Alzheimer and is a major life threat after cancer and cardiovascular diseases (Hebert et al., 2003). AD pathogenesis include synaptic deficiency of essential neurotransmitter (acetylcholine, ACh) which is implicated in the neurotransmission (Sadiq et al., 2015). Other aspects of AD include accumulation of amyloid beta (Aβ), neurofibrillary tangles (NFTs), and free radicals induced neurodegeneration (McLean et al., 1999; Zeb et al., 2014a; Ahmad et al., 2016). The inhibition of cholinesterase is a vital biochemical target involved in the degradation of ACh which increases its accumulation in the synaptic region. Among the five clinically approved anti-Alzheimer drugs, four are cholinesterase inhibitors while the fifth drug memantine is glutametergic system modifier. Despite the fact that several anti-amyloid and anti-NTFs drugs are in clinical trials, but, till date, no one is approved for clinical use. Furthermore, administration of free radical scavengers is also an important strategy, as Aβ is potent generator of free radicals and a mitochondrial poison. Plants are a source of mutli-potent drugs, including anti-AD drugs. Among the currently available anti-AD drugs, physostigmine, and galanthamine are derived from medicinal plants (Ahmad et al., 2016). Furthermore, natural products are free radicals' scavengers and can be effective on multiple pathways (Ayaz et al., 2017c).
Isodon rugosus Wall. ex. Benth. is a well-known species of family Labiateae. The bark of I. rugosus is used ethnomedicinally in the treatment of dysentery and curing of body pain (Shuaib et al., 2015). Folklorically, the fresh leaves' extract of I. rugosus is applied to the effected skin and is also used for earache (Sabeen and Ahmad, 2009). Moreover, the dried leaves of this plant can be used for the treatment of teeth pain (Akhtar et al., 2013). The plant has also been reported to posses potential effectiveness in gastric and abdominal pains (Ahmad et al., 2014). Moreover, other traditional uses of I. rugosus are attributed to its possible use against infectious diseases, pyrexia, blood pressure, rheumatism, and in pain associated with teeth (Khan and Khatoon, 2007; Adnan et al., 2012; Shuaib et al., 2014). The extracts of Isodon rugosus have been previously published to posses certain biological potentials like anti-diarrheal, analgesic, antimicrobial, anticholinesterase, antioxidant, cytotoxic, phytotoxic, hypoglycemic, and as bronchodilator (Sher et al., 2011; Ajmal et al., 2012; Janbaz et al., 2014; Zeb et al., 2014a,b, 2016, 2017).
Based on the ethnomedicinal importance and our previously published work, this piece of research is designed to investigate the in-vivo analgesic mechanism, in-vitro antioxidant, and anti-cholinesterase activities of essential oils of Isodon rugosus.
Methods
Plant Sample Collection & Isolation of Essential Oil
Isodon rugosus was collected from Dir (L), KP, Pakistan in July. The name Isodon rugosus was confirmed by Dr. Ali Hazrat, Department of Botany, Shaheed Benazir Bhutto University Dir (U), KP, Pakistan. The plant sample was stored for future record at the herbarium with voucher specimen number 1016AZ. The essential oils were extracted by hydrodistillation with the help of a Clevenger type apparatus (Lambert et al., 2001). The isolated essential oils were stored in refrigerator.
Gas Chromatography Analysis
The phytocomponents of essential oils were separated using the same GC instrument as we previously reported (Ahmad et al., 2016). A capillary column having dimensions of 30 m × 0.25 mm with film thickness of 0.25 μm in combination with a flame ionization detector was used. The initial temperature was 70°C for 1 min, which was raised gradually to 180°C with 6°C/min increase for 5 min. Finally, the oven temperature was increased to 280°C with 5°C/min increase for 20 min. Temperature of the injector port was 220°C while that of detector was maintained at 290°C. Helium was used as a carrier gas. The sample was diluted in n-pentane (1/1,000, v/v) of 1 μl (Ayaz et al., 2016).
GC-MS Analysis
The GC-MS analysis of essential oil isolated from Isodon rugosus was determined with the previously reported parameters (Ayaz et al., 2015).
Identification of Components
The retention times and spectra of separated compounds by GC-MS were compared with the standard compounds for identifications. The mass spectrum of each separated compound with its fragmentation pattern was compared with the reported compounds (Stein et al., 2002; Adams, 2007).
Experimental Animals
The Swiss albino mice of either sex were used in analgesic experiments which were obtained from research laboratory of National Institute of Health, Islamabad, Pakistan. The animals were used as per the approval of the ethical committee, Department of Pharmacy, University of Malakand, Pakistan according to the animals Bye-Laws 2008 (Scientific Procedure Issue-1).
Acute Toxicity
Swiss albino mice were taken in various groups, having 5 test animals in each group. The essential oil samples were administered to the animals orally in different doses (250–2,000 mg/kg). To increase the aqueous solubility of essential oil, 0.1% v/v tween-80 (Sigma Aldrich)- was used. After administration of the doses, animals were critically observed for 72 h for hypersensitivity, abnormal behavior, and death. The experimental animals were observed for 20 days for sub-chronic effects and lethality (Hosseinzadeh et al., 2000).
Analgesic Activities
Acetic Acid-Induced Writhing Test
In acetic acid induced writhing test, the essential oil was administered orally (PO) in the same concentrations as mentioned in above section. After 30 min of interval, acetic acid (0.6%, 10 ml/kg) was injected into the mice intra-peritoneally. Tween-80 (0.5%, 3 ml/kg) was administered to Group I animals. The Group I was used as a negative control. The standard drug diclofenac sodium was administered to Group II with a dose of 10 mg/kg. The essential oil samples were administered to Groups III and IV in concentrations of 50 and 100 mg/kg respectively. After administration of acetic acid, the number of writhes were counted for 30 min (Franzotti et al., 2000).
Formalin Test
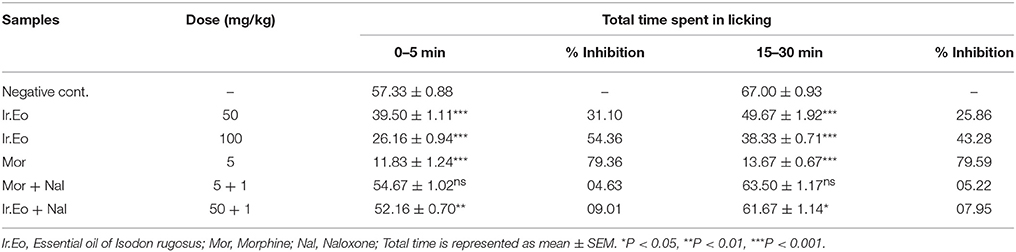
The formalin-induced licking test of Ir.EO was carried out using Swiss albino mice weighing 25–30 gm. The test was performed in a controlled environmental temperature (23 ± 2°C) with light-dark cycle of 12 h each. Food and water was freely available to the test animals throughout the investigations. The essential oil was administered intraperitonially (I/P) to the experimental animals at various concentrations. After 30 min, 20 μl formalin (2.5%, v/v in distilled water) was injected subcutuneously (S/C) into the plantar surface of the hind paw. Tween-80 (0.5%, 3 ml/kg), a negative control in the experiment was administered to the Group I. Morphine (5 mg/kg), a standard drug, was administered to Group II animals. The animals in Groups III and IV were injected Ir.EO at concentrations of 50 and 100 mg/kg respectively. The nociceptive behavior was designated by formalin-induced licking of paw. The total time taken in the behavioral changes of the mice responses to nociception was recorded, such as licking and/or biting of the injected paw. The time taken was recorded for 30 min. The initial 5 min were considered as early phase, while 2nd period (15–30 min) as the late phase of the response. The early and late phase are termed as neurogenic and inflammatory phase, respectively (Sulaiman et al., 2008).
Hot Plate Test
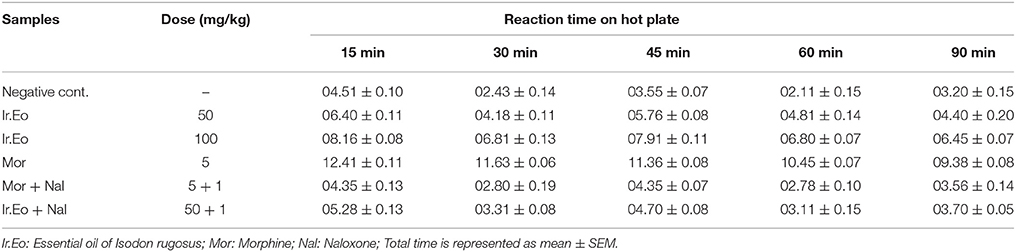
The hot plate test method was assessed for the antinociception potential of essential oil isolated from I. rugosus as per the reported procedure (Zeb et al., 2016). In this method, a heated surface of a hot plate analgesia meter (Ugo Basile, model-7280) was maintained at 55 ± 0.2°C. The animals were kept over a heated surface in a closed glass cylinder. The time of the animals' placement and licking of hind paw or jumping over the heated surface were recorded as response latency. These are the parameters as a result of the thermal reactions. The oil samples, in concentrations of 50 and 100 mg/kg, while morphine 5 mg/kg, i.p., were administered 30 min before the beginning of the assessment. Mice were observed before administration of samples, and then at 30, 60 and 90 min after the samples taken. The cut-off time was 20 s.
Involvement of Opioid Receptors
This experiment was carried out to confirm the possible involvement of opioid receptors in the essential oil-induced antinociception. The procedure was evaluated using a hot plate and formalin test method as mentioned earlier. In this method, different groups of experimental mice (n = 6) were pretreated with naloxone (5 mg/kg, S/C), which is a non-selective opioid receptor antagonist. Naloxone was injected 15 min before the administration of Ir.EO and morphine.
Antioxidant Assays
DPPH Assay
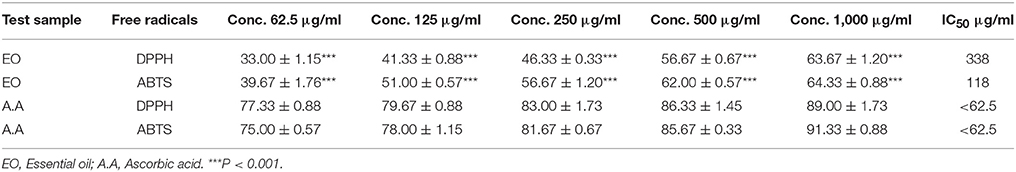
The DPPH free radicals scavenging effect was figured out for Ir.EO as previously published (Shah et al., 2015b). The DPPH solution (0.004%) in methanol was prepared which appeared with a deep violet color. Initially, the stock solution of essential oil with a known concentration of 1,000 μg/mL was prepared in ethanol. Then, this solution was diluted serially to obtain different concentrations from 62.5 to 1,000 μg/mL. Afterwards, 0.1 mL of the serially diluted concentration was added to 3.0 mL of DPPH solutions. This mixture was stored at dark place for 30 min at 23°C. After 30 min, the absorbance of each oil sample was measured by using double beam spectrophotometer at a wavelength of 517 nm. Ascorbic acid served as a positive control. The percent activity of all the samples was recorded as mean ± SEM. The percent radical scavenging potential was figured out using the following formula;
ABTS Assay
Antioxidant potential of Ir.EO was also investigated using free radicals of 2, 2-azinobis [3-ethylbenzthiazoline]-6-sulfonic acid (ABTS) (Ullah et al., 2017). Solutions of ABTS (7 mM) and potassium persulfate (2.45 mM) were prepared and mixed thoroughly. The prepared solution was stored in a dark place overnight to generate free radicals. The absorbance of this solution was adjusted at 745 nm to 0.7 by addition methanol (50%). ABTS solution 3 mL was added to the test tubes containing samples having volume of 300 μL. The solution was transferred to the sample holder and absorbance was recorded for 6 min by using a double beam spectrophotometer. Ascorbic acid was used as a standard. The percent ABTS free radicals scavenging potential of the oil sample was measured by using the given formula;
Anticholinesterase Assays
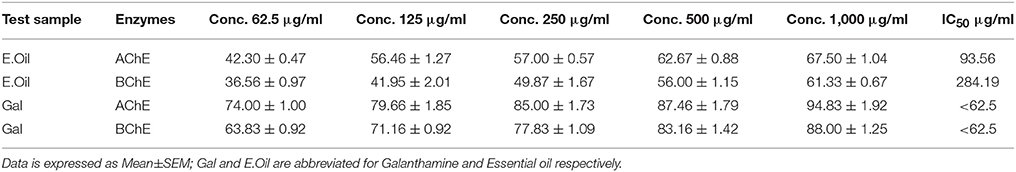
Cholinesterases inhibitory potentials of Ir.EO was evaluated following Ellman's assay (Ellman et al., 1961). This procedure is based on enzymatic breakdown of substrates like acetylthiocholine iodide and butyrylthiocholine iodide by AChE and BChE respectively to form 5-thio-2-nitrobenzoate anions. The resultant anions consequently form a complex with DTNB and are converted into UV detectable yellow color compound. The formation of this compound is quantified in the presence and absence of inhibitor agents. In brief, 5 μL enzyme solution was added to each well of micro plate with subsequent addition of 5 μL DTNB solution. The resulting mixture was incubated for fifteen min at 30°C in water bath, and finally 5 μl substrate solution was added to it. At the end, absorbances were recorded at 412 nm. The control samples were the same as above mentioned but were without inhibitors. The change in absorbance was observed beside reaction time. The activity of enzymes and its inhibitory activities were determined for control as well as test samples from the rate of absorption with change in time as, V = Δ Abs /Δ t, and enzyme inhibition as;
Where, Vmax is enzyme activity in the absence of inhibitor agent.
Estimation of IC50 Values
The median inhibitory concentration (IC50) values of DPPH, ABTS, AChE, and BChE inhibitory assays were find out by linear regression analysis of the percent inhibition versus concentrations of the test samples through MS Excel program (Shah et al., 2015a; Sadiq et al., 2016).
Statistical Data Analysis
The values of all the tests were tabulated as mean ± S.E.M. Significant differences of the percent inhibitions of various test samples were analyzed via one way ANOVA following Bonferroni's post-test using GraphPad Prism software in which the P < 0.05 were considered significant.
Results
GC-MS Analysis
The essential oil of Isodon rugosus was subjected to GC-MS analysis and total of 141 compounds were identified. On the given GC method, the retention times of the identified compounds were from 6.057 to 81.661 min. The details of all identified compounds are given in Table 1.
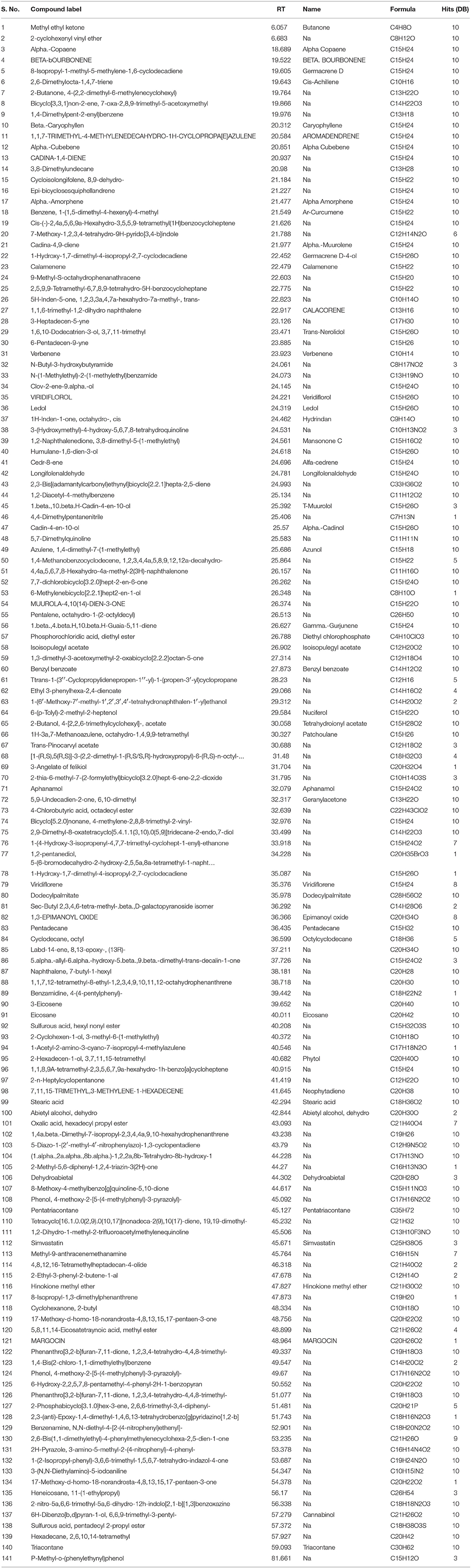
Table 1. List of all the compounds identified in the GC-MS analysis of essential oil of Isodon rugosus.
Acute Toxicity
No mortality and behavioral change were observed at specified doses to confirm acute toxicity of the samples. According to the assay, dose up to 2,000 mg/kg was considered as safe for essential oil of Isodon rugosus.
Writhing Test
A dose dependent response was observed in acetic acid induced writhing test for the assessment of analgesic activity. The mean writhes of the standard drug at 10 mg/kg, was 21.83 ± 0.60 with 70.29% inhibition. The essential oil sample exhibited mean inhibition of 31.50 ± 1.28 with 57.14% at 100 mg/kg, while, at 50 mg/kg it exhibited mean inhibition of 41.00 ± 0.57 with 44.21%. At 100 mg/kg, Ir.EO and positive control exhibited a response of 57.14 and 70.29% respectively as shown in Table 2.
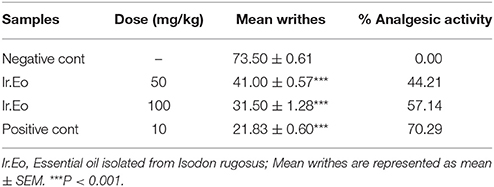
Table 2. Percent anti-nociceptive potential of essential oil following acetic acid induced writhing model.
Formalin Test
The results obtained from formalin test are shown in the Table 3. The formalin injection (2%, i.p) to the animals revealed a typical biphasic licking response. In the control group, duration of licking was observed as 57.33 ± 0.88 and 67.00 ± 0.93 s for early (0–5 min) and late phase (15–30 min) respectively. Pre-treatment of mice with various concentrations of essential oil (50 and 100 mg/kg) produced a significant effect on the duration of licking in both phases. A dose of 100 mg/kg of Ir.EO brought a significant reduction in paw licking of 54.36 and 43.28% in early and late phase respectively. In comparison, the standard drug morphine (5 mg/kg i.p.) demonstrated overwhelming reduction in both phases, i.e., 79.36% (early phase/neurogenic pain) and 79.59% (late phase/inflammatory pain). The morphine in combination with naloxone exhibited 04.63 and 05.22% activity in early and late phase respectively. In comparison, Ir.EO in combination with naloxone revealed 09.01% (early phase) and 07.95% (late phase) pain inhibitions. So, the naloxone reversed the antinociceptive effect of essential oil considerably at dose of 100 mg/kg in both phases as those of morphine.
Hot Plate Test
The results obtained in the hot plate assay are shown in Table 4. The Ir.EO revealed a dose dependent increase in the latency time as that of positive control. At 15 min, the mean reaction times for 50 and 100 mg/kg body weights of essential oil were observed as 06.40 ± 0.11 and 08.16 ± 0.08 min respectively. At 90 min, i.e., last interval, the mean reaction times of the same two doses were recorded as 04.40 ± 0.20 and 06.45 ± 0.07 min respectively. In comparison, the standard drug morphine exhibited reaction times of 12.41 ± 0.11 and 09.38 ± 0.08 min at initial and last interval respectively.
Moreover, the recorded mean reaction time for Ir.EO with naloxone (50: 1 mg/kg) at 15 min was 05.28 ± 0.13 min. Similarly, for morphine and naloxone (5: 1 mg/kg), the mean reaction time observed was 04.35 ± 0.13 min at initial 15 min. In our experiment, we found a distinct reduction in reaction time with the administration of naloxone.
Involvement of Opioid Receptors
In both hot plate and formalin models, we noticed that Ir.EO revealed a similar activity as that of morphine. The potency of Ir.EO was reduced effectively by opioid antagonist naloxone. With the use of naloxone, the decreased in reaction time in hot plate method and reversing the paw licking in formalin assay confirmed the possible involvement of opioid receptors.
Antioxidant Assays
The antioxidant potential of Ir.EO using DPPH and ABTS free radicals scavenging methods are shown in Table 5.
DPPH Assay
The observed percent inhibitions for Ir.EO using DPPH free radicals was 63.67 ± 1.20, 56.67 ± 0.67, 46.33 ± 0.33, 41.33 ± 0.88, and 33.00 ± 1.15% at concentrations of 1,000, 500, 250, 125, and 62.5 μg/ml respectively. The calculated IC50 value from the dose response curve was 338 μg/ml. In comparison, the standard drug ascorbic acid exhibited 89.00 ± 1.73, 86.33 ± 1.45, 83.00 ± 1.73, 79.67 ± 0.88, and 77.33 ± 0.88% inhibitions at 1,000, 500, 250, 125, and 62.5 μg/ml respectively with an IC50 value of <0.1 μg/ml.
ABTS Assay
In ABTS assay, Ir.EO attained 64.33 ± 0.88, 62.00 ± 0.57, 56.67 ± 1.20, 51.00 ± 0.57, and 39.67 ± 1.76% inhibitions at 1,000, 500, 250, 125, and 62.5 μg/ml respectively. The calculated IC50 for Ir.EO in scavenging ABTS free radicals was 118 μg/ml. In this assay, ascorbic acid demonstrated 91.33 ± 0.88, 85.67 ± 0.33, 81.67 ± 0.67, 78.00 ± 1.15, and 75.00 ± 0.57% inhibitions at 1,000, 500, 250, 125, and 62.5 μg/ml respectively attaining an IC50 value of <0.1 μg/ml.
Cholinesterase Inhibition Assay
In AChE inhibitory assay, Ir.EO exhibited concentration dependent inhibitions against the enzymes (Table 6). Ir.EO showed 67.50 ± 1.04% AChE inhibition at 1.0 mg/ml concentration with IC50 of 93.56 μg/ml. Similarly, the observed inhibitory potential against BChE at the same tested concentration as AChE was 61.33 ± 0.67% with an IC50 of 284.19 μg/ml. In comparison, the standard drug galanthamine exhibited 0.371 and 3.324 μg/ml IC50 against AChE and BChE respectively.
Discussion
In our designed work, the essential oil of I. rugosus was evaluated for antinociceptive, antioxidant, and anticholinestease potentials. The essential oils of plants are sources of wide variety of bioactive compounds (Dehpour et al., 2009). The pharmacological potentials of essential oil can be attributed to the hydropholic nature of its components and the same nature of our body cell membranes (Ait-Ouazzou et al., 2011). Various components of essential oils can easily get distributed to different compartments of our body including the central nervous system (Lambert et al., 2001; Vyas et al., 2008). In our current investigational study, the antinociceptive potential of essential oil was recorded with significant results. The possible mechanism of antinociceptive activity of Ir.EO was figured out as the central pathway due to involvement of opioid receptors. Recently, we have also reported the antinociceptive potential of chloroform fraction of I. rugosus following the same mechanism. The antinociceptive potential of essential oil may be due to the presence of large number of bioactive compounds as obvious from its GC-MS analysis. Among the identified compounds, we also observed some of the bioactive compounds previously reported with analgesic potentials. These compounds include α-copaene, germacrene D, β-caryophyllene, α-caryophyllene, aromadendrene, calamenene, viridiflorol, mansonone C, t-muurolol, α-cadinol, azunol, phytol, neophytadiene, and simvastatin. In short, α-copaene has been reported to possess strong analgesic and antioxidant potentials (Him et al., 2008; Chen et al., 2011; Costa et al., 2011). Likewise, germacrene D also possesses analgesic and antioxidant effects (Del-Vechio-Vieira et al., 2009; Victoria et al., 2012). β-Caryophyllene is also reported with its analgesic and antioxidant potentials (Calleja et al., 2013; Klauke et al., 2014). The antinociceptive activity of aromadendrene has also been demonstrated (Cruz et al., 2011). Similarly, α-caryophyllene has been reported for the treatment of body inflammatory pain (Pianowski et al., 2004). The analgesic and antioxidant effects of calamenene have also been demonstrated with significant results (Azevedo et al., 2013; Imam et al., 2014). Viridiflorol, a well-known bioactive compound is also reported to possess analgesic and radical scavenging potentials (Perry et al., 1997; do Amaral et al., 2007). Moreover, Mansonone C (including its reduced form) is also responsible for direct antioxidant activity (Villamil et al., 1990). T-muurolol has been verified for inhibitory activity against DPPH free radicals (Cheng et al., 2004). The analgesic activity of α-cadinol has also been reported with notable results (Boutaghane et al., 2011). The antinociceptive aspects of azunol has also been published previously (Ushiyama et al., 2009). In the same way, ledene has also been reported to possess analgesic activity (Alagammal et al., 2012). A well-known compound, i.e., phytol, is famous for its antioxidant potential along with its antinoceptive potential (Santos et al., 2013). Neophytadiene is also among the famous analgesic and antioxidant candidates (Jayashree et al., 2015). Similarly, simvastatin is also previously reported with its analgesic and antioxidant potentials (Carneado et al., 2002; Chen et al., 2013).
Literature review and the results of our current investigations go parallel with sound correlation. The traditional use of I. rugosus as analgesic is efficiently verified in the current research project, along with the identification of bioactive compounds.
Beside the antioxidant potential of Ir.EO, we also evaluated its AChE and BChE inhibitory potentials. Among other pathological targets of Alzheimer disease, inhibitions of cholinesterase and free radicals are also vital targets. Among the clinically approved anti-Alzheimer drugs, four are cholinesterase inhibitors, which signify the importance of this target in the symptomatic management of the disease. In the current study, we observed a moderate in-vitro cholinesterase inhibitory activity of Ir.EO. In AChE and BChE inhibitory assays, Ir.EO showed concentration dependent inhibitions against the enzymes with IC50 values of 93.56 and 284.19 μg/ml respectively. Though the in-vitro enzyme inhibitory activity of essential oil was low in comparison to galanthamine, yet, we hypothesize that it will have more availability at the target site. However, further studies are required regarding in-vivo efficacy of our tested essential oil.
Conclusion
Based on the literature survey regarding the medicinal aspects of I. rugosus and the results of current investigational study, it may be deduced that the essential oil of I. rugosus is a good source of natural bioactive compounds containing numerous analgesic and antioxidant agents. Its antioxidant potentials along with cholinesterase inhibitory activity will be potentially effective in the management of Alzheimer's disease patients. It may also be inferred that further exploitation of essential oil of I. rugosus may lead to the development of new analgesic and/or anti-Alzheimer drug candidates.
Author Contributions
AZ and SA carried out experimental work, data collection and literature search under the supervision of AS. FU helped as co-supervision of the research work. MA, NM and UR drafted the manuscript for publication. AS supervise the overall project and make the final version of publication. All the authors have read and approved the final manuscript for publication.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Ali Hazrat, Department of Botany, Shaheed Benazir Bhutto University, Sheringal Dir (U), KPK, Pakistan for the identification of plant. We are also grateful to Department of Pharmacy, University of Malakand, Pakistan for providing the laboratory facilities to conduct the experiments.
References
Adams, R. (2007). Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Carol Stream, IL: Allured Publishing.
Adnan, M., Begum, S., Khan, A. L., Tareen, A. M., and Lee, I.-J. (2012). Medicinal plants and their uses in selected temperate zones of Pakistani Hindukush-Himalaya. J. Med. Plants Res. 6, 4113–4127. doi: 10.5897/JMPR12.656
Ahmad, M., Sultana, S., Fazl-i-Hadi, S., ben Hadda, T., Rashid, S., Zafar, M., et al. (2014). An ethnobotanical study of medicinal plants in high mountainous region of Chail valley (District Swat-Pakistan). J. Ethnobiol. Ethnomed. 10:1. doi: 10.1186/1746-4269-10-36
Ahmad, S., Ullah, F., Ayaz, M., Sadiq, A., and Imran, M. (2015). Antioxidant and anticholinesterase investigations of Rumex hastatus D. Don: potential effectiveness in oxidative stress and neurological disorders. Biol. Res. 48, 1–8. doi: 10.1186/s40659-015-0010-2
Ahmad, S., Ullah, F., Sadiq, A., Ayaz, M., Imran, M., Ali, I., et al. (2016). Chemical composition, antioxidant and anticholinesterase potentials of essential oil of Rumex hastatus D. Don collected from the North West of Pakistan. BMC Compl. Alter. Med. 16:29. doi: 10.1186/s12906-016-0998-z
Ait-Ouazzou, A., Cherrat, L., Espina, L., Lorán, S., Rota, C., and Pagán, R. (2011). The antimicrobial activity of hydrophobic essential oil constituents acting alone or in combined processes of food preservation. Innov. Food Sci. Emerg. Technol. 12, 320–329. doi: 10.1016/j.ifset.2011.04.004
Ajmal, S., Mohammad, S., Zahid, K., Bakht, Z., Habib, A., and Alam, M. (2012). Ethnomedicinal and phytoeconomic elaboration of Lilownai valley, district Shangla Pakistan. Int. Res. J. Pharm. 3, 164–169.
Akhtar, N., Rashid, A., Murad, W., and Bergmeier, E. (2013). Diversity and use of ethno-medicinal plants in the region of Swat, North Pakistan. J. Ethnobiol. Ethnomed. 9:1. doi: 10.1186/1746-4269-9-25
Alagammal, M., Tresina, P., and Mohan, V. (2012). GC-MS determination of bioactive components of polygala javana dc. Int. J. Curr. Pharm. Res. 4, 42–44.
Ali, M., Muhammad, S., Shah, M. R., Khan, A., Rashid, U., Farooq, U., et al. (2017). Neurologically potent molecules from crataegus oxyacantha; isolation, anticholinesterase inhibition, and molecular docking. Front. Pharmacol. 8:327. doi: 10.3389/fphar.2017.00327
Ayaz, M., Junaid, M., Ullah, F., Sadiq, A., Khan, M. A., Ahmad, W., et al. (2015). Comparative chemical profiling, cholinesterase inhibitions and anti-radicals properties of essential oils from Polygonum hydropiper L: a preliminary anti-Alzheimer's study. Lipids Health Dis. 14:141. doi: 10.1186/s12944-015-0145-8
Ayaz, M., Junaid, M., Ullah, F., Sadiq, A., Ovais, M., Ahmad, W., et al. (2016). Chemical profiling, antimicrobial and insecticidal evaluations of Polygonum hydropiper L. BMC Compl. Alter. Med. 16:502. doi: 10.1186/s12906-016-1491-4
Ayaz, M., Junaid, M., Ullah, F., Sadiq, A., Shahid, M., Ahmad, W., et al. (2017a). GC-MS Analysis and gastroprotective evaluations of crude extracts, isolated saponins, and essential oil from Polygonum hydropiper L. Front. Chem. 5:58. doi: 10.3389/fchem.2017.00058
Ayaz, M., Junaid, M., Ullah, F., Subhan, F., Sadiq, A., Ali, G., et al. (2017b). Anti-Alzheimer's studies on beta-sitosterol isolated from Polygonum hydropiper L. Front. Pharmacol. 8:697. doi: 10.3389/fphar.2017.00697
Ayaz, M., Sadiq, A., Junaid, M., Ullah, F., Subhan, F., and Ahmed, J. (2017c). Neuroprotective and anti-aging potentials of essential oils from aromatic and medicinal plants. Front. Aging Neurosci. 9:168. doi: 10.3389/fnagi.2017.00168
Azevedo, M., Chaves, F., Almeida, C. A., Bizzo, H. R., Duarte, R. S., Campos-Takaki, G. M., et al. (2013). Antioxidant and antimicrobial activities of 7-hydroxy-calamenene-rich essential oils from Croton cajucara Benth. Molecules 18, 1128–1137. doi: 10.3390/molecules18011128
Boutaghane, N., Kabouche, A., Touzani, R., Maklad, Y. A., El-Azzouny, A., Bruneau, C., et al. (2011). GC/MS analysis and analgesic effect of the essential oil of Matricaria pubescens from Algeria. Nat. Prod. Commun. 6, 251–252.
Calixto, J. B., Beirith, A., Ferreira, J., Santos, A. R., Filho, V. C., and Yunes, R. A. (2000). Naturally occurring antinociceptive substances from plants. Phytother. Res. 14, 401–418. doi: 10.1002/1099-1573(200009)14:63.0.CO;2-H
Calleja, M. A., Vieites, J. M., Montero-Meterdez, T., Torres, M. I., Faus, M. J., Gil, A., et al. (2013). The antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br. J. Nutr. 109, 394–401. doi: 10.1017/S0007114512001298
Carneado, J., Alvarez de Sotomayor, M., Perez-Guerrero, C., Jimenez, L., Herrera, M. D., Pamies, E., et al. (2002). Simvastatin improves endothelial function in spontaneously hypertensive rats through a superoxide dismutase mediated antioxidant effect. J. Hyperten. 20, 429–437. doi: 10.1097/00004872-200203000-00018
Chen, X.-Y., Li, K., Light, A. R., and Fu, K.-Y. (2013). Simvastatin attenuates formalin-induced nociceptive behaviors by inhibiting microglial RhoA and p38 MAPK activation. J. Pain 14, 1310–1319. doi: 10.1016/j.jpain.2013.05.011
Chen, Y., Zhao, Y., Wang, X., Liu, J., Huang, L., and Peng, C. (2011). [GC-MS analysis and analgesic activity of essential oil from fresh rhizoma of Cyperus rotundus]. J. Chin. Med. Mater. 34, 1225–1229.
Cheng, S.-S., Wu, C.-L., Chang, H.-T., Kao, Y.-T., and Chang, S.-T. (2004). Antitermitic and antifungal activities of essential oil of Calocedrus formosana leaf and its composition. J. Chem. Ecol. 30, 1957–1967. doi: 10.1023/B:JOEC.0000045588.67710.74
Costa, E. V., Dutra, L. M., de Jesus, H., Nogueira, P., Moraes, V., Salvador, M. J., et al. (2011). Chemical composition and antioxidant, antimicrobial, and larvicidal activities of the essential oils of Annona salzmannii and A. pickelii (Annonaceae). Nat. Prod. Commun. 6, 907–912.
Cruz, S., Cáceres, A., Álvarez, L., Apel, M., and Henriques, A. (2011). “Chemical diversity of essential oils of 15 Piper species from Guatemala,” in International Symposium on Medicinal and Aromatic Plants IMAPS2010 and History of Mayan Ethnopharmacology IMAPS2011 964, 39–46.
Cuzzocrea, S., Riley, D. P., Caputi, A. P., and Salvemini, D. (2001). Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol. Rev. 53, 135–159.
Dehpour, A. A., Ebrahimzadeh, M. A., Seyed Fazel, N., and Seyed Mohammad, N. (2009). Antioxidant activity of the methanol extract of Ferula assafoetida and its essential oil composition. Gras. Aceites 60, 405–412. doi: 10.3989/gya.010109
Del-Vechio-Vieira, G., Sousa, O. V. D., Miranda, M. A., Senna-Valle, L., and Kaplan, M. A. C. (2009). Analgesic and anti-inflammatory properties of essential oil from Ageratum fastigiatum. Braz. Arch. Biol. Technol. 52, 1115–1121. doi: 10.1590/S1516-89132009000500008
do Amaral, J. F., Silva, M. I. G., de Aquino Neto, M. R. A., Neto, P. F. T., Moura, B. A., de Melo, C. T. V., et al. (2007). Antinociceptive effect of the monoterpene R-(+)-limonene in mice. Biol. Pharm. Bull. 30, 1217–1220. doi: 10.1248/bpb.30.1217
Ellman, G. L., Courtney, K. D., Andres, V., and Featherstone, R. M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7, 88IN191-9095. doi: 10.1016/0006-2952(61)90145-9
Franzotti, E. M., Santos, C., Rodrigues, H., Mourao, R., Andrade, M., and Antoniolli, A. (2000). Anti-inflammatory, analgesic activity and acute toxicity of Sida cordifolia L.(Malva-branca). J. Ethnopharmacol. 72, 273–277. doi: 10.1016/S0378-8741(00)00205-1
Gupta, M., Mazumder, U., Gomathi, P., and Selvan, V. T. (2006). Antiinflammatory evaluation of leaves of Plumeria acuminata. BMC Compl. Alter. Med. 6:36. doi: 10.1186/1472-6882-6-36
Hebert, L. E., Scherr, P. A., Bienias, J. L., Bennett, D. A., and Evans, D. A. (2003). Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch. Neurol. 60, 1119–1122. doi: 10.1001/archneur.60.8.1119
Him, A., Ozbek, H., Turel, I., and Oner, A. C. (2008). Antinociceptive activity of alpha-pinene and fenchone. Pharmacol. Online 3, 363–369.
Hosseinzadeh, H., Ramezani, M., and Salmani, G. (2000). Antinociceptive, anti-inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. J. Ethnopharmacol. 73, 379–385. doi: 10.1016/S0378-8741(00)00238-5
Imam, H., Sofi, G., Seikh, A., and Lone, A. (2014). The incredible benefits of Nagarmotha (Cyperus rotundus). Int. J. Nutr. Pharm. Neurol. Dis. 4:23. doi: 10.4103/2231-0738.124611
Janbaz, K. H., Arif, J., Saqib, F., Imran, I., Ashraf, M., Zia-Ul-Haq, M., et al. (2014). In-vitro and in-vivo validation of ethnopharmacological uses of methanol extract of Isodon rugosus Wall. ex Benth.(Lamiaceae). BMC Compl. Alter. Med. 14:71. doi: 10.1186/1472-6882-14-71
Jayashree, I., Geetha, D., and Rajeswari, M. (2015). GC-MS Analysis of bioactive constituents of glochidion ellipticum WT. Int. J. Pharm. Sci. Res. 6:2546. doi: 10.13040/IJPSR.0975-8232.6(6).2546-50
Khalil, Z., Liu, T., and Helme, R. D. (1999). Free radicals contribute to the reduction in peripheral vascular responses and the maintenance of thermal hyperalgesia in rats with chronic constriction injury. Pain 79, 31–37. doi: 10.1016/S0304-3959(98)00143-2
Khan, S. W., and Khatoon, S. (2007). Ethnobotanical studies on useful trees and shrubs of haramosh and bugrote valleys in gilgit northern areas of Pakistan. Pak. J. Bot. 39, 699–710.
Klauke, A.-L., Racz, I., Pradier, B., Markert, A., Zimmer, A., Gertsch, J., et al. (2014). The cannabinoid CB 2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur. Neuropsychopharmacol. 24, 608–620. doi: 10.1016/j.euroneuro.2013.10.008
Lambert, R. J., Skandamis, P. N., Coote, P. J., and Nychas, G. J. (2001). A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 91, 453–462. doi: 10.1046/j.1365-2672.2001.01428.x
McLean, C. A., Cherny, R. A., Fraser, F. W., Fuller, S. J., Smith, M. J., Vbeyreuther, K., et al. (1999). Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann. Neurol. 46, 860–866.
Perry, N. B., Brennan, N. J., Van Klink, J. W., Harris, W., Douglas, M. H., McGimpsey, J. A., et al. (1997). Essential oils from New Zealand manuka and kanuka: chemotaxonomy of Leptospermum. Phytochemistry 44, 1485–1494. doi: 10.1016/S0031-9422(96)00743-1
Pianowski, L. F., Calixto, J. B., and Brandao, D. D.C. (2004). Use of Carophyllenes in the Manufacture of Medicaments and Treatment of Bodily Conditions of Inflammation and Inflammatory Pain. Google Patents.
Rates, S. M. (2001). Plants as source of drugs. Toxicon 39, 603–613. doi: 10.1016/S0041-0101(00)00154-9
Sabeen, M., and Ahmad, S. S. (2009). Exploring the folk medicinal flora of Abbotabad city, Pakistan. Ethnobot. Leaflets 3:810–833.
Sadiq, A., Ahmad, S., Ali, R., Ahmad, F., Ahmad, S., Zeb, A., et al. (2016). Antibacterial and antifungal potentials of the solvents extracts from Eryngium caeruleum, Notholirion thomsonianum and Allium consanguineum. BMC Compl. Alter. Med. 16:478. doi: 10.1186/s12906-016-1465-6
Sadiq, A., Mahmood, F., Ullah, F., Ayaz, M., Ahmad, S., Haq, F. U., et al. (2015). Synthesis, anticholinesterase and antioxidant potentials of ketoesters derivatives of succinimides: a possible role in the management of Alzheimer’s. Chem. Cent. J. 9:31. doi: 10.1186/s13065-015-0107-2
Santos, C. C., Salvadori, M. S., Mota, V. G., Costa, L. M., de Almeida, A. A. C., de Oliveira, G. A. L., et al. (2013). Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neurosci. J. 2013:949452. doi: 10.1155/2013/949452
Shah, S. M., Ayaz, M., Khan, A. U., Ullah, F., Farhan Shah, A. U., et al. (2015a). 1,1-Diphenyl,2-picrylhydrazyl free radical scavenging, bactericidal, fungicidal and leishmanicidal properties of Teucrium stocksianum. Toxicol. Ind. Health 31, 1037–1043. doi: 10.1177/0748233713487250
Shah, S. M., Shah, S. M. M., Ahmad, Z., Yaseen, M., Shah, R., Sadiq, A., et al. (2015b). Phytochemicals, in vitro antioxidant, total phenolic contents and phytotoxic activity of Cornus macrophylla Wall bark collected from the North-West of Pakistan. Pak. J. Pharm. Sci. 28, 23–28.
Sher, Z., Khan, Z., and Hussain, F. (2011). Ethnobotanical studies of some plants of Chagharzai valley, district Buner, Pakistan. Pak. J. Bot. 43, 1445–1452.
Shuaib, M., Khan, I., and Sharifullah Khan, M. (2015). Study of Medicinal Plants of Lower Dir, Timergara, Tehsil Balambat, Khyber Paktunkhaw-Pakistan. Am. Eurasian J. Agric. Environ. Sci. 15, 2088–2094. doi: 10.5829/idosi.aejaes.2015.15.10.12811
Shuaib, M., Khan, I., Sharifullah, R. K., Hashmatullah, S. M., and Naz, R. (2014). Ethnobotanical studies of spring flora of Dir Lower, Khyber Pakhtunkhwa, Pakistan. Pak. J. Weed Sci. Res. 20, 37–49.
Stein, S., Mirokhin, D., and Tchekhovskoi, D, and G., M. (2002). The NIST Mass Spectral Search Program for the NIST/EPA/NIH Mass Spectra Library; Standard Reference Data Program of the National Institute of Standards and Technology. Gaithersburg, MD.
Sulaiman, M. R., Hussain, M., Zakaria, Z. A., Somchit, M., Moin, S., Mohamad, A., et al. (2008). Evaluation of the antinociceptive activity of Ficus deltoidea aqueous extract. Fitoterapia 79, 557–561. doi: 10.1016/j.fitote.2008.06.005
Ullah, F., Ayaz, M., Sadiq, A., Hussain, A., Ahmad, S., Imran, M., et al. (2016). Phenolic, flavonoid contents, anticholinesterase and antioxidant evaluation of Iris germanica var; florentina. Nat. Prod. Res. 30, 1440–1444. doi: 10.1080/14786419.2015.1057585
Ullah, F., Iqbal, N., Ayaz, M., Sadiq, A., Ullah, I., Ahmad, S., et al. (2017). DPPH, ABTS free radical scavenging, antibacterial and phytochemical evaluation of crude methanolic extract and subsequent fractions of Chenopodium botrys aerial parts. Pak. J. Pharm. Sci. 30, 761–766.
Ushiyama, M., Ikeda, R., Nitta, T., Tazitsu, Y., Miyawaki, A., Nishizawa, Y., et al. (2009). Stability of hospital preparations of Azunol Water Gargles for pain relief in oral cancer patients with oral mucositis. Cancer Ther. 7, 277–281.
Victoria, F. N., Lenardão, E. J., Savegnago, L., Perin, G., Jacob, R. G., Alves, D., et al. (2012). Essential oil of the leaves of Eugenia uniflora L.: antioxidant and antimicrobial properties. Food Chem. Toxicol. 50, 2668–2674. doi: 10.1016/j.fct.2012.05.002
Villamil, S. F., Dubin, M., Galeffi, C., and Stoppani, A. O. (1990). Effects of mansonones on lipid peroxidation, P450 monooxygenase activity, and superoxide anion generation by rat liver microsomes. Biochem. Pharmacol. 40, 2343–2351. doi: 10.1016/0006-2952(90)90732-Z
Vyas, T. K., Shahiwala, A., and Amiji, M. M. (2008). Improved oral bioavailability and brain transport of Saquinavir upon administration in novel nanoemulsion formulations. Int. J. Pharm. 347, 93–101. doi: 10.1016/j.ijpharm.2007.06.016
Zarin, D. A., Tse, T., and Ide, N. C. (2005). Trial registration at ClinicalTrials. gov between May and October 2005. N. Engl. J. Med. 353, 2779–2787. doi: 10.1056/NEJMsa053234
Zeb, A., Ahmad, S., Ullah, F., Ayaz, M., and Sadiq, A. (2016). anti-nociceptive activity of ethnomedicinally important analgesic plant Isodon rugosus Wall. ex Benth: Mechanistic Study and Identifications of Bioactive Compounds. Front. Pharmacol. 7:200. doi: 10.3389/fphar.2016.00200
Zeb, A., Sadiq, A., Ullah, F., Ahmad, S., and Ayaz, M. (2014a). Investigations of anticholinesterase and antioxidant potentials of methanolic extract, subsequent fractions, crude saponins and flavonoids isolated from Isodon rugosus. Biol. Res. 47:76. doi: 10.1186/0717-6287-47-76
Zeb, A., Sadiq, A., Ullah, F., Ahmad, S., and Ayaz, M. (2014b). Phytochemical and toxicological investigations of crude methanolic extracts, subsequent fractions and crude saponins of Isodon rugosus. Biol. Res. 47:57. doi: 10.1186/0717-6287-47-57
Zeb, A., Ullah, F., Ayaz, M., Ahmad, S., and Sadiq, A. (2017). Demonstration of biological activities of extracts from Isodon rugosus Wall. Ex Benth: Separation and identification of bioactive phytoconstituents by GC-MS analysis in the ethyl acetate extract. BMC Compl. Alter. Med. 17:284. doi: 10.1186/s12906-017-1798-9
Keywords: essential oil, GC-MS, Isodon rugosus, antinociception, opioid receptors, antioxidant, anticholinesterase
Citation: Sadiq A, Zeb A, Ullah F, Ahmad S, Ayaz M, Rashid U and Muhammad N (2018) Chemical Characterization, Analgesic, Antioxidant, and Anticholinesterase Potentials of Essential Oils From Isodon rugosus Wall. ex. Benth. Front. Pharmacol. 9:623. doi: 10.3389/fphar.2018.00623
Received: 11 June 2017; Accepted: 24 May 2018;
Published: 13 June 2018.
Edited by:
Lyndy Joy McGaw, University of Pretoria, South AfricaReviewed by:
Kannan R. R. Rengasamy, Alagappa University, IndiaAbdel-Tawab H. Mossa, National Research Centre, Egypt
Copyright © 2018 Sadiq, Zeb, Ullah, Ahmad, Ayaz, Rashid and Muhammad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdul Sadiq, sadiquom@yahoo.com
 Abdul Sadiq
Abdul Sadiq Anwar Zeb
Anwar Zeb Farhat Ullah
Farhat Ullah Sajjad Ahmad
Sajjad Ahmad Muhammad Ayaz
Muhammad Ayaz Umer Rashid
Umer Rashid Noor Muhammad3
Noor Muhammad3