- Institute of Complementary Medicine, University of Bern, Bern, Switzerland
An increasing cancer incidence affecting any age and social class is putting serious strain on populations and health care systems around the world. This systematic literature search aims (i) to examine the correlation of heart rate variability (HRV) and cancer patients’ prognosis, (ii) to examine the relationship of HRV and clinicopathological features, and (iii) to compare HRV between different patient groups, and between patient and control groups. We conducted a systematic literature review following the PRISMA Statement. We searched the PubMed and EMBASE databases for publications released by December 2017. The search terms were: “cancer” AND “heart rate variability” AND “human” NOT “animal” NOT “review.” A total of 19 studies were finally included in this review. Most publications were high-quality observational studies. The studies showed that higher HRV correlated positively with patients’ progression of disease and outcome. Thus, we conclude that individuals with higher HRV and advanced coping mechanisms seem to have a better prognosis in cancer progression. HRV appears to be a useful aspect to access the general health status of cancer patients.
Introduction
With approximately 8.8 million cancer-related deaths and 14 million new cancer cases per year, at present neoplastic diseases are a significant cause of morbidity and mortality worldwide. The number of new cases of cancer is expected to rise by about 70% over the next two decades (Stewart et al., 2014) as key risk factors increase, including exposure to physical (e.g., ionizing and non-ionizing radiation) and chemical carcinogens (e.g., acrylamide, aflatoxin, endocrine disruptors), lifestyle choices (e.g., tobacco use, alcohol use, unhealthy diet, physical inactivity and circadian disruption) and infections (e.g., human papillomavirus, hepatitis B-virus, helicobacter pylori).
Clinical research spans many different fields with the aim of improving diagnosis, treatment, and prognosis through increased knowledge about cancer pathophysiology. Although guidelines support cancer care, the management of cancer patients requires predictions and decisions on an individualized rather than generalized basis, taking into account the patient’s clinicopathological and psychological situation. Hence, data on prognostic factors are integral to improve patients’ specific prognosis (Gidron et al., 2014). Prognostic factors such as tumor stage and tumor markers [e.g., prostate-specific antigen (PSA), alpha-fetoprotein (AFP), human chorionic gonadotropin (hCG)] have been shown to correlate with the course of disease and/or prognosis (Mead and Stenning, 1997; Gospodarowicz and O’Sullivan, 2003). Currently, there is much interest around such prognostic factors but their reliability remains a subject of debate. Additionally, there are many other host-related or environmental factors (e.g., pollution, nutrition) which may affect the outcome. Host-related factors include general variables such as age, sex and ethnicity, inflammatory markers (e.g., C-reactive protein) and organ functioning (forced expiratory volume in one second in lung cancer), as well as immune status and personal coping mechanisms (Moreno-Smith et al., 2010). These, together with potentially undiscovered factors, may have an impact on disease control.
Heart rate variability (HRV) is a biomarker of the autonomic nervous system (ANS) function and provides a measure of ANS through sympathetic and parasympathetic modulation of cardiac function (Karvinen et al., 2013). The vagal nerve is the main component of the parasympathetic system and the main modulator of the parasympathetic innervation of the heart. HRV analysis has already been used in recreational sports, sports medicine and other clinical fields to monitor the level of physical fitness and biofeedback procedures (Park and Thayer, 2014; Prinsloo et al., 2014; Dong, 2016; Penzel et al., 2016). HRV analysis has the potential to provide additional valuable insights into multiple physiological and pathological conditions (Malik et al., 1996). It further serves as a potential marker of stress and health in functions of an organism associated with adaptability and health (Thayer et al., 2012). In a recent review by Arab et al. (2016), chemotherapy or surgery was shown to have an important effect on HRV, which indicated an impairment of the patients’ autonomic function, i.e., an autonomic dysfunction. In another recent review that also included a meta-analysis, the overall survival of cancer patients was found to be significantly longer in a group with higher HRV compared to a group with lower HRV (Zhou et al., 2016).
The aim of this systematic literature search was to provide and appraise an overview of publications which report on the following three research questions: (i) What is the role of HRV as a biomarker and prognostic factor in cancer disease? (ii) How is HRV correlated with cancer progression? and (iii) What is the value of HRV in predicting cancer patients’ prognosis and survival outcome in comparison to different patient groups, or healthy individuals? The search focused on adult cancer patients with any kind of cancer disease whose HRV was measured during a follow-up period.
Materials and Methods
A systematic review was conducted according to the PRISMA Statement (Moher et al., 2009). The literature search was performed on the databases PubMed and EMBASE. The search included all publications until December 2017 and was conducted independently by two of the authors (KB and EK). Only studies on humans were included. The search terms were: “cancer” AND “heart rate variability” AND “human” NOT “animal” NOT “review.”
The process of the literature search is depicted in Figure 1A.
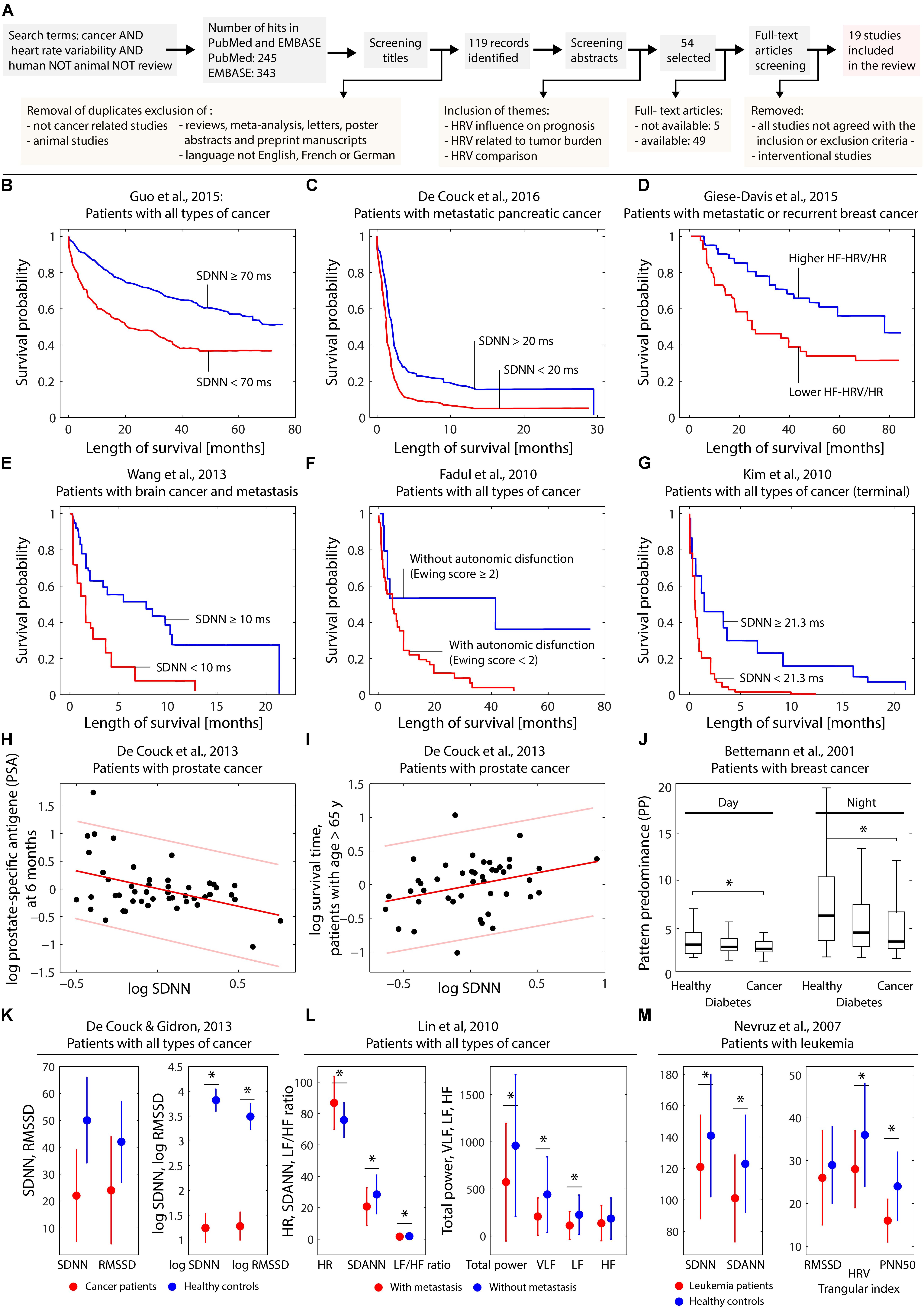
FIGURE 1. (A) Visualization of the search process leading to the 19 publications selected for the review. (B–G) Kaplan–Meier plots obtained by different studies investigating the effect of several HRV parameters on the survival probability. (H,I) Dependence on log SDNN on patient characteristics in case of prostate cancer. (J) Dependence of heart rate complexity in breast cancer patients compared to healthy controls and subjects with diabetes. (K–M) Differences of HRV parameters comparing healthy controls and cancer patients (K–M) and cancer patients with or without metastasis (L). The subfigures (B–M) were created based on digitally extracting the numerical data from the published figures in the original published papers. For subfigure (H) and (I) the linear fit with predictions bounds was recalculated using Matlab (Natick, MA, United States). Statistically significant (p < 0.05) differences in the parameters comparing groups (see J,K–M) are indicated (∗).
Data Collection
Publications were included when meeting the following criteria: study participants were adult cancer patients with any kind of cancer. Any number of participants and any year of publication were accepted. The prognostic factor HRV had to be assessed at least once and set in relation to other variables (e.g., progression-free survival or survival time), to prognosis or compared to healthy individuals. Meta-analysis and letters, poster abstracts or unpublished manuscripts were excluded. However, the reference lists of reviews were screened for additional publications of relevance for this systematic review.
The aim of the included studies had to be at least one of the following: (i) Investigating the correlation between HRV and patients’ prognosis, (ii) Examining the relationship of HRV with clinicopathological features, or (iii) Comparing HRV in different patient groups, or between patient and control groups.
While screening titles and abstracts, duplicates were removed. Studies were instantly excluded if they were not related to cancer patients, if the test species were not human and if the language of the publication was not English, French, or German. Publications with abstracts not related to the topic or not meeting the inclusion criteria were excluded. The full texts were read, and data extracted and appraised by two researchers (KB and EK) independently. Publications had to be available either via journals or on other scientific platforms which provided access to the Swiss University libraries. Authors were contacted if publications were not accessible through these routes. If there was no response within the period the literature search was conducted, the respective publications were excluded.
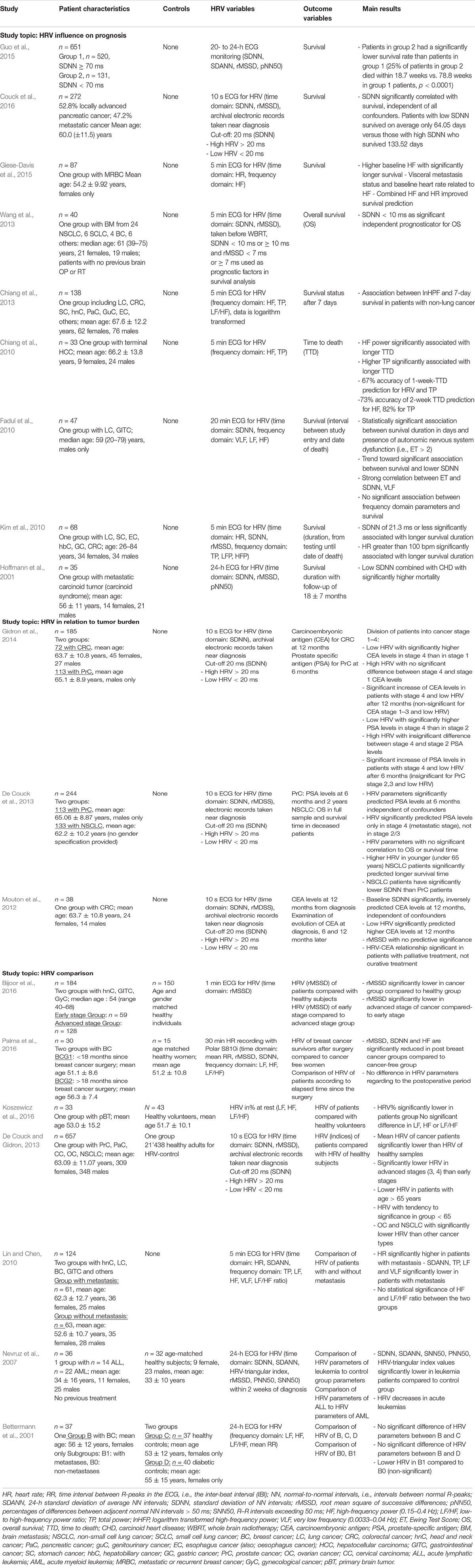
Extracted data were sorted and categorized according to the patient characteristics, HRV variables, and outcome variables. The main results are listed in Table 1.
Classification of bias and a Grading of Recommendations, Assessment, Development and Evaluations (GRADE) scoring evaluation were performed in accordance with the Cochrane guidelines (Higgins and Altman, 2008).
Results
A total of 588 matches were screened. Following the inclusion and exclusion criteria described above, 19 studies were finally included in this systematic review. Of these 19 studies, 6 were retrospective studies and 13 prospective studies. The number of patients per study varied between 30 and 657 (mean: 154.68, median: 68 patients). HRV in each patient was described, but different indices in time or frequency domain were determined in the different studies. Table 1 provides a description of the publications included in this systematic review. Figures 1B–M visualizes the key findings of the studies reviewed.
The studies included in this systematic review were performed in patients with various types of cancer, i.e., breast cancer (BC): with a total of 196 patients; prostate cancer (PrC): 113; colorectal cancer (CRC): 208; non-small cell lung cancer (NSCLC): 133; stomach and esophagus cancer: 28; bladder cancer: 3; lung cancer: 51; pancreatic cancer: 332; hepatocellular- and hepatobiliary cancer: 43; genitourinary cancer: 22; gynecologic cancers (uterus, cervix, ovary): 125; head and neck cancer: 127; brain metastasis: 40; primary brain tumor: 33; leukemia non-acute: 14, acute: 22; metastatic carcinoid tumor: 35; liver cancer: 36; gastrointestinal: 91; and sarcoma: 4. In 704 patients, the type of cancer was not defined.
In the six retrospective studies (Mouton et al., 2012; De Couck and Gidron, 2013; De Couck et al., 2013; Couck et al., 2016; Gidron et al., 2014; Guo et al., 2015), the main outcome measure was HRV in correlation with survival or tumor marker levels. Additionally, HRV of groups with and without cancer, and HRV in patients with and without metastases were compared. Within the 13 prospective studies, the main outcome measures were HRV in correlation to overall survival, time to death, 7-day survival and comparison of HRV within different groups. All studies reported at least a tendency toward higher HRV signifying a useful indicator for longer survival. This was true irrespective of measurement methods, e.g., 24-h electrocardiogram (ECG) or 10 s ECG, and regardless of HRV indices, e.g., the standard deviation of normal-to-normal interval (SDNN), high-frequency (HF) range (0.15–0.4 Hz), heart rate, the root of the mean squared differences of successive normal-to-normal interval (rMSSD).
Our first research question (i) investigated the correlation between HRV and patients’ prognosis. Giese-Davis et al. (2015), described a model of overall survival prediction. They suggested that the correlation between high-frequency power and overall survival indicates that efferent cardiac vagal activity may represent overall afferent and efferent information transfer between the vagal and the immune system and may provide early clinical prognoses in cancer patients. Guo et al. (2015), demonstrated with 24-h ECG monitoring a significantly lower survival rate in patients with SDNN < 70 ms compared to patients with SDNN > 70 ms. The same conclusion, i.e., lower SDNN associated with lower survival, was reached in the studies of Fadul et al. (2010), Kim et al. (2010), Wang et al. (2013), and also De Couck et al. (2016). Chiang et al. (2013), investigated a group of patients in a palliative care setting in their last weeks of life, although a much shorter ECG time frame (10 s to 5 min ECGs) was applied. This study also showed that patients with high-frequency power less than two dimensions had a higher risk of survival of less than 7 days.
The second research question (ii) focused on the correlation between HRV and tumor burden. Gidron, in Mouton et al. (2012), De Couck et al. (2013), and Gidron et al. (2014), analyzed data of PrC, CRC, and NSCLC patients with different cancer stages and described significantly higher tumor marker levels in patients with lower HRV.
The third research question (iii) explored the comparison between different groups of patients and healthy individuals. Among all studies related to this topic (Bettermann et al., 2001; Nevruz et al., 2007; Lin and Chen, 2010; De Couck and Gidron, 2013; Gidron et al., 2014; Bijoor et al., 2016; Koszewicz et al., 2016; Palma et al., 2016), significant differences in the HRV values were found. HRV was shown to be significantly lower in cancer patients compared to healthy individuals and also significantly lower in patients with metastases or generally with advanced stages III or IV compared to non-metastatic patients. Irrespective of the HRV measurement duration and variables, all studies showed a reduced HRV in the groups with more severely affected disease. Wang et al. (2013) and De Couck et al. (2016) evaluated the difference of patients with advanced stages compared to patients with primary and secondary stages as well as compared to healthy individuals. They reported that disease severity affects HRV, as patients in early stages showed a significantly higher vagal nerve activity than patients in later stages.
Discussion
In recent years HRV analysis has attracted increasing interest as a diagnostic tool in cardiology. In this field, decreased HRV is a predictor of adverse outcome in myocardial infarction, sudden cardiac death and congestive heart failure (Kleiger et al., 1987; Casolo et al., 1989; Cripps et al., 1991; Ponikowski et al., 1997; La Rovere et al., 1998, 2003; Makikallio et al., 2001). HRV may also be applied as an early indication for diabetic neuropathy (De Couck et al., 2012) and is progressively employed by athletes to assess the level of physical fitness and stress coping ability (De Meersman, 1993; Chen et al., 2011; Teisala et al., 2014).
For this systematic review, all study designs including observational studies were considered. According to the GRADE score, an observational study has an evidence level between low and moderate. Since most of the publications included here were observational studies, the overall score of the studies in this review was between low and moderate. It is important to note that no strong biases were detected in any of the prospective and retrospective observational studies included, and that the quality of all publications was good.
Based on the studies included in this review, HRV may be a useful non-invasive tool to evaluate prognosis of cancer patients. However, Fadul et al. (2010), Kim et al. (2010), and Mouton et al. (2012), still consider it a poor prognosticator, particularly in patients with advanced cancer. This prognostic function of HRV, specifically for patients with metastases, has been discussed in several studies. The main hypothesis is that a lower HRV is associated with tumor growth through three pathways, i.e., inflammation, oxidative stress, and sympathetic nerve activation. De Couck et al. (2013), argued that in earlier tumor stages, commonly provided treatments such as surgery and radiotherapy are successful in reducing the tumor burden, possibly leaving less of a margin for vagal nerve activity to contribute to the process. By contrast, these treatments may have less impact in later metastatic stages, where vagal nerve activity might possibly be of even more importance. HRV-lowering effects of chemotherapy and radiotherapy appeared to be reversible with treatment cessation. This effect may therefore not be relevant for a patient’s prognosis. In a recent study by Kim et al. (2015), HRV indices were compared to other clinical variables to capture overall survival in patients with advanced NSCLC. SDNN significantly correlated with poor survival, but was not an independent prognosticator for survival. This led to the conclusion that HRV, as a stand- alone method, might be a useful tool to monitor the general wellbeing of a patient, rather than to predict overall survival. Future studies are needed to clarify these findings.
This systematic review has several limitations. The design of the six retrospective studies included might be a limiting factor since HRV may change instantaneously in stressful situations, meaning that it may be affected by a patient’s state of mind at the time of measurement. This is particularly relevant considering the fact that short-term ECGs of seconds to minutes were often measured in the context of medical consultation, a potentially stressful situation for the patient depending on the topic (e.g., diagnosis, progression of disease) addressed. The HRV measurement may be influenced by stress of the situation thus not reflecting the patient’s average HRV behavior overall. At the same time, a high HRV within the context of a medical consultation may indicate good resilience or coping abilities. Given the fact that stressful situations – like medical consultations – may influence HRV measurement, we recommend that HRV measurements in cancer patients be carried out as 24-h Holter ECG.
In five of the studies, time domain measurements of HRV, such as SDNN and rMSSD, were based on 10 s archival ECG recordings. The authors mentioned the short ECG of 10 s as a possible limitation but also referred to two studies in which HRV obtained from 10 s ECGs was found to be similar to HRV obtained from 5 and 20 min ECGs (Hamilton et al., 2004; Nussinovitch et al., 2011). Although both publications suggest a good correlation of rMSSD derived from a 10 s ECG to the rMSSD of 5 min ECG, this is not the case for SDNN. In the publication by Hamilton et al. (2004), SDNN was predictive, but to a lesser extent than rMSSD. It was concluded that it was unclear whether SDNN of 10 s ECG should be applied. Nevertheless, the authors had applied the 10 s ECG variable and defined high and low HRV with an SDNN above and below 20 ms.
Further studies are needed to clarify the correlation of HRV with cancer prognosis. It might be that individuals with advanced coping abilities have better prognosis of cancer. It would be worth testing whether patients could acquire skills to diminish acute and long-term stress reactions, supporting their healing process not only on the physiological but also the psychological level (Haurand and Aatz, 2015). Options to improve resilience could include developing the personal coping capabilities of each patient through HRV biofeedback (Karavidas et al., 2007) or enhancing vagal activity through vagal stimulating drugs (Bernik et al., 2002) or vagal nerve stimulators (Murphy and Patil, 2003). Relaxation exercises have also been found to positively affect HRV (Asher et al., 2010). In addition, physical exercise and therapeutic eurhythmy (Seifert et al., 2012) have been shown to exert an enhancing effect on HRV, which was suggested to be a goal in cancer treatment due to the association of higher HRV variables with prolonged survival in cancer patients (Niederer et al., 2013, 2015). Finally, improvement of nutrition has been shown to positively affect HRV, too (Hansen et al., 2014).
Conclusion
This manuscript is the first to systematically compile and appraise on how HRV is associated with cancer progression and the value of HRV in predicting cancer patients’ prognosis. The majority of the studies indicate that a decreased HRV is common in cancer patients, likely reflecting autonomic dysfunction associated with the disease. Additionally, the publications reported a correlation between HRV and the progression and overall survival of cancer patients. A higher HRV is hereby associated with a better prognosis for cancer patients. HRV might be a valuable biomarker in assessing patients’ progression and outcome and further research on this topic should be conducted.
More severely affected persons exhibit lower HRV, as demonstrated in this literature search by a comparison of different patient groups or by the comparison between patients and healthy individuals. For a healthy individual, a higher level of HRV is desirable. It indicates a state of calm, relaxation and capacities to rest and regenerate. There are physical and psychological illnesses, like heart diseases, diabetes or depression, where parasympathetic activity including HRV is clearly and severely reduced (De Couck et al., 2012). Based on the literature, cancer may be added to the list of illnesses where HRV is reduced.
Author Contributions
UW: initiation of the project and supervision of all steps. KB and EK: literature search, data extraction, and appraisal. EK, KB, SK, FS, and UW: contributed substantially to the writing and revision of the manuscript and approved its final version.
Funding
The salary of EK is mainly supported by a research grant from the support association of the Paracelsus-Spital Richterswil, Switzerland. It had no influence whatsoever on this research.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Arab, C., Dias, D. P., Barbosa, R. T., Carvalho, T. D., Valenti, V. E., Crocetta, T. B., et al. (2016). Heart rate variability measure in breast cancer patients and survivors: a systematic review. Psychoneuroendocrinology 68, 57–68. doi: 10.1016/j.psyneuen.2016.02.018
Asher, A., Lynn Palmer, J., Yadav, R. R., Yusuf, S. W., Konzen, B., Bruera, E., et al. (2010). The effects of a brief relaxation program on symptom distress and heart rate variability in cancer patients. PM R 2, 636–641. doi: 10.1016/j.pmrj.2010.04.026
Bernik, T. R., Friedman, S. G., Ochani, M., Diraimo, R., Ulloa, L., Yang, H., et al. (2002). Pharmacological stimulation of the cholinergic antiinflammatory pathway. J. Exp. Med. 195, 781–788. doi: 10.1084/jem.20011714
Bettermann, H., Kröz, M., Girke, M., and Heckmann, C. (2001). Heart rate dynamics and cardiorespiratory coordination in diabetic and breast cancer patients. Clin. Physiol. 21, 411–420. doi: 10.1046/j.1365-2281.2001.00342.x
Bijoor, S. N., Subbalakshmi, N. K., and Banerjee, S. (2016). Influence of cancer and its severity on vagal nerve activity assessed by time domain measures of heart rate variability. Res. J. Pharm. Biol. Chem. Sci. 7, 1215–1220.
Casolo, G., Balli, E., Taddei, T., Amuhasi, J., and Gori, C. (1989). Decreased spontaneous heart rate variability in congestive heart failure. Am. J. Cardiol. 64, 1162–1167. doi: 10.1016/0002-9149(89)90871-0
Chen, J. L., Yeh, D. P., Lee, J. P., Chen, C. Y., Huang, C. Y., Lee, S. D., et al. (2011). Parasympathetic nervous activity mirrors recovery status in weightlifting performance after training. J. Strength Cond. Res. 25, 1546–1552. doi: 10.1519/JSC.0b013e3181da7858
Chiang, J. K., Koo, M., Kuo, T. B., and Fu, C. H. (2010). Association between cardiovascular autonomic functions and time to death in patients with terminal hepatocellular carcinoma. J. Pain Symptom Manage. 39, 673–679. doi: 10.1016/j.jpainsymman.2009.09.014
Chiang, J. K., Kuo, T. B., Fu, C. H., and Koo, M. (2013). Predicting 7-day survival using heart rate variability in hospice patients with non-lung cancers. PLoS One 8:e69482. doi: 10.1371/journal.pone.0069482
Couck, M. D., Marechal, R., Moorthamers, S., Laethem, J. L., and Gidron, Y. (2016). Vagal nerve activity predicts overall survival in metastatic pancreatic cancer, mediated by inflammation. Cancer Epidemiol. 40, 47–51. doi: 10.1016/j.canep.2015.11.007
Cripps, T. R., Malik, M., Farrell, T. G., and Camm, A. J. (1991). Prognostic value of reduced heart rate variability after myocardial infarction: clinical evaluation of a new analysis method. Br. Heart J. 65, 14–19. doi: 10.1136/hrt.65.1.14
De Couck, M., and Gidron, Y. (2013). Norms of vagal nerve activity, indexed by Heart Rate Variability, in cancer patients. Cancer Epidemiol. 37, 737–741. doi: 10.1016/j.canep.2013.04.016
De Couck, M., Marechal, R., Moorthamers, S., Van Laethem, J. L., and Gidron, Y. (2016). Vagal nerve activity predicts overall survival in metastatic pancreatic cancer, mediated by inflammation. Cancer Epidemiol. 40, 47–51. doi: 10.1016/j.canep.2015.11.007
De Couck, M., Mravec, B., and Gidron, Y. (2012). You may need the vagus nerve to understand pathophysiology and to treat diseases. Clin. Sci. 122, 323–328. doi: 10.1042/CS20110299
De Couck, M., Van Brummelen, D., Schallier, D., De Greve, J., and Gidron, Y. (2013). The relationship between vagal nerve activity and clinical outcomes in prostate and non-small cell lung cancer patients. Oncol. Rep. 30, 2435–2441. doi: 10.3892/or.2013.2725
De Meersman, R. E. (1993). Heart rate variability and aerobic fitness. Am. Heart J. 125, 726–731. doi: 10.1016/0002-8703(93)90164-5
Dong, J. G. (2016). The role of heart rate variability in sports physiology. Exp. Ther. Med. 11, 1531–1536. doi: 10.3892/etm.2016.3104
Fadul, N., Strasser, F., Palmer, J. L., Yusuf, S. W., Guo, Y., Li, Z., et al. (2010). The association between autonomic dysfunction and survival in male patients with advanced cancer: a preliminary report. J. Pain Symptom Manage. 39, 283–290. doi: 10.1016/j.jpainsymman.2009.06.014
Gidron, Y., De Couck, M., and De Greve, J. (2014). If you have an active vagus nerve, cancer stage may no longer be important. J. Biol. Regul. Homeost. Agents 28, 195–201.
Giese-Davis, J., Wilhelm, F. H., Tamagawa, R., Palesh, O., Neri, E., Taylor, C. B., et al. (2015). Higher vagal activity as related to survival in patients with advanced breast cancer: an analysis of autonomic dysregulation. Psychosom. Med. 77, 346–355. doi: 10.1097/PSY.0000000000000167
Gospodarowicz, M., and O’Sullivan, B. (2003). Prognostic factors in cancer. Semin. Surg. Oncol. 21, 13–18. doi: 10.1002/ssu.10016
Guo, Y., Koshy, S., Hui, D., Palmer, J. L., Shin, K., Bozkurt, M., et al. (2015). Prognostic value of heart rate variability in patients with cancer. J. Clin. Neurophysiol. 32, 516–520. doi: 10.1097/WNP.0000000000000210
Hamilton, R. M., Mckechnie, P. S., and Macfarlane, P. W. (2004). Can cardiac vagal tone be estimated from the 10-second ECG? Int. J. Cardiol. 95, 109–115.
Hansen, A. L., Dahl, L., Olson, G., Thornton, D., Graff, I. E., Froyland, L., et al. (2014). Fish consumption, sleep, daily functioning, and heart rate variability. J. Clin. Sleep Med. 10, 567–575. doi: 10.5664/jcsm.3714
Haurand, C., and Aatz, H. (2015). Stressmedizin Beratung, Vorbeugung, Behandlung. Berlin: MWV Medizinisch Wissenschaftliche Verlagsgesellschaft.
Higgins, J. P. T., and Altman, D. G. (2008). “Chapter 8: Assessing risk of bias in included studies,” in Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series, 1, eds J. P. T. Higgins and S. Green (Chichester: Wiley-Blackwell). doi: 10.1002/9780470712184
Hoffmann, J., Grimm, W., Menz, V., Wied, M., Sprenger, A., Arnold, R., et al. (2001). Prognostic value of heart rate variability analysis in patients with carcinoid syndrome. Digestion 63, 35–42. doi: 10.1159/000051870
Karavidas, M. K., Lehrer, P. M., Vaschillo, E., Vaschillo, B., Marin, H., Buyske, S., et al. (2007). Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl. Psychophysiol. Biofeedback 32, 19–30. doi: 10.1007/s10484-006-9029-z
Karvinen, K. H., Murray, N. P., Arastu, H., and Allison, R. R. (2013). Stress reactivity, health behaviors, and compliance to medical care in breast cancer survivors. Oncol. Nurs. Forum 40, 149–156. doi: 10.1188/13.ONF.149-156
Kim, D. H., Kim, J. A., Choi, Y. S., Kim, S. H., Lee, J. Y., and Kim, Y. E. (2010). Heart rate variability and length of survival in hospice cancer patients. J. Korean Med. Sci. 25, 1140–1145. doi: 10.3346/jkms.2010.25.8.1140
Kim, K., Chae, J., and Lee, S. (2015). The role of heart rate variability in advanced non-small-cell lung cancer patients. J. Palliat. Care 31, 103–108. doi: 10.1177/082585971503100206
Kleiger, R. E., Miller, J. P., Bigger, J. T. Jr., and Moss, A. J. (1987). Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol. 59, 256–262. doi: 10.1016/0002-9149(87)90795-8
Koszewicz, M., Michalak, S., Bilinska, M., Budrewicz, S., Zaborowski, M., Slotwinski, K., et al. (2016). Profile of autonomic dysfunctions in patients with primary brain tumor and possible autoimmunity. Clin. Neurol. Neurosurg. 151, 51–54. doi: 10.1016/j.clineuro.2016.10.013
La Rovere, M. T., Bigger, J. T. Jr., Marcus, F. I., Mortara, A., and Schwartz, P. J. (1998). Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (autonomic tone and reflexes after myocardial infarction) investigators. Lancet 351, 478–484. doi: 10.1016/S0140-6736(97)11144-8
La Rovere, M. T., Pinna, G. D., Maestri, R., Mortara, A., Capomolla, S., Febo, O., et al. (2003). Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 107, 565–570. doi: 10.1161/01.CIR.0000047275.25795.17
Lin, S. C., and Chen, M. F. (2010). Increased yin-deficient symptoms and aggravated autonomic nervous system function in patients with metastatic cancer. J. Altern. Complement. Med. 16, 1059–1063. doi: 10.1089/acm.2009.0487
Makikallio, T. H., Huikuri, H. V., Makikallio, A., Sourander, L. B., Mitrani, R. D., Castellanos, A., et al. (2001). Prediction of sudden cardiac death by fractal analysis of heart rate variability in elderly subjects. J. Am. Coll. Cardiol. 37, 1395–1402. doi: 10.1016/S0735-1097(01)01171-8
Malik, M., Bigger, J. T., Camm, A. J., Kleiger, R. E., Malliani, A., Moss, A. J., Schwartz, P. J. (1996). Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 17, 354–381. doi: 10.1093/oxfordjournals.eurheartj.a014868
Mead, G. M., and Stenning, S. P. (1997). The International Germ Cell Consensus Classification: a new prognostic factor-based staging classification for metastatic germ cell tumours. Clin. Oncol. 9, 207–209. doi: 10.1016/S0936-6555(97)80001-5
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Group, P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 151, 264–269, W264. doi: 10.7326/0003-4819-151-4-200908180-00135
Moreno-Smith, M., Lutgendorf, S. K., and Sood, A. K. (2010). Impact of stress on cancer metastasis. Future Oncol. 6, 1863–1881. doi: 10.2217/fon.10.142
Mouton, C., Ronson, A., Razavi, D., Delhaye, F., Kupper, N., Paesmans, M., et al. (2012). The relationship between heart rate variability and time-course of carcinoembryonic antigen in colorectal cancer. Auton. Neurosci. 166, 96–99. doi: 10.1016/j.autneu.2011.10.002
Murphy, J. V., and Patil, A. (2003). Stimulation of the nervous system for the management of seizures: current and future developments. CNS Drugs 17, 101–115. doi: 10.2165/00023210-200317020-00003
Nevruz, O., Yokusoglu, M., Uzun, M., Demirkol, S., Avcu, F., Baysan, O., et al. (2007). Cardiac autonomic functions are altered in patients with acute leukemia, assessed by heart rate variability. Tohoku J. Exp. Med. 211, 121–126. doi: 10.1620/tjem.211.121
Niederer, D., Vogt, L., Gonzalez-Rivera, J., Schmidt, K., and Banzer, W. (2015). Heart rate recovery and aerobic endurance capacity in cancer survivors: interdependence and exercise-induced improvements. Support Care Cancer 23, 3513–3520. doi: 10.1007/s00520-015-2719-4
Niederer, D., Vogt, L., Thiel, C., Schmidt, K., Bernhörster, M., Lungwitz, A., et al. (2013). Exercise effects on HRV in cancer patients. Int. J. Sports Med. 34, 68–73. doi: 10.1055/s-0032-1314816
Nussinovitch, U., Elishkevitz, K. P., Katz, K., Nussinovitch, M., Segev, S., Volovitz, B., et al. (2011). Reliability of ultra-short ECG indices for heart rate variability. Ann. Noninvasive Electrocardiol. 16, 117–122. doi: 10.1111/j.1542-474X.2011.00417.x
Palma, M. R., Vanderlei, L. C., Ribeiro, F. E., Mantovani, A. M., Christofaro, D. G., and Fregonesi, C. E. (2016). The relationship between post-operative time and cardiac autonomic modulation in breast cancer survivors. Int. J. Cardiol. 224, 360–365. doi: 10.1016/j.ijcard.2016.09.053
Park, G., and Thayer, J. F. (2014). From the heart to the mind: cardiac vagal tone modulates top-down and bottom-up visual perception and attention to emotional stimuli. Front. Psychol. 5:278. doi: 10.3389/fpsyg.2014.00278
Penzel, T., Kantelhardt, J. W., Bartsch, R. P., Riedl, M., Kraemer, J. F., Wessel, N., et al. (2016). Modulations of heart rate, ECG, and cardio-respiratory coupling observed in polysomnography. Front. Physiol. 7:460. doi: 10.3389/fphys.2016.00460
Ponikowski, P., Anker, S. D., Chua, T. P., Szelemej, R., Piepoli, M., Adamopoulos, S., et al. (1997). Depressed heart rate variability as an independent predictor of death in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 79, 1645–1650. doi: 10.1016/S0002-9149(97)00215-4
Prinsloo, G. E., Rauch, H. G., and Derman, W. E. (2014). A brief review and clinical application of heart rate variability biofeedback in sports, exercise, and rehabilitation medicine. Phys. Sportsmed. 42, 88–99. doi: 10.3810/psm.2014.05.2061
Seifert, G., Kanitz, J. L., Pretzer, K., Henze, G., Witt, K., Reulecke, S., et al. (2012). Improvement of heart rate variability by eurythmy therapy after a 6-week eurythmy therapy training. Integr. Cancer Ther. 11, 111–119. doi: 10.1177/1534735411413263
Stewart, B. W., Weltgesundheitsorganisation and Centre International De Recherche Sur Le Cancer (2014). World Cancer Report. Lyon: IARC Press.
Teisala, T., Mutikainen, S., Tolvanen, A., Rottensteiner, M., Leskinen, T., Kaprio, J., et al. (2014). Associations of physical activity, fitness, and body composition with heart rate variability-based indicators of stress and recovery on workdays: a cross-sectional study. J. Occup. Med. Toxicol. 9:16. doi: 10.1186/1745-6673-9-16
Thayer, J. F., Ahs, F., Fredrikson, M., Sollers, J. J. III, and Wager, T. D. (2012). A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 36, 747–756. doi: 10.1016/j.neubiorev.2011.11.009
Wang, Y. M., Wu, H. T., Huang, E. Y., Kou, Y. R., and Hseu, S. S. (2013). Heart rate variability is associated with survival in patients with brain metastasis: a preliminary report. BioMed Res. Int. 2013:503421. doi: 10.1155/2013/503421
Keywords: HRV, tumor, vagal nerve, malignancy, prognosis
Citation: Kloter E, Barrueto K, Klein SD, Scholkmann F and Wolf U (2018) Heart Rate Variability as a Prognostic Factor for Cancer Survival – A Systematic Review. Front. Physiol. 9:623. doi: 10.3389/fphys.2018.00623
Received: 13 February 2018; Accepted: 08 May 2018;
Published: 29 May 2018.
Edited by:
Yih-Kuen Jan, University of Illinois at Urbana–Champaign, United StatesReviewed by:
Alessandro Tonacci, Istituto di Fisiologia Clinica (IFC), ItalyChia-Hua Kuo, University of Taipei, Taiwan
Copyright © 2018 Kloter, Barrueto, Klein, Scholkmann and Wolf. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Evelyne Kloter, evelyne.kloter@ikom.unibe.ch
 Evelyne Kloter
Evelyne Kloter Katja Barrueto
Katja Barrueto Sabine D. Klein
Sabine D. Klein Felix Scholkmann
Felix Scholkmann Ursula Wolf
Ursula Wolf