- College of Basic Medical Science, Zhejiang Chinese Medical University, Hangzhou, China
Rheumatoid arthritis (RA) is an autoimmune disease mainly characterized by chronic polyarthritis. Many types of cells play pivotal roles in the pathogenicity of RA, such as T cells, B cells, macrophages, dendritic cells (DCs), osteoclasts (OCs), and fibroblast-like synoviocytes (FLS). Tripterygium wilfordii Hook f. (TwHf) and its ingredients are able to control disease activity by regulating the functions of cells mentioned above, and the clinical studies have highlighted the importance of TwHf ingredients in RA treatment. They have been demonstrated to improve the RA symptoms of animal models and patients. In this review, we discussed the effect of TwHf ingredients on pathogenicity cells, including disease/cell phenotypes and molecular mechanisms. Here, we constructed a cell-cell interaction network to visualize the effect of TwHf ingredients. We found that TwHf ingredients could inhibit the differentiation and proliferation of the pathogenicity cells. Besides, the components could decrease the levels of pathogenicity cytokines [i.e., interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α)]. Many signaling pathways are involved in the underlying mechanisms, such as PI3K, NF-κB, and MAPK signaling pathways.
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease mainly characterized by chronic persistent synovitis, which causes the destruction of articular cartilage and bone, eventually leading to joint deformity and finally loss of function. The incidence of RA in mainland China is about 0.42%, and the disability rate of this disease course over 15 years is 61.3%. The clinical studies have shown that the effectiveness of the anchoring drug methotrexate is only 15%–25% (Lopez‐Olivo et al., 2014; Chinese Rheumatology Association, 2018). While the addition of Tripterygium wilfordii Hook. f. (TwHf) or Tripterygium hypoglaucum (Levl.) Hutch is able to control RA disease activity more effectively by regulating immune cell functions (Jun et al., 2016; Xiao-yue et al., 2019). In the separable components of TwHf and Levl. Hutch, there are many similar but different active component, such as Wilforlide, Celastrol (Cel), and Triptolide (TP) (Xianguang and Li, 2006; Chao, 2015; Chen-qiong et al., 2015). These active components can regulate the pathogenicity immune cells and connective tissue cells of RA. For example, they can reduce the secretion of inflammatory cytokines (such as TNF-α, IL-1β, and IL-6) of macrophages, the proliferation, and differentiation of pathogenic T cells, and the bone destruction which mediated by the fibroblast-like synoviocytes (FLS) and the osteoclasts (Peng et al., 2014; Wang et al., 2014; Te et al., 2019).
Tripterygium genus alleviates disease activity by regulating RA-related cells by multiple targeting approaches. Up to date, there is no literature review for TwHf or its active ingredients on RA-related cell dysfunction. Meanwhile, there are signal transduction interactions between various immune cells and connective tissue cells in the process of RA, which jointly promote the occurrence and development of RA. Therefore, we conducted a literature review (search strategy is available in Supplementary Materials 1) to summarize the effects of Tripterygium genus active ingredients on RA-related cells. Furthermore, we constructed signal pathways and cell-cell interaction networks to summarize their molecular mechanisms and to speculate the potential target cells and proteins.
The Functions of Tripterygium Ingredients on T Cells and the Molecule Mechanisms
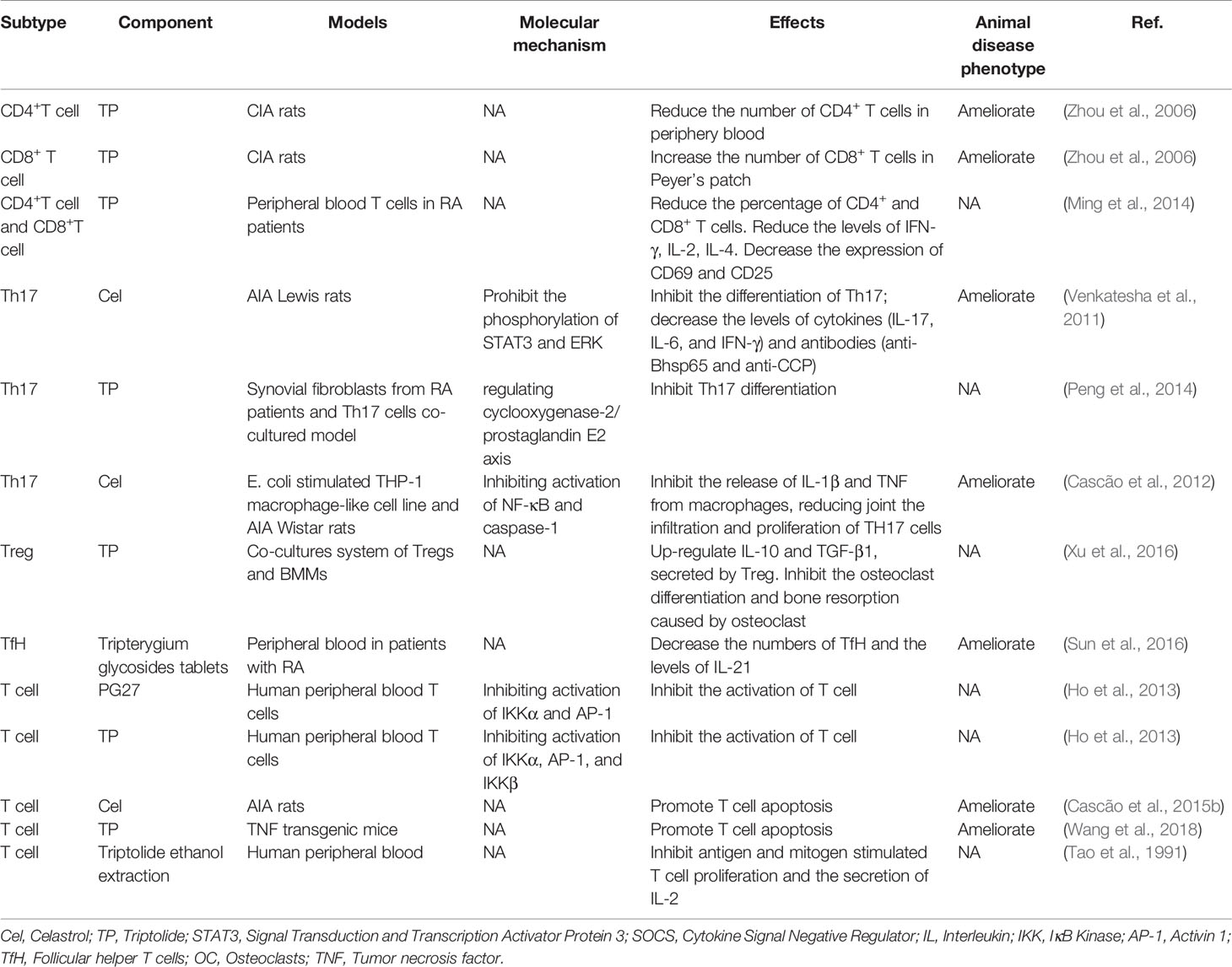
T cells play a crucial role in various adaptive immune responses. During RA, T cells received antigens will be activated and proliferate (Smolen et al., 2018). Ho et al. (2013) found that PG27, one of the ingredients of TwHf, inhibited the T cell activation via targeting NF-κB and AP-1 pathways. PG27 can inhibit IKKα/IκBα/NF-κB and mitogen-activated protein kinase (MAPK)-AP-1 signaling pathways, while IKKβ activity was less sensitive for the inhibition of PG27. By contrast, the purified component of TwHf, PG490 (triptolide), similarly suppressed the above pathways. Similar results were demonstrated in RA animal models and patients but lacking molecule mechanisms. Triptolide reduced the numbers of CD4+ cells in the periphery and increased the numbers of CD8+ cells in Peyer’s patch (Zhou et al., 2006). When triptolide was used to treat T cell isolated from peripheral blood of RA patients, the percentage of CD4+ and CD8+T cells secreting IFN-γ, IL-2, and IL-4 was decreased, and the percentage of CD4+ and CD8+T cells expressing CD69 and CD25 was also reduced (Ming et al., 2014). Besides, Tripterygium active compounds have been demonstrated in vivo and in vitro to reduce T cell number by promoting T cell apoptosis as well as suppressing T cell proliferation and cytokine secretion, while the mechanism is unknown (Tao et al., 1991; Cascão et al., 2015b; Wang et al., 2018).
CD4+ T cells can activate and polarize into various T helper cell subsets, including T helper 1 (Th1), T helper 2 (Th2), regulatory T (Treg), T helper 9 (Th9), T follicular helper cells (Tfh), T helper 17 (Th17), or T helper 22 (Th22) cells. Th17 cell numbers were increased in the peripheral blood, inflamed synovial tissue, and synovial fluid of RA patients (Leipe et al., 2010; van Hamburg et al., 2011; Penatti et al., 2017). Th17 cells promote the development of RA through the secretion of various inflammatory cytokines and chemokines. TGF-β/SMADs/RORγt and IL-6/STAT3 pathways are involved in mediating Th17 cell differentiation and mediating the expression of IL-17A, IL-17F, and IL-21 (Ivanov et al., 2006; Nishihara et al., 2007; Yang et al., 2008). The Cel, one of the Tripterygium ingredients, has been proved to have anti-arthritic activity by inhibiting IL-6/STAT3 signal and finally reduce the secretion of Th17-related pro-inflammatory cytokines (Venkatesha et al., 2011). Moreover, Cel inhibits the activation of NF- κB, and caspase-1 in macrophages, resulting in the reduced release of IL-1β and TNF-α, and finally decreased the infiltration and proliferation of joint Th17 cells (Cascão et al., 2012) because IL-1β is able to promote the polarization of Th17 cells through inducing the expression of the transcription factors IFR4 and RORγ (Vallières et al., 2019). In addition, TP inhibits the expression of COX2 and the secretion of PGE2 in the co-culture models of RA synovial fibroblasts (RASFs) and RA CD4+ T cells, blocking the differentiation of Th17 cells in vitro (Peng et al., 2014).
Similar to Th17, Tfh cells also promote RA progression by secreting IL-21 (Vinuesa et al., 2016). However, there is less research on the effects of TwHf on Tfh. In patients with RA treated with TwHf, the number of tenderness joints, the number of swollen joints, and the evaluation score of overall RA in the experimental group were lower than those in the control group. Consistently, the levels of Tfh cells and IL-21 were lower than those in the control group, and the levels of Tfh cells and IL-21 were positively correlated with DAS28 score (Sun et al., 2016).
Treg cells act as protective cells during RA. Enhancing the function or improving the number of Treg cells has been proved to alleviate the RA activity in varying degrees (Cooles et al., 2013). So far, research focused on the effects of TwHf on Treg cells in RA were limited. In the co-culture system of bone marrow macrophages and Tregs, TP up-regulated IL-10 and TGF-β1 produced by Treg cells, resulting in the inhibition of osteoclast differentiation and bone resorption (Xu et al., 2016).
The role of tripterygium ingredients for T cells and the molecule mechanisms were summarized in Table 1. The molecule mechanisms of CD8+ cell and Th17 are available in Figures 1, 2 (Th1, Th2, Treg, and Tfh are not available because insufficient study describes their molecule mechanism).
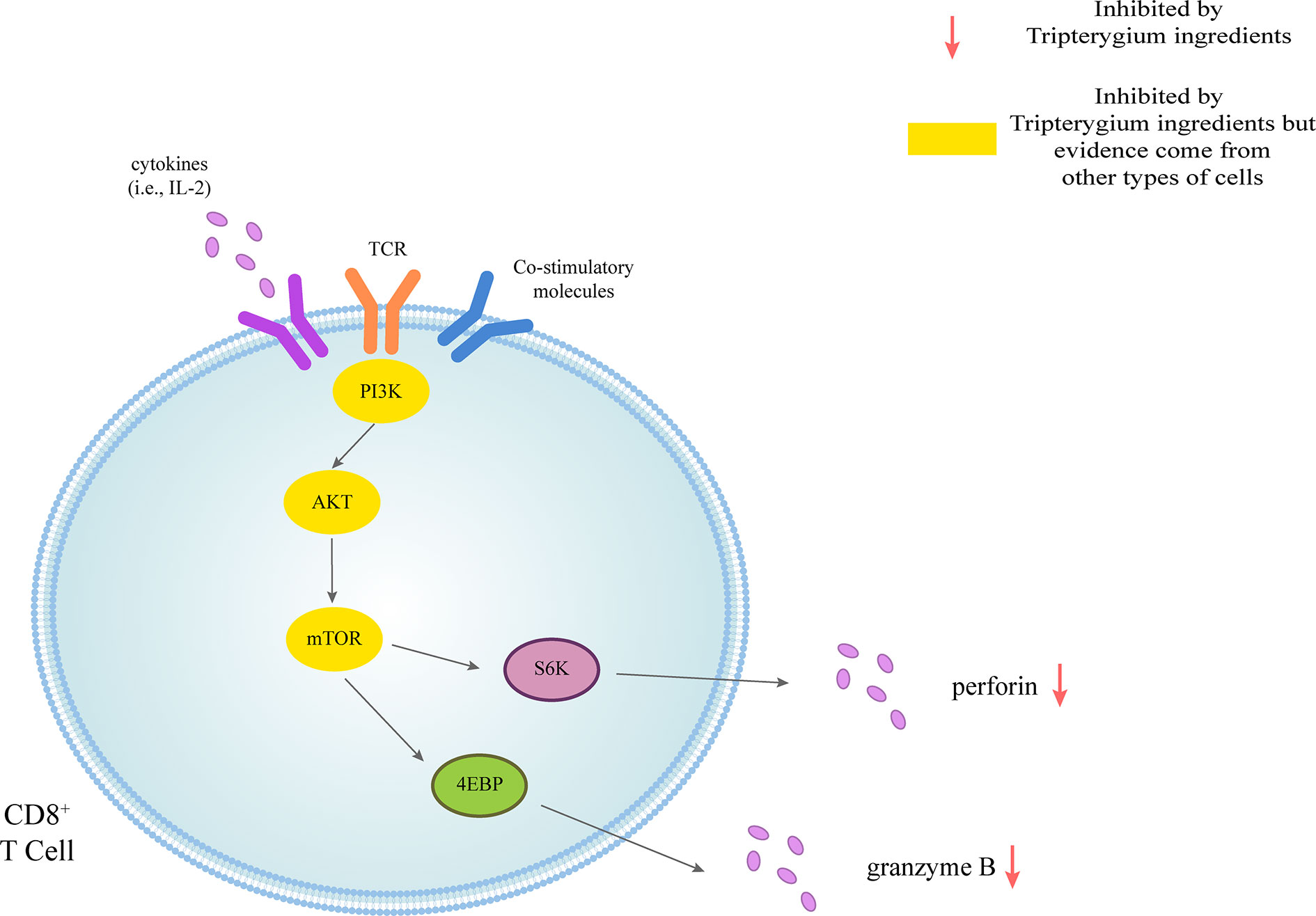
Figure 1 Tripterygium ingredients act on the CD8+ T cell. CD8+ T cells could be activated through three signals. The first signal of T cell activation comes from the specific binding of its receptor TCR to the antigen. The second signal of T cell activation comes from costimulatory molecules. Cytokines promote the full activation of T cells. Perforin and granzyme B are secreted to exert cellular immunity via the PI3K signaling pathway. In other cell types, tripterygium ingredients act on the PI3K signaling pathway to prohibit the activation of CD8+ T cells. Note: AKT (also known as PKB), Protein kinase B; PI3K, Phosphoinositide 3-kinase; mTOR, Mechanistic target of rapamycin kinase; S6K, Ribosomal protein S6 kinase; TCR, T-cell receptor; 4EBP, 4E-binding protein.
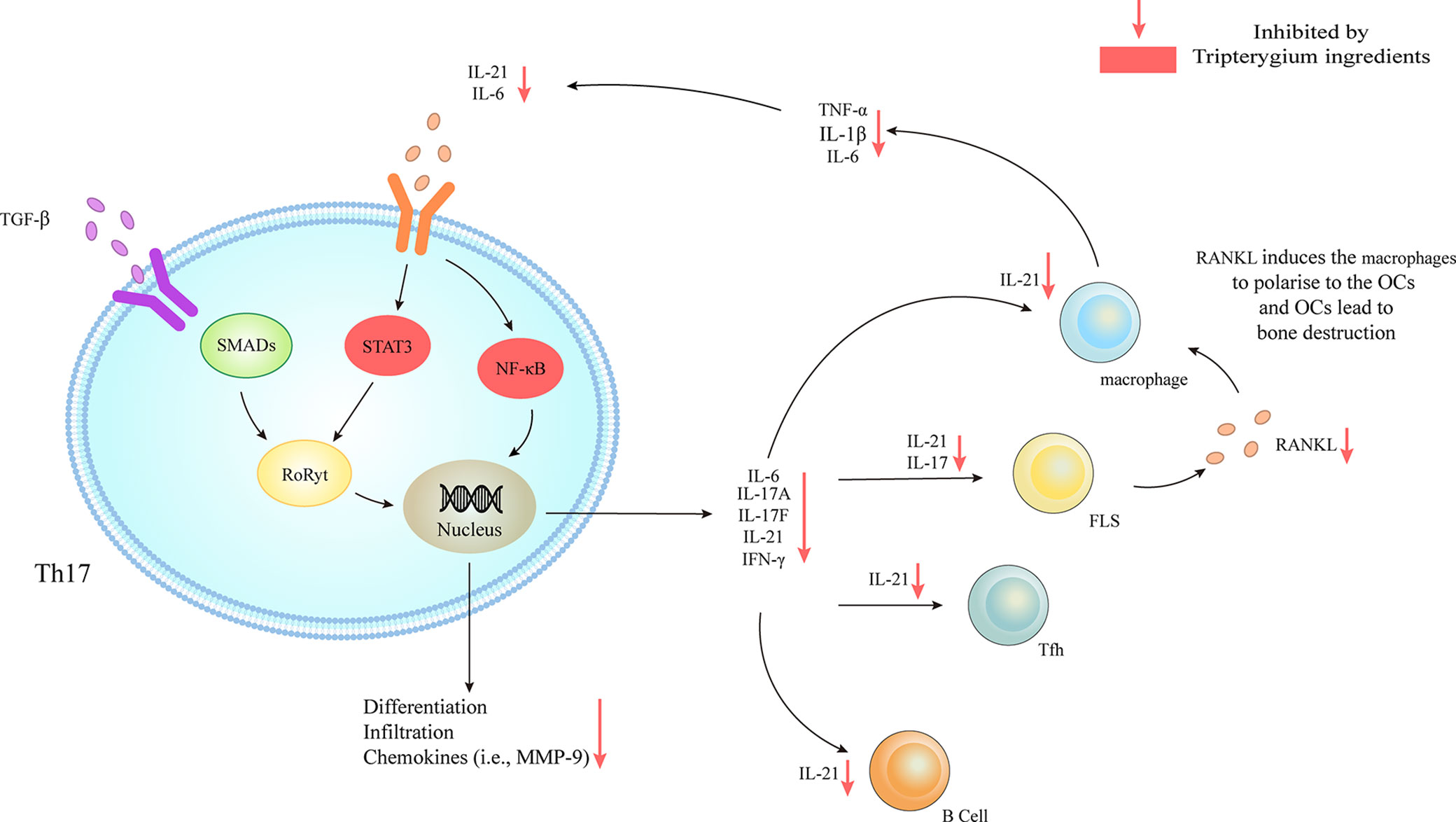
Figure 2 Tripterygium ingredients act on Th17. TGF-β/SMADs/RORγt, IL-6/STAT3/RORγt, and IL-6/NF-κβ signaling pathways are responsible for the differentiation and the secretion of chemokines and cytokines of Th17. The downstream effector cells include B cells, Tfh, FLS, and macrophages. The activation of FLS releases RANKL to promote the secretion of chemokines (i.e., IL-6, IL-1β, and TNF-α) of macrophages, which could target Th17 and further active Th17. Tripterygium ingredients inhibit STAT3 and NF-κβ, resulting in the reduction of many cytokines (i.e., IL-6 and IL-21) to negatively regulates Th17 and downstream effector cells. Note: FLS, Fibroblast-like synoviocytes; IFN-γ, Interferon-gamma; IL, Interleukin; NF-κβ, Nuclear factor-kappa B; RANKL, Receptor activator of nuclear factor-κB ligand; RORγt, Retinoic acid-related orphan receptor gamma t; SMAD, Suppressor of mothers against decapentaplegic; STAT, Signal transducer and activator of transcription; TGF-β, Transforming growth factor-beta; TNF-α, Tumor necrosis factor-alpha.
The Effects of Tripterygium Ingredients on B Cells
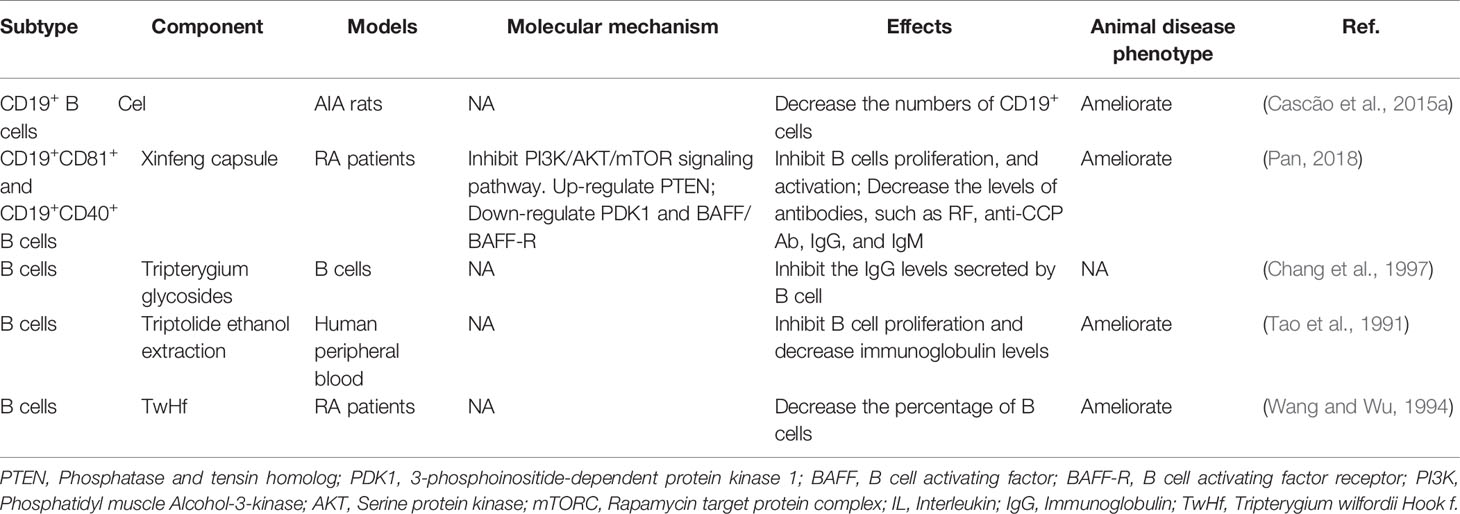
B cells are also critical in the development of RA. B cells can be used as antigen-presenting cells (APC) to provide synergistic stimulation and then activate T cells. In addition, B cells are able to secrete autoantibodies, such as rheumatoid factor (RF) and anti-citrullinated protein antibody (ACPA) (Scherer et al., 2018; Smolen et al., 2018). ACPAs, RF, or their immune complexes interact with immune cells such as macrophages, neutrophils, and osteoclasts to promote joint inflammation of RA. The differentiation and activation of B cells could be mediated by BAFF/BAFF-R-ATK-mTOR, and TACI-NF-κB (Niiro and Clark, 2002; Schmidlin et al., 2009; Pieper et al., 2013). Many studies have demonstrated that Tripterygium ingredients could inhibit the proliferation and the antibody production of B cells while the molecular mechanisms remained unknown (Tao et al., 1991; Wang and Wu, 1994; Chang et al., 1997; Cascão et al., 2015b). Only one study revealed the molecular mechanisms. Pan etc. found Xinfeng capsule, a proprietary Chinese medicine mainly composed of TwHf, can up-regulate the PTEN level of B cells while down-regulate PDK1 and BAFF/BAFF-R to suppress the activation of PI3K/AKT/mTORC signal pathway and finally inhibit the proliferation and activation of B cells (Pan, 2018). Besides, the levels of related antibodies such as RF, anti-cyclic citrullinated peptide antibody (anti-CCP Ab), IgG, and IgM, were also inhibited. The effects of Tripterygium ingredients on B cells and the molecule mechanisms are summarized in Table 2. The molecule mechanisms of B cell are summarized in Figure 3.
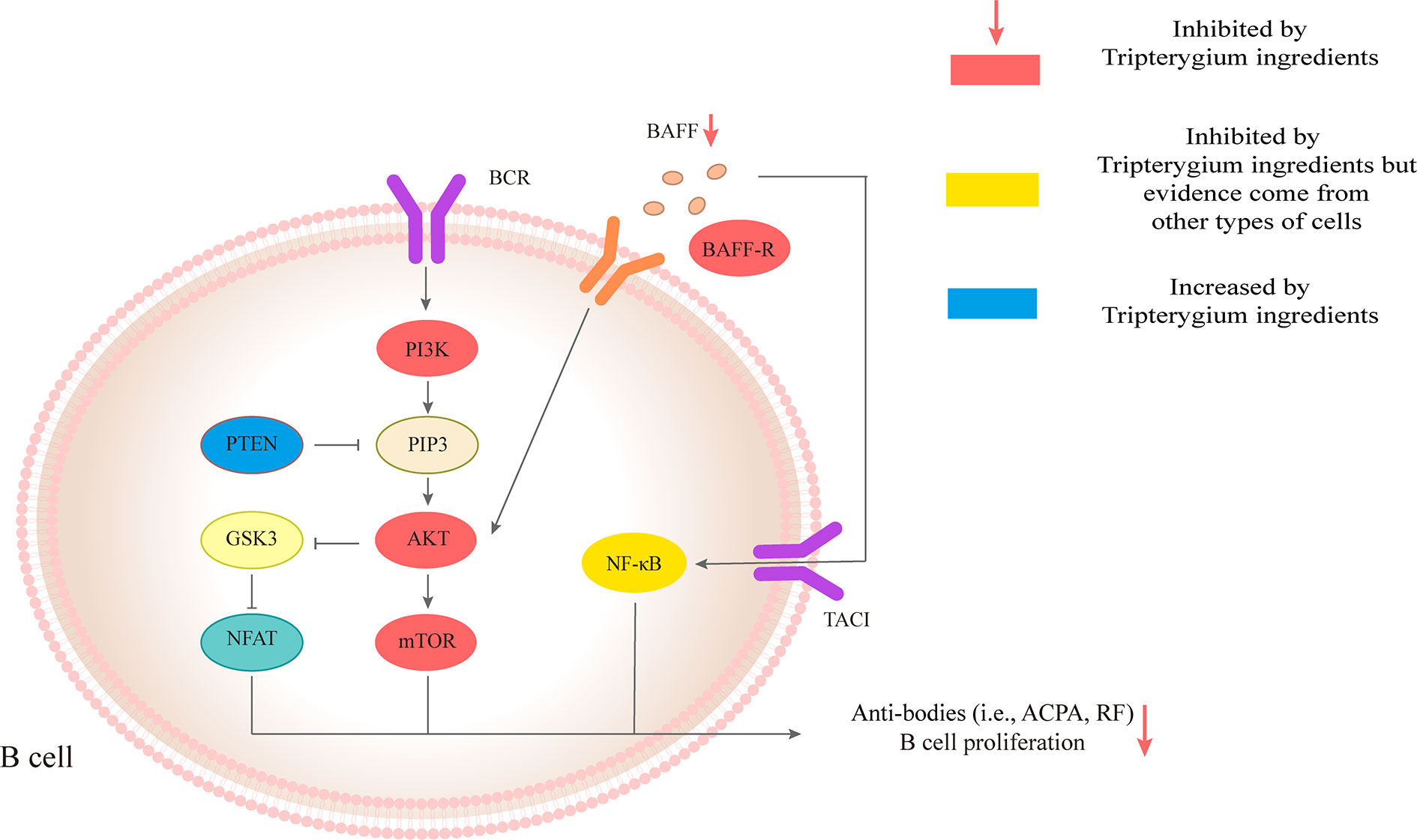
Figure 3 Tripterygium ingredients act on the B cell. The anti-bodies production and proliferation of B cells are associated with BAFF/AKT/mTOR, PI3K/AKT/mTOR, and TACI/NF-κβ signaling pathways. PTEN negatively regulates PI3K/AKT/mTOR signaling pathways through dephosphorylating the lipid signaling intermediate PIP3. Tripterygium ingredients could increase the levels of PTEN and decrease the levels of molecules (red nodes) in the three pathways mentioned above to inhibit the anti-bodies production and proliferation of B cells. Note: AKT (also known as PKB), Protein kinase B; BCR, B cell receptor; BAFF, B cell-activating factor; BAFF-R, B cell-activating factor receptor; GSK3. Glycogen synthase kinase-3; mTOR, Mechanistic target of rapamycin kinase; NF-κβ, Nuclear factor-kappa B; NFAT, Nuclear factor of activated T-cells; PI3K, Phosphoinositide 3-kinase; PIP3, phosphatidylinositol-3,4,5-trisphosphate; PTEN, Phosphatase and tensin homolog.
The Role of Tripterygium Ingredients on Macrophage and the Molecule Mechanism
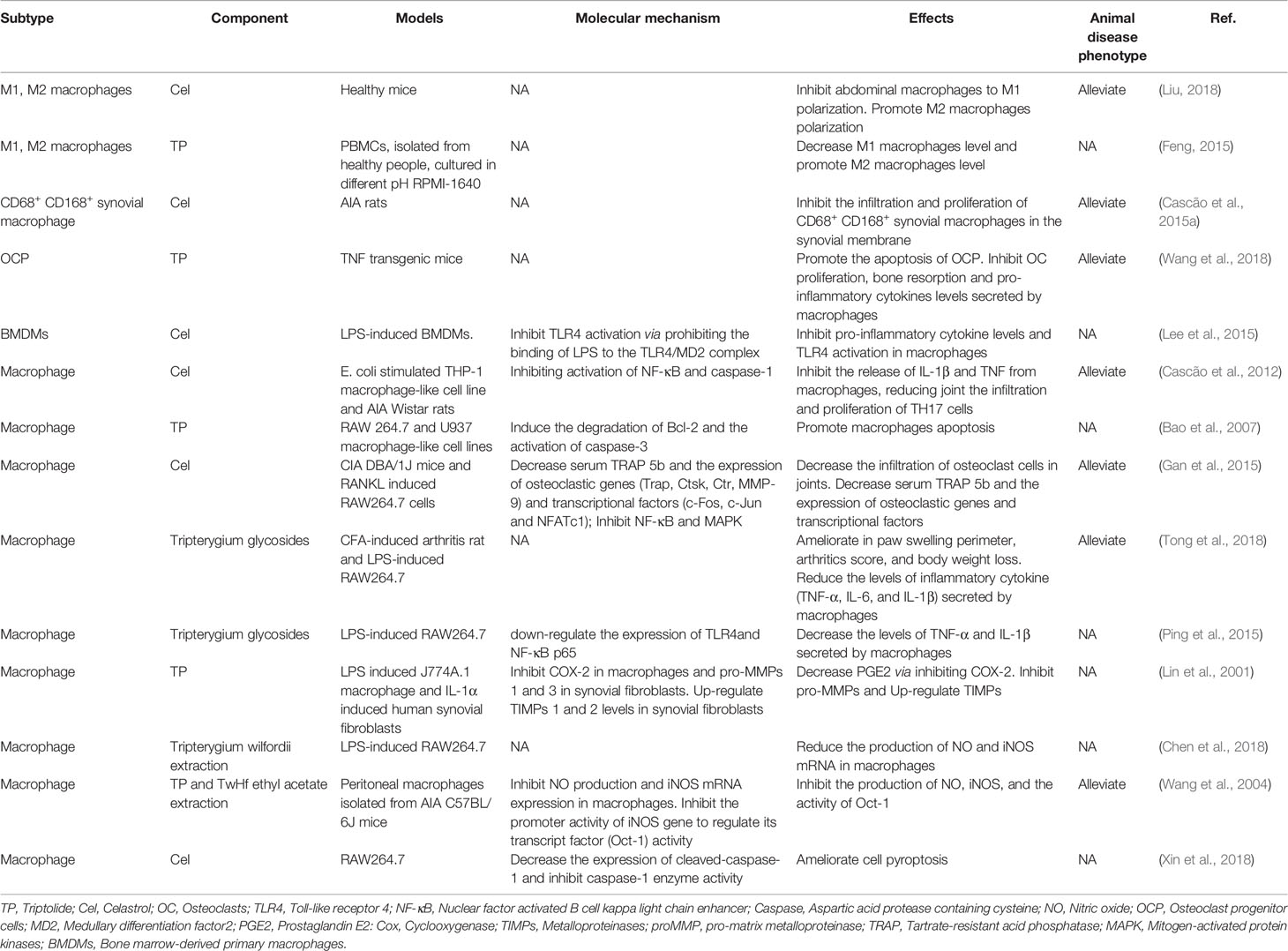
Macrophages are mainly divided into classical activated M1 type and selective activated M2 type. The immuno-inflammatory reaction in RA patients directly affects the polarization of macrophages in peripheral blood, synovium, and synovial fluid, resulting in the continuous increase of M1-type macrophages and disrupting the balance of M1/M2 (Laria et al., 2016). Macrophages also promote RA and participate in the bone destruction of RA through antigen presentation. The degree of synovial macrophage infiltration was positively correlated with the bone destruction and clinical symptoms of RA (Gierut et al., 2010).
Tripterygium ingredients have been reported to inhibit the M1 polarization and promote the polarization of M2 type macrophages, resulting in the rebalance of the pro-inflammatory and anti-inflammatory cytokines (Feng, 2015; Liu, 2018). Besides, tripterygium ingredients could alleviate synovial macrophage infiltration. However, the molecular mechanism was not explained (Bao et al., 2007; Cascão et al., 2015a; Cascão et al., 2015b; Feng, 2015; Gan et al., 2015; Liu, 2018; Tong et al., 2018; Wang et al., 2018). M1 polarization is mediated by JAK-STAT1 signaling stimulated with IFNγ and characterized by increased iNOS, IL-1β, and TNF-α. Another M1 activated signaling pathway is the TLR4/NF-κB signaling pathway (Lawrence and Natoli, 2011). In the AIA rat model, Cel exerted the anti-inflammatory properties via down-regulating the NF-κB and the caspase-1 activation, leading to a decrease of IL-1β and TNF-α secretion in macrophages. Furthermore, the proliferation of Th17 was also inhibited because of lacking cytokines stimulation (Cascão et al., 2012). In addition, research has indicated that Cel blocked the binding of lipopolysaccharides (LPS) to a myeloid differentiation factor2 (MD2) and then inhibited the M1 activation, which was measured by the expression of inflammatory cytokines including TNF-α, IL-6, and IL-1β (Lee et al., 2015). Some researchers (Lin et al., 2001; Ping et al., 2015) also found that Tripterygium ingredients decreased the production of TNF-α, IL-1β, and IL-6 via inhibiting the expression of the TLR4, NF-κB, and prostaglandin E2 (PGE2). Besides, NO production and iNOS expression in macrophages were significantly inhibited by Tripterygium ingredients (Wang et al., 2004; Chen et al., 2018). Furthermore, TP inhibited the promoter activity of the iNOS gene and the inducible activity of iNOS transcriptional regulator Oct-1 (Wang et al., 2004). Pyroptosis is a unique and newly discovered mode of programmed cell death, which is triggered by the activation of Caspase-1 (Bergsbaken et al., 2009). It has been found that Cel can inhibit the pyroptosis induced by LPS and ATP via inhibiting the enzyme activities of cleaved-Caspase1 and Caspase-1, and finally blocking the secretion of IL-1β in macrophages (Xin et al., 2018). The effect of tripterygium ingredients on macrophages and the molecule mechanisms are summarized in Table 3. The figure for the molecule mechanism of macrophages is available in Figure 4.
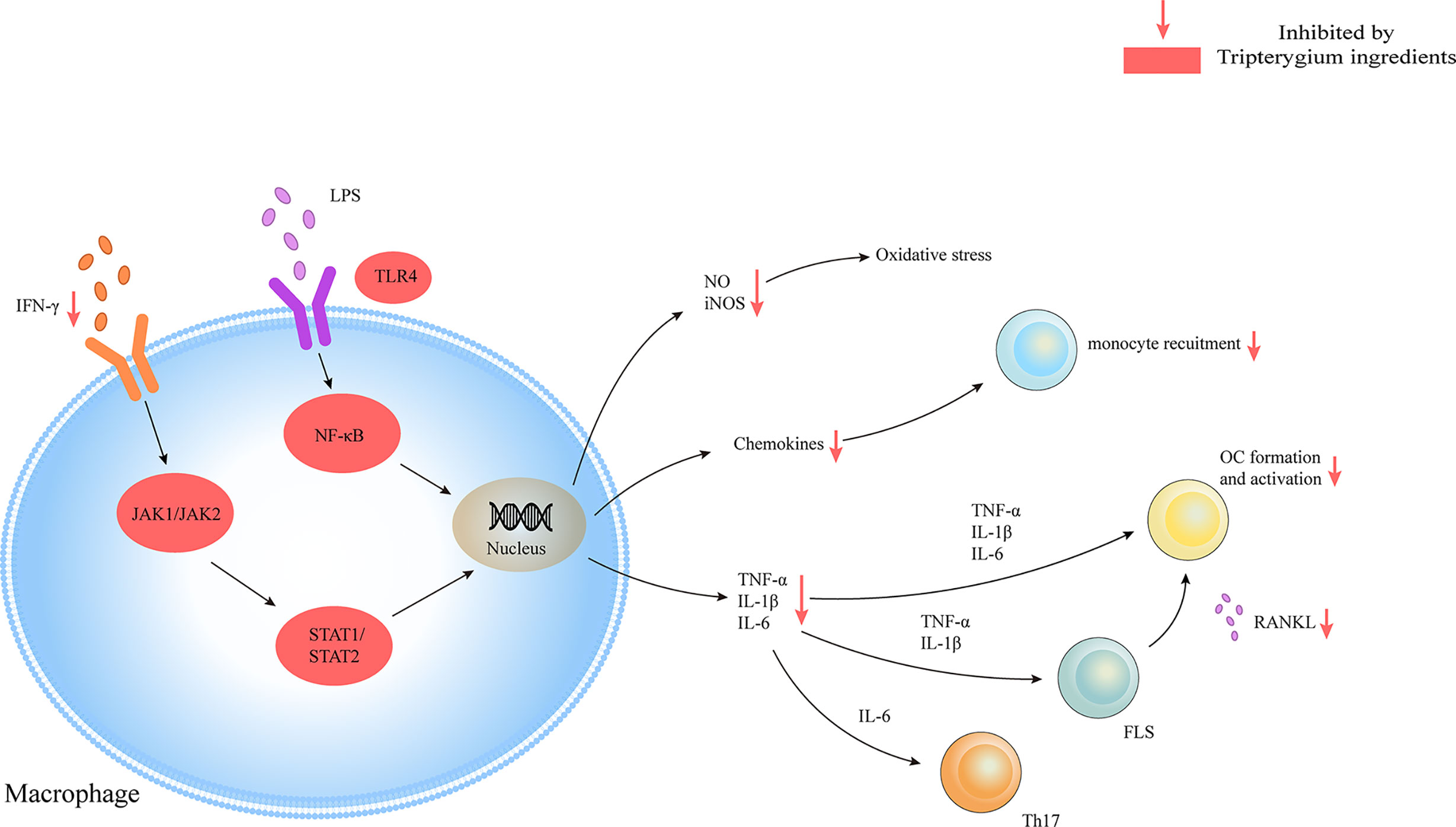
Figure 4 Tripterygium ingredients act on the macrophage. Macrophages play as pro-inflammatory cells in RA with the release of chemokines, cytokines, and oxidative stress molecules through JAK/STAT and TLR/NF-κβ signaling pathways. Tripterygium ingredients block the signaling pathways to lead to the reduction of cytokines, which would inhibit the activation of effector cells. Note: FLS, Fibroblast-like synoviocytes; IFN-γ, Interferon-gamma; IL, Interleukin; JAK, Janus kinase; NF-κβ, Nuclear factor-kappa B; OC, Osteoclast; RANKL, Receptor activator of nuclear factor-κB ligand; STAT, Signal transducer and activator of transcription; TLR, Toll-like receptor; TNF-α, Tumor necrosis factor alpha.
The Effects of Tripterygium Ingredients on Dendritic Cells (DCS)
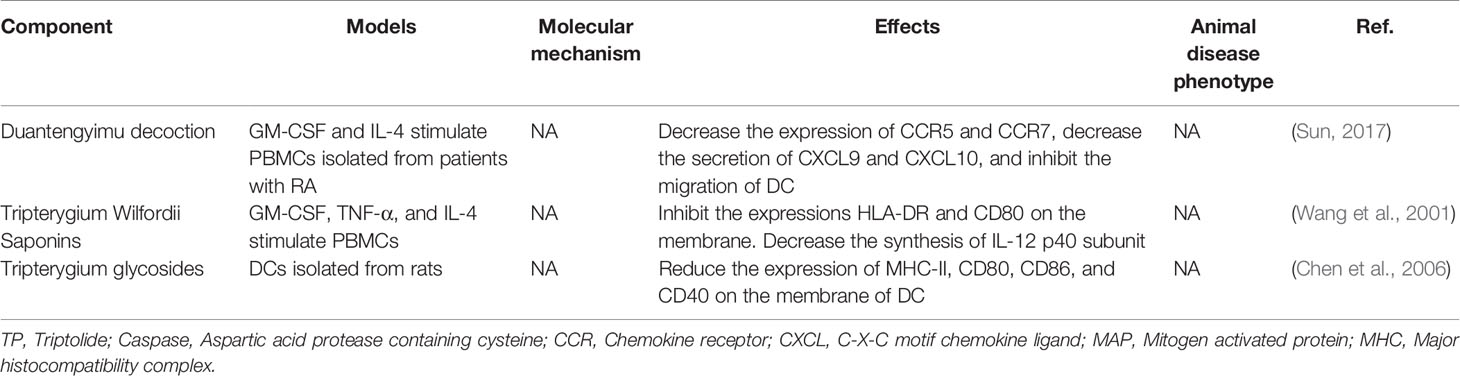
TwHf is reported to inhibit DC development and induce DC apoptosis, finally decreasing the DC number, which resulted in the blocking of the naïve T cell activation and ultimately reduced the differentiation of the autoinflammatory T cells (Wang et al., 2001; Chen et al., 2006; Sun, 2017). Also, TP inhibits DC-related chemokines and reduces the sharing of DCs with MHC molecules and co-stimulatory factors of T and B cells, thereby blockade T and B cell activation. Antigenic peptide on MHC molecules, co-stimulatory molecules (CD80, CD86), and IL-12 of DCs promote the differentiation of Th1 cells, which produce IFN-γ and IL-2, required for cell-mediated immunity. Th1 cells directly modulate B cell differentiation into plasma cells. Besides, DCs also mediate the proliferation of these antibody-producing cells by producing BAFF (Khan et al., 2009). Unfortunately, the specific molecule mechanisms were not involved in these studies. Tripterygium ingredients for DCs are summarized in Table 4.
The Functions of Tripterygium Ingredients on Osteoclasts (OCS) and the Molecule Mechanism
OC-mediated bone resorption is one of the typical manifestations of RA. OCs, giant multinucleated cells derived from the monocyte lineage, are the only cells capable of resorbing bone (Teitelbaum and Ross, 2003). Receptor activator of nuclear factor kappa-B (RANK)/Receptor activator of nuclear factor kappa-B ligand (RANKL)/Osteoprotegerin (OPG) is the most crucial pathway of OC differentiation. Leibbrandt A etc. has demonstrated that RANKL is the critical mediator of OC activation and joint destruction; In a rat model of arthritis, osteoblasts and bone marrow stromal cells produce RANKL, which then triggers local development and activation of OCs. This finding has now become the basis for osteoimmunology (Leibbrandt and Penninger, 2009). Multiple studies have confirmed that Tripterygium ingredients can inhibit the expression of RANK and RANKL, thereby increasing the proportion of OPG, which can antagonize the function of RANK and finally inhibit the differentiation of OCs and reduce bone destruction (Nanjundaiah et al., 2012; Feng et al., 2013; Liu et al., 2013; Wang, 2015). Youn-Kwan Jung et al. (Jung et al., 2019) reviewed the roles of inflammatory signal pathways, including IL-1β/Myd88/TRAF6/NF-κB, TNF-α/TRADD/TRAF2/NF-κB, IL-6/STAT3/MAPK, and RANKL/RANK signal transduction. In these inflammatory pathways, TwHf or its active components impaired the release of cytokines/chemokines, reduced osteoclast differentiation, and activation, then finally blocked bone erosion in mice with collagen-induced arthritis through inhibiting the phosphorylation of NF- κB p65, MAPK (ERK, JNK, and p38) and reducing the expression of transcription factors c-Fos, c-Jun, and NFATcl (Gan, 2013; Qian et al., 2015). TwHf or its active components can also promote the apoptosis of OCs and osteoclast precursor (OCP) (Wang et al., 2018; Wang S. et al., 2019). The mechanism may be due to the inhibition of cIAP2 (the positive regulatory protein of TNF and NF-κB signaling pathway). Furthermore, TP has been reported to block OC differentiation by down-regulating the receptor for advanced glycation end-products (RAGE) and the high-mobility group box chromosomal protein 1 (HMGB1) (Wang et al., 2017). RAGE and its ligands (i.e., HMGB1) are necessary for the skeletal homeostasis and related-disease onset/progression (Plotkin et al., 2019). The elevated levels of RAGE and HMGB1 induce osteoblast apoptosis and OC differentiation/activity. Tripterygium ingredients on OC and the molecule mechanisms are summarized in Table 5. The figures for the molecule mechanism of OCs are available in Figure 5.
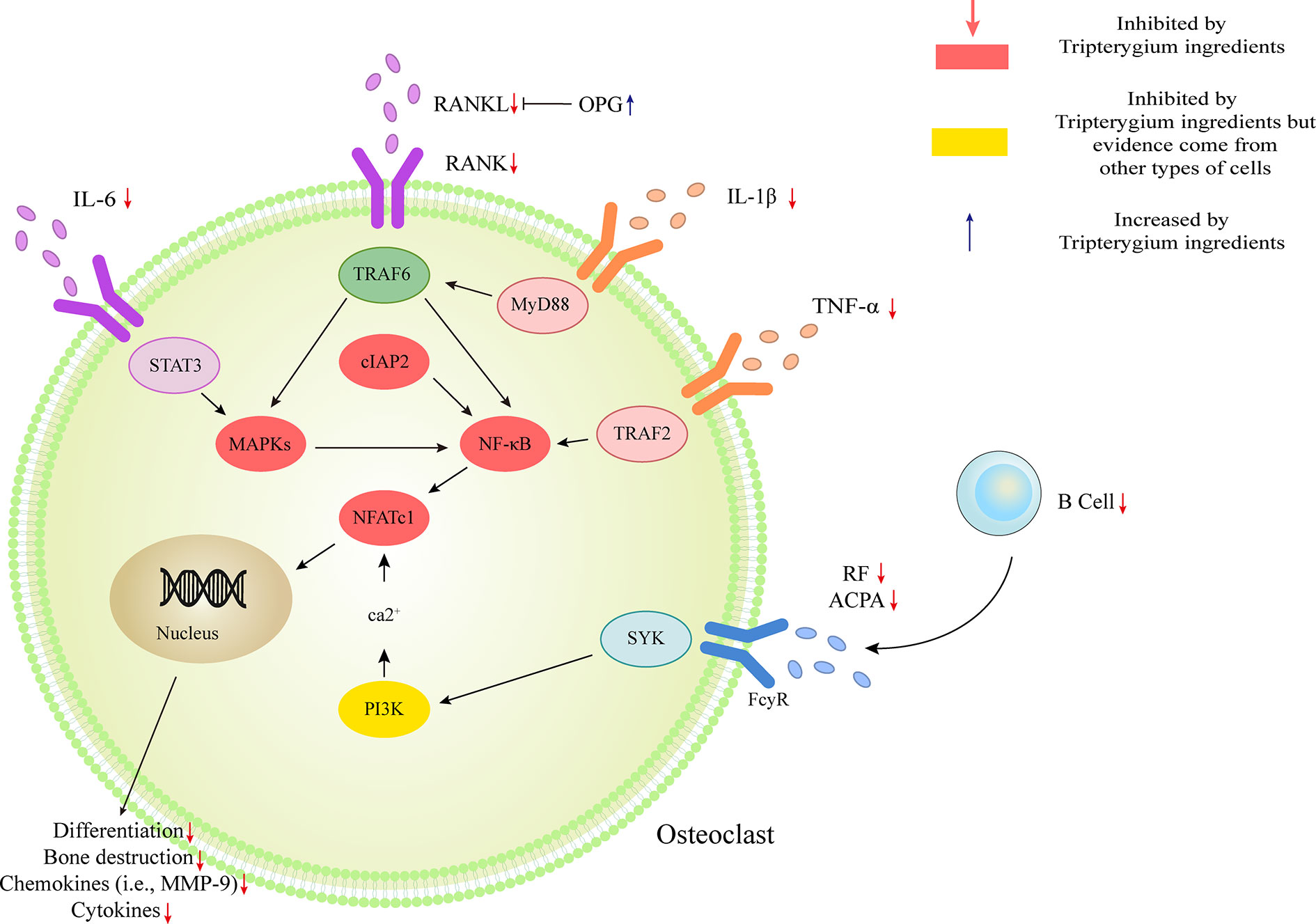
Figure 5 Tripterygium ingredients act on osteoclast. The activation of osteoclast mostly involved IL-1β/Myd88/TRAF6/NF-κB, TNF-α/TRADD/TRAF2/NF-κB, IL-6/STAT3/MAPK, and RANKL/RANK signal transduction. Besides, the anti-bodies released by B cells would promote the activation of osteoclast via PI3K signaling. OPG takes part as a decoy receptor for RANKL and inhibiting RANKL-RANK binding. By rebalancing the RANKL and OPG levels, and inhibiting the pathways mentioned above, tripterygium ingredients negatively regulate the differentiation, bone destruction, cytokines and chemokines expression of osteoclast. Note: ACPA, Anti-citrullinated protein antibodies; cIAP2, Cellular inhibitor of apoptosis 2; IL, Interleukin; MAPK, Mitogen-activated protein kinase; MYD88, Myeloid differentiation primary response 88; NFATc1, Nuclear factor of activated T cells 1; RANK, Receptor activator of nuclear factor-κB; RANKL, Receptor activator of nuclear factor-κB ligand; RF, Rheumatoid factor; STAT, Signal transducer and activator of transcription; SYK, Spleen tyrosine kinase; TNF-α, Tumor necrosis factor-alpha; TRAF, Tumor necrosis factor receptor-associated factor.
The Role of Tripterygium Ingredients on FLS and the Molecule Mechanism
Synovial inflammation and synovial cell hyperplasia is a distinctive feature of RA. Synovial cells are composed of two types of cells, including type A and type B. Type A cells have a phagocytic function and are macrophage-like cells; type B cells are fibroblast-like, called FLS (Junqueira and Mescher, 2013). FLS is abundant in the endoplasmic reticulum and can secrete protein complexes (mucin) and hyaluronic acid in synovial fluid. FLS contributes mainly to the exacerbation of RA by attaching to, followed by invading into, and finally degrading cartilage and bone (Lefèvre et al., 2009). FLS are the primary cells leading to joint destruction in RA (Bartok and Firestein, 2010).
The molecular pathologic basis RA-FLS includes the MAPK and NF-κB pathways. These pathways are the most widely studied to mediate the aggressiveness of FLS in RA (Bottini and Firestein, 2013; Ganesan and Rasool, 2017). NF-κB pathway, a significant regulator of pro-inflammatory cytokine production, activates NF-κB kinase (IKK) subunit β (IKKβ) in the cytosol through IL-1β, TNF-α, and TLR signaling. The activation of IKKβ results in the NF-κB family inhibitor proteins (IκB) degradation, promoting NF-κB to migrate freely into the nucleus and initiate gene transcription (Bottini and Firestein, 2013). Cel inhibited the translocation of NF-κβ p65 and reduced the phosphorylation of IKBα and IKK in FLSs from patients with RA, resulting in the decreased expression of several chemokines (i.e., CCR2, CXCR4, CCL2, CXCL10, and CXCL12), cytokines (i.e., IL-6, IL-8, and MCP-1), and matrix metalloproteinase-9 (MMP-9) (Fang et al., 2017). Besides, HIF-1α binding to the CXCR4 promoter would increase the transcriptional activity of CXCR4, consequently leading to FLS migration and invasion. However, it could be reversed by Cel treatment (Li et al., 2013b). Guo et al. (Li et al., 2012) found that Cel inhibited IκBα phosphorylation and nuclear translocation of NF-κB. Cel also has been found to inhibit the expression of MMP-9 by suppressing the binding activity of NF-κB to the MMP-9 promoter (Li et al., 2012; Li et al., 2013a). Furthermore, MMP-9 suppression was also related to the inhibition of the TLR4/MyD88/NF-κB pathway (Li et al., 2013a). As a result, Cel changes the phenotype of FLS migration and invasion via the molecule mechanism mentioned above. RA-FLS releases important inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-21, IL-22, and IL-32), chemokines (CXCL1, CXCL5, MCP-1, G-CSF, and IL-8) and Inflammatory mediators (TLR-2, TLR-3, TLR-4, iNOS, and COX-2), which promotes the infiltration of monocytes, macrophages, neutrophils, DCs, T cells, and B cells into joints and results in chronic inflammation and joint destruction (Bottini and Firestein, 2013; Ganesan and Rasool, 2017). Additionally, TP also was found to reduce the FLS migration and invasion by targeting JNK/MAPK signaling pathway (Yang et al., 2016). Moreover, LLDT-8, a Tripterygium derivative, decreased the secretion of chemokines in FLS (Ping et al., 2016; Jia et al., 2017).
Many studies showed the Tripterygium ingredients have the properties to promote FLS apoptosis and cell cycle arrest and inhibit FLS autophagy (Xu, 2013; Xu et al., 2013; Lei et al., 2015; Su et al., 2017; Wong et al., 2019). It may be relevant to the increased expression of Bax/Bcl-2, Caspase-3, Caspase-9, and regulating by Ca2+/calmodulin-dependent protein kinases beta (CaMKK)-AMPK-mTOR signaling pathway. Tripterygium ingredients for FLS and the molecule mechanism are summarized in Table 6. The figures for the molecule mechanism of FLS are available in Figure 6.
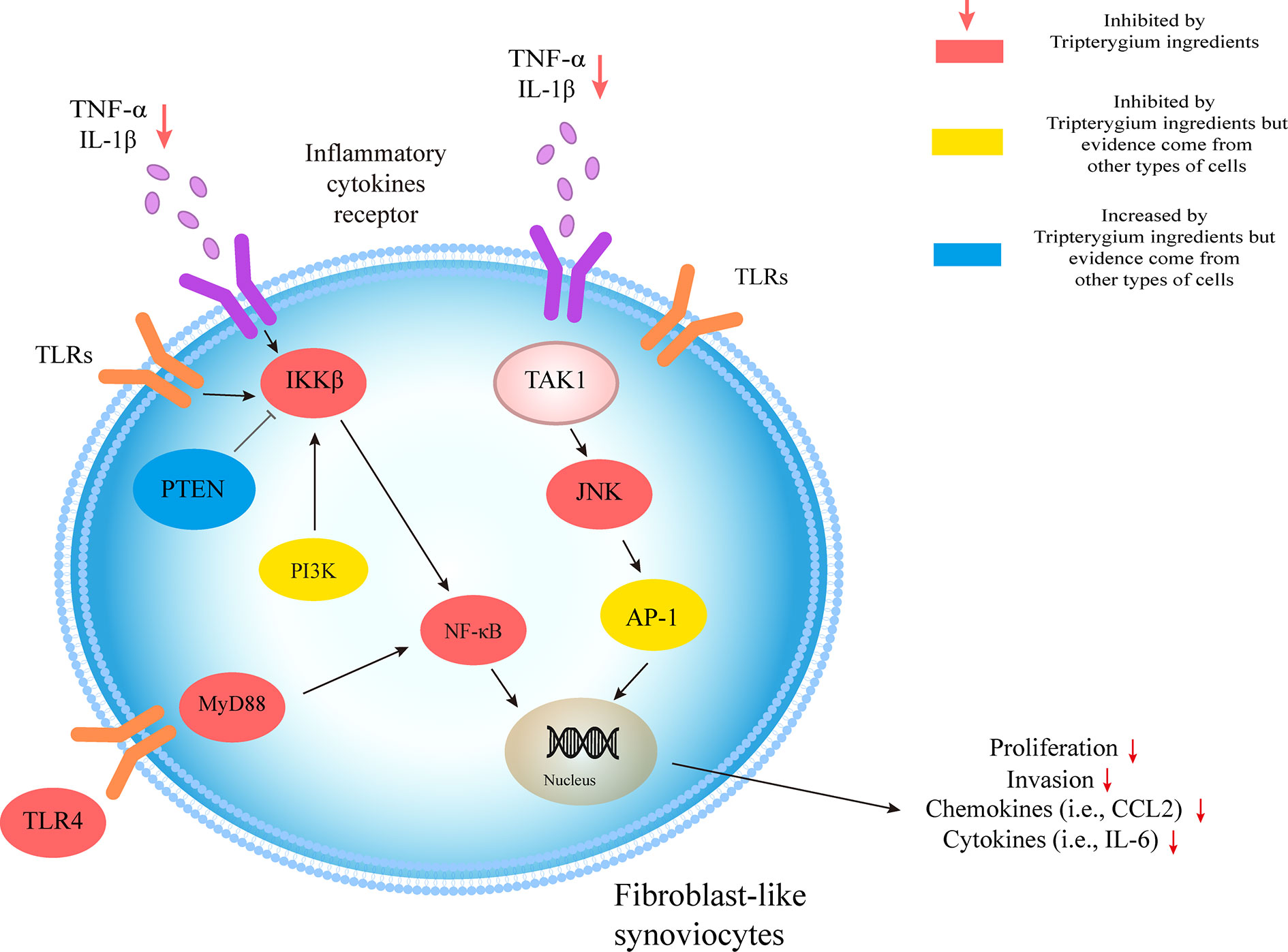
Figure 6 Tripterygium ingredients act on FLS. The molecular pathologic basis FLS includes the MAPK and NF-κB pathways. By prohibiting the signal transduction in the pathways, tripterygium ingredients alleviate the proliferation, invasion of FLS, which would promote bone erosion in RA. PTEN and PI3K, which inhibit and promote the activity of IKKβ, respectively, also are related in the networks of FLS. We suppose tripterygium ingredients could increase PTEN and decrease PI3K to alleviate the pathogenicity of FLS. Note: AP-1, Activator protein 1; FLS, Fibroblast-like synoviocytes; JNK, c-Jun N-terminal kinase; IL, Interleukin; IKKβ, IκB Kinase β; MYD88, Myeloid differentiation primary response 88; NF-κβ, Nuclear factor-kappa B; PI3K, Phosphoinositide 3-kinase; PTEN, Phosphatase and tensin homolog; TNF-α, Tumor necrosis factor-alpha; TLR, Toll-like receptor; TAK1, Transforming growth factor beta-activated kinase 1.
Discussion
We systematically summarized the role of tripterygium ingredients in the RA treatment as well as explained the therapeutic mechanism (Figure 7). The NF-κB pathway is a common pathway involved in TwHf-treated RA. It has been involved in mediating multiple genes, such as the genes of cytokines (i.e., IL-6, IL-17, and TNF-α), chemokines (i.e., CCL2 and CXCL5), growth factors (i.e., GM-CSF and M-CSF), regulators of apoptosis (i.e., Bcl-2) and transcription factors (i.e., HIF-1α), to regulate cell function, cell death and survival, and proliferation (Mitchell and Carmody, 2018). Experimental inhibitors targeted the IKK kinases to inhibit the activation of NF-κB, but they failed due to toxicity in genetic models (Mitchell and Carmody, 2018). The failure indicated that a broad blockade of NF-κB activation maybe an impracticable approach. Thus, some drugs focus on the non-canonical NF-κB pathway in RA, such as BAFF/NF-κB, RANK/NF-κB signaling (Noort et al., 2015). A phase II trial showed that belimumab [a biologics target B lymphocyte stimulator (BLyS)] was efficacy and well-tolerated in patients with RA (Stohl et al., 2013). Denosumab is a monoclonal antibody neutralizing RANKL. Up to date, many clinical studies have demonstrated that denosumab could inhibit the progression of joint destruction and increase bone mineral density, including a double-blind, placebo-controlled phase 3 trial (Deodhar et al., 2010; Dore et al., 2010; Sharp et al., 2010; Takeuchi et al., 2016; Yue et al., 2017; Takeuchi et al., 2019). However, none of the studies reported there is any benefit in improving disease activity. It is also regrettable that none of the studies test the expression of cytokines, chemokines, and RA-related pathogenicity cells. NF-κB pathways, including canonical and non-canonical pathways, are critical targets of tripterygium ingredients. In the experimental and clinical dimensions, these could explain why tripterygium ingredients could reduce the levels of many chemokines, cytokines, and growth factors in different cells to improve disease activity as well as inhibit the progression of joint destruction. TwHf and its ingredients could be regarded as one of DMARDs. Thus, they are widely used in treating RA in China. The last 24 weeks, open-label, multicentre, randomized controlled trial demonstrated MTX+TwHF was better than MTX monotherapy (Lv et al., 2015). Furthermore, three meta-analyses (the trial mentioned above included) showed that MTX+TwHF had advantages in improving the laboratory index (CRP, RF, ESR), clinical symptoms, and clinical efficacy, compared with MTX alone (assessed in ACR20, ACR50, and ACR70) (Li et al., 2019; Wang X. et al., 2019; Chen et al., 2020). Another small sample meta-analyses showed that TwHF could decrease bone destruction scores (Zhu et al., 2019).
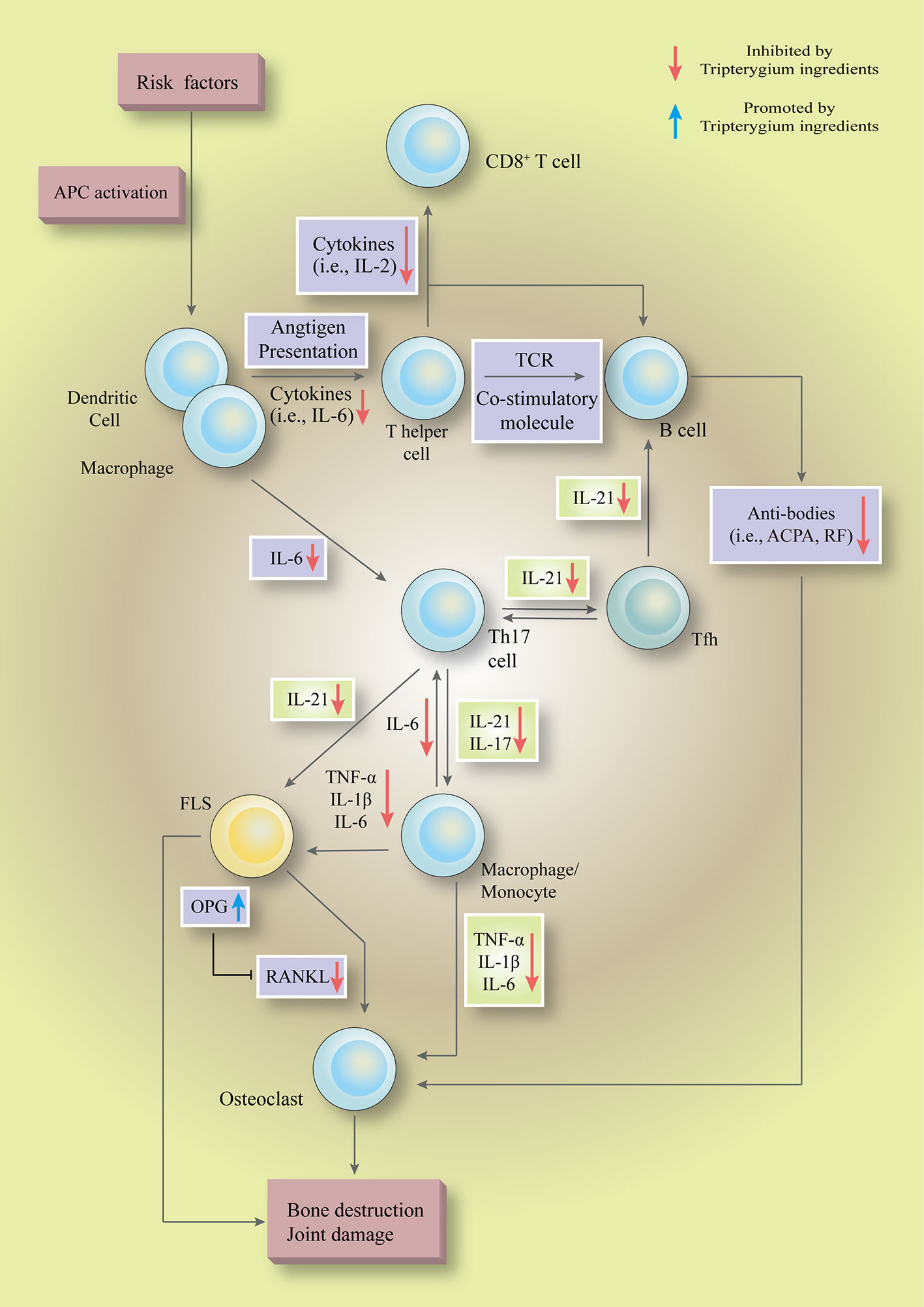
Figure 7 Tripterygium ingredients could inhibit multiple pathways, such as the NF-κB pathway, JAK-STAT pathway, and PI3K-mTOR pathway, to regulate the hyperactive as well as pathogenicity biological functions in a various type of cells ( to ). In the initial phase, tripterygium ingredients inhibit the immunological recognition functions of APCs to block the pathogenicity signals which are responsible for activating the lymphocytes. In the secondary stage, tripterygium ingredients could inhibit the humoral immunity and cellular immunity of lymphocytes. Meanwhile, the pro-inflammatory signals are amplified by pro-inflammatory cells, such as macrophages. The pro-inflammatory cells release inflammatory cytokines and chemokines to recruit and activate immune cells (i.e., APCs and T cells), connective tissue cells (i.e., macrophages and FLS) and OCs to infiltrate into the joints. Besides, the pro-inflammatory cells lead to a systemic inflammatory response, further promoting the pathogenicity signals of lymphocytes in central and peripheral immune organs. Tripterygium ingredients prohibit the pro-inflammatory signals, alleviate the infiltration and activation of pathogenicity cells in the joint, and finally interrupt the vicious circle which formed by pro-inflammatory cells and immune cells. In the final stage, tripterygium ingredients relieve the joint damage and bone destruction by mediating the expression of OPG and RANKL. Note: APC, Antigen-presenting cell; ACPA, Anti-citrullinated protein antibodies; FLS, Fibroblast-like synoviocytes; IL, Interleukin; OPG, Osteoprotegerin; RANKL, Receptor activator of nuclear factor-κB ligand; RF, Rheumatoid factor; TCR, T-cell receptor; TNF-α, Tumor necrosis factor-alpha.
Our previous research using a bioinformatics approach demonstrated that Kunxian Capsule (a Traditional Chinese Medicine (TCM) patent prescription mainly comprises Levl. Hutch) could target at PI3K/AKT/mTOR signaling pathway (Tang et al., 2020). Therefore, we speculate that some proteins in the PI3K-AKT-mTOR signal pathway are the most likely direct targets of tripterygium ingredients. The signaling pathway is an intracellular signaling pathway and performs multiple physiological functions, such as regulating the cell cycle, survival, and growth (Yap et al., 2008; Ersahin et al., 2015). By far, no clinical study has reported PI3K inhibitors approved by the FDA (idelalisib, copanlisib, duvelisib, and alpelisib) in treating RA. Some drugs were demonstrated they were effective in treating RA models via PI3K signaling pathway in vivo and in vitro, including one PI3K inhibitor (Boyle et al., 2014; Feng and Qiu, 2018; Qi et al., 2019).
The two pathways mentioned above have a variety of biological functions. Both of them control cell death, survival, and proliferation. We found that the therapeutic effects of tripterygium ingredients are mostly related to the reduction of the absolute number of cells. At the same time, there are some differences in stimulating signals, signal receptors, and transcription factors required for cascade reactions in different cells, which is related to the multi-target of TwHf. Considering the adverse effects (AEs) of these drugs, a variety of healthy cells in multiple systems (i.e., liver cells) are also be affected. Therefore, the AEs of tripterygium ingredients could be due to the inhibition of NF-κB and PI3K pathways. Ameliorating AEs through drug matching may be a feasible strategy (Tang et al., 2020). For example, some Chinese researchers matched the TwHf with Cistanche deserticola Ma or Cuscuta chinensis Lam, to reduce the reproductive toxicity of TwHf (Dong et al., 2009; Jing and He, 2013). Nevertheless, whether drug matching would impair the curative effect, still needed to be discovered.
There are many deficiencies in this review. The studies on the drugs/ingredients are all indirect mechanism studies, even with only cell phenotypes but no specific molecular mechanism. Besides, most of them are normal phenotypes in this field, such as inhibition of the apoptosis and differentiation of T cells or impaired proliferation, migration, and invasion of FLS. Moreover, none of the studies analyzed the direct interaction between the drugs and proteins via bioinformatics and mass spectrometry approaches. For example, computer simulation and electrospray mass spectrometry (ESI-MS) were used to explore the inhibitory effect of paclitaxel and aryl ether ketone on farpentine diphosphate synthetase by binding to isoprene diphosphate site (Liu et al., 2014), and explain the anticancer and anti-infective drug mechanism of paclitaxel and aryl et her ketone in the direct interaction mechanism. Therefore, we could follow the methods which were used in the study, as mentioned above. For example, we could use bioinformatics to identify whether TP and Cel could bind to some sites of PI3K, ATK, and NF-κB. Subsequently, we could use ESI-MS to validate it.
Author Contributions
YT: evidence collection, manuscript preparation and write the central part of the manuscript. QL: evidence collection and manuscript editing. FY: evidence collection and write the minor part of the manuscript. YiZ: evidence collection, figures preparation. XZ: gave many professional suggestions and project funding. CW: ideas, reviewed the manuscript, and project funding. YuZ: critically reviewed the manuscript, study initiation, and project funding. All authors contributed to the article and approved the submitted version.
Funding
Research was funded by National Key R&D Program of China (2018YFC1705500) to CW, National Natural Science Foundation of Zhejiang Province (No.LY20H270007), TCM Science and Technology Plan of Zhejiang Province (No.2020ZQ012) to YZ, and National Natural Science Foundation of China (81673623) to ZX.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Yujie Tang for the modification of the figures.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.583171/full#supplementary-material
References
Bao, X., Cui, J., Wu, Y., Han, X., Gao, C., Hua, Z., et al. (2007). The roles of endogenous reactive oxygen species and nitric oxide in triptolide-induced apoptotic cell death in macrophages. J. Mol. Med. (Berl) 85 (1), 85–98. doi: 10.1007/s00109-006-0113-x
Bartok, B., Firestein, G. S. (2010). Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev. 233 (1), 233–255. doi: 10.1111/j.0105-2896.2009.00859.x
Bergsbaken, T., Fink, S. L., Cookson, B. T. (2009). Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7 (2), 99–109. doi: 10.1038/nrmicro2070
Bottini, N., Firestein, G. S. (2013). Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat. Rev. Rheumatol. 9 (1), 24. doi: 10.1038/nrrheum.2012.190
Boyle, D. L., Kim, H. R., Topolewski, K., Bartok, B., Firestein, G. S. (2014). Novel phosphoinositide 3-kinase δ,γ inhibitor: potent anti-inflammatory effects and joint protection in models of rheumatoid arthritis. J. Pharmacol. Exp. Ther. 348 (2), 271–280. doi: 10.1124/jpet.113.205955
Cascão, R., Vidal, B., Raquel, H., Neves-Costa, A., Figueiredo, N., Gupta, V., et al. (2012). Effective treatment of rat adjuvant-induced arthritis by celastrol. Autoimmun. Rev. 11 (12), 856–862. doi: 10.1016/j.autrev.2012.02.022
Cascão, R., Vidal, B., Lopes, I. P., Paisana, E., Rino, J., Moita, L. F., et al. (2015a). Decrease of CD68 Synovial Macrophages in Celastrol Treated Arthritic Rats. PloS One 10 (12), e0142448. doi: 10.1371/journal.pone.0142448
Cascão, R., Vidal, B., Lopes, I. P., Paisana, E., Rino, J., Moita, L. F., et al. (2015b). Decrease of CD68 synovial macrophages in celastrol treated arthritic rats. PloS One 10 (12), e0142448. doi: 10.1371/journal.pone.0142448
Chang, D. M., Chang, W. Y., Kuo, S. Y., Chang, M. L. (1997). The effects of traditional antirheumatic herbal medicines on immune response cells. J. Rheumatol. 24 (3), 436–441.
Chao, L. (2015). Study on the differences between Tripterygium wilfordii Hook.f. and Tripterygium Hypoglaucum (Level) Hutch Based on Genetics and chemical methods (China Academy of Chinese Medical Sciences: Dissertation).
Chen, T., Xu, H., Wang, H., Ji, M., Wu, W. (2006). Single and conjoined effects of tripterygium wilfordii and IL-10 on the immuna function of rat dendritic cells in vitro. Acta Universit. Med. Nanjing (Natural Sci.) 07), 520–522. doi: 10.1007/s11664-006-0095-z
Chen, X.-L., Liu, F., Xiao, X.-R., Yang, X.-W., Li, F. (2018). Anti-inflammatory abietanes diterpenoids isolated from Tripterygium hypoglaucum. Phytochemistry 156, 167–175. doi: 10.1016/j.phytochem.2018.10.001
Chen, W., Li, T., Wang, X., Xue, Z., Lv, C., Li, H., et al. (2020). Meta-analysis of RCT studies on clinical efficacy of single administration of Tripterygium Glycosides Tablets or combined administration with methotrexate against rheumatoid arthritis. China J. Chin. Mater. Med. 45 (04), 791–797. doi: 10.19540/j.cnki.cjcmm.20191115.503
Chen-qiong, X., Ping, Z., Xiang, L., Jian-wei, C. (2015). Research progress on chemical constituents, pharmacological effects, and clinical application of Tripterygium hypoglaucum. Chin. Tradit. Herbal Drugs 46 (13), 1996–2010. doi: 10.7501/j.issn.0253-2670.2015.13.024
Chinese Rheumatology Association (2018). 2018 Chinese rheumatoid arthritis diagnosis and treatment guide. Clin. Res. Pract. 57 (4), 242–251. doi: 10.3760/cma.j.issn.0578-1426.2018.04.004
Cooles, F. A., Isaacs, J. D., Anderson, A. E. (2013). Treg cells in rheumatoid arthritis: an update. Curr. Rheumatol. Rep. 15 (9), 352. doi: 10.1007/s11926-013-0352-0
Deodhar, A., Dore, R. K., Mandel, D., Schechtman, J., Shergy, W., Trapp, R., et al. (2010). Denosumab-mediated increase in hand bone mineral density associated with decreased progression of bone erosion in rheumatoid arthritis patients. Arthritis Care Res. (Hoboken) 62 (4), 569–574. doi: 10.1002/acr.20004
Dong, F., Li, J., Huang, D., He, L. (2009). Effects of Glycosides of Tripterygium Wilfordii on Reproduction Capacity and Its Intervention by Caulis Cistanchi in Male Mice. Shanghai J. Tradit. Chin. Med. 43 (08), 64–66. doi: 10.16305/j.1007-1334.2009.08.025
Dore, R. K., Cohen, S. B., Lane, N. E., Palmer, W., Shergy, W., Zhou, L., et al. (2010). Effects of denosumab on bone mineral density and bone turnover in patients with rheumatoid arthritis receiving concurrent glucocorticoids or bisphosphonates. Ann. Rheum Dis. 69 (5), 872–875. doi: 10.1136/ard.2009.112920
Ersahin, T., Tuncbag, N., Cetin-Atalay, R. (2015). The PI3K/AKT/mTOR interactive pathway. Mol. Biosyst. 11 (7), 1946–1954. doi: 10.1039/c5mb00101c
Fang, Z., He, D., Yu, B., Liu, F., Zuo, J., Li, Y., et al. (2017). High-Throughput Study of the Effects of Celastrol on Activated Fibroblast-Like Synoviocytes from Patients with Rheumatoid Arthritis. Genes (Basel) 8 (9), 221. doi: 10.3390/genes8090221
Feng, F. B., Qiu, H. Y. (2018). Effects of Artesunate on chondrocyte proliferation, apoptosis and autophagy through the PI3K/AKT/mTOR signaling pathway in rat models with rheumatoid arthritis. BioMed. Pharmacother. 102, 1209–1220. doi: 10.1016/j.biopha.2018.03.142
Feng, X., Tan, W., Wan, F., Gan, K., Zhang, M., Zhang, Q. (2013). The effect of Celastrol on the expressions of RANKL, OPG, IL-6, TNF-α and IL-8 in human rheumatoid synoviocyte MH7A. Acta Universit. Med. Nanjing (Natural Sci.) 33 (06), 759–765. doi: 10.7655/NYDXBNS20130609
Feng, H. (2015). Influence of Acid-microenvironment on Monocyte-macrophages, Tca8113 Cells and the Role of Triptolide. dissertation (Guizhou: Guizhou Medical University).
Gan, K., Xu, L., Feng, X., Zhang, Q., Wang, F., Zhang, M., et al. (2015). Celastrol attenuates bone erosion in collagen-Induced arthritis mice and inhibits osteoclast differentiation and function in RANKL-induced RAW264. 7. Int. Immunopharmacol. 24 (2), 239–246. doi: 10.1016/j.intimp.2014.12.012
Gan, K. (2013). The role of Celastrol on osteoclastogenesis and bone erosion in collagen-induced arthritis doctor (Nanjing: Nanjing University of Chinese Medicine).
Ganesan, R., Rasool, M. (2017). Fibroblast-like synoviocytes-dependent effector molecules as a critical mediator for rheumatoid arthritis: Current status and future directions. Int. Rev. Immunol. 36 (1), 20–30. doi: 10.1080/08830185.2016.1269175
Gierut, A., Perlman, H., Pope, R. M. (2010). Innate immunity and rheumatoid arthritis. Rheumatic Dis. Clinics 36 (2), 271–296. doi: 10.1016/j.rdc.2010.03.004
Ho, L. J., Chang, W. L., Chen, A., Chao, P., Lai, J. H. (2013). Differential immunomodulatory effects by Tripterygium wilfordii Hook f-derived refined extract PG27 and its purified component PG490 (triptolide) in human peripheral blood T cells: potential therapeutics for arthritis and possible mechanisms explaining in part Chinese herbal theory “Junn-Chenn-Zuou-SS”. J. Transl. Med. 11:294. doi: 10.1186/1479-5876-11-294
Ivanov, I. I., McKenzie, B. S., Zhou, L., Tadokoro, C. E., Lepelley, A., Lafaille, J. J., et al. (2006). The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126 (6), 1121–1133. doi: 10.1016/j.cell.2006.07.035
Jia, L., Ping, T., Dongyi, H. (2017). Effects of (5R) -5-Hydroxytriptolide (LLDT-8) on Gene Expressions in Fibroblast-like Synoviocytes of Rheumatoid Arthritis. Acta Universit. Tradit. Med. Sinensis Pharmacol. Shanghai 31 (06), 70–75. doi: 10.16306/j.1008-861x.2017.06.017
Jing, X., He, L. (2013). Effects of GTW on expression of EGF and the intervention effect of Tusizi flavones in male juvenile rats. China J. Tradit. Chin. Med. Pharm. 28 (06), 1884–1886.
Jun, Z., Wei, X., Rui, W., Xiaole, S. (2016). Effectiveness and Safety of Kunxian Capsule for Rueumatoid Arthritis:A Systematic Review. J. Liaoning Univ. Tradit. Chin. Med. 18 (10), 122–126. doi: 10.13194/j.issn.1673-842x.2016.10.037
Jung, Y.-K., Kang, Y.-M., Han, S. (2019). Osteoclasts in the inflammatory arthritis: Implications for pathologic osteolysis. Immune Netw. 19 (1), e2. doi: 10.4110/in.2019.19.e2
Junqueira, L. C., Mescher, A. L. (2013). Junqueira’s basic histology: text & atlas/Anthony L. Mescher (New York [etc.]: McGraw-Hill Medical).
Khan, S., Greenberg, J. D., Bhardwaj, N. (2009). Dendritic cells as targets for therapy in rheumatoid arthritis. Nat. Rev. Rheumatol. 5 (10), 566. doi: 10.1038/nrrheum.2009.185
Laria, A., Lurati, A., Marrazza, M., Mazzocchi, D., Re, K. A., Scarpellini, M. (2016). The macrophages in rheumatic diseases. J. Inflammation Res. 9, 1–11. doi: 10.2147/JIR.S82320
Lawrence, T., Natoli, G. (2011). Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 11 (11), 750–761. doi: 10.1038/nri3088
Lee, J. Y., Lee, B. H., Kim, N. D., Lee, J. Y. (2015). Celastrol blocks binding of lipopolysaccharides to a Toll-like receptor4/myeloid differentiation factor2 complex in a thiol-dependent manner. J. Ethnopharmacol. 172, 254–260. doi: 10.1016/j.jep.2015.06.028
Lefèvre, S., Knedla, A., Tennie, C., Kampmann, A., Wunrau, C., Dinser, R., et al. (2009). Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat. Med. 15 (12), 1414–1420. doi: 10.1038/nm.2050
Lei, Y., Shuang, J., Wenping, P. (2015). Study on Inhibitory Effects of Triptolide on the Proliferation of Fibroblast-like Synovial Cells from Patients with Rheumatoid Arthritis in vitro. China Pharm. 26 (31), 4357–4359. doi: 10.6039/j.issn.1001-0408.2015.31.13
Leibbrandt, A., Penninger, J. M. (2009). RANKL/RANK as key factors for osteoclast development and bone loss in arthropathies. Adv. Exp. Med. Biol. 649, 100–113. doi: 10.1007/978-1-4419-0298-6_7
Leipe, J., Grunke, M., Dechant, C., Reindl, C., Kerzendorf, U., Schulze-Koops, H., et al. (2010). Role of Th17 cells in human autoimmune arthritis. Arthritis Rheumatism 62 (10), 2876–2885. doi: 10.1002/art.27622
Li, X., He, L. (2006). Pharmacological Control Study between Tripterygium. J. Kunming Med. Coll. 02), 107–110.
Li, G. Q., Zhang, Y., Liu, D., Qian, Y. Y., Zhang, H., Guo, S. Y., et al. (2012). Celastrol inhibits interleukin-17A-stimulated rheumatoid fibroblast-like synoviocyte migration and invasion through suppression of NF-kappaB-mediated matrix metalloproteinase-9 expression. Int. Immunopharmacol. 14 (4), 422–431. doi: 10.1016/j.intimp.2012.08.016
Li, G., Liu, D., Zhang, Y., Qian, Y., Zhang, H., Guo, S., et al. (2013a). Celastrol inhibits lipopolysaccharide-stimulated rheumatoid fibroblast-like synoviocyte invasion through suppression of TLR4/NF-kappaB-mediated matrix metalloproteinase-9 expression. PloS One 8 (7), e68905. doi: 10.1371/journal.pone.0068905
Li, G. Q., Liu, D., Zhang, Y., Qian, Y. Y., Zhu, Y. D., Guo, S. Y., et al. (2013b). Anti-invasive effects of celastrol in hypoxia-induced fibroblast-like synoviocyte through suppressing of HIF-1alpha/CXCR4 signaling pathway. Int. Immunopharmacol. 17 (4), 1028–1036. doi: 10.1016/j.intimp.2013.10.006
Li, T., Wang, X., Xue, Z., Lv, C., Li, H., Fan, Y., et al. (2019). Meta-analysis of laboratory index of Tripterygium Glycosides Tablets in treatment of rheumatoid arthritis. China J. Chin. Mater. Med. 44 (16), 3542–3550. doi: 10.19540/j.cnki.cjcmm.20190612.503
Lin, N., Sato, T., Ito, A. (2001). Triptolide, a novel diterpenoid triepoxide from Tripterygium wilfordii Hook. f., suppresses the production and gene expression of pro-matrix metalloproteinases 1 and 3 and augments those of tissue inhibitors of metalloproteinases 1 and 2 in human synovial fibroblasts. Arthritis Rheum 44 (9), 2193–2200. doi: 10.1002/1529-0131(200109)44:9<2193::aid-art373>3.0.co;2-5
Liu, C., Zhang, Y., Kong, X., Zhu, L., Pang, J., Xu, Y., et al. (2013). Triptolide prevents bone destruction in the collagen-induced arthritis model of rheumatoid arthritis by targeting RANKL/RANK/OPG signal pathway. Evidence-Based Complement. Altern. Med. 2013, 626038. doi: 10.1155/2013/626038
Liu, Y. L., Lindert, S., Zhu, W., Wang, K., McCammon, J. A., Oldfield, E. (2014). Taxodione and arenarone inhibit farnesyl diphosphate synthase by binding to the isopentenyl diphosphate site. Proc. Natl. Acad. Sci. U.S.A. 111 (25), E2530–E2539. doi: 10.1073/pnas.1409061111
Liu, X. (2018). Impact of celastrol on polarization of mouse peritoneal macrophage. Basic Clin. Med. 38 (05), 643–648. doi: 10.16352/j.issn.1001-6325.2018.05.011
Lopez-Olivo, M. A., Siddhanamatha, H. R., Shea, B., Tugwell, P., Wells, G. A., Suarez-Almazor, M. E. (2014). Methotrexate for treating rheumatoid arthritis. Cochrane Database Systemat. Rev. 2014 (6), CD000957. doi: 10.1002/14651858.CD000957.pub2
Lv, Q. W., Zhang, W., Shi, Q., Zheng, W. J., Li, X., Chen, H., et al. (2015). Comparison of Tripterygium wilfordii Hook F with methotrexate in the treatment of active rheumatoid arthritis (TRIFRA): a randomised, controlled clinical trial. Ann. Rheum Dis. 74 (6), 1078–1086. doi: 10.1136/annrheumdis-2013-204807
Mao, X., Sun, S., Pei, Z., Zhang, L. (2009). Inhibitory effect of triptolide on interleukin-18 and its receptor in rheumatoid arthritis synovial fibroblasts. Chin. J. Cell. Mol. Immunol. 25 (07), 606–608+611. doi: 10.1007/s00011-007-7128-9
Ming, Z., Lihua, M., Ying, C., Jun, X. (2014). Inhibitory Effects of Triptolide on Immune Function of Peripheral Blood T Cells in Rheumatoid Arthritis Patients. China Pharm. 25 (47), 4441–4443. doi: 10.6039/j.issn.1001-0408.2014.47.08
Mitchell, J. P., Carmody, R. J. (2018). NF-κB and the Transcriptional Control of Inflammation. Int. Rev. Cell Mol. Biol. 335, 41–84. doi: 10.1016/bs.ircmb.2017.07.007
Nanjundaiah, S. M., Venkatesha, S. H., Yu, H., Tong, L., Stains, J. P., Moudgil, K. D. (2012). Celastrus and its bioactive celastrol protect against bone damage in autoimmune arthritis by modulating osteoimmune cross-talk. J. Biol. Chem. 287 (26), 22216–22226. doi: 10.1074/jbc.M112.356816
Niiro, H., Clark, E. A. (2002). Regulation of B-cell fate by antigen-receptor signals. Nat. Rev. Immunol. 2 (12), 945–956. doi: 10.1038/nri955
Nishihara, M., Ogura, H., Ueda, N., Tsuruoka, M., Kitabayashi, C., Tsuji, F., et al. (2007). IL-6–gp130–STAT3 in T cells directs the development of IL-17+ Th with a minimum effect on that of Treg in the steady state. Int. Immunol. 19 (6), 695–702. doi: 10.1093/intimm/dxm045
Noort, A. R., Tak, P. P., Tas, S. W. (2015). Non-canonical NF-κB signaling in rheumatoid arthritis: Dr Jekyll and Mr Hyde? Arthritis Res. Ther. 17 (1):15. doi: 10.1186/s13075-015-0527-3
Pan, H. (2018). Mechanism Research of Xinfeng Capsule on rheumatoid arthritis by PI3K / AKT / mTOR signal pathway mediated by BAFF / BAFF-R. master (Hefei: Anhui University of Chinese Medicine).
Penatti, A., Facciotti, F., De Matteis, R., Larghi, P., Paroni, M., Murgo, A., et al. (2017). Differences in serum and synovial CD4+ T cells and cytokine profiles to stratify patients with inflammatory osteoarthritis and rheumatoid arthritis. Arthritis Res. Ther. 19 (1), 103. doi: 10.1186/s13075-017-1305-1
Peng, A., Wang, X., Zhuang, J. (2014). Triptolide inhibites Th17 cell differentiation via regulating cyclooxygenase-2/prostaglandin E2 axis in synovial fibroblasts from rheumatoid arthritis. China J. Chin. Mater. Med. 39 (3), 536–539. doi: 10.4268/cjcmm20140334
Pieper, K., Grimbacher, B., Eibel, H. (2013). B-cell biology and development. J. Allergy Clin. Immunol. 131 (4), 959–971. doi: 10.1016/j.jaci.2013.01.046
Ping, Q., Zhou, Y., Zhang, S., Cao, J., Xu, L., Fang, G., et al. (2015). Study on effects of Tripterygium wilfordii polycoride in resisting macrophage inflammation and regulating inflammation via TLR4/NF-κB. China J. Chin. Mater. Med. 40 (16), 3256–3261. doi: 10.4268/cjcmm20151626
Ping, T., Jia, L., Dongyi, H. (2016). Effect of (5R)-5-hydroxytriptolide(LLDT-8) on chemotactic factor in fibroblast-like synoviocytes. Curr. Immunol. 36 (06), 448–454.
Plotkin, L. I., Essex, A. L., Davis, H. M. (2019). RAGE Signaling in Skeletal Biology. Curr. Osteoporos Rep. 17 (1), 16–25. doi: 10.1007/s11914-019-00499-w
Qi, W., Lin, C., Fan, K., Chen, Z., Liu, L., Feng, X., et al. (2019). Hesperidin inhibits synovial cell inflammation and macrophage polarization through suppression of the PI3K/AKT pathway in complete Freund’s adjuvant-induced arthritis in mice. Chem. Biol. Interact. 306, 19–28. doi: 10.1016/j.cbi.2019.04.002
Qian, C., Peng, L., Feng, X., Tan, W., Zhang, Q. (2015). Celastrol Inhibiting Osteoclast Formation by Reducing Chemokine CCl4. Liaoning J. Tradit. Chin. Med. 42 (02), 415–417+447. doi: 10.13192/j.issn.1000-1719.2015.02.078
Scherer, H. U., Huizinga, T. W., Krönke, G., Schett, G., Toes, R. E. (2018). The B cell response to citrullinated antigens in the development of rheumatoid arthritis. Nat. Rev. Rheumatol. 14 (3), 157. doi: 10.1038/nrrheum.2018.10
Schmidlin, H., Diehl, S. A., Blom, B. (2009). New insights into the regulation of human B-cell differentiation. Trends Immunol. 30 (6), 277–285. doi: 10.1016/j.it.2009.03.008
Sharp, J. T., Tsuji, W., Ory, P., Harper-Barek, C., Wang, H., Newmark, R. (2010). Denosumab prevents metacarpal shaft cortical bone loss in patients with erosive rheumatoid arthritis. Arthritis Care Res. (Hoboken) 62 (4), 537–544. doi: 10.1002/acr.20172
Smolen, J. S., Aletaha, D., Barton, A., Burmester, G. R., Emery, P., Firestein, G. S., et al. (2018). Rheumatoid arthritis. Nat. Rev. Dis. Primers 4, 18001. doi: 10.1038/nrdp.2018.1
Stohl, W., Merrill, J. T., McKay, J. D., Lisse, J. R., Zhong, Z. J., Freimuth, W. W., et al. (2013). Efficacy and safety of belimumab in patients with rheumatoid arthritis: a phase II, randomized, double-blind, placebo-controlled, dose-ranging Study. J. Rheumatol. 40 (5), 579–589. doi: 10.3899/jrheum.120886
Su, Z., Sun, H., Ao, M., Zhao, C. (2017). Atomic Force Microscopy Study of the Anti-inflammatory Effects of Triptolide on Rheumatoid Arthritis Fibroblast-like Synoviocytes. Microsc. Microanal. 23 (5), 1002–1012. doi: 10.1017/s1431927617012399
Sun, F., Jiang, S., Ping, L., Feng, H., Dai, L. (2016). Effect of Tripterygium Wilfordii on Follicular Helper T Cells and IL-21 of Rheumatoid Arthritis Patients. Med. Recapitulate 22 (03), 566–569. doi: 10.3969/j.issn.1006-2084.2016.03.044
Sun, J. (2017). The effects of Duantengyimu decoction on the expression of chemokine receptor and secretion of chemokine in dendritic cells of patients with rheumatoid arthritis. dissertation (Guangzhou: Guangzhou University of Chinese Medicine).
Takeuchi, T., Tanaka, Y., Ishiguro, N., Yamanaka, H., Yoneda, T., Ohira, T., et al. (2016). Effect of denosumab on Japanese patients with rheumatoid arthritis: a dose-response study of AMG 162 (Denosumab) in patients with RheumatoId arthritis on methotrexate to Validate inhibitory effect on bone Erosion (DRIVE)-a 12-month, multicentre, randomised, double-blind, placebo-controlled, phase II clinical trial. Ann. Rheum Dis. 75 (6), 983–990. doi: 10.1136/annrheumdis-2015-208052
Takeuchi, T., Tanaka, Y., Soen, S., Yamanaka, H., Yoneda, T., Tanaka, S., et al. (2019). Effects of the anti-RANKL antibody denosumab on joint structural damage in patients with rheumatoid arthritis treated with conventional synthetic disease-modifying antirheumatic drugs (DESIRABLE study): a randomised, double-blind, placebo-controlled phase 3 trial. Ann. Rheum Dis. 78 (7), 899–907. doi: 10.1136/annrheumdis-2018-214827
Tang, Y., Zhang, Y., Li, L., Xie, Z., Wen, C., Huang, L. (2020). Kunxian Capsule for Rheumatoid Arthritis: Inhibition of Inflammatory Network and Reducing Adverse Reactions Through Drug Matching. Front. Pharmacol. 11, 485. doi: 10.3389/fphar.2020.00485
Tao, X., Davis, L. S., Lipsky, P. E. (1991). Effect of an extract of the Chinese herbal remedy Tripterygium wilfordii Hook F on human immune responsiveness. Arthritis Rheum 34 (10), 1274–1281. doi: 10.1002/art.1780341011
Te, W., Zhao-fu, L., Tao, L., Xiao-you, Y., Li-ping, Z. (2019). Research Progress on the Mechanism of Kunmingshanhaitang in the Treatment of Rheumatoid Arthritis. Rheumatism Arthritis 08), 60–63. doi: 10.3969/j.issn.2095-4174.2019.08.014
Teitelbaum, S. L., Ross, F. P. (2003). Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4 (8), 638. doi: 10.1038/nrg1122
Tong, Z., Cheng, L., Song, J., Wang, M., Yuan, J., Li, X., et al. (2018). Therapeutic effects of Caesalpinia minax Hance on complete Freund’s adjuvant (CFA)-induced arthritis and the anti-inflammatory activity of cassane diterpenes as main active components. J. Ethnopharmacol. 226, 90–96. doi: 10.1016/j.jep.2018.08.011
Vallières, F., Durocher, I., Girard, D. (2019). Biological activities of interleukin (IL)-21 in human monocytes and macrophages. Cell. Immunol. 337, 62–70. doi: 10.1016/j.cellimm.2019.02.002
van Hamburg, J. P., Asmawidjaja, P., Davelaar, N., Mus, A., Colin, E., Hazes, J., et al. (2011). Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheumatism 63 (1), 73–83. doi: 10.1002/art.30093
Venkatesha, S. H., Yu, H., Rajaiah, R., Tong, L., Moudgil, K. D. (2011). Celastrus-derived celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses. J. Biol. Chem. 286 (17), 15138–15146. doi: 10.1074/jbc.M111.226365
Vinuesa, C. G., Linterman, M. A., Yu, D., MacLennan, I. C. (2016). Follicular Helper T Cells. Annu. Rev. Immunol. 34, 335–368. doi: 10.1146/annurev-immunol-041015-055605
Wang, G., Wu, D. (1994). Effect of tripterygium wilfordii on lymphocyte subsets in patients with rheumatoid arthritis. Chin. J. Internal Med. 01), 41–42.
Wang, S. J., Yao, K., Xie, F. D., Ji, X. H. (2001). Effects of Tripterygium wilfordii saponins and interleukin-10 on dendritic cells from human peripheral blood. Acta Pharmacol. Sin. 22 (8).
Wang, B., Ma, L., Tao, X., Lipsky, P. (2004). Triptolide, an active component of the Chinese herbal remedy Tripterygium wilfordii Hook F, inhibits production of nitric oxide by decreasing inducible nitric oxide synthase gene transcription. Arthritis Rheumatism: Off. J. Am. Coll. Rheumatol. 50 (9), 2995–3003. doi: 10.1002/art.20459
Wang, X., Zhang, L., Duan, W., Liu, B., Gong, P., Ding, Y., et al. (2014). Anti−inflammatory effects of triptolide by inhibiting the NF−κB signalling pathway in LPS−induced acute lung injury in a murine model. Mol. Med. Rep. 10 (1), 447–452. doi: 10.3892/mmr.2014.2191
Wang, G., Guo, M., Xu, H., Huang, J., Lv, S., Zhao, H., et al. (2017). The effect of triptolide on differentiation of osteoclasts induced by HMGB1. J. China-Japan Friendship Hosp. 31 (02), 102–106+130. doi: 10.3969/j.issn.1001-0025.2017.02.010
Wang, S., Zuo, S., Liu, Z., Ji, X., Yao, Z., Wang, X. (2018). Study on the efficacy and mechanism of triptolide on treating TNF transgenic mice with rheumatoid arthritis. BioMed. Pharmacother. 106, 813–820. doi: 10.1016/j.biopha.2018.07.021
Wang, S., Liu, Z., Wang, J., Wang, Y., Liu, J., Ji, X., et al. (2019). The triptolide-induced apoptosis of osteoclast precursor by degradation of cIAP2 and treatment of rheumatoid arthritis of TNF-transgenic mice. Phytother. Res. 33 (2), 342–349. doi: 10.1002/ptr.6224
Wang, X., Li, T., Xue, Z., Lv, C., Li, H., Fan, Y., et al. (2019). Clinical symptoms effect of Tripterygium Glycosides Tablets alone or combined with methotrexate in treatment of rheumatoid arthritis: a Meta-analysis. China J. Chin. Mater. Med. 44 (16), 3533–3541. doi: 10.19540/j.cnki.cjcmm.20190605.501
Wang, X. (2015). Effects of Triptolide on RANKL Expression in the Epidermal Microenvironment of CIA Mice. dissertation (Nanjing: Nanjing Medical University).
Wong, V. K. W., Qiu, C., Xu, S. W., Law, B. Y. K., Zeng, W., Wang, H., et al. (2019). Ca(2+) signalling plays a role in celastrol-mediated suppression of synovial fibroblasts of rheumatoid arthritis patients and experimental arthritis in rats. Br. J. Pharmacol. 176 (16), 2922–2944. doi: 10.1111/bph.14718
Xiao-yue, W., Tai-xian, L., Zhi-peng, X., Cheng, L., Hui-zhen, L., Yuan-fang, F., et al. (2019). Clinical symptoms effect of Tripterygium Glycosides Tablets Tablets alone or combined with methotrexate in treatment of rheumatoid arthritis: a Meta-analysis. China J. Chin. Mater. Med. 44 (16), 3533–3541. doi: 10.19540/j.cnki.cjcmm.20190605.501
Xin, W., Wei, Z., Zhang, Y., Sun, Y., Zhang, D. (2018). Effect of celastrol on pyroptosis of macrophages RAW264.7. Chin. Tradit. Herbal Drugs 49 (05), 1087–1091. doi: 10.7501/j.issn.0253-2670.2018.05.015
Xu, Z., Wu, G., Wei, X., Chen, X., Wang, Y., Chen, L. (2013). Celastrol induced DNA damage, cell cycle arrest, and apoptosis in human rheumatoid fibroblast-like synovial cells. Am. J. Chin. Med. 41 (3), 615–628. doi: 10.1142/s0192415x13500432
Xu, H., Zhao, H., Lu, C., Qiu, Q., Wang, G., Huang, J., et al. (2016). Triptolide Inhibits Osteoclast Differentiation and Bone Resorption In Vitro via Enhancing the Production of IL-10 and TGF-β1 by Regulatory T Cells. Mediators Inflammation 2016, 8048170. doi: 10.1155/2016/8048170
Xu, Z. (2013). Celastrol-Introduced Apoptosis, cell Cycle arrest and DNA Damage in Rheumatoid Arthritis Fibroblast-like synovial dissertation (Fuzhou: Fujian University of Traditional Chinese Medicine).
Xu, H. (2016). Effects of Triptolide on OC Differentiation and Bone Resorption in Tregs-OC Co-culture System. dissertation (Beijing: Beijing University of Chinese Medicine).
Yang, X. O., Pappu, B. P., Nurieva, R., Akimzhanov, A., Kang, H. S., Chung, Y., et al. (2008). T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity 28 (1), 29–39. doi: 10.1016/j.immuni.2007.11.016
Yang, Y., Ye, Y., Qiu, Q., Xiao, Y., Huang, M., Shi, M., et al. (2016). Triptolide inhibits the migration and invasion of rheumatoid fibroblast-like synoviocytes by blocking the activation of the JNK MAPK pathway. Int. Immunopharmacol. 41, 8–16. doi: 10.1016/j.intimp.2016.10.005
Yap, T. A., Garrett, M. D., Walton, M. I., Raynaud, F., de Bono, J. S., Workman, P. (2008). Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and promises. Curr. Opin. Pharmacol. 8 (4), 393–412. doi: 10.1016/j.coph.2008.08.004
Yue, J., Griffith, J. F., Xiao, F., Shi, L., Wang, D., Shen, J., et al. (2017). Repair of Bone Erosion in Rheumatoid Arthritis by Denosumab: A High-Resolution Peripheral Quantitative Computed Tomography Study. Arthritis Care Res. (Hoboken) 69 (8), 1156–1163. doi: 10.1002/acr.23133
Zhang, Q., Shi, Y., Tan, W., Wang, F. (2008). Effect of triptolide on the changes of VEGF and MMP-9 levels in the rheumatoid arthritis fibroblast-like synoviocyte line, MH7A. Acta Universit. Med. Nanjing (Natural Sci.) 07), 902–905. doi: 10.3724/SP.J.1141.2008.00459
Zhou, J., Xiao, C., Zhao, L., Jia, H., Zhao, N., Lu, C., et al. (2006). The effect of triptolide on CD4+ and CD8+ cells in Peyer’s patch of SD rats with collagen induced arthritis. Int. Immunopharmacol. 6 (2), 198–203. doi: 10.1016/j.intimp.2005.08.011
Keywords: Tripterygium wilfordii Hook f., rheumatoid arthritis, immune cell, mechanism, review
Citation: Tang Y, Liu Q, Feng Y, Zhang Y, Xu Z, Wen C and Zhang Y (2020) Tripterygium Ingredients for Pathogenicity Cells in Rheumatoid Arthritis. Front. Pharmacol. 11:583171. doi: 10.3389/fphar.2020.583171
Received: 14 July 2020; Accepted: 03 September 2020;
Published: 02 October 2020.
Edited by:
Yanqiong Zhang, China Academy of Chinese Medical Sciences, ChinaReviewed by:
Jinxia Zhao, Peking University Third Hospital, ChinaGanesan Ramamoorthi, Moffitt Cancer Center, United States
Copyright © 2020 Tang, Liu, Feng, Zhang, Xu, Wen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengping Wen, wengcp@163.com; Yun Zhang, zhangyun@zcmu.edu.cn
 Yujun Tang
Yujun Tang Qiuping Liu
Qiuping Liu Yuxiang Feng
Yuxiang Feng Yi Zhang
Yi Zhang Zhenghao Xu
Zhenghao Xu Yun Zhang
Yun Zhang