- Department of Laboratory Medicine, Zhongnan Hospital of Wuhan University, Wuhan, China
Background: Human mutL homolog 1 (hMLH1) is located on chromosome 3q21-23. As a classic tumor suppressor gene, many researchers have studied the association between hMLH1 promoter methylation and gastric cancer, but their conclusions were not always consistent. Therefore, we performed a meta-analysis to make a more integrated and precise estimate of the associations.
Method: PubMed, EMBASE, and Cochrane Library were retrieved without language restrictions. Data were analyzed by Review Manager 5.2 and Stata 12.0 software. Odds ratio (OR) with 95% confidence interval (95%CI) was used to assess the statistical associations.
Result: A total of 39 studies published before January 20, 2018 were included in this study. The results indicated that the frequency of hMLH1 promoter methylation in gastric cancers was substantially higher than that in non-cancer controls (OR = 7.94, 95%CI = 4.32–14.58, P < 0.001). Furthermore, hMLH1 promoter methylation had considerable associations with lymph node metastasis, microsatellite instability (MSI), and low expression of hMLH1 protein (OR = 1.53, 95%CI = 1.04–2.26, P = 0.03; OR = 15.33, 95%CI = 9.26–25.36, P < 0.001; OR = 37.86, 95%CI = 18.03–79.50, P < 0.001, respectively). No association was found between hMLH1 promoter methylation and Lauren classification or Helicobacter pylori (HP) infection status.
Conclusion: The present study provides evidence that promoter methylation of hMLH1 is a major causative event in the occurrence and development of human gastric cancer.
Introduction
Gastric cancer, also known as stomach cancer, continues to be a vital heath threat as the fifth leading cause of cancer and the third leading cause of death from carcinoma globally according to World Health Organization in 2014 (Stewart and Wild, 2014). Owing to inadequate early diagnosis and unclear pathogenesis, gastric cancer was considered to be a high-mortality disease and its 5-year survival rate was reported to be <10% (Orditura et al., 2014). Although the pathogenic mechanisms have not been fully elucidated, some factors including Helicobacter pylori (HP) infection, smoking, and excessive drinking, have been identified to contribute to the tumorigenesis of gastric cancer. In addition, epigenetic silencing of tumor suppressor genes was also thought to play an important part in the genesis of gastric cancer (Tahara and Arisawa, 2015).
DNA methylation is the most common epigenetic mechanism. It is catalyzed by a family of DNA methyltransferases (DNMT) that transfer a methyl group from S-adenyl methionine (SAM) to the fifth carbon of a cytosine residue to form 5- methyl cytosine (5 mC), which is unstable and can spontaneously deaminate to form thymine, thereby affecting gene expression (Moore et al., 2013). Much evidence showed that hypermethylation of normally unmethylated CpG islands in the promoter regions of tumor suppressor genes was strongly related to carcinomas, for instance, BRCA1 promoter methylation in breast cancer (Zhang and Long, 2015), GSTP1 promoter methylation in prostate cancer (Jerónimo et al., 2001), hMLH1 methylation in gastroenteric tumors (Arai et al., 2010). hMLH1 is one of the human mismatched repair (MMR) genes, which is located on chromosome 3q21-23. As a classic anti-oncogene, the protein encoded by this gene is a component of the DNA mismatch repair pathway which can effectively repair mismatched bases and prevent the accumulation of DNA damage.
During the past decades, the associations between hMLH1 promoter methylation and the risk or clinicopathological characteristics of stomach cancer have been reported by many researchers. However, the conclusions were not always consistent and some results were unconvincing because of the small sample size. Therefore, we performed a meta-analysis to clarify the role of hMLH1 gene promoter methylation in the tumorigenesis and development of gastric cancer.
Methods
Literature Search Strategy
PubMed, EMBASE, and Cochrane Library were retrieved to obtain literatures concerning the association between gastric cancer and hMLH1 promoter methylation without language restrictions. We used the terms as follows: (“hMLH1” or “human mutL homolog 1”) and (“methylation”) and (“stomach” or “gastric”) and (“cancer” or “neoplasms” or “carcinoma”). The search results were updated until January 20, 2018. In addition, we also performed manual search for other relevant literatures.
Selection Criteria
The following criteria were used in selecting eligible articles: (a) articles dealing with the association of hMLH1 promoter methylation with gastric cancers; (b) case-control studies. The major reasons for exclusion of studies: (a) reviews, letters, or case-only articles (b) articles with insufficient data or duplicated data.
Data Extraction and Quality Assessment
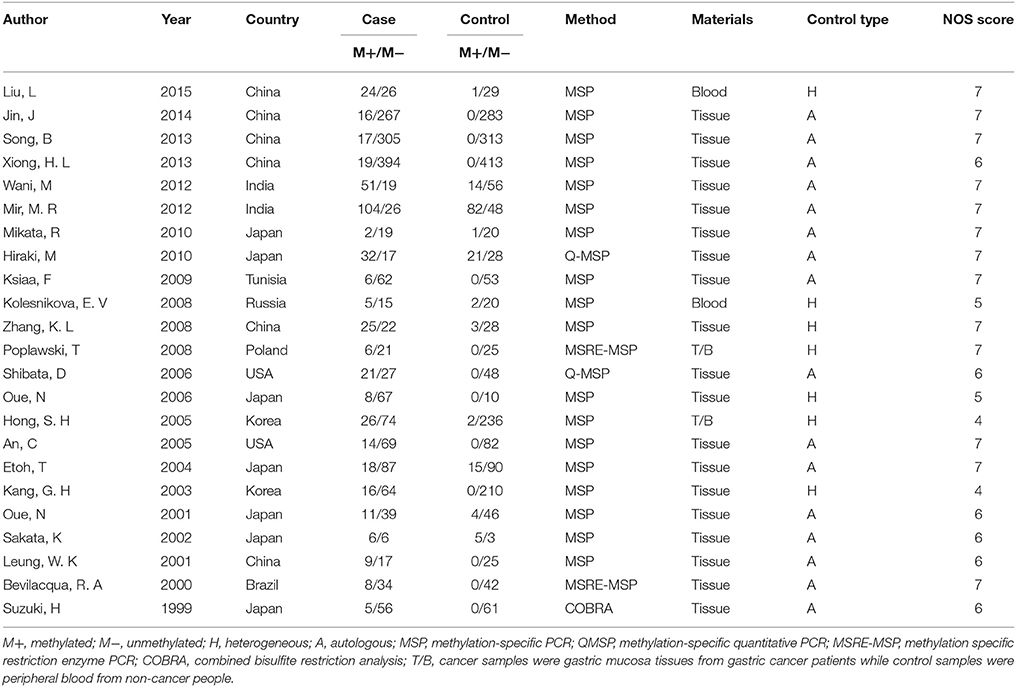
Articles were screened first by reading titles and abstracts, then two reviewers (Y Shi and P Ye) read the entire articles that seemed to fit the inclusion criteria and extracted basic information including the first authors' names, publication years, countries, methylation detection methods, sample sizes, and number of methylation in cases and controls from every eligible study. All included studies concerning hMLH1 methylation and gastric cancers risk were evaluated by the modified Newcastle-Ottawa scale (NOS) assessment (Jadad et al., 1996) (available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). The latest NOS assessment for case-control studies consists of seven items of methodology which are grouped into three major categories: cases and controls selection, comparability of cases and controls, and ascertainment of exposure. The total score ranges from 0 to 8, and studies with more than four points are considered as qualified. Disagreement was resolved by discussion and consensus.
Statistical Analysis
Data were analyzed by Review Manager 5.2 and Stata 12.0. The strength of the association between hMLH1 promoter methylation and gastric cancers risk or clinicopathologic characteristics was assessed by pooled OR with corresponding 95% CI. Chi-square test based Q-test and I2-test were performed to assess heterogeneity among studies. It indicated a lack of heterogeneity if P > 0.10 and I2 < 50%, then the pooled OR would be calculated by using the fixed-effect model in line with the Mantel-Haenszel method. Otherwise, the random-effect model would be used according to the DerSimonian-Laird method. The subgroup analysis was further conducted based on different ethnicities, types of controls, specimen materials, and methods of detecting methylation. The stability of the pooled result was evaluated by sensitivity analysis and potential publication bias was assessed by Begg's test.
Results
Study Characteristics
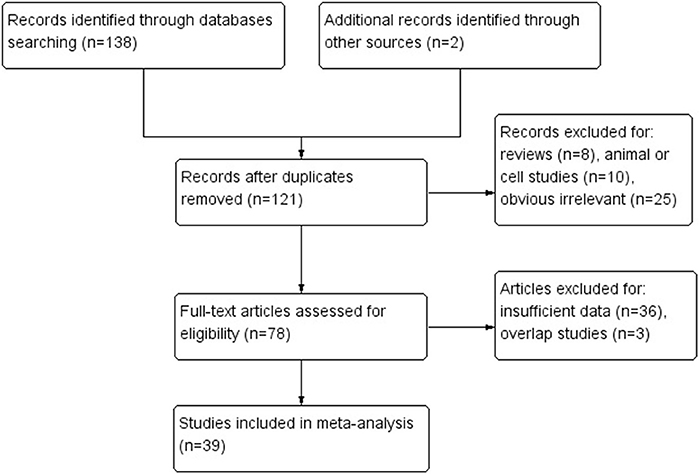
Based on above selection criteria, 39 studies were included in our research. Twenty-three of them (Suzuki et al., 1999; Bevilacqua and Simpson, 2000; Leung et al., 2001; Oue et al., 2001, 2006; Sakata et al., 2002; Kang et al., 2003; Etoh et al., 2004; An et al., 2005; Hong et al., 2005; Shibata et al., 2006; Kolesnikova et al., 2008; Poplawski et al., 2008; Zhang et al., 2008; Ksiaa et al., 2009; Hiraki et al., 2010; Mikata et al., 2010; Mir et al., 2012; Wani et al., 2012; Song et al., 2013; Xiong et al., 2013; Jin et al., 2014; Liu and Yang, 2015) evaluated the association between hMLIH1 promoter methylation and gastric cancer risk, including 2,182 cases and 2,319 controls; 27 of them (Fleisher et al., 1999; Leung et al., 1999; Suzuki et al., 1999; Toyota et al., 1999; Pinto et al., 2000; Nakajima et al., 2001; Oue et al., 2001, 2003; Sakata et al., 2002; Fang et al., 2003; Sugai et al., 2004; Wu et al., 2004; An et al., 2005; Hong et al., 2005; Kim et al., 2005, 2010; Nan et al., 2005; Kolesnikova et al., 2008; Ferrasi et al., 2010; Hiraki et al., 2010; Mikata et al., 2010; Alves et al., 2011; Song et al., 2013; Xiong et al., 2013; Jin et al., 2014; Moghbeli et al., 2014; Kawanaka et al., 2016) with 2,713 patients investigated the associations of hMLIH1 methylation with clinicopathological features including lymph node metastasis, Lauren's histological type, microsatellite status, Helicobacter pylori (HP) infection status, and hMLH1 protein expression in gastric cancer patients. The flow chart (Figure 1) summarized the study screening process. The main characteristics of these included studies were listed in Table 1.
Meta-Analysis Results
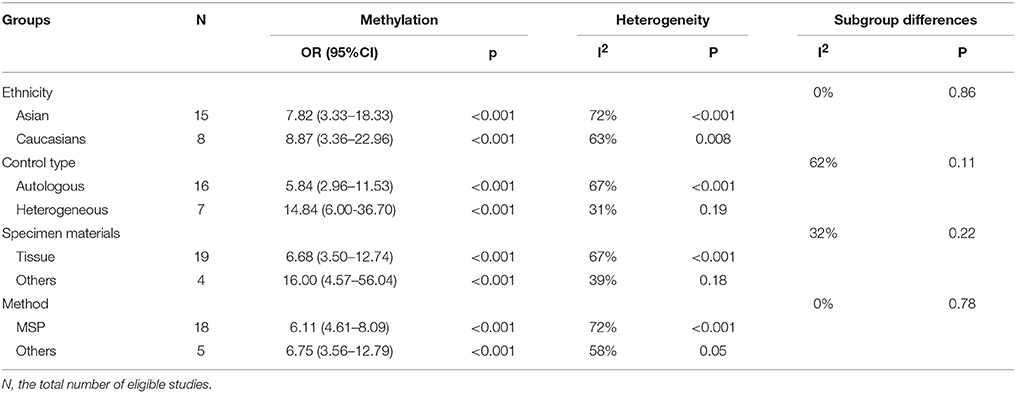
Firstly, we analyzed the association between hMLH1 promoter methylation and risk of gastric cancer. Significant heterogeneity of studies (I2 = 68%, P < 0.001) was detected by X2 test, so we employed random-effect model. In general, our result showed that the hMLH1 methylation frequency in gastric cancer was obviously higher compared with non-cancer controls (OR = 7.94, 95%CI = 4.32–14.58, P < 0.001, Figure 2). We explored the sources of heterogeneity through subgroup analysis about the ethnicity, type of controls, specimen materials, and methods of detecting methylation (Table 2). There was no significant heterogeneity between different races (Asians subgroup and Caucasians subgroup), different specimen materials (tissue subgroup or other materials subgroup), or different methylation detection methods (MSP subgroup and other methods subgroup). However, remarkable heterogeneity between subgroup whose controls were autologous and subgroup whose controls were heterogeneous has been observed (I2 = 61.7%, P = 0.11).
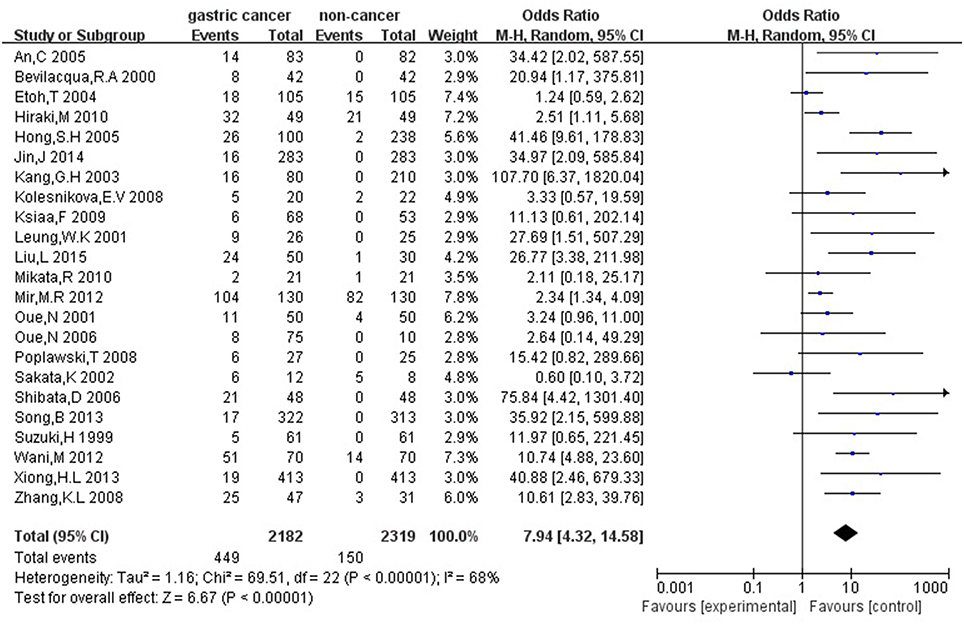
Figure 2. Forest plot concerning hMLH1 methylation and gastric cancer risk. The squares and horizontal lines represent the corresponding OR and 95% CI for each study. The area of the squares reflects the weight of each study. The diamond represents the pooled OR and 95% CI. Random-effect model was used.
Then we explored the associations between hMLH1 promoter methylation and clinicopathological characteristics of gastric cancer. In short, there were significant statistical associations between hMLH1 methylation and lymph node metastasis (OR = 1.53, 95%CI = 1.04–2.26, P = 0.03, fixed-effect model), microsatellite status (OR = 15.33, 95%CI = 9.26–25.36, P < 0.001, fixed-effect model) and low hMLH1 protein expression (OR = 37.86, 95%CI = 18.03–79.50, P < 0.001, fixed-effect model) in gastric cancer patients, but not with Lauren classification (OR = 1.48, 95%CI = 0.86–2.55, P = 0.16, fixed-effect model) or HP infection status (OR = 1.18, 95%CI = 0.69-2.01, P = 0.54, fixed-effect model). The results were listed in Table 3 and the forest plots were shown in Figures 3–7.
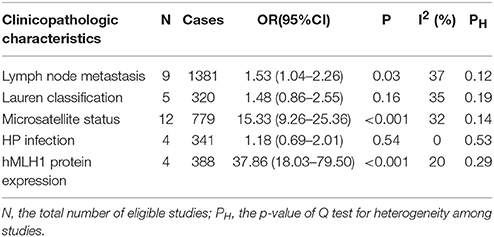
Table 3. The association between hMLH1 promoter methylation and clinicopathological characteristics of gastric cancer.
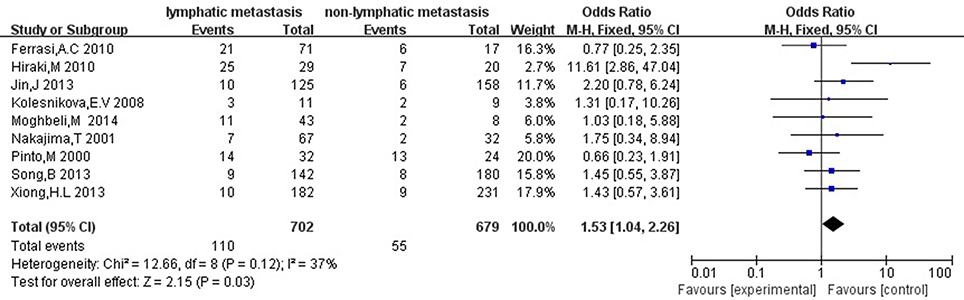
Figure 3. Forest plot concerning hMLH1 methylation and lymph node metastasis. Fixed-effect model was used.
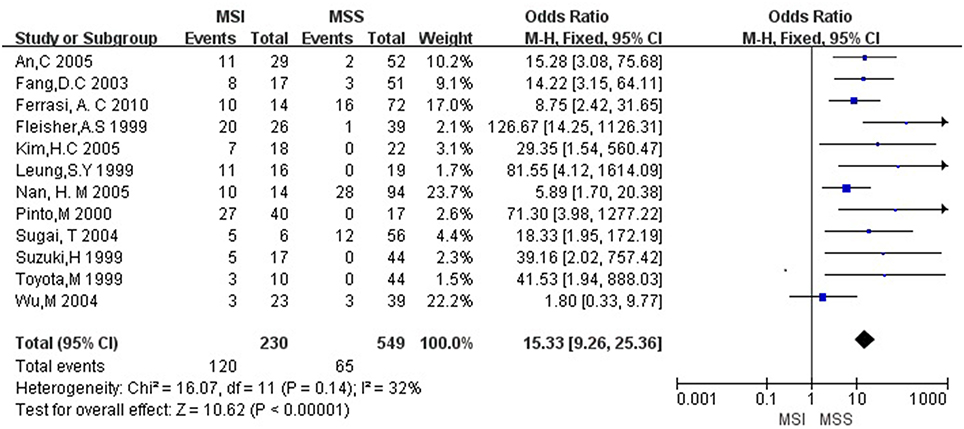
Figure 4. Forest plot concerning hMLH1 methylation and MSI. MSI, microsatellite instability. Fixed-effect model was used.
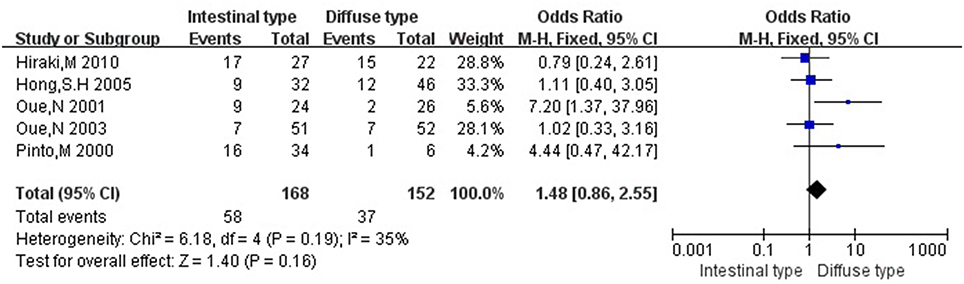
Figure 5. Forest plot concerning hMLH1 methylation and Lauren classification. Fixed-effect model was used.
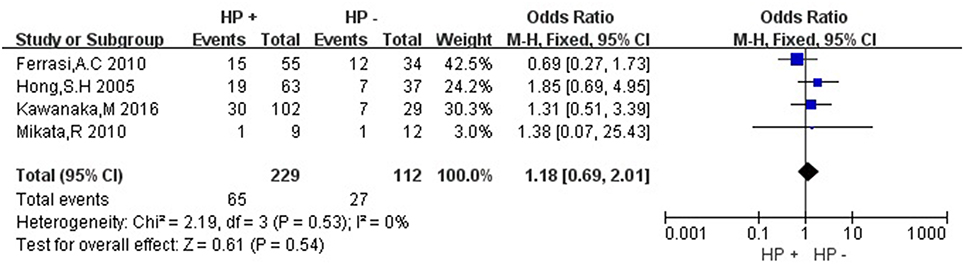
Figure 6. Forest plot concerning hMLH1 methylation and Helicobacter pylori infection. HP, Helicobacter pylori. Fixed-effect model was used.
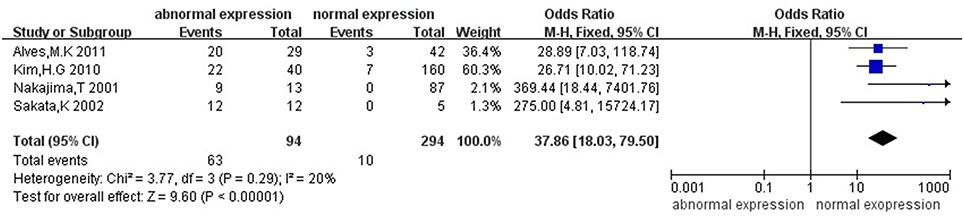
Figure 7. Forest plot concerning hMLH1 methylation and hMLH1 protein expression. Fixed-effect model was used.
Sensitivity Analysis and Publication Bias
We conducted a sensitivity analysis to evaluate the stability of our result by sequentially omitting every study from pooled analysis. The sensitivity analysis confirmed that the result was stable since omission of each single study could not significantly alter the pooled OR (Figure 8). We applied Begg's funnel plot to assess potential publication bias of these eligible articles. The shape of funnel plot was symmetric and P = 0.561, which indicated that no obvious publication bias was found. The funnel plot for evaluating the association of hMLH1 promoter methylation with stomach cancer risk was shown in Figure 9.
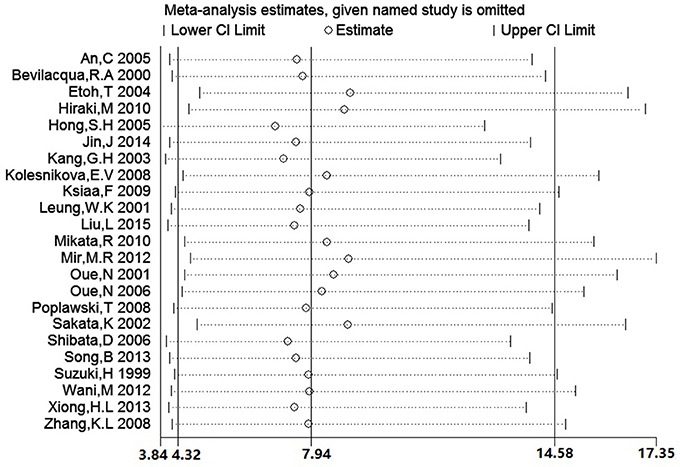
Figure 8. The plot of sensitivity analysis for evaluating the association between hMLH1 methylation and gastric risk. The circle and horizontal dashed line represent the pooled OR and 95% CI after omitting the corresponding study.
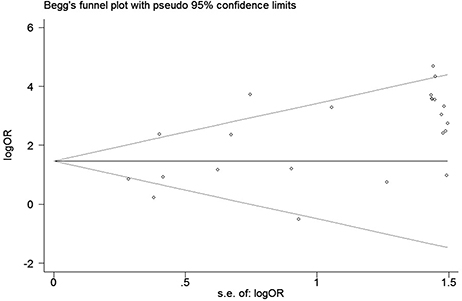
Figure 9. Funnel plot for evaluating the association of hMLH1 methylation with gastric cancer risk. Each circle represents one specific study.
Discussion
Mismatch repairing is essential to ensure DNA replication fidelity, and mismatch repair deficiency will increase the chances of DNA mutation, which is important to the development of a tumor from normal cells (Thomas et al., 1996). Mismatch repair genes are a group of highly conserved housekeeping genes playing a major role in the maintenance of genetic and epigenetic stability (Li, 2008). hMLH1 gene is one of the most widely studied. In recent years, some studies on cell lines suggested that methylation of hMLH1 gene promoter is directly related to lack of hMLH1 protein expression, which is an important event in initiation of many kinds of sporadic tumors (Kane et al., 1997; Yao et al., 2004).
Previous studies have investigated the association between aberrant hMLH1 promoter methylation and gastric cancer, yet the results were not consistent probably due to different ethnic groups, types of control, methylation detection methods and specimen materials. In order to obtain consistent results, we performed a meta-analysis involving 2,182 cases of tumors and 2,319 controls. The results showed that aberrant methylation of hMLH1 promoter in stomach cancer tissues or bloods was positively associated with gastric cancer risk. Moreover, the frequency of hMLH1 methylation was higher in gastric cancer with lymph node metastasis, microsatellite instability and absence of hMLH1 protein expression, suggesting that hMLH1 promoter methylation might play a critical role in gastric cancer initiation and progression.
We confirmed that the frequency of hMLH1 promoter methylation in gastric cancer was 7.94-fold higher than that in control groups, meanwhile, the expression of hMLH1 protein substantially decreased in stomach cancer patients with hMLH1 hypermethylation. Our conclusion was similar to some results reported in other types of carcinomas (Mitchell et al., 2002; Han et al., 2016). hMLH1 promoter methylation often leads to transcriptional silencing accompanied by down-regulation of mRNA expression, resulting in decrease of hMLH1 protein expression and mismatch repair dysfunction which contribute to tumorigenesis. Because of the heterogeneity of the studies, we conducted a subgroup analysis to explore the sources of heterogeneity. We found that there was heterogeneity between the autologous controls group and the heterogeneous controls group. The pooled OR was higher when the heterogeneous samples from non-cancer patients were used as the control group. There may be two explanations: (1) Gastric cancer is a systemic disease, certain changes may occur in healthy tissues more or less. (2) Different sampling methods among the studies: some used adjacent normal tissues as their autologous controls while the others chose remote normal tissues. We also found that hMLH1 methylation was more frequent in gastric cancer patients with lymph node metastasis, indicated that hMLH1 methylation may be implicated in the invasion and metastasis of gastric cancer.
Our meta-analysis also demonstrated that aberrant methylation of hMLH1 was closely related to microsatellite instability. MSI was first found in hereditary nonpolyposis colorectal cancer (HNPCC) (Aaltonen et al., 1993), while gastric cancer possessed the highest prevalence of MSI (Ottini et al., 1997; Keller et al., 1998). It comprises length mutations in tandem oligonucleotide repeats which was believed to be caused by the inability of the MMR protein to fix a DNA replication error (Lynch and de la Chapelle, 2003). Indeed, MSI can be a molecular hallmark of mismatch-repair-deficient-tumors and even serve as a tool for the classification of gastric cancer (Simpson et al., 2001). We didn't find associations between hMLH1 methylation and HP infection or Lauren classification, upon which researchers had different views. Nevertheless, the outcomes might be due to small sample sizes and need to be confirmed by more studies with larger samples in the future.
The sensitivity analysis and publication bias analysis results demonstrated that this meta-analysis was stable and had no obvious publication bias. However, our meta-analysis might still have some limitations. First of all, there was significant heterogeneity among these studies which were used to analyze the prevalence of hMLH1 promoter methylation in gastric cancers, but we could not provide a good solution about sources of heterogeneity; Also, there may be differential effects in hMLH1 methylation among different races, but these eligible studies did not contain all races, more researches are also needed to determine whether our outcomes are in consistent with studies about other ethnicities; Thirdly, we were not able to evaluate the associations between hMLH1 promoter methylation and other clinicopathological features because of insufficient data, prospective population-based studies are necessary for further research.
Above all, this is the first meta-analysis focused on the association between aberrant hMLH1 promoter methylation and gastric cancer, which provides evidence that silencing of the hMLH1 gene by promoter hypermethylation is a major causative event in the occurrence and development of human gastric cancer. Nevertheless, more efforts are still needed to be made before regarding hMLH1 promoter methylation as a potential diagnostic or prognostic biomarker.
Author Contributions
The literature searching, data extraction, statistical analysis, and paper writing were conducted by PY and YS. AL reviewed the manuscript. All authors approved the final version of the manuscript.
Funding
This work was supported by grants from Natural Science Foundation of Hubei Province (2015CKB741).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SM and handling Editor declared their shared affiliation.
References
Aaltonen, L. A., Peltomaki, P., Leach, F. S., Sistonen, P., Pylkkanen, L., Mecklin, J. P., et al. (1993). Clues to the pathogenesis of familial colorectal cancer. Science 260, 812–816. doi: 10.1126/science.8484121
Alves, M. K., Ferrasi, A. C., Lima, V. P., Ferreira, M. V., de Moura Campos Pardini, M. I., and Rabenhorst, S. H. (2011). Inactivation of COX-2, HMLH1 and CDKN2A gene by promoter methylation in gastric cancer: relationship with histological subtype, tumor location and Helicobacter pylori genotype. Pathobiology 78, 266–276. doi: 10.1159/000329475
An, C., Choi, I. S., Yao, J. C., Worah, S., Xie, K., Mansfield, P. F., et al. (2005). Prognostic significance of CpG island methylator phenotype and microsatellite instability in gastric carcinoma. Clin. Cancer Res. 11(2 Pt 1), 656–663.
Arai, T., Kasahara, I., Sawabe, M., Honma, N., Aida, J., and Tabubo, K. (2010). Role of methylation of the hMLH1 gene promoter in the development of gastric and colorectal carcinoma in the elderly. Geriatr. Gerontol. Int. 10(Suppl. 1), S207–S212. doi: 10.1111/j.1447-0594.2010.00590.x
Bevilacqua, R. A., and Simpson, A. J. (2000). Methylation of the hMLH1 promoter but no hMLH1 mutations in sporadic gastric carcinomas with high-level microsatellite instability. Int. J. Cancer 87, 200–203. doi: 10.1002/1097-0215(20000715)87:2<200::AID-IJC7>3.0.CO;2-I
Etoh, T., Kanai, Y., Ushijima, S., Nakagawa, T., Nakanishi, Y., Sasako, M., et al. (2004). Increased DNA methyltransferase 1 (DNMT1) protein expression correlates significantly with poorer tumor differentiation and frequent DNA hypermethylation of multiple CpG islands in gastric cancers. Am. J. Pathol. 164, 689–699. doi: 10.1016/S0002-9440(10)63156-2
Fang, D. C., Wang, R. Q., Yang, S. M., Yang, J. M., Liu, H. F., Peng, G. Y., et al. (2003). Mutation and methylation of hMLH1 in gastric carcinomas with microsatellite instability. World J. Gastroenterol. 9, 655–659. doi: 10.3748/wjg.v9.i4.655
Ferrasi, A. C., Pinheiro, N. A., Rabenhorst, S. H., Caballero, O. L., Rodrigues, M. A., de Carvalho, F., et al. (2010). Helicobacter pylori and EBV in gastric carcinomas: methylation status and microsatellite instability. World J. Gastroenterol. 16, 312–319. doi: 10.3748/wjg.v16.i3.312
Fleisher, A. S., Esteller, M., Wang, S., Tamura, G., Suzuki, H., Yin, J., et al. (1999). Hypermethylation of the hMLH1 gene promoter in human gastric cancers with microsatellite instability. Cancer Res. 59, 1090–1095.
Han, Y., Shi, K., Zhou, S. J., Yu, D. P., and Liu, Z. D. (2016). The clinicopathological significance of hMLH1 hypermethylation in non-small-cell lung cancer: a meta-analysis and literature review. Onco. Targets. Ther. 9, 5081–5090. doi: 10.2147/OTT.S106345
Hiraki, M., Kitajima, Y., Sato, S., Mitsuno, M., Koga, Y., Nakamura, J., et al. (2010). Aberrant gene methylation in the lymph nodes provides a possible marker for diagnosing micrometastasis in gastric cancer. Ann. Surg. Oncol. 17, 1177–1186. doi: 10.1245/s10434-009-0815-8
Hong, S. H., Kim, H. G., Chung, W. B., Kim, E. Y., Lee, J. Y., Yoon, S. M., et al. (2005). DNA hypermethylation of tumor-related genes in gastric carcinoma. J. Korean Med. Sci. 20, 236–241. doi: 10.3346/jkms.2005.20.2.236
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J., Gavaghan, D. J., et al. (1996). Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control. Clin. Trials 17, 1–12. doi: 10.1016/0197-2456(95)00134-4
Jerónimo, C., Usadel, H., Henrique, R., Oliveira, J., Lopes, C., Nelson, W. G., et al. (2001). Quantitation of GSTP1 methylation in non-neoplastic prostatic tissue and organ-confined prostate adenocarcinoma. J. Natl. Cancer Inst. 93, 1747–1752. doi: 10.1093/jnci/93.22.1747
Jin, J., Xie, L., Xie, C. H., and Zhou, Y. F. (2014). Aberrant DNA methylation of MGMT and hMLH1 genes in prediction of gastric cancer. Genet. Mol. Res. 13, 4140–4145. doi: 10.4238/2014.May.30.9
Kane, M. F., Loda, M., Gaida, G. M., Lipman, J., Mishra, R., Goldman, H., et al. (1997). Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 57, 808–811.
Kang, G. H., Lee, S., Kim, J. S., and Jung, H. Y. (2003). Profile of aberrant CpG island methylation along the multistep pathway of gastric carcinogenesis. Lab. Invest. 83, 635–641. doi: 10.1097/01.LAB.0000067481.08984.3F
Kawanaka, M., Watari, J., Kamiya, N., Yamasaki, T., Kondo, T., Toyoshima, F., et al. (2016). Effects of Helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic treatment: analysis of molecular alterations by a randomised controlled trial. Br. J. Cancer 114, 21–29. doi: 10.1038/bjc.2015.418
Keller, G., Rudelius, M., Vogelsang, H., Grimm, V., Wilhelm, M. G., Mueller, J., et al. (1998). Microsatellite instability and loss of heterozygosity in gastric carcinoma in comparison to family history. Am. J. Pathol. 152, 1281–1289.
Kim, H. C., Kim, J. C., Roh, S. A., Yu, C. S., Yook, J. H., Oh, S. T., et al. (2005). Aberrant CpG island methylation in early-onset sporadic gastric carcinoma. J. Cancer Res. Clin. Oncol. 131, 733–740. doi: 10.1007/s00432-005-0017-0
Kim, H. G., Lee, S., Kim, D. Y., Ryu, S. Y., Joo, J. K., Kim, J. C., et al. (2010). Aberrant methylation of DNA mismatch repair genes in elderly patients with sporadic gastric carcinoma: a comparison with younger patients. J. Surg. Oncol. 101, 28–35. doi: 10.1002/jso.21432
Kolesnikova, E. V., Tamkovich, S. N., Bryzgunova, O. E., Shelestyuk, P. I., Permyakova, V. I., Vlassov, V. V., et al. (2008). Circulating DNA in the blood of gastric cancer patients. Ann. N. Y. Acad. Sci. 1137, 226–231. doi: 10.1196/annals.1448.009
Ksiaa, F., Ziadi, S., Amara, K., Korbi, S., and Trimeche, M. (2009). Biological significance of promoter hypermethylation of tumor-related genes in patients with gastric carcinoma. Clin. Chim. Acta 404, 128–133. doi: 10.1016/j.cca.2009.03.044
Leung, S. Y., Yuen, S. T., Chung, L. P., Chu, K. M., Chan, A. S., and Ho, J. C. (1999). hMLH1 promoter methylation and lack of hMLH1 expression in sporadic gastric carcinomas with high-frequency microsatellite instability. Cancer Res. 59, 159–164.
Leung, W. K., Yu, J., Ng, E. K., To, K. F., Ma, P. K., Lee, T. L., et al. (2001). Concurrent hypermethylation of multiple tumor-related genes in gastric carcinoma and adjacent normal tissues. Cancer 91, 2294–2301. doi: 10.1002/1097-0142(20010615)91:12<2294::AID-CNCR1261>3.0.CO;2-G
Li, G. M. (2008). Mechanisms and functions of DNA mismatch repair. Cell Res. 18, 85–98. doi: 10.1038/cr.2007.115
Liu, L., and Yang, X. (2015). Implication of Reprimo and hMLH1 gene methylation in early diagnosis of gastric carcinoma. Int. J. Clin. Exp. Pathol. 8, 14977–14982.
Lynch, H. T., and de la Chapelle, A. (2003). Hereditary colorectal cancer. N. Engl. J. Med. 348, 919–932. doi: 10.1056/NEJMra012242
Mikata, R., Fukai, K., Imazeki, F., Arai, M., Fujiwara, K., Yonemitsu, Y., et al. (2010). BCL2L10 is frequently silenced by promoter hypermethylation in gastric cancer. Oncol. Rep. 23, 1701–1708.
Mir, M. R., Shabir, N., Wani, K. A., Shaff, S., Hussain, I., Banday, M. A., et al. (2012). Association between p16, hMLH1 and E-cadherin promoter hypermethylation and intake of local hot salted tea and sun-dried foods in Kashmiris with gastric tumors. Asian Pac. J. Cancer Prev. 13, 181–186. doi: 10.7314/APJCP.2012.13.1.181
Mitchell, R. J., Farrington, S. M., Dunlop, M. G., and Campbell, H. (2002). Mismatch repair genes hMLH1 and hMSH2 and colorectal cancer: a HuGE review. Am. J. Epidemiol. 156, 885–902. doi: 10.1093/aje/kwf139
Moghbeli, M., Moaven, O., Memar, B., Raziei, H. R., Aarabi, A., Dadkhah, E., et al. (2014). Role of hMLH1 and E-cadherin promoter methylation in gastric cancer progression. J. Gastrointest. Cancer 45, 40–47. doi: 10.1007/s12029-013-9548-9
Moore, L. D., Le, T., and Fan, G. (2013). DNA methylation and its basic function. Neuropsychopharmacology 38, 23–38. doi: 10.1038/npp.2012.112
Nakajima, T., Akiyama, Y., Shiraishi, J., Arai, T., Yanagisawa, Y., Ara, M., et al. (2001). Age-related hypermethylation of the hMLH1 promoter in gastric cancers. Int. J. Cancer 94, 208–211. doi: 10.1002/ijc.1454
Nan, H. M., Song, Y. J., Yun, H. Y., Park, J. S., and Kim, H. (2005). Effects of dietary intake and genetic factors on hypermethylation of the hMLH1 gene promoter in gastric cancer. World J. Gastroenterol. 11, 3834–3841. doi: 10.3748/wjg.v11.i25.3834
Orditura, M., Galizia, G., Sforza, V., Gambardella, V., Fabozzi, A., Laterza, M. M., et al. (2014). Treatment of gastric cancer. World J. Gastroenterol. 20, 1635–1649. doi: 10.3748/wjg.v20.i7.1635
Ottini, L., Palli, D., Falchetti, M., D'Amico, C., Amorosi, A., Saieva, C., et al. (1997). Microsatellite instability in gastric cancer is associated with tumor location and family history in a high-risk population from Tuscany. Cancer Res. 57, 4523–4529.
Oue, N., Mitani, Y., Motoshita, J., Matsumura, S., Yoshida, K., Kuniyasu, H., et al. (2006). Accumulation of DNA methylation is associated with tumor stage in gastric cancer. Cancer 106, 1250–1259. doi: 10.1002/cncr.21754
Oue, N., Oshimo, Y., Nakayama, H., Ito, R., Yoshida, K., Matsusaki, K., et al. (2003). DNA methylation of multiple genes in gastric carcinoma: association with histological type and CpG island methylator phenotype. Cancer Sci. 94, 901–905. doi: 10.1111/j.1349-7006.2003.tb01373.x
Oue, N., Sentani, K., Yokozaki, H., Kitadai, Y., Ito, R., and Yasui, W. (2001). Promoter methylation status of the DNA repair genes hMLH1 and MGMT in gastric carcinoma and metaplastic mucosa. Pathobiology 69, 143–149. doi: 10.1159/000048769
Pinto, M., Oliveira, C., Machado, J. C., Cirnes, L., Tavares, J., Carneiro, F., et al. (2000). MSI-L gastric carcinomas share the hMLH1 methylation status of MSI-H carcinomas but not their clinicopathological profile. Lab. Invest. 80, 1915–1923. doi: 10.1038/labinvest.3780201
Poplawski, T., Tomaszewska, K., Galicki, M., Morawiec, Z., and Blasiak, J. (2008). Promoter methylation of cancer-related genes in gastric carcinoma. Exp. Oncol. 30, 112–116.
Sakata, K., Tamura, G., Endoh, Y., Ohmura, K., Ogata, S., and Motoyama, T. (2002). Hypermethylation of the hMLH1 gene promoter in solitary and multiple gastric cancers with microsatellite instability. Br. J. Cancer 86, 564–567. doi: 10.1038/sj.bjc.6600076
Shibata, D., Mori, Y., Cai, K., Zhang, L., Yin, J., Elahi, A., et al. (2006). RAB32 hypermethylation and microsatellite instability in gastric and endometrial adenocarcinomas. Int. J. Cancer 119, 801–806. doi: 10.1002/ijc.21912
Simpson, A. J., Caballero, O. L., and Pena, S. D. (2001). Microsatellite instability as a tool for the classification of gastric cancer. Trends Mol. Med. 7, 76–80. doi: 10.1016/S1471-4914(01)01916-5
Song, B., Ai, J., Kong, X., Liu, D., and Li, J. (2013). Aberrant DNA methylation of P16, MGMT, and hMLH1 genes in combination with MTHFR C677T genetic Polymorphism in gastric cancer. Pak. J. Med. Sci. 29, 1338–1343.
Sugai, T., Habano, W., Uesugi, N., Jao, Y. F., Nakamura, S., Abe, K., et al. (2004). Three independent genetic profiles based on mucin expression in early differentiated-type gastric cancers–a new concept of genetic carcinogenesis of early differentiated-type adenocarcinomas. Mod. Pathol. 17, 1223–1234. doi: 10.1038/modpathol.3800170
Suzuki, H., Itoh, F., Toyota, M., Kikuchi, T., Kakiuchi, H., Hinoda, Y., et al. (1999). Distinct methylation pattern and microsatellite instability in sporadic gastric cancer. Int. J. Cancer 83, 309–313. doi: 10.1002/(SICI)1097-0215(19991029)83:3<309::AID-IJC4>3.0.CO;2-Z
Tahara, T., and Arisawa, T. (2015). DNA methylation as a molecular biomarker in gastric cancer. Epigenomics 7, 475–486. doi: 10.2217/epi.15.4
Thomas, D. C., Umar, A., and Kunkel, T. A. (1996). Microsatellite instability and mismatch repair defects in cancer. Mutat. Res. 350, 201–205. doi: 10.1016/0027-5107(95)00112-3
Toyota, M., Ahuja, N., Suzuki, H., Itoh, F., Ohe-Toyota, M., Imai, K., et al. (1999). Aberrant methylation in gastric cancer associated with the CpG island methylator phenotype. Cancer Res. 59, 5438–5442.
Wani, M., Afroze, D., Makhdoomi, M., Hamid, I., Wani, B., Bhat, G., et al. (2012). Promoter methylation status of DNA repair gene (hMLH1) in gastric carcinoma patients of the Kashmir valley. Asian Pac. J. Cancer Prev. 13, 4177–4181. doi: 10.7314/APJCP.2012.13.8.4177
Wu, M., Semba, S., Oue, N., Ikehara, N., Yasui, W., and Yokozaki, H. (2004). BRAF/K-ras mutation, microsatellite instability, and promoter hypermethylation of hMLH1/MGMT in human gastric carcinomas. Gastric Cancer 7, 246–253. doi: 10.1007/s10120-004-0300-9
Xiong, H. L., Liu, X. Q., Sun, A. H., He, Y., Li, J., and Xia, Y. (2013). Aberrant DNA methylation of P16, MGMT, hMLH1 and hMSH2 genes in combination with the MTHFR C677T genetic polymorphism in gastric cancer. Asian Pac. J. Cancer Prev. 14, 3139–3142. doi: 10.7314/APJCP.2013.14.5.3139
Yao, Y., Tao, H., Kim, J. J., Burkhead, B., Carloni, E., Gasbarrini, A., et al. (2004). Alterations of DNA mismatch repair proteins and microsatellite instability levels in gastric cancer cell lines. Lab. Invest. 84, 915–922. doi: 10.1038/labinvest.3700117
Zhang, K. L., Sun, Y., Li, Y., Liu, M., Qu, B., Cui, S. H., et al. (2008). Increased frequency of CpG island methylator phenotype and CDH1 methylation in a gastric cancer high-risk region of china. Transl. Oncol. 1, 28–35. doi: 10.1593/tlo.07103
Keywords: gastric cancer, hMLH1, methylation, meta-analysis, MSI
Citation: Ye P, Shi Y and Li A (2018) Association Between hMLH1 Promoter Methylation and Risk of Gastric Cancer: A Meta-Analysis. Front. Physiol. 9:368. doi: 10.3389/fphys.2018.00368
Received: 24 January 2018; Accepted: 27 March 2018;
Published: 17 April 2018.
Edited by:
Mouad Edderkaoui, Cedars-Sinai Medical Center, United StatesReviewed by:
Sudarshan Malla, Cedars-Sinai Medical Center, United StatesFatima Zohra Chenni, University of Sidi-Bel-Abbès, Algeria
Copyright © 2018 Ye, Shi and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anling Li, 1306218011@qq.com
†These authors have contributed equally to this work.
 Peng Ye
Peng Ye Yu Shi
Yu Shi Anling Li
Anling Li