- 1Department of Radiation and Medical Oncology, Zhongnan Hospital of Wuhan University, Wuhan, China
- 2Hubei Key Laboratory of Tumor Biological Behaviors Zhongnan Hospital of Wuhan University, Wuhan, China
- 3Hubei Cancer Clinical Study Center Zhongnan Hospital of Wuhan University, Wuhan, China
Background: Tumor mutational burden (TMB) is a genomic biomarker that can predict favorable responses to immune checkpoint inhibitors (ICIs). Although we have better understanding of TMB in cancer immunity and cancer immunotherapy, the relationship between TMB and the clinical efficacy of ICIs remains unknown in the treatment of melanoma patients. Here, we conduct a systematic review and meta-analysis to evaluate the predictive value of TMB on the efficacy of ICIs in patients with melanoma.
Methods: We systematically collected data from PubMed, Embase, Cochrane Library, CNKI, China Biomedical Database (CBM), and Wanfang Database. The end date was set to 26 June 2021. We included retrospective studies or clinical trials of ICIs that reported hazard ratios (HRs) for overall survival and/or progression-free survival according to TMB. Data for 1,493 patients from 15 studies were included. In addition, pooled effect size, heterogeneity analysis, sensitivity analysis, publication bias detection, and subgroup analysis were performed based on the included data.
Results: Patients with high TMB showed significantly improved OS (HR = 0.49, 95% CI: 0.33, 0.73; p = 0.001) and PFS (HR = 0.47, 95% CI: 0.33, 0.68; p < 0.001) compared with patients with low TMB. This association was very good in patients treated with monotherapy, that is, anti-CTLA-4 or anti-PD-(L)-1 inhibitors, but not for the patients treated with a combination of the two drugs. The subgroup analysis results showed that heterogeneity was substantial in the targeted next-generation sequencing (NGS) group. Publication bias was detected, and the results were visualized using the funnel chart. And sensitivity analysis and trim-and-fill method analysis showed that our results were stable and reliable.
Conclusion: High TMB is associated with improved OS and PFS in melanoma patients treated with mono-drug ICIs. TMB determined by NGS should be standardized to eliminate heterogeneity. Therefore, the role of TMB in identifying melanoma patients who may benefit from ICI should be further determined in more randomized controlled trials in the future.
Introduction
As a highly aggressive type of skin cancer, melanoma is the leading cause of skin cancer-related deaths, causing nearly 60,000 deaths worldwide each year (Karimkhani et al., 2017). The results of the latest global cancer statistics in 2020 showed that there were more than 320,000 new skin melanoma patients and more than 57,000 deaths (Sung et al., 2021). At present, the basic principle of clinical treatment of melanoma is extensive local surgical resection, but the outstanding feature of melanoma is that it is prone to distant metastasis in the early stage of onset. Meantime, sensitivity to traditional radiotherapy and chemotherapy of melanoma is very low, and drug resistance is prone to occur. Due to the poor efficacy of existing programs, the 5-year survival rate of melanoma patients is less than 10% (Kaufman et al., 2018; Franke and van Akkooi, 2019).
With the deepening of research, the emergence of immune checkpoint inhibitors (ICIs) has completely changed the treatment prospects for patients with stage III/IV melanoma. A variety of ICIs have been proven to have a good effect on patients with unresectable or metastatic melanoma. These drugs, whether used as a monotherapy or in combination, produce lasting improvement in survival rates and potential cure rates for patients with advanced-stage III and IV melanomas (Eggermont et al., 2016; Schachter et al., 2017; Larkin et al., 2019). However, it should be noted that not all melanoma patients can benefit from immunotherapy (Lugowska et al., 2018). Melanoma patients urgently need effective biomarkers that can indicate the potential benefits of immunotherapy.
Tumor mutational burden (TMB) is defined as the total number of somatic mutations per megabase or the non-synonymous mutations in tumor tissues, including replacement and insertion–deletion mutations, and it is likely to be a promising biomarker. According to reports, in melanoma, non-small-cell lung cancer and urothelial cell carcinoma patients with high TMB had a better response to ICI and survival rate than patients with low TMB (Rosenberg et al., 2016; Teo et al., 2018; Alborelli et al., 2020; Huang et al., 2020; Valero et al., 2021a; Gogas et al., 2021). Meanwhile, Liu et al. pointed out that compared with copy number alteration (CNA) alone, CNA combined with TMB as a new biomarker showed better prediction for ICI efficacy (L. Liu D. et al., 2019). Furthermore, TMB is also a potential predictor for ICI efficacy in liver cancer and biliary tract cancer (Rizzo and Brandi, 2021; Rizzo and Ricci, 2021; Rizzo et al., 2021). However, TMB, as a biomarker, still has some controversy in the evaluation of the outcome of melanoma patients with ICI therapy. Therefore, we conducted a comprehensive systematic review and meta-analysis to evaluate the effect of TMB on the efficacy of immune checkpoint inhibitors in melanoma patients, and overall subgroup analysis and sensitivity analysis to identify potential sources of heterogeneity.
Materials and Methods
Literature Search
This meta-analysis follows the PRISMA statement (Moher et al., 2009). As of 26 June 2021, systematic literature searches had been conducted on PubMed, Embase, Cochrane Library, China Knowledge Network (CNKI), China Biomedical Database (CBM), and Wanfang Database. The search terms were (“Immune Checkpoint Inhibitors” OR “Immune Checkpoint Inhibitor” OR “Immune Checkpoint Blockers” OR “Immune Checkpoint Blockade” OR “Immune Checkpoint Inhibition” OR “PD-L1 Inhibitors” OR “PD-L1 Inhibitor” OR ″ Programmed Death-Ligand 1 Inhibitors” OR “CTLA-4 Inhibitors” OR “CTLA-4 Inhibitor” OR “Cytotoxic T-Lymphocyte-Associated Protein 4 Inhibitors” OR “Cytotoxic T-Lymphocyte-Associated Protein 4 Inhibitor” OR “PD-1 Inhibitors” OR “PD-1 Inhibitor” OR “Programmed Cell Death Protein 1 Inhibitor” OR “Programmed Cell Death Protein 1 Inhibitors” OR “PD-1-PD-L1 Blockade” OR “Ipilimumab” OR “Tremelimumab” OR “Nivolumab” OR “Pembrolizumab” OR “Lambrolizumab” OR “Atezolizumab” OR “Avelumab” OR “Durvalumab”) AND (“Melanoma” OR “Melanomas” OR “Malignant Melanoma” OR “Malignant Melanomas”) AND (“mutation burden” OR “mutational Burden” OR “mutation load” OR “mutational load” OR “TMB” OR “TML”) and the corresponding Chinese search terms. In addition, we manually searched the references of selected articles to obtain all possible relevant studies. All searched documents were not restricted to languages.
Literature Inclusion and Exclusion Criteria
In order to meet the conditions, the study must meet the following inclusion criteria: 1) pathologically confirmed melanoma; 2) cohort studies or clinical trials used TMB with cutoff values to evaluate the clinical outcome of melanoma patients who were treated with PD-1/PD-L1, CTLA-4, or their combined inhibitor; 3) hazard ratio (HR) of overall survival (OS) or progression-free survival (PFS), and their 95% confidence interval (95% CI) were given in the article, or there were enough data to calculate them; and 4) the number of evaluable patients was not less than 20. Exclusion criteria were as follows: insufficient information and data, non-original research (such as reviews and meta-analysis), repeated research, letters, editorials, comments, conference abstracts, and case reports.
Data Extraction
Two researchers independently extracted data from the included studies, and any inconsistencies were resolved through consultation with all researchers. The following information were extracted from each study: title, first author, year of publication, study type, sample size evaluable for TMB, immune checkpoint inhibitor category, TMB sequencing method, TMB cutoff value, and results (PFS, OS).
Literature Quality Evaluation
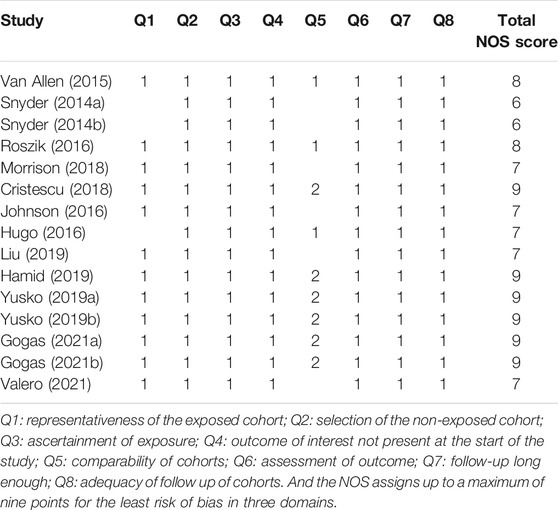
The Newcastle–Ottawa Scale (NOS) quality assessment scale was used to assess the quality of the included studies or cohorts (Stang, 2010). The total score ranged from 0 to 9, with 8-9 points indicating high research quality, 5-7 points indicating medium quality, and less than 5 points indicating poor research quality.
Statistical Methods and Data Analysis
The main goal of this meta-analysis was to compare the efficacy of ICIs between the TMB high group and the TMB low group, measured by HR of OS or PFS. If the chi-square test p < 0.1 or I2>50%, it was considered that there was significant heterogeneity among included studies (Higgins et al., 2003). If heterogeneity was significant, the random-effects model was used to reduce the impact of heterogeneity on the results, otherwise, the fixed-effects model was used. In addition, a funnel chart was constructed, and Begg’s test and Egger’s test were performed to assess publication bias (p > 0.1 was considered to have no obvious publication bias). On the other hand, the trim-and-fill method analysis and sensitivity analysis were used to test the result’s stability of our meta-analysis. In order to further explore the source of heterogeneity, a subgroup analysis was performed based on immune checkpoint inhibitor type, TMB sequencing method, study type, and the number of patients included in the study. STATA15.1 software was used for statistical analysis.
In addition, several articles provided raw data or graphs but did not report HR values and 95% CI. For original survival data, SPSS23.0 was used to calculate the HR value and 95% CI using the Cox proportional hazard regression model. For the Kaplan–Meier curve, Engauge Digitizer was used to extract survival data from the graph, and then HR value was estimated using the method reported by Tierney et al. (2007).
Results
Literature Searching and Results Screening
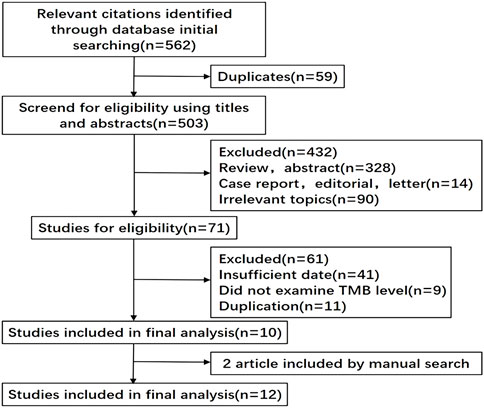
We initially retrieved 562 records with the keywords. Excluding 59 duplicate documents, we screened the remaining 503 documents by reading the titles and abstracts, and then only 71 documents were left. After reading the full-text 12 documents including fifteen studies were eligible for the final analysis. The publication year ranged from 2014 to 2021, and the number of patients in each study ranged from 23 to 298, with a total of 1,493 patients (Figure 1) (Snyder et al., 2014; Van Allen et al., 2015; Johnson et al., 2016; Roszik et al., 2016; Hugo et al., 2016; Cristescu et al., 2018; Morrison et al., 2018; Hamid et al., 2019; Liu L. et al., 2019; Yusko et al., 2019; Gogas et al., 2021; Valero et al., 2021b).
Basic Situation and Quality Assessment of Selected Documents
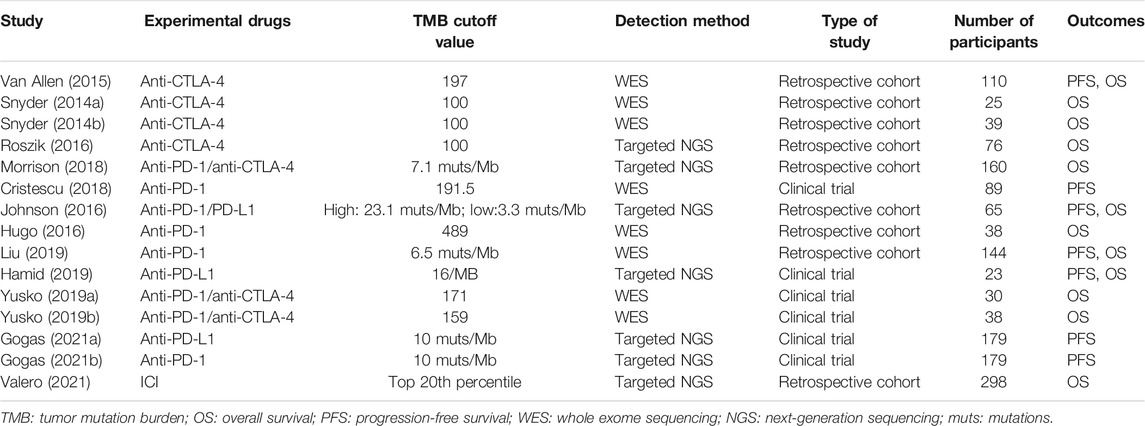
The basic characteristics of the studies included in our study are shown in Table 1 and Appendix Supplementary Table S1 in the Supplementary Material. And results of the NOS quality assessment scale are shown in Table 2. Eight studies had high quality, and the rest showed medium quality, which ensured a high quality of the included studies and improved the reliability of our meta-analysis.
Analysis of the Relationship Between TMB and ICI Efficacy
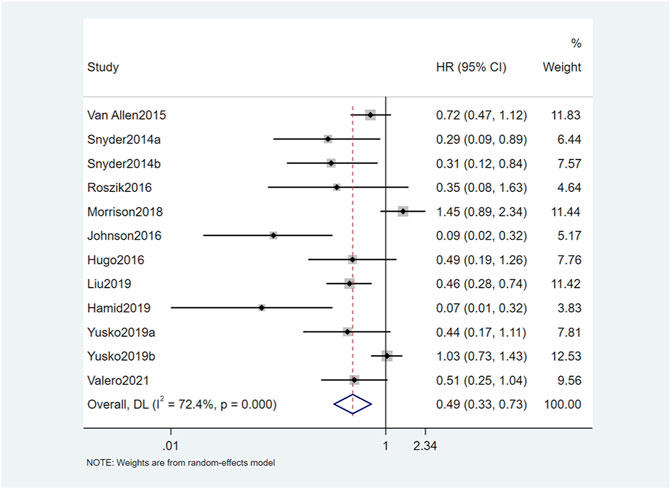
The results of this study showed that OS and PFS were significantly improved in patients with high TMB. A total of 12 studies reported a relationship between TMB and OS. The OS of patients with high TMB was significantly better than patients with low TMB (HR = 0.49, 95% CI: 0.33, 0.73; p = 0.001) (Figure 2). Seven studies reported the relationship between TMB and PFS, and the result was similar to OS. The PFS of TMB high group was significantly improved (HR = 0.47, 95% CI: 0.33, 0.68; p < 0.001) (Figure 3). Obvious heterogeneity could be observed in the two groups, OS group (I2 = 72.4%, p = 0.001) and PFS group (I2 = 66.3%, p < 0.001). Therefore, both groups used the random-effects model to pool effect sizes to reduce the impact of heterogeneity on results.
Publication Bias
This study evaluated publication bias by analyzing Egger’s test and Begg’s test. There was evidence of publication bias, with asymmetry in the funnel plots (Figures 4A,B). And Egger’s test p-values were, respectively, 0.002 and 0.012. The trim-and-fill method analysis resulted that no studies were clipped or new studies were added, suggesting that the result of our study was stable and reliable (Figures 5A,B).
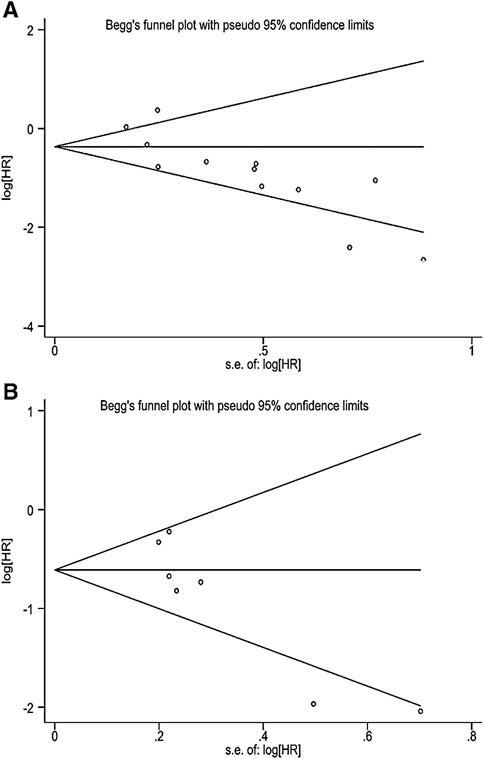
FIGURE 4. (A) Begg’s funnel plot of correlation between TMB and OS. (B) Begg’s funnel plot of correlation between TMB and PFS.
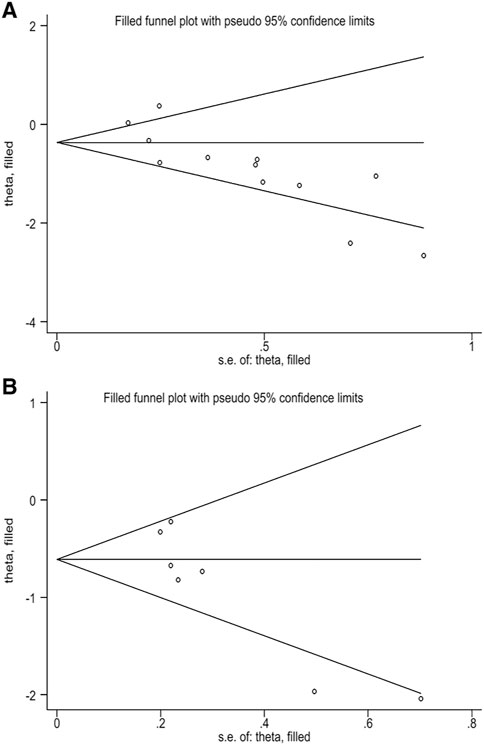
FIGURE 5. (A) Filled funnel plot of correlation between TMB and OS. (B) Filled funnel plot of correlation between TMB and PFS.
Sensitivity Analysis and Subgroup Analysis
Sensitivity analysis showed a good stability of the pooled HR. Relatively speaking, Morrison,[22] Johnson,[24] and Hamid [27] had high heterogeneity. After excluding the three documents, I2 of the OS group dropped to 48.7%, HR = 0.56 (95% CI: 0.40, 0.77; p < 0.001); And I2 of the PFS group dropped to 27.0%, HR = 0.59 (95% CI: 0.46, 0.74; p < 0.001) (Appendix Supplementary Figure S1, 2 in the Supplementary Material).
The results of subgroup analysis are shown in Table 3. For different treatment strategies, when anti-CTLA-4 or anti-PD-(L)1 was used alone, the OS and PFS of patients with high TMB were dramatically better than patients with low TMB. However, when combining these two drugs, the OS and PFS showed no difference between the two groups. Second, a subgroup analysis of TMB detection methods showed that regardless of whether WES or targeted NGS, patients with high TMB were associated with prolonged OS and PFS. Compared with the WES detection method, the targeted NGS method showed obvious heterogeneity. In addition, subgroup analysis was performed on the study type and number of participants in each study. The correlation between the TMB level and OS was not statistically different among the clinical trial group and the number of participants ≥100 group, while the correlation between the TMB level and PFS was not statistically different in the cohort group (Appendix Supplementary Figure S3–10 in the Supplementary Material).
Discussion
The primary target of this meta-analysis was to assess the association between TMB and OS or PFS in melanoma patients treated with ICIs. Our pooled analysis integrated the data of 1,493 melanoma patients, and results showed that compared with the low-TMB group, the risk of death in the high-TMB group was reduced by 51%, and the risk of disease progression was reduced by 53%. In patients receiving treatment other than ICIs, no such difference in survival based on TMB levels was found (Cao et al., 2019). Since most of the studies we included were conducted in Western countries, the role of TMB in Asian melanoma patients needs more relevant clinical studies. In addition, the results of this article showed that TMB was clinically significant in melanoma patients who were treated with a single ICI, while the result in the non-monotherapy group was opposite. Due to an insufficient number of patients included in our study, whether TMB could predict the efficacy of combination therapy (anti-PD-(L)1 plus anti-CTLA-4) in melanoma patients required more studies to further confirm.
TMB, a quantitative biomarker, is defined by the total number of somatic mutations in the coding region of genes in tumor cells, which may reflect both mutation status and neoantigen load (Zehir et al., 2017). Neoantigen load has been shown to be associated with the clinical response to immunotherapy in several studies (Rizvi et al., 2015; Rooney et al., 2015). Although TMB is not completely equivalent to the neoantigen load produced via many mechanisms, tumors harboring more mutations generate more neoantigens and have a greater likelihood of being recognized by the immune system. When the PD-1/PD-L1 pathway is activated, it can inhibit the proliferation of T lymphocytes and suppress the immune function of T cells (Sharpe and Pauken, 2018). Thus, TMB can reasonably be assumed to be a proxy for neoantigen load to predict the response to immunity therapy.
The quantitative detection of TMB currently uses mainly WES and the targeted NGS method. Interestingly, in our subgroup analysis, whether for OS or PFS, significant heterogeneity was concentrated in the targeted NGS group; and sensitivity analysis found that the three documents with greater heterogeneity all detected TMB by the using targeted NGS method. So different TMB sequencing methods might clarify most of the heterogeneities. Currently, simultaneous detection of tumors and matched blood or normal tissue using WES is considered the gold standard for TMB quantification, and many initial studies have been performed on this method (Snyder et al., 2014; Hugo et al., 2016; Riaz et al., 2017). However, measuring TMB by WES has some limitations in daily clinical practice due to the tissue processing difficulty, time- and labor-intensiveness due to its large sequencing capacity, and subsequent high costs. With relatively cheap costs and simple operation, NGS panels consisting of only hundreds of genes are more suitable for clinical needs than WES (Horak et al., 2016). So far, the FDA has approved two targeted NGS panels for TMB analysis: Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) panel and Foundation One CDx (F1CDx) panel. However, our results showed that there was significant heterogeneity in different targeted NGS panels, which might affect the accuracy and stability of TMB prediction. In fact, panel-based TMB evaluation is also affected by several experimental factors (e.g., tumor purity or sequencing depth) and variant calling pipeline, which need to be standardized among different targeted NGS panels (Chan et al., 2019; Fancello et al., 2019).
One of the most critical issues of TMB is the optimal threshold for predicting the effect of immunotherapy. As TMB varies greatly in different tumors, there may not be a universal TMB cutoff value for all cancer types, especially cancers with high TMB levels, such as NSCLC and melanoma (Chalmers et al., 2017; Samstein et al., 2019). In order to determine the best TMB cutoff value, a Foundation Medicine officially divided TMB into three groups: low (1–five Mut/Mb), intermediate (6–19 Mut/Mb), and high (≥20 Mut/Mb) (Goodman et al., 2017). In addition, in 2020, based on the results of the Keynote158 trial, the FDA approved anti-PD-1 therapy for any type of solid tumor with TMB ≥10 mut/Mb (Marabelle et al., 2020). Although studies have confirmed that patients with TMB ≥10 mut/Mb have generally higher response rates to ICI treatment in many tumors (Valero et al., 2021a), a new study suggested that simply defining a certain threshold value as “high TMB” was not suitable for predicting the effect of immunotherapy for each type of tumor (McGrail et al., 2021). In our meta-analysis, the detection of TMB in each study was based on different detection technologies and different gene panel platforms, in addition, TMB may come from tissue or blood samples, these factors would all affect the optimal TMB cutoff value. Nevertheless, while TMB testing has important guiding significance in immunotherapy strategies, a number of clinical trials are in progress in the context of TMB assessment in diverse cancers (Chan et al., 2019), and these trials are expected to provide more high-quality data to help us determine appropriate TMB cutoff value for certain cancer types. In addition, according to a recent study, Vega et al. developed a calibration tool based on panel assays from 16 participating laboratories which will help improve the consistency and reliability of panel tissue TMB estimation across platforms and facilitate the use of this complex biomarker in clinical decision making (Vega et al., 2021).
Although the result of our study suggested that TMB was indeed related to the efficacy of immunotherapy and had good predictive value in some patients, not all patients with high TMB could benefit from it. The expression of programmed cell death receptor 1 ligand (PD-L1) is another major biomarker of response to ICIs. However, most randomized clinical trials have confirmed that PD-L1 expression remains only moderately predictive, being dynamic, heterogeneous, and unable to distinguish adaptive and constitutive patterns of expression and neglecting variable characteristics of the tumor immune microenvironment (Chan et al., 2019). According to previous reports, other biomarkers, such as lactate dehydrogenase (LDH) and driver mutations in NRAS and NF1, although related to the response to ICIs, could not independently predict benefit to ICIs (Johnson et al., 2015; Axelrod et al., 2018; Wagner et al., 2018). In this context, we propose to combine these different biomarkers for evaluation to develop a multivariate prediction model and scoring system (Hur et al., 2016; Zhai et al., 2017; Beukinga et al., 2018; Jiang et al., 2020). Moreover, Langen et al. summarized the potential value of positron emission tomography (PET) in predicting and evaluating the treatment response to immunotherapy (Dimitrakopoulou-Strauss, 2019; Borm et al., 2021). Therefore, other biomarkers like FDG PET-CT or PD-1 or PD L-1 imaging in combination with TMB and laboratory parameters should be used in the future (as prognostic markers and also for therapy monitoring) to get a holistic approach of tumor biology including heterogeneity of the metastatic disease and in order to tailor therapy on a personalized basis.
In addition, this meta-analysis has some limitations. First of all, there were differences in the sample size of included studies, leading to large differences in the sample size between different subgroups. Some studies with small sample sizes may be the main source of publication bias in this meta-analysis. Second, the HRs and corresponding 95% CIs were not reported in some studies, and we were unable to obtain their original data; therefore, these studies were excluded, which may also lead to potential publication bias. Third, some important clinical features, which were reported to be related to the efficacy of ICIs, such as age and gender (Chalmers et al., 2017; Conforti et al., 2018; Wu et al., 2019), have been ignored due to insufficient data. Finally, TMB is still a controversial biomarker in clinical practice. There are still many problems in the standardization of TMB detection. In the future, more relevant studies are needed to define and clarify the optimal TMB threshold.
Overall, this study is the first large-sample meta-analysis on the effect of TMB in the efficacy of ICI therapy in patients with melanoma, which is of important reference value for future research studies on the relationship between TMB and immunotherapy and the clinical application of TMB in melanoma patients. Although there are limitations, we conducted subgroup analysis from some aspects and found most of the sources of heterogeneity. In addition, sensitivity analysis and trim-and-fill method analysis showed that our results have good stability. Therefore, despite the current technical and practical obstacles, we believe that after the standardized TMB cutoff value is determined in the future, it may become a preferable biomarker for screening melanoma patients who are most suitable for ICI treatment. Additionally, we consider that a comprehensive prediction model of multiple biomarkers (such as TMB, PD-L1, LDH, and PET) may be beneficial. Finally, we also take the view that TMB may be used as a predictor not only in ICI therapy but also play a predictive role in new immunotherapies, such as therapeutic vaccines and chimeric antigen receptor T-cell therapy.
Conclusion
Our meta-analysis result shows that high TMB can predict the improvement of ICI efficacy in patients with melanoma, indicating that TMB can be used as a new potential predictive biomarker for mono-immunotherapy strategy in melanoma. In the future, more large-sample, standardized design studies are needed to further verify the predictive value of TMB in subgroups, such as combination therapy (anti-PD-(L)1 plus anti-CTLA-4). In addition, the clinically targeted NGS used to quantify TMB should be standardized to eliminate the influence of heterogeneity. Finally, combining TMB with eligible biomarkers may expand the choice of patients who will benefit from immune checkpoint inhibitors.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
BN contributed to the conception and design of the work; BN and YXL contributed to the writing of the draft manuscript; BN, YXL, and MW carried out the literature search and data collection; MW, YL, and TX contributed to data analysis and interpreted the data; and YW contributed to supervision and critical revision of the manuscript. All authors contributed to the interpretation of the results and approved the final version.
Funding
This study was supported by the Science and Technology Innovation Cultivation Fund of Zhongnan Hospital of Wuhan University (grant no. ZNJC201910).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.748674/full#supplementary-material
References
Alborelli, I., Leonards, K., Rothschild, S. I., Leuenberger, L. P., Savic Prince, S., Mertz, K. D., et al. (2020). Tumor Mutational burden Assessed by Targeted NGS Predicts Clinical Benefit from Immune Checkpoint Inhibitors in Non-small Cell Lung Cancer. J. Pathol. 250 (1), 19–29. doi:10.1002/path.5344
Axelrod, M. L., Johnson, D. B., and Balko, J. M. (2018). Emerging Biomarkers for Cancer Immunotherapy in Melanoma. Semin. Cancer Biol. 52 (Pt 2), 207–215. doi:10.1016/j.semcancer.2017.09.004
Beukinga, R. J., Hulshoff, J. B., Mul, V. E. M., Noordzij, W., Kats-Ugurlu, G., Slart, R. H. J. A., et al. (2018). Prediction of Response to Neoadjuvant Chemotherapy and Radiation Therapy with Baseline and Restaging 18F-FDG PET Imaging Biomarkers in Patients with Esophageal Cancer. Radiology 287 (3), 983–992. doi:10.1148/radiol.2018172229
Borm, F. J., Smit, J., Oprea-Lager, D. E., Wondergem, M., Haanen, J. B. A. G., Smit, E. F., et al. (2021). Response Prediction and Evaluation Using PET in Patients with Solid Tumors Treated with Immunotherapy. Cancers (Basel) 13 (12), 3083. doi:10.3390/cancers13123083
Cao, D., Xu, H., Xu, X., Guo, T., and Ge, W. (2019). High Tumor Mutation burden Predicts Better Efficacy of Immunotherapy: a Pooled Analysis of 103078 Cancer Patients. OncoImmunology 8 (9), e1629258. doi:10.1080/2162402X.2019.1629258
Chalmers, Z. R., Connelly, C. F., Fabrizio, D., Gay, L., Ali, S. M., Ennis, R., et al. (2017). Analysis of 100,000 Human Cancer Genomes Reveals the Landscape of Tumor Mutational burden. Genome Med. 9 (1), 34. doi:10.1186/s13073-017-0424-2
Chan, T. A., Yarchoan, M., Jaffee, E., Swanton, C., Quezada, S. A., Stenzinger, A., et al. (2019). Development of Tumor Mutation burden as an Immunotherapy Biomarker: Utility for the Oncology Clinic. Ann. Oncol. 30 (1), 44–56. doi:10.1093/annonc/mdy495
Conforti, F., Pala, L., Bagnardi, V., De Pas, T., Martinetti, M., Viale, G., et al. (2018). Cancer Immunotherapy Efficacy and Patients' Sex: a Systematic Review and Meta-Analysis. Lancet Oncol. 19 (6), 737–746. doi:10.1016/S1470-2045(18)30261-4
Cristescu, R., Mogg, R., Ayers, M., Albright, A., Murphy, E., Yearley, J., et al. (2018). Pan-tumor Genomic Biomarkers for PD-1 Checkpoint Blockade-Based Immunotherapy. Science 362 (6411), eaar3593. doi:10.1126/science.aar3593
Dimitrakopoulou-Strauss, A. (2019). Monitoring of Patients with Metastatic Melanoma Treated with Immune Checkpoint Inhibitors Using PET-CT. Cancer Immunol. Immunother. 68 (5), 813–822. doi:10.1007/s00262-018-2229-6
Eggermont, A. M., Chiarion-Sileni, V., Grob, J. J., Dummer, R., Wolchok, J. D., Schmidt, H., et al. (2016). Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N. Engl. J. Med. 375 (19), 1845–1855. doi:10.1056/NEJMoa1611299
Fancello, L., Gandini, S., Pelicci, P. G., and Mazzarella, L. (2019). Tumor Mutational burden Quantification from Targeted Gene Panels: Major Advancements and Challenges. J. Immunother. Cancer 7 (1), 183. doi:10.1186/s40425-019-0647-4
Franke, V., and van Akkooi, A. C. J. (2019). The Extent of Surgery for Stage III Melanoma: How Much Is Appropriate? Lancet Oncol. 20 (3), e167–e174. doi:10.1016/s1470-2045(19)30099-3
Gogas, H., Dréno, B., Larkin, J., Demidov, L., Stroyakovskiy, D., Eroglu, Z., et al. (2021). Cobimetinib Plus Atezolizumab in BRAFV600 Wild-type Melanoma: Primary Results from the Randomized Phase III IMspire170 Study. Ann. Oncol. 32 (3), 384–394. doi:10.1016/j.annonc.2020.12.004
Goodman, A. M., Kato, S., Bazhenova, L., Patel, S. P., Frampton, G. M., Miller, V., et al. (2017). Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 16 (11), 2598–2608. doi:10.1158/1535-7163.MCT-17-0386
Hamid, O., Molinero, L., Bolen, C. R., Sosman, J. A., Muñoz-Couselo, E., Kluger, H. M., et al. (2019). Safety, Clinical Activity, and Biological Correlates of Response in Patients with Metastatic Melanoma: Results from a Phase I Trial of Atezolizumab. Clin. Cancer Res. 25 (20), 6061–6072. doi:10.1158/1078-0432.CCR-18-3488
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring Inconsistency in Meta-Analyses. BMJ 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Horak, P., Fröhling, S., and Glimm, H. (2016). Integrating Next-Generation Sequencing into Clinical Oncology: Strategies, Promises and Pitfalls. ESMO Open 1 (5), e000094. doi:10.1136/esmoopen-2016-000094
Huang, D., Zhang, F., Tao, H., Zhang, S., Ma, J., Wang, J., et al. (2020). Tumor Mutation Burden as a Potential Biomarker for PD-1/pd-L1 Inhibition in Advanced Non-small Cell Lung Cancer. Target. Oncol. 15 (1), 93–100. doi:10.1007/s11523-020-00703-3
Hugo, W., Zaretsky, J. M., Sun, L., Song, C., Moreno, B. H., Hu-Lieskovan, S., et al. (2016). Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 165 (1), 35–44. doi:10.1016/j.cell.2016.02.065
Hur, H., Tulina, I., Cho, M. S., Min, B. S., Koom, W. S., Lim, J. S., et al. (2016). Biomarker-Based Scoring System for Prediction of Tumor Response after Preoperative Chemoradiotherapy in Rectal Cancer by Reverse Transcriptase Polymerase Chain Reaction Analysis. Dis. Colon Rectum 59 (12), 1174–1182. doi:10.1097/DCR.0000000000000711
Jiang, J., Ding, Y., Wu, M., Chen, Y., Lyu, X., Lu, J., et al. (2020). Integrated Genomic Analysis Identifies a Genetic Mutation Model Predicting Response to Immune Checkpoint Inhibitors in Melanoma. Cancer Med. 9 (22), 8498–8518. doi:10.1002/cam4.3481
Johnson, D. B., Frampton, G. M., Rioth, M. J., Yusko, E., Xu, Y., Guo, X., et al. (2016). Targeted Next Generation Sequencing Identifies Markers of Response to PD-1 Blockade. Cancer Immunol. Res. 4 (11), 959–967. doi:10.1158/2326-6066.CIR-16-0143
Johnson, D. B., Lovly, C. M., Flavin, M., Panageas, K. S., Ayers, G. D., Zhao, Z., et al. (2015). Impact of NRAS Mutations for Patients with Advanced Melanoma Treated with Immune Therapies. Cancer Immunol. Res. 3 (3), 288–295. doi:10.1158/2326-6066.CIR-14-0207
Karimkhani, C., Green, A. C., Nijsten, T., Weinstock, M. A., Dellavalle, R. P., Naghavi, M., et al. (2017). The Global burden of Melanoma: Results from the Global Burden of Disease Study 2015. Br. J. Dermatol. 177 (1), 134–140. doi:10.1111/bjd.15510
Kaufman, H. L., Margolin, K., and Sullivan, R. (2018). Management of Metastatic Melanoma in 2018. JAMA Oncol. 4 (6), 857–858. doi:10.1001/jamaoncol.2018.0170
Larkin, J., Chiarion-Sileni, V., Gonzalez, R., Grob, J. J., Rutkowski, P., Lao, C. D., et al. (2019). Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 381 (16), 1535–1546. doi:10.1056/NEJMoa1910836
Liu, D., Schilling, B., Liu, D., Sucker, A., Livingstone, E., Jerby-Arnon, L., et al. (2019a). Integrative Molecular and Clinical Modeling of Clinical Outcomes to PD1 Blockade in Patients with Metastatic Melanoma. Nat. Med. 25 (12), 1916–1927. doi:10.1038/s41591-019-0654-5
Liu, L., Bai, X., Wang, J., Tang, X. R., Wu, D. H., Du, S. S., et al. (2019b). Combination of TMB and CNA Stratifies Prognostic and Predictive Responses to Immunotherapy across Metastatic Cancer. Clin. Cancer Res. 25 (24), 7413–7423. doi:10.1158/1078-0432.CCR-19-0558
Lugowska, I., Teterycz, P., and Rutkowski, P. (2018). Immunotherapy of Melanoma. Contemp. Oncol. (Pozn) 22 (1A), 61–67. doi:10.5114/wo.2018.73889
Marabelle, A., Fakih, M., Lopez, J., Shah, M., Shapira-Frommer, R., Nakagawa, K., et al. (2020). Association of Tumour Mutational burden with Outcomes in Patients with Advanced Solid Tumours Treated with Pembrolizumab: Prospective Biomarker Analysis of the Multicohort, Open-Label, Phase 2 KEYNOTE-158 Study. Lancet Oncol. 21 (10), 1353–1365. doi:10.1016/S1470-2045(20)30445-9
McGrail, D. J., Pilié, P. G., Rashid, N. U., Voorwerk, L., Slagter, M., Kok, M., et al. (2021). High Tumor Mutation burden Fails to Predict Immune Checkpoint Blockade Response across All Cancer Types. Ann. Oncol. 32 (5), 661–672. doi:10.1016/j.annonc.2021.02.006
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Group, P. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. BMJ 339 (7), b2535. doi:10.1371/journal.pmed.100009710.1136/bmj.b2535
Morrison, C., Pabla, S., Conroy, J. M., Nesline, M. K., Glenn, S. T., Dressman, D., et al. (2018). Predicting Response to Checkpoint Inhibitors in Melanoma beyond PD-L1 and Mutational burden. J. Immunother. Cancer 6 (1), 32. doi:10.1186/s40425-018-0344-8
Riaz, N., Havel, J. J., Makarov, V., Desrichard, A., Urba, W. J., Sims, J. S., et al. (2017). Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell 171 (4), 934–e16. doi:10.1016/j.cell.2017.09.028
Rizvi, N. A., Hellmann, M. D., Snyder, A., Kvistborg, P., Makarov, V., Havel, J. J., et al. (2015). Cancer Immunology. Mutational Landscape Determines Sensitivity to PD-1 Blockade in Non-small Cell Lung Cancer. Science 348 (6230), 124–128. doi:10.1126/science.aaa1348
Rizzo, A., and Brandi, G. (2021). Biochemical Predictors of Response to Immune Checkpoint Inhibitors in Unresectable Hepatocellular Carcinoma. Cancer Treat. Res. Commun. 27, 100328. doi:10.1016/j.ctarc.2021.100328
Rizzo, A., Ricci, A. D., and Brandi, G. (2021). PD-L1, TMB, MSI, and Other Predictors of Response to Immune Checkpoint Inhibitors in Biliary Tract Cancer. Cancers 13 (3), 558. doi:10.3390/cancers13030558
Rizzo, A., and Ricci, A. D. (2021). PD-L1, TMB, and Other Potential Predictors of Response to Immunotherapy for Hepatocellular Carcinoma: How Can They Assist Drug Clinical Trials. Expert Opin. Investig. Drugs, 1–9. doi:10.1080/13543784.2021.1972969
Rooney, M. S., Shukla, S. A., Wu, C. J., Getz, G., and Hacohen, N. (2015). Molecular and Genetic Properties of Tumors Associated with Local Immune Cytolytic Activity. Cell 160 (1-2), 48–61. doi:10.1016/j.cell.2014.12.033
Rosenberg, J. E., Hoffman-Censits, J., Powles, T., van der Heijden, M. S., Balar, A. V., Necchi, A., et al. (2016). Atezolizumab in Patients with Locally Advanced and Metastatic Urothelial Carcinoma Who Have Progressed Following Treatment with Platinum-Based Chemotherapy: a Single-Arm, Multicentre, Phase 2 Trial. Lancet 387 (10031), 1909–1920. doi:10.1016/s0140-6736(16)00561-4
Roszik, J., Haydu, L. E., Hess, K. R., Oba, J., Joon, A. Y., Siroy, A. E., et al. (2016). Novel Algorithmic Approach Predicts Tumor Mutation Load and Correlates with Immunotherapy Clinical Outcomes Using a Defined Gene Mutation Set. BMC Med. 14 (1), 168. doi:10.1186/s12916-016-0705-4
Samstein, R. M., Lee, C. H., Shoushtari, A. N., Hellmann, M. D., Shen, R., Janjigian, Y. Y., et al. (2019). Tumor Mutational Load Predicts Survival after Immunotherapy across Multiple Cancer Types. Nat. Genet. 51 (2), 202–206. doi:10.1038/s41588-018-0312-8
Schachter, J., Ribas, A., Long, G. V., Arance, A., Grob, J. J., Mortier, L., et al. (2017). Pembrolizumab versus Ipilimumab for Advanced Melanoma: Final Overall Survival Results of a Multicentre, Randomised, Open-Label Phase 3 Study (KEYNOTE-006). Lancet 390 (10105), 1853–1862. doi:10.1016/s0140-6736(17)31601-x
Sharpe, A. H., and Pauken, K. E. (2018). The Diverse Functions of the PD1 Inhibitory Pathway. Nat. Rev. Immunol. 18 (3), 153–167. doi:10.1038/nri.2017.108
Snyder, A., Makarov, V., Merghoub, T., Yuan, J., Zaretsky, J. M., Desrichard, A., et al. (2014). Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N. Engl. J. Med. 371 (23), 2189–2199. doi:10.1056/NEJMoa1406498
Stang, A. (2010). Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 25 (9), 603–605. doi:10.1007/s10654-010-9491-z
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Teo, M. Y., Seier, K., Ostrovnaya, I., Regazzi, A. M., Kania, B. E., Moran, M. M., et al. (2018). Alterations in DNA Damage Response and Repair Genes as Potential Marker of Clinical Benefit from PD-1/pd-L1 Blockade in Advanced Urothelial Cancers. J. Clin. Oncol. 36 (17), 1685–1694. doi:10.1200/JCO.2017.75.7740
Tierney, J. F., Stewart, L. A., Ghersi, D., Burdett, S., and Sydes, M. R. (2007). Practical Methods for Incorporating Summary Time-To-Event Data into Meta-Analysis. Trials 8, 16. doi:10.1186/1745-6215-8-16
Valero, C., Lee, M., Hoen, D., Wang, J., Nadeem, Z., Patel, N., et al. (2021a). The Association between Tumor Mutational burden and Prognosis Is Dependent on Treatment Context. Nat. Genet. 53 (1), 11–15. doi:10.1038/s41588-020-00752-4
Valero, C., Lee, M., Hoen, D., Zehir, A., Berger, M. F., Seshan, V. E., et al. (2021b). Response Rates to Anti-PD-1 Immunotherapy in Microsatellite-Stable Solid Tumors with 10 or More Mutations Per Megabase. JAMA Oncol. 7 (5), 739–743. doi:10.1001/jamaoncol.2020.7684
Van Allen, E. M., Miao, D., Schilling, B., Shukla, S. A., Blank, C., Zimmer, L., et al. (2015). Erratum for the Report "Genomic Correlates of Response to CTLA-4 Blockade in Metastatic Melanoma" by E. M. Van Allen, D. Miao, B. Schilling, S. A. Shukla, C. Blank, L. Zimmer, A. Sucker, U. Hillen, M. H. Geukes Foppen, S. M. Goldinger, J. Utikal, J. C. Hassel, B. Weide, K. C. Kaehler, C. Loquai, P. Mohr, R. Gutzmer, R. Dummer, S. Gabriel, C. J. Wu, D. Schadendorf, L. A. Garraway. Science 350 (6257), aad8366–211. doi:10.1126/science.aad009510.1126/science.aad8366
Vega, D. M., Yee, L. M., McShane, L. M., Williams, P. M., Chen, L., Vilimas, T., et al. (2021). Aligning Tumor Mutational burden (TMB) Quantification across Diagnostic Platforms: Phase II of the Friends of Cancer Research TMB Harmonization Project. Ann. Oncol. 32 (12), 1626–1636. doi:10.1016/j.annonc.2021.09.016
Wagner, N. B., Forschner, A., Leiter, U., Garbe, C., and Eigentler, T. K. (2018). S100B and LDH as Early Prognostic Markers for Response and Overall Survival in Melanoma Patients Treated with Anti-PD-1 or Combined Anti-PD-1 Plus Anti-CTLA-4 Antibodies. Br. J. Cancer 119 (3), 339–346. doi:10.1038/s41416-018-0167-x
Wu, Q., Wang, Q., Tang, X., Xu, R., Zhang, L., Chen, X., et al. (2019). Correlation between Patients' Age and Cancer Immunotherapy Efficacy. OncoImmunology 8 (4), e1568810. doi:10.1080/2162402X.2019.1568810
Yusko, E., Vignali, M., Wilson, R. K., Mardis, E. R., Hodi, F. S., Horak, C., et al. (2019). Association of Tumor Microenvironment T-Cell Repertoire and Mutational Load with Clinical Outcome after Sequential Checkpoint Blockade in Melanoma. Cancer Immunol. Res. 7 (3), 458–465. doi:10.1158/2326-6066.CIR-18-0226
Zehir, A., Benayed, R., Shah, R. H., Syed, A., Middha, S., Kim, H. R., et al. (2017). Mutational Landscape of Metastatic Cancer Revealed from Prospective Clinical Sequencing of 10,000 Patients. Nat. Med. 23 (6), 703–713. doi:10.1038/nm.4333
Zhai, T. T., van Dijk, L. V., Huang, B. T., Lin, Z. X., Ribeiro, C. O., Brouwer, C. L., et al. (2017). Improving the Prediction of Overall Survival for Head and Neck Cancer Patients Using Image Biomarkers in Combination with Clinical Parameters. Radiother. Oncol. 124 (2), 256–262. doi:10.1016/j.radonc.2017.07.013
Keywords: tumor mutation burden, immune checkpoint inhibitor, OS, PFS, melanoma, meta-analysis
Citation: Ning B, Liu Y, Wang M, Li Y, Xu T and Wei Y (2022) The Predictive Value of Tumor Mutation Burden on Clinical Efficacy of Immune Checkpoint Inhibitors in Melanoma: A Systematic Review and Meta-Analysis. Front. Pharmacol. 13:748674. doi: 10.3389/fphar.2022.748674
Received: 28 July 2021; Accepted: 10 February 2022;
Published: 09 March 2022.
Edited by:
Robert Clarke, University of Minnesota Twin Cities, United StatesReviewed by:
Alessandro Rizzo, National Cancer Institute Foundation (IRCCS), ItalyAntonia Dimitrakopoulou-Strauss, German Cancer Research Center, Germany
Copyright © 2022 Ning, Liu, Wang, Li, Xu and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongchang Wei, weiyongchang@whu.edu.cn
 Biao Ning1,2,3
Biao Ning1,2,3 Miao Wang
Miao Wang Yi Li
Yi Li Yongchang Wei
Yongchang Wei