- 1Center of Molecular Immunology, Havana, Cuba
- 2Department of Biochemistry, Faculty of Medicine & Dentistry, University of Alberta, Edmonton, AB, Canada
Bone is a dynamic organ that undergoes constant remodeling, an energetically costly process by which old bone is replaced and localized bone defects are repaired to renew the skeleton over time, thereby maintaining skeletal health. This review provides a general overview of bone’s main players (bone lining cells, osteocytes, osteoclasts, reversal cells, and osteoblasts) that participate in bone remodeling. Placing emphasis on the family of extracellular matrix metalloproteinases (MMPs), we describe how: (i) Convergence of multiple protease families (including MMPs and cysteine proteinases) ensures complexity and robustness of the bone remodeling process, (ii) Enzymatic activity of MMPs affects bone physiology at the molecular and cellular levels and (iii) Either overexpression or deficiency/insufficiency of individual MMPs impairs healthy bone remodeling and systemic metabolism. Today, it is generally accepted that proteolytic activity is required for the degradation of bone tissue in osteoarthritis and osteoporosis. However, it is increasingly evident that inactivating mutations in MMP genes can also lead to bone pathology including osteolysis and metabolic abnormalities such as delayed growth. We argue that there remains a need to rethink the role played by proteases in bone physiology and pathology.
Introduction
Bone is a hard, dense, rigid form of highly specialized connective tissue making up the skeleton of vertebrates. Bone protects internal organs, supports body structures, and aids in locomotion (Maffioli and Derosa, 2015). In addition, bone provides an environment for hematopoiesis (i.e., formation and development of blood cells) in the bone marrow, and acts as a homeostatic reservoir of calcium, phosphorus, insulin-like growth factors, transforming growth factor-β, and cytokines. Bone buffers the blood against drastic pH changes, thus detoxifying the circulation from heavy metals (Rauner et al., 2012). Bone develops by intramembranous ossification (e.g., bone of the clavicle, some skull bones), endochondral ossification (e.g., the appendicular and axial skeleton) or pseudo-metamorphic ossification (Rauner et al., 2012).
Bone remodeling is a complex process involving the sequential resorption of bone tissue and deposition of new bone at the same site (Kerschan-Schindl and Ebenbichler, 2012). Together with bone structure, geometry, size, and density, remodeling determines bone’s overall mechanical properties (e.g., the strength) (Mosekilde et al., 1993; Jiang et al., 1997; Ikeda et al., 2003; Shahnazari et al., 2009) as well as enables the repair of damaged bone and the adaption of bone to changing biomechanical forces (Kerschan-Schindl and Ebenbichler, 2012).
We review here the prevailing view of the bone remodeling process with an emphasis on well-accepted and newly emerging roles played by matrix metalloproteinases (MMPs) and cysteine proteinases in this process. Finally, we review the increasing number of instances in which inactivating mutations in MMP genes are found to lead to bone pathology including osteolysis and metabolic abnormalities such as delayed growth.
General Overview on the Cycle of Bone Remodeling
The bone remodeling process consists of four distinct consecutive phases spanning over 3–6 months (Datta et al., 2008).
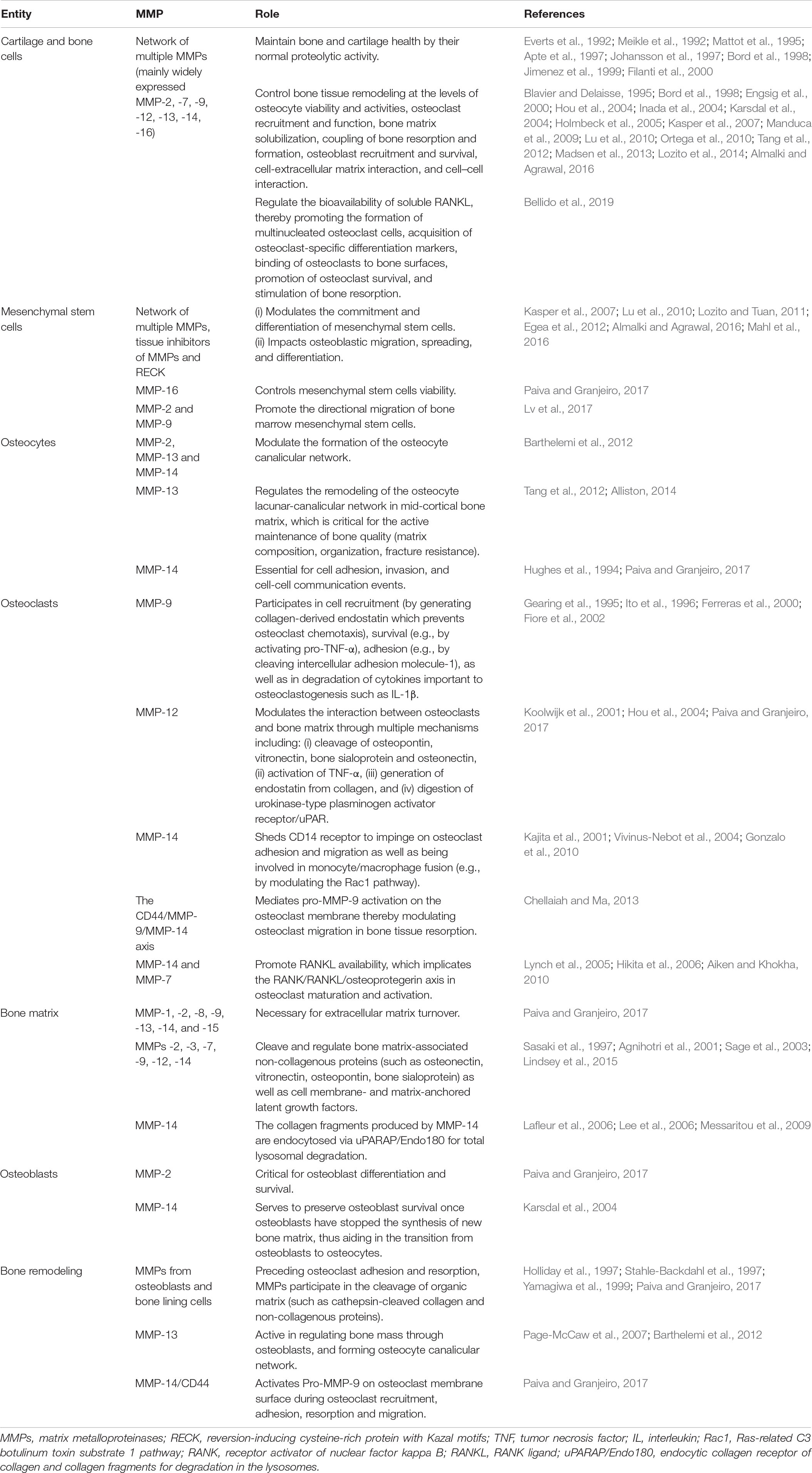
The first phase of bone remodeling is known as the ‘activation phase’ and can be triggered by mechanical and nutritional stress on the bone as well as by hormones (e.g., parathyroid hormone, estrogen) (Parra-Torres et al., 2013). As described in Table 1, terminally differentiated osteocyte cell is a key player in the activation phase (Rauner et al., 2012; Parra-Torres et al., 2013).
The second phase lasts 8–10 days (Teitelbaum, 2007) and is called the ‘bone resorption phase’ – a process by which large multinucleated osteoclast cells break down old bone organic matrix impregnated with minerals (e.g., calcium phosphate nanocrystals), as described in Table 2.
The third ‘reversal’ phase connects osteoclastic bone tissue resorption and osteoblastic bone tissue formation (Delaisse, 2014) and lasts 7–14 days (Pettit et al., 2008; Hienz et al., 2015). After departure of the osteoclast from a cavity in bones undergoing resorption, which is a resorptive lacuna known as the Howship’s lacuna, bone lining cells occupy the Howship’s lacuna and clean it (Everts et al., 2002). The cleaning process occurs by enwrapping and digesting non-mineralized collagenous proteins protruding from the bone surface left by osteoclasts. This cleaning process is a requirement for the subsequent deposition of a first layer of collagen along the Howship’s lacuna (Everts et al., 2002). Four types of osteoclast-derived coupling factors stimulate bone formation during the reversal phase: (i) Matrix-derived factors including transforming growth factor-β, bone morphogenetic protein-2, platelet-derived growth factor, and insulin-like growth factors, which are released during bone tissue resorption, (ii) Osteoclast-secreted factors, including cardiotrophin-1, sphingosine-1-phosphate, collagen triple helix repeat containing 1, and complement factor 3a, (iii) Osteoclast membrane-bound factors such as EphrinB2 and Semaphorin D, and (iv) Structural changes brought about by the osteoclast on the bone tissue surface (Sims and Martin, 2014). Reversal cells originating from pre-osteoblast cells (Andersen et al., 2013) colonize the osteoclast-eroded surface and respond to osteoclast-derived messages and coupling factors along with fibroblast-like cells covering the surface of bone (known as bone lining cells), osteoblast precursors, and canopy cells (Delaisse, 2014; Sims and Martin, 2014; Lassen et al., 2017; Pirapaharan et al., 2019).
The fourth phase of the bone remodeling cycle is ‘formation,’ when mononucleate osteoblast cells synthesize new bone organic matrix formed by collagen fibers and non-collagenous proteins (e.g., bone sialoprotein, osteopontin, osteocalcin, proteoglycans) that later becomes surrounded and impregnated with mineral deposit mainly in the form of calcium hydroxyapatite. A summary of osteoblastogenesis, the roles played by osteoblasts during this last phase, and the fate of osteoblasts is described in Table 3.
While bone formation surpasses resorption during childhood, bone formation and resorption are in balance during young adulthood. However, an unbalanced bone loss occurs with aging (Datta et al., 2008; Rauner et al., 2012; Brandi and Piscitelli, 2013) and could predispose an individual to skeletal disorders including: (i) inflammatory bone loss in periodontal disease, (ii) arthritis (stimulation of bone resorption and inhibition of bone formation by prostaglandins and cytokines), (iii) osteoporosis (bone resorption outpaces bone formation), (iv) hyperparathyroidism and hyperthyroidism (greatly increased rate of bone resorption and formation), (v) Paget’s disease (increased and abnormal [shape, weakness, and brittleness] bone formation), (vi) osteomalacia (delayed/defficient bone mineralization), and (vi) osteopetrosis (failure of osteoclasts to resorb bone) (Roodman et al., 1992; Delmas, 1995; Gallagher, 1997; Mills and Frausto, 1997; Raisz, 1997; Charles and Key, 1998; Schneider et al., 1998; Siris, 1998; Kini and Nandeesh, 2012).
Matrix Metalloproteinases: Modulators of Bone Remodeling
Matrix metalloproteinases are a family of at least 24 highly homologous, multi-domain enzymes (Figure 1) with the capacity to degrade virtually all extracellular matrix components including collagen, aggrecan, elastin, and fibronectin (Lu et al., 2011; Fernandez-Patron et al., 2016).
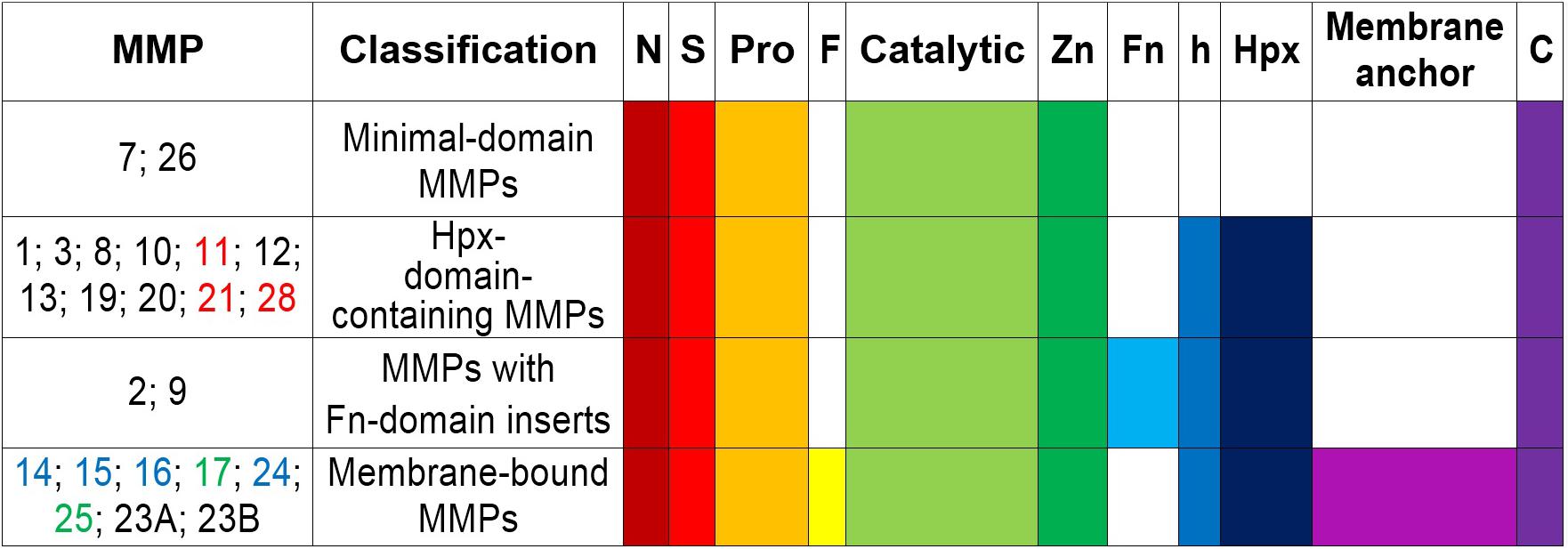
Figure 1. Schematic structure and classification of matrix metalloproteinases. S, amino-terminal signal sequence; Pro, pro-peptide; Zn(II)-binding site; h, hinge region; Hpx, hemopexin; FN, collagen-binding type II repeats of fibronectin; F, furin; MT-MMPs, membrane-type MMPs; N, N-terminus; C, C- terminus. MMPs -11, -21, and -28 (all in red) contain a Furin-like cleavage domain. MMPs -17 and -25 (both in green) contain a glycophosphatidyl inositol-anchoring sequence. MMPs -14, -15, -16, and -24 (all in blue) comprise a transmembrane domain with a cytosolic tail. MMP23A and MMP23B lack the signal peptide, the cysteine-switch motif and the hemopexin-like domain, but they contain a unique cysteine-rich domain, an immunoglobulin-like domain and an N-terminal type II transmembrane domain (Velasco et al., 1999).
All MMP family members are synthesized as catalytically inactive (latent) pro-enzymes (pro-MMPs) that contain a: signal N-terminal peptide sequence (∼20 amino acids), pro-peptide domain (∼80 amino acids), catalytic domain (approximately 160 amino acids), hinge (linker peptide) region of variable length (10–30 amino acids), and a hemopexin-like C-terminal domain (Hpx) (∼210 amino acids). The smallest MMPs (MMP-7 and MMP-26) lack the hinge and hemopexin domains, and therefore exhibit a reduced affinity for gelatin. MMP-23 has unique domains (such as the cysteine array, IgG-like domain, interleukin-1 type II receptor-like domains) instead of the hemopexin domain (Massova et al., 1998; Pei et al., 2000; Bode and Maskos, 2003; Visse and Nagase, 2003; Nagase et al., 2006; Piccard et al., 2007; Lopez-Otin et al., 2009; Bonnans et al., 2014; Vandooren et al., 2014; Vandenbroucke and Libert, 2014; Cui et al., 2017). The amino-terminal signal peptide targets the pro-MMPs to the rough endoplasmic reticulum, whereas the C-terminus harbors a cysteine residue and a furin cleavage site (PRCGXPD), both of which are important for conversion into the mature, active enzyme (Bonnans et al., 2014). Presence of an intact pro-peptide accounts for the latency of pro-MMPs, which can be overriden through the activation of a “cysteine-switch” mechanism (Van Wart and Birkedal-Hansen, 1990). The pro-peptide contains a cysteine residue that prevents catalytic activity when it is coordinated with a Zn(II)-ion in the catalytic domain (Springman et al., 1990; Van Wart and Birkedal-Hansen, 1990). The cysteine-Zn(II) interaction can be disrupted by alkylating compounds such as the organomercurial 4-aminophenylmercuric acetate as well as by serine proteases and other MMPs such as membrane-type MMPs, which act at the cell surface to which they anchor through their transmembrane domain/short cytoplasmic tail or by glycosylphosphatidylinositol linkage (Bonnans et al., 2014). MMP autolysis is another mechanism of activation mediated by allosteric perturbation of the inactive proenzyme (Springman et al., 1990; Van Wart and Birkedal-Hansen, 1990; Pei and Weiss, 1995; Pei et al., 2000; Meng et al., 2016). The catalytic domain harbors the Zn(II)-binding motif HEXXHXXGXXH, a catalytic Zn(II), a structural Zn(II), specific pockets related to specificity (S1, S2,…Sn and S1′, S2′,…Sn′) and coordinated Ca(II) ions which confer stabilization. The catalytic Zn(II) is coordinated by three histidine residues (Bode and Maskos, 2003; Bonnans et al., 2014; Vandenbroucke and Libert, 2014). The hinge domain is flexible and mediates interactions with substrates, cell-surface proteins, and tissue inhibitors (Cui et al., 2017; Liu and Khalil, 2017). The hemopexin domain modulates substrate recognition and specificity, binding to cell-surface receptors and inhibitors, activation of MMPs, and cellular MMP internalization for degradation (Visse and Nagase, 2003; Nagase et al., 2006; Piccard et al., 2007).
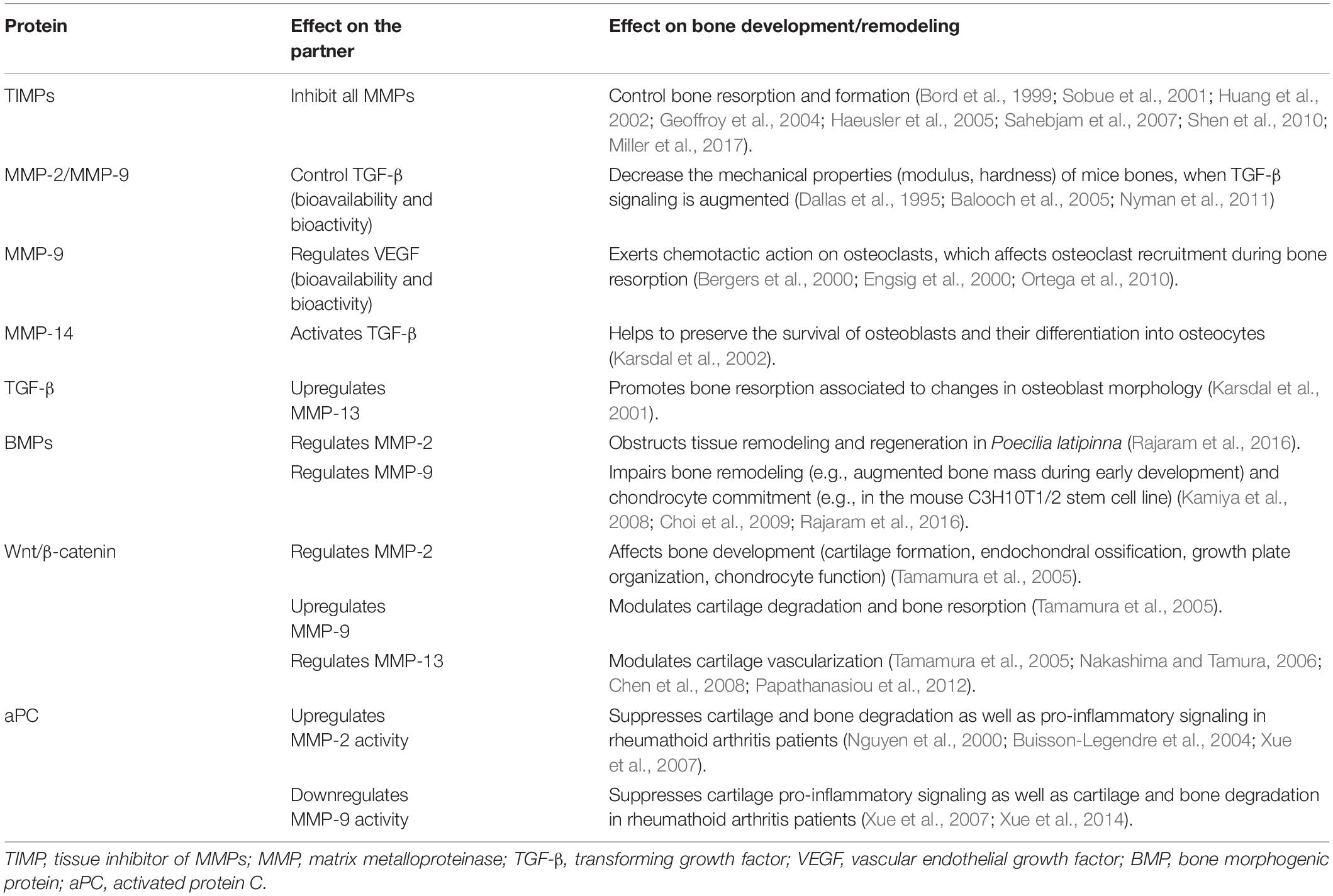
Matrix metalloproteinases expression and activity are tightly regulated at various levels: gene transcription, translation and secretion of the inactive enzyme precursor, proteolytic activation of the zymogen, spatial localization, interaction with specific extracellular matrix proteins, and inhibition by endogenous inhibitors (such as tissue inhibitors of MMPs [TIMPs 1-4], α2-macroglobulin, and human fibrinogen) (Sottrup-Jensen, 1989; Overall et al., 1991; Kusano et al., 1998; Zeng et al., 1998; Sternlicht and Werb, 2001; Han et al., 2003; Greenlee et al., 2007; Clark et al., 2008; Fanjul-Fernandez et al., 2010; Hadler-Olsen et al., 2011; Arpino et al., 2015; Sarker et al., 2019). Despite their similar names, TIMPs 1-4 exhibit large differences in their primary sequence, tissue expression, transcriptional regulation and in their inhibitory spectrum (Brew et al., 2000). In bone, TIMP-2 and TIMP-3, unlike TIMP-1, are effective inhibitors of the membrane-type MMPs (e.g., MMP-14), while TIMP-3 displays the broadest inhibitory actions of all TIMPs against metalloproteinases. Unlike TIMP-1, -2, and -4, which are soluble, TIMP-3 has basic amino acid residues in its C- and N-termini through which TIMP-3 attaches to heparan and chondroitin sulfate in the extracellular matrix and inhibits both MMPs and members of ‘a disintegrin and metalloproteinase’ (ADAM) and ‘a disintegrin and metalloproteinase with thrombospondin domains’ (ADAMTS) family including ADAM-17 and ADAMTS-4/-5 (Porter et al., 2005; Javaheri et al., 2016). Deficiency of tissue inhibitors (TIMP-1, -2, or -4) has minor impact on bone phenotype. However, both Timp3 deficiency and transgenic overexpression alters craniofacial bones of endochondral and intramembranous origins in mice, while the growth plates appear normal in these mice (Javaheri et al., 2016). Paradoxically, mice deficient in RECK (an MMP inhibitor anchored on the cell membrane with inhibitory actions against MMP-2, -9, and -14 and ADAM-10) die in utero displaying a perturbed extracellular matrix organization (Javaheri et al., 2016).
These observations suggest that bone remodeling may not be solely defined by the balance/imbalance between MMPs and TIMPs. Rather, other molecules expressed and released in the settings of bone physiology and pathology such as RECK (Paiva and Granjeiro, 2014) and some acute phase reactants (alpha 2-macroglobulin, fibrinogen) may regulate/dysregulate MMP activity in inflammatory conditions thus perturbing the normal bone remodeling process (Cook et al., 2018; Sarker et al., 2019). A consequence implied by the latter notion is that MMPs, ADAMs and ADAMTS molecules may be released from bone or non-bone tissues to influence bone remodeling through autocrine and paracrine actions. In other words, MMPs likely circulate bound to non-classical inhibitors (such as acute phase reactants) being recruited to sites of active bone remodeling, where local substrates act as chemoattractants and local activators (other proteases, reactive oxygen species) activate them.
The aforementioned levels of regulation effectively dissociate MMP expression from MMP activity (e.g., since overexpression of endogenous MMP inhibitors would effectively reduce MMP activity). Current biochemical techniques for assessing MMP activity are non-reliable. However, as research requires a proxy, MMP expression is often used as a surrogate (albeit incorrectly) for MMP activity. There remains an urgent need for highly sensitive, specific, and robust methods for assessing the activity potential of individual MMPs such that therapeutic strategies can be designed to specifically reduce the activity of overactive MMPs (i.e., those whose activity levels are above baseline) or to increase the activity of underactive MMPs (i.e., those whose activity levels are below baseline).
Roles of MMPs Associated to Bone Development and Remodeling
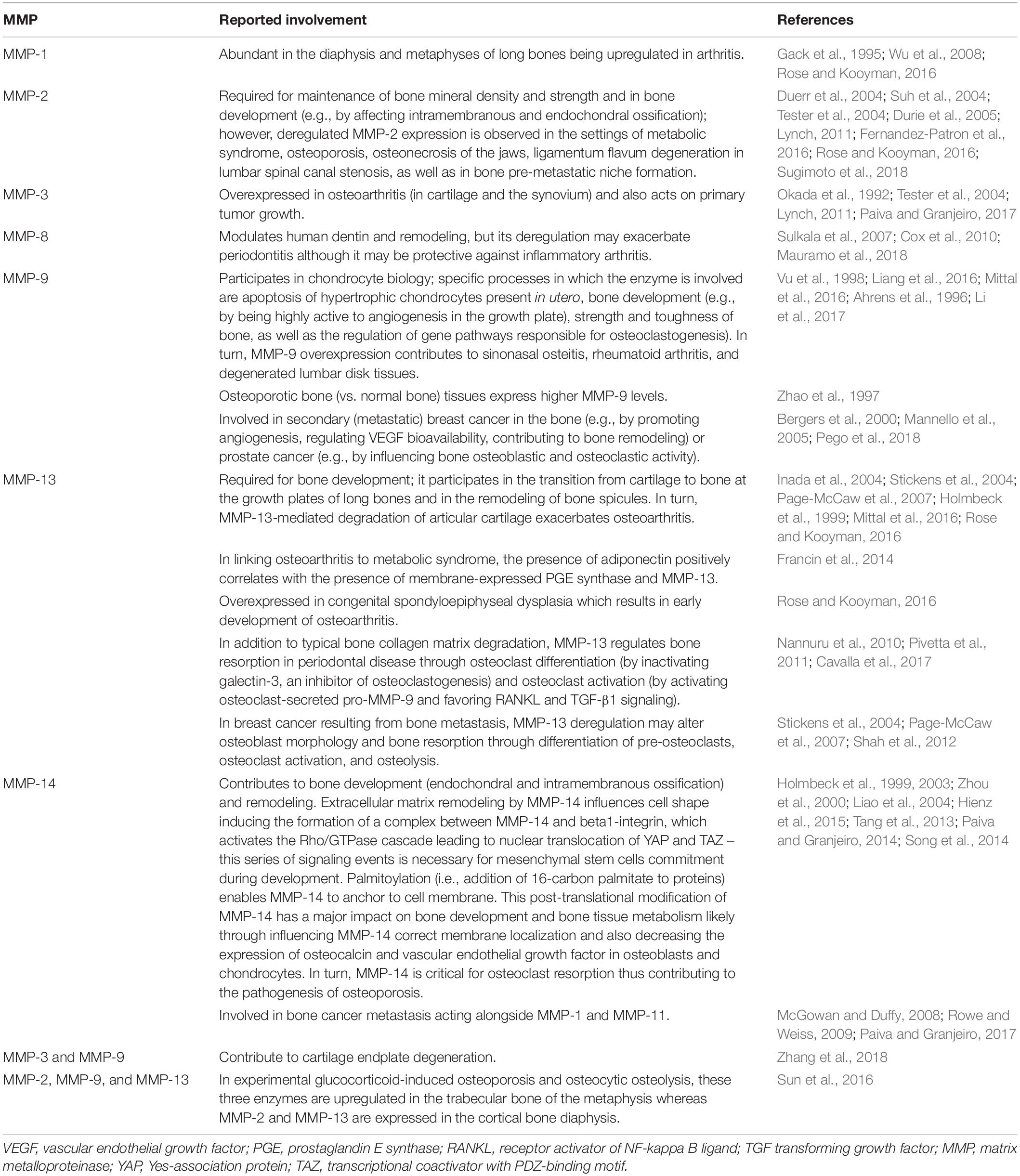
The biochemical actions of MMPs are intimately linked to their cells of origin. Table 4 describes cell-specific roles of MMPs in physiological bone remodeling. Osteoclast-mediated bone resorption in calvaria and long bones requires normal enzymatic activity of MMPs and cysteine proteinases such as cathepsin K whose deficiency impairs bone remodeling (Everts et al., 1999; Delaisse et al., 2003). This is evidenced in osteoclasts from patients with pycnodysostosis (an osteopetrosis-like bone disease related to loss-of-function mutations in the cathepsin K gene) and osteoclasts from cathepsin K-deficient mice which are unable to efficiently digest organic bone matrix, resulting in large, mineral-free areas of bone matrix (Everts et al., 1998, 2009). Cysteine proteinases synthesized and used by the different osteoclasts for bone matrix digestion (Everts et al., 2006) can degrade intramembranous bones as well as osteoclast-derived MMPs (Everts et al., 2009). Cysteine proteinases are secreted to act in the low pH environments formed by osteoclasts in the resorption sites, with MMPs degrading the rest of the bone matrix when the pH increases (Everts et al., 1998) as well as contributing to the digestion of fibrillar, non-mineralized collagen in Howship’s lacunae abandoned by osteoclast cells (Everts et al., 2002). These complementary and overlapping contributions of the MMP and cysteine proteinase families make the process of bone tissue remodeling both complex and robust.
The involvement of MMPs in bone remodeling has become clear with the aid of animal models such as MMP-deficient mice, which show a variety of bone abnormalities (Table 5). Impaired bone tissue remodeling in Mmp2–/– mice (Table 5, row 2) is characterized by a reduced number of osteoblasts and osteoclasts, disruption of the canicular network exacerbating osteocyte death, disruption of the MMP-2-osteopontin-bone sialoprotein axis, and promotion of osteolysis (Martignetti et al., 2001; Inoue et al., 2006; Mosig et al., 2007; Malaponte et al., 2016). MMP-9-deficient mice show alterations in cartilage-bone replacement during endochondral ossification (Vu et al., 1998) (Table 5, row 3). This phenotype may be explained by an inefficient degradation of the cartilage matrix, which leads to a diminished bioavailability of extracellular matrix-derived vascular endothelial growth factor and consequently effects osteoclasts and endothelial cells movement into the cartilage (Ortega et al., 2010). Bone tissue modeling and remodeling processes are altered in MMP-13 deficient mice (Table 5, row 4) (Inada et al., 2004; Stickens et al., 2004; Ortega et al., 2005). MMP-14 deficiency (Table 5, row 5), which is associated with high lethality, results in the most drastic skeletal phenotype among MMP-deficient mice (Holmbeck et al., 1999; Zhou et al., 2000). Double gene-deficient mice lacking at least one MMP gene have been engineered and their bone phenotype have been studied. For instance, double-knockout mice lacking MMP-2 and uPARAP/Endo180 (endocytic receptor of collagen and collagen fragments for degradation in the lysosomes) show reduced bone mineral density, short long bones, and poor trabecular bone quality (Madsen et al., 2013). MMP-8 and MMP-13 double-deficient mice have abnormal growth plate as well as augmented metaphyseal trabecular bone mineral density (Inada et al., 2001, 2002; Stickens et al., 2004). Double knockout mice lacking MMP-9 and MMP-13 exhibit expanded growth plates, disorganized hypertrophic chondrocyte zone, increased number of end-differentiated hypertrophic cells, and delayed formation of the bone marrow cavity (Kennedy et al., 2005; Paiva and Granjeiro, 2014). The bone phenotype of mice with a double knockout for MMP-14 and MMP-2 reassembles that of MMP-14-deficient mice (Oh et al., 2004). MMP-14 and MMP-16 double-knockout mice develop a bone phenotype that affects ossification (intramembranous and endochondral) and is characterized by severe irregularities, including (i) high mortality associated to developmental defects, (ii) noticeable craniofacial malformations such as cleft palate, thinner cranial vault bones, deficiently developed parietal, as well as frontal and nasal bones, (iii) altered growth plate, and (iv) cortical bone shortening (Paiva and Granjeiro, 2014). MMP-14 and uPARAP/Endo180 double-knockout mice die soon after birth (Wagenaar-Miller et al., 2007). As listed in Table 6, MMP activity contributes to numerous bone pathologies including arthritis, osteoporosis, osteonecrosis, periodontitis, sinonasal osteitis, degenerated lumbar disk tissues, and bone cancer metastasis (Aiken and Khokha, 2010; Koskinen et al., 2011; Mittal et al., 2016; Rose and Kooyman, 2016; Lazarus et al., 2017; Paiva and Granjeiro, 2017; Tauro and Lynch, 2018; Zhang et al., 2018). The roles played by MMPs in these pathologies are influenced by non-matrix proteins such as TIMPs, transforming growth factor, vascular endothelial growth factor, bone morphogenic proteins, activated protein C, and the Wnt [Wingless-type MMTV integration site family]/β-catenin (Table 7).
MMPs as Sheddases
Beyond the direct degradation of extracellular matrix substrates (e.g., collagen), MMP-mediated cleavage of substrates can lead to the release (shedding) into the extracellular matrix of soluble fragments of cell membrane-anchored receptor ligands. This extracellular event enables ligand-mediated activation of cognate receptors and elicits downstream intracellular signal transduction cascades which modify gene transcription and, ultimately, cell behavior. A prominent example pertinent to osteoblasts is the release of RANKL, which is the ligand of receptor activator of nuclear factor kappa B (RANK), by MMP-14. This MMP-14/RANKL/RANK/signal transduction axis regulates osteoblastogenesis and osteoclastogenesis, making MMP-14 crucial for normal bone formation (Bonfil et al., 2007; Thiolloy et al., 2009; Sabbota et al., 2010; Bonfil and Cher, 2011). The ligand shedding activity of MMPs influences the propensity to cancer metastasis and bone disease. For instance, MMP-14-mediated shedding of RANKL and downstream activation of RANK in the left supraclavicular lymph node cells of the prostate stimulates the non-receptor tyrosine kinase, SRC, to effectively increase the migration of prostate tumor cells which can metastasize to bone (Sabbota et al., 2010). Similarly, osteoclast-derived MMP-7 solubilizes osteoblast-bound RANKL whose release into the tumor-bone microenvironment promotes osteoclast activation in bone metastatic sites contributing to prostate and mammary tumor-induced osteolysis (Lynch et al., 2005; Thiolloy et al., 2009).
MMP-Generated Neoepitopes
The proteolytic action of MMPs on extracellular matrix macromolecules can result in the exposure of neo-epitopes (i.e., unique bioactive MMP-generated fragments). Compared to healthy subject controls, patients with ankylosing spondylitis (which is a form of arthritis that causes inflammation of the vertebrae) show significantly higher levels of different neo-epitopes such as C1M, C2M, C3M, C4M, C5M, C6M, and C7M from collagen type I, II, III, IV, V, VI, and VII (Veidal et al., 2012; Genovese and Karsdal, 2016). Some of these neo-epitopes have been combined (e.g., C2M, C3M, and C6M) for diagnostic purposes (Bay-Jensen et al., 2012). IPEN341-342FFGV is an MMP cleavage site which could be useful as diagnostic and prognostic makers for osteoarthritis (Bay-Jensen et al., 2011). Similarly, other MMP-generated neo-epitopes derived from collagen type II (e.g., C2C, C2M, C-terminal telopeptide of type II collagen (CTX-II), and TIINE) hold biomarker potential for osteoarthritis (Karsdal et al., 2010; Qvist et al., 2010; Karsdal et al., 2011).
Over-Overexpression of MMPs
Over-expression of MMPs is frequently reported in arthritis (Burrage et al., 2006; Tokito and Jougasaki, 2016). Collagenolytic MMPs (such as MMP-1, -2, -8, -13, and -14) are expressed in the arthritic joint and likely participate in the degradation of cartilage type II collagen, while MMP-3, -7, and -9 can degrade aggrecan leading to joint destruction (Puliti et al., 2012; Tokito and Jougasaki, 2016). Such a pathological mechanism has been proposed for MMP-3 and MMP-13 in degenerative joint disease in the elderly (Neuhold et al., 2001; Troeberg and Nagase, 2012; Jackson et al., 2014; Pap and Korb-Pap, 2015). Other contributions to osteoarthritis from activities related to MMP-3 include MMP-3-mediated activation of MMP-1 and MMP-13 (Mancini and di Battista, 2006; Tokito and Jougasaki, 2016). In rheumatoid arthritis, MMP-14 is greatly expressed in fibroblast-like synoviocytes and macrophages, and it could be an effector to cartilage destruction (Pap et al., 2000; Sabeh et al., 2010). MMP-1 and MMP-3 likely participate in cartilage destruction in rheumatoid arthritis and osteoarthritis (Burrage et al., 2006; Fiedorczyk et al., 2006; Tokito and Jougasaki, 2016). As a result, MMP overexpression could be therapeutically targeted in arthritis (Tokito and Jougasaki, 2016). Whether reducing MMP expression (or activity) levels provides a clinical benefit is unclear. In experimental models, many synthetic MMP inhibitors have shown positive effects (Ishikawa et al., 2005). At the clinical level, however, all efforts with MMP inhibitors to block the damaging activity of MMPs in arthritis and other non-neoplastic conditions were regrettably unsuccessful (Burrage et al., 2006; Tokito and Jougasaki, 2016). Reasons for these failures include: (i) deficient clinical trial designs (Burrage et al., 2006), (ii) unwanted characteristics of MMP inhibitors (side effects including musculoskeletal pain, low oral bioavailability, short in vivo half-lives, and lack of selectivity [Iyer et al., 2012; Fields, 2015; Tokito and Jougasaki, 2016]), (iii) inability of MMP inhibitors to infiltrate the cartilage/bone/synovial interface (Burrage et al., 2006), (iv) neglect of the highly complex functions served by MMPs in physiological and disease states (Iyer et al., 2012; Li et al., 2013; Sawicki, 2013) and (v) broad tissue distribution and substrate promiscuity exhibited by MMPs and their substrates (Burrage et al., 2006; Tokito and Jougasaki, 2016). To date, there remains a need for highly selective MMP inhibitors and for better information on the disease-specific substrates, which could be therapeutically targeted as shown by recent studies with MMP-13 in osteoarthritis (Li et al., 2011) as well as for more efficient and reliable techniques to sensitively measure condition-specific MMP activity potential (not just MMP expression levels).
MMP Gene Polymorphism
A nucleotide polymorphism, by which an additional guanine creates an ETS transcription factor binding site (5′-GGA-3′) at position 1607 in the promoter sequence of the MMP-1 gene, has been related to bone mineral density (BMD) (Rutter et al., 1998). This polymorphism is associated with increased transcription of the MMP-1 gene and elevated MMP-1 activity. Among 819 postmenopausal Japanese women, BMD (e.g., D50, D100) for the distal radius had a lower value in women with the GG/GG genotype (47.9%) than in those with other (e.g., G/GG [41.9%], G/G [10.3%], G/G + G/GG [52.1%]) genotypes. A -1562C3 thymine polymorphism in the MMP-9 gene has been related to BMD in a population-based study (1114 Japanese men and 1087 women). It seems that the T allele (e.g., in men with CT or TT genotypes) of MMP-9, which shows greater transcriptional activity than the C allele (e.g., in men with CC genotype), is linked to decreased bone mass, and has a predominant effect on BMD (Zhang et al., 1999; Yamada et al., 2004). A single nucleotide polymorphism rs17576 may be involved in the pathogenesis of lumbar disk herniation (Jing et al., 2018); while the G allele of rs17576 appears to correlate with more severe stages of disk degeneration.
MMP Deficiency and Insufficiency in Humans
Having discussed the roles of MMPs under physiological and pathological conditions, we will next discuss how their deficiency and insufficiency relates to bone metabolic abnormalities.
MMP-2 gene deficiency leads to a rare human skeletal disorder1, which was first reported in consanguineous Saudi Arabian families, and is characterized by severe bone alterations (Martignetti et al., 2001). Osteolytic and metabolic changes linked to MMP-2 deficiency affect tarsal, carpal, and phalangeal bones, cause severe arthropathy, osteoporosis, fibrous nodules, distinctive craniofacial defects such as exophthalmos, brachycephaly, and flattened nasal bridges and dwarfism (Al-Aqeel et al., 2000; Al-Mayouf et al., 2000; Al-Aqeel, 2005; Mosig et al., 2007; Page-McCaw et al., 2007; Castberg et al., 2013). This complex syndrome is currently categorized as a form of Torg syndrome and results from homoallelic mutations in the gene for MMP-2 located at 16q12-21 (Martignetti et al., 2001; Liang et al., 2016). A Tyr codon in the MMP-2 prodomain is replaced with the Y244X stop codon and an Arg is replaced with a His (R101H) in the cysteine-containing domain (PRCGNPD substituted by PHCGNPD). The R101H mutation is suggested to perturb coordination of Cys102 to the catalytic Zn(II) domain, consequently activating intracellular pro-MMP-2 and leading to its auto-degradation (Kennedy et al., 2005; Krane and Inada, 2008). A homoallelic missense mutation in the catalytic Zn(II) domain (E404K) has been revealed in Winchester syndrome (another variant of multicentric osteolysis) (Zankl et al., 2005). These rare Torg and Winchester arthritic syndromes together with others (such as multicentric osteolysis with nodulosis and arthropathy [known as MONA]) belong to a general family of hereditary autosomal dominant and recessive skeletal disorders with progressive bone loss and joint destruction (Al-Mayouf et al., 2000; Martignetti et al., 2001; Al-Aqeel, 2005; Zankl et al., 2005; Rouzier et al., 2006; Mosig et al., 2007; Tuysuz et al., 2009).
Similar to MMP-2, a homozygous dominant mutation (Ser substituted by Phe [F56S]) in the pro-region domain of MMP-13 also results in a bone development disorder known as spondyloepimetaphyseal dysplasia-Missouri type (Kennedy et al., 2005)2. This disorder, which appears to spontaneously resolve by adolescence, is characterized by anomalous modeling of long bones, mild defects in epiphysis, moderate to severe changes in the metaphysis morphology, pear-shaped vertebrae, femoral and tibial bowing, genu varum deformities, and osteoarthritis. While the biochemical mechanisms linking MMP-13 to these bone abnormalities remain unclear, the phenotype of MMP-13 deficiency could be due to a late exit of chondrocyte cells from the growth plate (Kennedy et al., 2005).
MMP-14 is widely considered one of the physiological activators of MMP-2 as it converts pro-MMP-2 into mature MMP-2 at the cell surface (Fernandez-Patron et al., 2016). An MMP-14 homoallelic mutation (T > R replacement in the signal peptide domain) destabilizes the interaction (e.g., recognition and binding) of the MMP-14 signal peptide with the signal recognition particle complex, thus affecting MMP-14 targeting to the plasma membrane (Evans et al., 2012). This MMP-14 homoallelic mutation causes an apparent deficiency of biochemically active MMP-14 at the cell membrane which impairs pro-MMP-2 activation and causes a condition of MMP-2 activity deficiency with Winchester syndrome (Evans et al., 2012)3.
A missense homozygous mutation (g.16250T > A, which replaces His226 of the Zn(II) catalytic domain with Gln [p.H226Q]), in the MMP20 gene disrupts the metal-binding site and prevents MMP-20 proteolytic activity regarding enamel matrix proteins (Ozdemir et al., 2005)4. This mutation may lead to autosomal-recessive hypomaturation amelogenesis imperfecta, a group of inherited heterogeneous diseases that alter enamel development (amount, composition, structure) in humans (Kim J.W. et al., 2005). Another mutation in the intron 6 splice acceptor (g.30561A > T) that causes this disease is specifically characterized by pigmented teeth with a mottled and rough surface (Kim J.W. et al., 2005).
Partial loss of MMP activity or impaired MMP secretion can lead to MMP activity insufficiency. A pervasive cause of MMP insufficiency can be medications with such MMP inhibitory actions including: (i) Statins (200 million prescriptions in the United States/year; 14 million prescriptions for lovastatin alone in 2014)5 which can cause myositis and rhabdomyolysis (Luan et al., 2003; Thompson et al., 2003). (ii) Doxycycline (7 million prescriptions in 2014)5 with side-effects including joint inflammation in humans and cardiac inflammation in mice (Berry et al., 2015). (iii) Therapeutic antibodies against MMPs and MMP inhibitor drugs for treating patients with rheumatoid arthritis, severely active Crohn’s disease, and cystic fibrosis6. If these antibodies reduce MMP activity below baseline levels, they would cause MMP insufficiency with unpredictable consequences. Pharmacological MMP-inhibitors in Phase 3 clinical trials conducted during 1997 and 1998 in patients with advanced cancers led to an as of yet poorly understood, very severe inflammatory musculoskeletal syndrome (Zucker et al., 2000; Coussens et al., 2002). Another common cause of MMP insufficiency could be the pathological elevation of endogenous MMP inhibitors (e.g., tissue inhibitors of MMPs, α-2-macroglobulin, RECK) (Mott et al., 2000; Oh et al., 2001; Nagase et al., 2006; Klein and Bischoff, 2011). In addition, there is fibrinogen, an acute phase reactant in arthritis, which our laboratory discovered recently to inhibit MMP-2 in a cohort of rheumatoid arthritis patients (Sarker et al., 2019).
Summary
In summary, bone lining cells, osteocytes, osteoclasts, reversal cells, and osteoblasts are responsible for constant bone tissue remodeling (Figure 2). The activation of this multicellular unit and the intense communication between the bone cells is tightly regulated by mechanical stimuli, apoptosis, as well as systemic and local factors such as hormones and cytokines including RANKL, CSF-M, IL-3, and IL-6. Proteases of the MMP and cysteine proteinase families converge in the modulation of bone remodeling. Whereas proteolytic activity has long been thought to be required for the degradation of bone tissue in osteoarthritis and osteoporosis, inactivating mutations in MMP genes can also lead to bone pathology including osteolysis and metabolic abnormalities such as delayed growth. Thus, there remains a need to rethink the role played by proteases in bone physiology and pathology. More specific information related to bone remodeling and presumed pathways by which proteases, in particular MMPs, contribute to bone tissue remodeling in health and disease is provided in previous excellent reviews (Kini and Nandeesh, 2012; Rauner et al., 2012; Hienz et al., 2015; Liang et al., 2016; Mittal et al., 2016; Franco et al., 2017; Paiva and Granjeiro, 2017; Tauro and Lynch, 2018; Plotkin and Bruzzaniti, 2019).
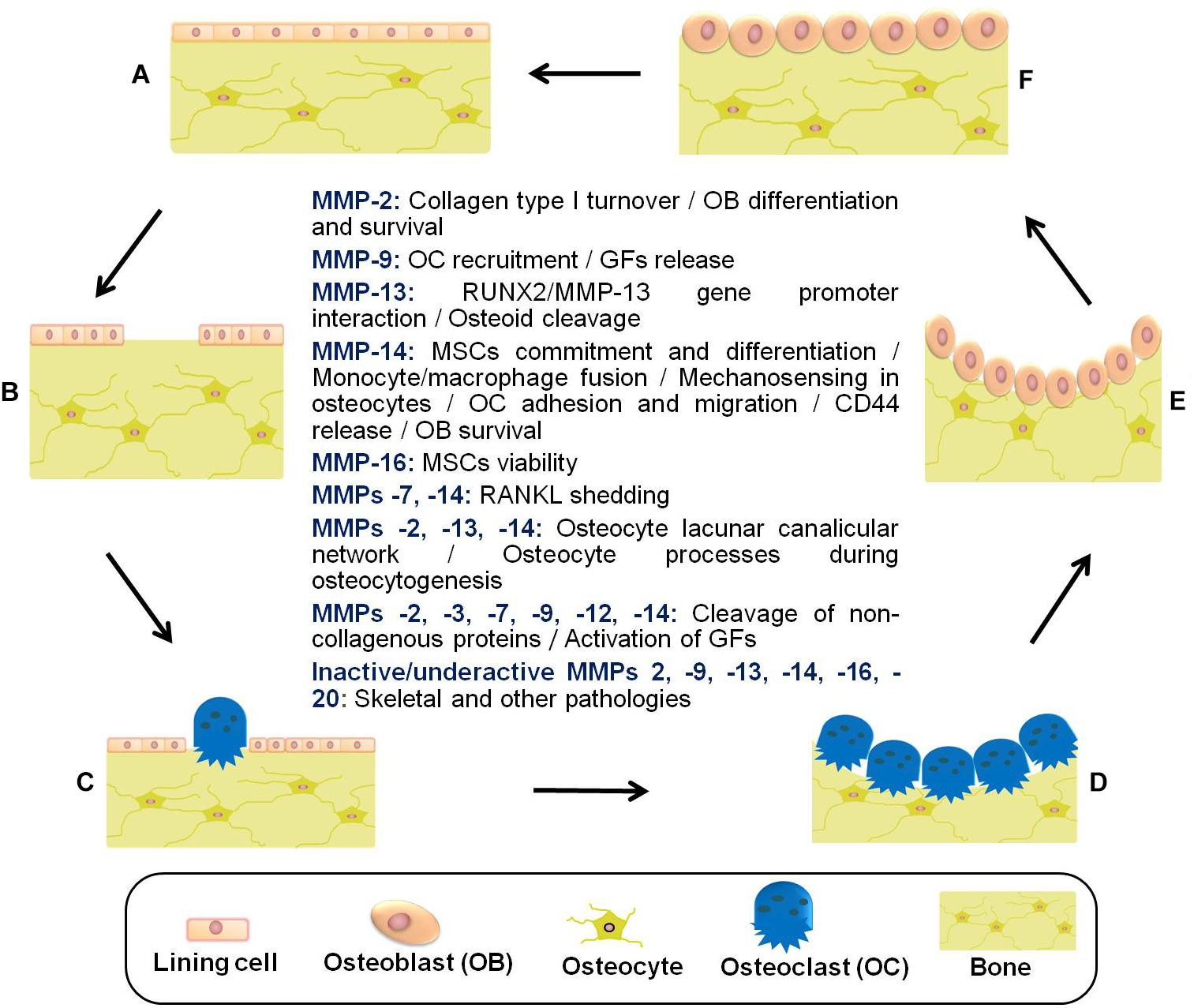
Figure 2. Schematic representation of the bone remodeling cycle with emphasis on the manifold roles played by matrix metalloproteinases. (A) Osteocytes detect mechanical stress or respond to biochemical stimuli. (B) Lining cells of the endosteal bone surface retract and proteases (e.g., MMPs) remove bone underlying membrane. (C) Osteoclasts are attracted and fused to become activated. (D) The underlying bone is digested by active multinucleated osteoclasts. (E) Osteoblasts are recruited to the bone resorption cavity. (F) New osteoid is formed by osteoblasts, and then mineralized (Datta et al., 2008; Fernandez-Patron et al., 2016; Paiva and Granjeiro, 2017; Cook et al., 2018). Other pathologies related to inactive/underactive MMPs are excessive inflammation, cardiovascular disorders, and metabolic dysregulation. MMP underactivity could also result from undesired side effects of common medications with MMP inhibitory actions (e.g., statins) (Cook et al., 2018). MSCs, mesenchymal stem cells; GFs, growth factors; RUNX2, runt-related transcription factor 2; RANKL, receptor activator of NF-kappa B ligand.
Author Contributions
EH and CF-P worked together on the conception, design, edition, revision, and approval of review manuscript.
Funding
CF-P was supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank MSc. Angela Sosa for help with Figure 2 illustration.
Footnotes
- ^ https://omim.org/entry/120360
- ^ https://www.omim.org/entry/600108
- ^ https://www.omim.org/entry/600754
- ^ https://www.omim.org/entry/604629
- ^ http://clincalc.com/DrugStats
- ^ http://www.gilead.com
References
Agnihotri, R., Crawford, H. C., Haro, H., Matrisian, L. M., Havrda, M. C., and Liaw, L. (2001). Osteopontin, a novel substrate for matrix metalloproteinase-3 (Stromelysin-1) and matrix metalloproteinase-7 (Matrilysin). J. Biol. Chem. 276, 28261–28267. doi: 10.1074/jbc.m103608200
Aguirre, J. I., Plotkin, L. I., Stewart, S. A., Weinstein, R. S., Parfitt, A. M., Manolagas, S. C., et al. (2006). Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J. Bone Miner. Res. 21, 605–615. doi: 10.1359/jbmr.060107
Ahrens, D., Koch, A. E., Pope, R. M., Stein-Picarella, M., and Niedbala, M. J. (1996). Expression of matrix metalloproteinase 9 (96-kd gelatinase B) in human rheumatoid arthritis. Arthritis Rheum. 39, 1576–1587. doi: 10.1002/art.1780390919
Aiken, A., and Khokha, R. (2010). Unraveling metalloproteinase function in skeletal biology and disease using genetically altered mice. Biochim. Biophys. Acta 1803, 121–132. doi: 10.1016/j.bbamcr.2009.07.002
Al-Aqeel, A., Al Sewairi, W., Edress, B., Gorlin, R. J., Desnick, R. J., and Martignetti, J. A. (2000). Inherited multicentric osteolysis with arthritis: a variant resembling Torg syndrome in a Saudi family. Am. J. Med. Genet. 93, 11–18. doi: 10.1002/1096-8628(20000703)93:1<11::aid-ajmg3>3.0.co;2-3
Al-Aqeel, A. I. (2005). Al-Aqeel Sewairi syndrome, a new autosomal recessive disorder with multicentric osteolysis, nodulosis and arthropathy. The first genetic defect of matrix metalloproteinase 2 gene. Saudi Med. J. 26, 24–30.
Alford, A. I., Jacobs, C. R., and Donahue, H. J. (2003). Oscillating fluid flow regulates gap junction communication in osteocytic MLO-Y4 cells by an ERK1/2 MAP kinase-dependent mechanism. Bone 33, 64–70. doi: 10.1016/s8756-3282(03)00167-4
Alliston, T. (2014). Biological regulation of bone quality. Curr. Osteoporos. Rep. 12, 366–375. doi: 10.1007/s11914-014-0213-4
Almalki, S. G., and Agrawal, D. K. (2016). Effects of matrix metalloproteinases on the fate of mesenchymal stem cells. Stem Cell. Res. Ther. 7, 1–12. doi: 10.1186/s13287-016-0393-1
Al-Mayouf, S. M., Majeed, M., Hugosson, C., and Bahabri, S. (2000). New form of idiopathic osteolysis: nodulosis, arthropathy and osteolysis (NAO) syndrome. Am. J. Med. Genet. 93, 5–10. doi: 10.1002/1096-8628(20000703)93:1<5::aid-ajmg2>3.0.co;2-y
Andersen, T. L., Abdelgawad, M. E., Kristensen, H. B., Hauge, E. M., Rolighed, L., Bollerslev, J., et al. (2013). Understanding coupling between bone resorption and formation: are reversal cells the missing link? Am. J. Pathol. 183, 235–246. doi: 10.1016/j.ajpath.2013.03.006
Apte, S. S., Fukai, N., Beier, D. R., and Olsen, B. R. (1997). The matrix metalloproteinase-14 (MMP-14) gene is structurally distinct from other MMP genes and is co-expressed with the TIMP-2 gene during mouse embryogenesis. J. Biol. Chem. 272, 25511–25517. doi: 10.1074/jbc.272.41.25511
Arpino, V., Brock, M., and Gill, S. E. (2015). The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 4, 247–254. doi: 10.1016/j.matbio.2015.03.005
Arumugam, B., Vairamani, M., Partridge, N. C., and Selvamurugan, N. (2018). Characterization of Runx2 phosphorylation sites required for TGF-β1-mediated stimulation of matrix metalloproteinase-13 expression in osteoblastic cells. J. Cell Physiol. 233, 1082–1094. doi: 10.1002/jcp.25964
Arumugam, B., Vishal, M., Shreya, S., Malavika, D., Rajpriya, V., He, Z., et al. (2019). Parathyroid hormone-stimulation of Runx2 during osteoblast differentiation via the regulation of lnc-SUPT3H-1:16 (RUNX2-AS1:32) and miR-6797-5p. Biochimie 158, 43–52. doi: 10.1016/j.biochi.2018.12.006
Balemans, W., Piters, E., Cleiren, E., Ai, M., Van Wesenbeeck, L., Warman, M. L., et al. (2008). The binding between sclerostin and LRP5 is altered by DKK1 and by high-bone mass LRP5 mutations. Calcif. Tissue Int. 82, 445–453. doi: 10.1007/s00223-008-9130-9
Balooch, G., Balooch, M., Nalla, R. K., Schilling, S., Filvaroff, E. H., Marshall, G. W., et al. (2005). TGF-beta regulates the mechanical properties and composition of bone matrix. Proc. Natl. Acad. Sci. U.S.A. 102, 18813–18818. doi: 10.1073/pnas.0507417102
Baron, R. (1989). Molecular mechanisms of bone resorption by the osteoclast. Anat. Rec. 224, 317–324. doi: 10.1002/ar.1092240220
Bar-Shavit, Z. (2007). Theosteoclast: amultinucleated, hematopoieticorigin, bone-resorbing osteoimmune cell. J. Cell Biochem. 102, 1130–1139. doi: 10.1002/jcb.21553
Barthelemi, S., Robinet, J., Garnotel, R., Antonicelli, F., Schittly, E., Hornebeck, W., et al. (2012). Mechanical forces-induced human osteoblasts differentiation involves MMP-2/MMP-13/MT1-MMP proteolytic cascade. J. Cell Biochem. 113, 760–772. doi: 10.1002/jcb.23401
Bay-Jensen, A. C., Leeming, D. J., Kleyer, A., Veidal, S. S., Schett, G., and Karsdal, M. A. (2012). Ankylosing spondylitis is characterized by an increased turnover of several different metalloproteinase-derived collagen species: a cross-sectional study. Rheumatol. Int. 32, 3565–3572. doi: 10.1007/s00296-011-2237-8
Bay-Jensen, A. C., Liu, Q., Byrjalsen, I., Li, Y., Wang, J., Pedersen, C., et al. (2011). Enzyme-linked immunosorbent assay (ELISAs) for metalloproteinase derived type II collagen neoepitope, CIIM–increased serum CIIM in subjects with severe radiographic osteoarthritis. Clin. Biochem. 44, 423–429. doi: 10.1016/j.clinbiochem.2011.01.001
Baylink, D. J., Finkelman, R. D., and Mohan, S. (1993). Growth factors to stimulate bone formation. J. Bone. Miner. Res. 8, 565–572.
Bellido, T. (2014). Osteocyte-driven bone remodeling. Calcif. Tissue Int. 94, 25–34. doi: 10.1007/s00223-013-9774-y
Bellido, T., and Gallant, K. M. H. (2019). “Hormonal effects on bone cells,” in Basic and Applied Bone Biology, eds D. B. Burr and M. R. Allen, (Cambridge, MA: Academic Press), 299–314. doi: 10.1016/b978-0-12-416015-6.00015-0
Bellido, T., Plotkin, L. I., and Bruzzaniti, A. (2019). “Bone cells,” in Basic and Applied Bone Biology, eds D. B. Burr and M. R. Allen, (Cambridge, MA: Academic Press), 37–55.
Bergers, G., Brekken, R., McMahon, G., Vu, T. H., Itoh, T., Tamaki, K., et al. (2000). Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell. Biol. 2, 737–744. doi: 10.1038/35036374
Berry, E., Hernandez-Anzaldo, S., Ghomashchi, F., Lehner, R., Murakami, M., Gelb, M. H., et al. (2015). Matrix metalloproteinase-2 negatively regulates cardiac secreted phospholipase A2 to modulate inflammation and fever. J. Am. Heart. Assoc. 4, 1–22. doi: 10.1161/JAHA.115.001868
Blair, H. C., Teitelbaum, S. L., Ghiselli, R., and Gluck, S. (1989). Osteoclastic bone resorption by a polarized vacuolar proton pump. Science 245, 855–857. doi: 10.1126/science.2528207
Blavier, L., and Delaisse, J. M. (1995). Matrix metalloproteinases are obligatory for the migration of preosteoclasts to the developing marrow cavity of primitive long bones. J. Cell Sci. 108, 3649–3659.
Bode, W., and Maskos, K. (2003). Structural basis of the matrix metalloproteinases and their physiological inhibitors, the tissue inhibitors of metalloproteinases. Biol. Chem. 384, 863–872.
Bodine, P. V., and Komm, B. S. (2006). Wnt signaling and osteoblastogenesis. Rev. Endocr. Metab. Disord. 7, 33–39. doi: 10.1007/s11154-006-9002-4
Bonewald, L. F. (2011). The amazing osteocyte. J. Bone Miner. Res. 26, 229–238. doi: 10.1002/jbmr.320
Bonewald, L. F., and Johnson, M. L. (2008). Osteocytes, mechanosensing and Wnt signaling. Bone 42, 606–615. doi: 10.1016/j.bone.2007.12.224
Bonewald, L. F., and Mundy, G. R. (1990). Role of transforming growth factor-beta in bone remodeling. Clin. Orthop. Relat. Res. 250, 261–276.
Bonfil, R. D., and Cher, M. L. (2011). The role of proteolytic enzymes in metastatic bone disease. IBMS BoneKEy 8, 16–36. doi: 10.1138/20110487
Bonfil, R. D., Dong, Z., Trindade Filho, J. C., Sabbota, A., Osenkowski, P., Nabha, S., et al. (2007). Prostate cancer-associated membrane type 1-matrix metalloproteinase: a pivotal role in bone response and intraosseous tumor growth. Am. J. Pathol. 170, 2100–2111.
Bonnans, C., Chou, J., and Werb, Z. (2014). Remodelling the extracellular matrix in development and disease. Nature Rev. Mol. Cell. Biol. 15, 786–801. doi: 10.1038/nrm3904
Bord, S., Horner, A., Beeton, C. A., Hembry, R. M., and Compston, J. E. (1999). Tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) distribution in normal and pathological human bone. Bone 24, 229–235. doi: 10.1016/s8756-3282(98)00174-4
Bord, S., Horner, A., Hembry, R. M., and Compston, J. E. (1998). Stromelysin-1 (MMP-3) and stromelysin-2 (MMP-10) expression in developing human bone: potential roles in skeletal development. Bone 23, 7–12. doi: 10.1016/s8756-3282(98)00064-7
Bord, S., Horner, A., Hembry, R. M., Reynolds, J. J., and Compston, J. E. (1996). Production of collagenase by human osteoblasts and osteoclasts in vivo. Bone 19, 35–40. doi: 10.1016/8756-3282(96)00106-8
Boyden, L. M., Mao, J., Belsky, J., Mitzner, L., Farhi, A., Mitnick, M. A., et al. (2002). High bone density due to a mutation in LDL receptor related protein 5. N. Engl. J. Med. 346, 1513–1521. doi: 10.1056/nejmoa013444
Boyle, W. J., Simonet, W. S., and Lacey, D. L. (2003). Osteoclast differentiation and activation. Nature 423, 337–342. doi: 10.1038/nature01658
Brandi, M. L., and Piscitelli, P. (2013). “Epidemiology of osteoporosis and fragility fractures,” in Osteoporosis and Bone Densitometry Measurements, ed. G. Guglielmi, (Berlin: Springer-Verlag), 1–4. doi: 10.1007/174_2012_747
Brew, K., Dinakarpandian, D., and Nagase, H. (2000). Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim. Biophys. Acta 1477, 267–283. doi: 10.1016/s0167-4838(99)00279-4
Bruzzaniti, A., and Baron, R. (2006). Molecular regulation of osteoclast activity. Rev. Endocr. Metab. Disord. 7, 123–139. doi: 10.1007/s11154-006-9009-x
Buisson-Legendre, N., Smith, S., March, L., and Jackson, C. (2004). Elevation of activated protein C in synovial joints in rheumatoid arthritis and its correlation with matrix metalloproteinase 2. Arthritis Rheum. 50, 2151–2156. doi: 10.1002/art.20313
Burger, E. H., and Klein-Nulend, J. (1999). Mechanotransduction in bone-role of the laculocanalicular network. FASEB 13, S101–S112.
Burrage, P. S., Mix, K. S., and Brinckerhoff, C. E. (2006). Matrix metalloproteinases: role in arthritis. Front. Biosci. 11:529–543.
Canalis, E. (2009). Growth factor control of bone mass. J. Cell Biochem. 108, 769–777. doi: 10.1002/jcb.22322
Canalis, E., Economides, A. N., and Gazzerro, E. (2003). Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr. Rev. 24, 218–235. doi: 10.1210/er.2002-0023
Castberg, F. C., Kjaergaard, S., Mosig, R. A., Lobl, M., Martignetti, C., Martignetti, J. A., et al. (2013). Multicentric osteolysis with nodulosis and arthropathy (MONA) with cardiac malformation, mimicking polyarticular juvenile idiopathic arthritis: case report and literature review. Eur. J. Pediatr. 172, 1657–1663. doi: 10.1007/s00431-013-2102-8
Cavalla, F., Hernandez-Rios, P., Sorsa, T., Biguetti, C., and Hernandez, M. (2017). Matrix metalloproteinases as regulators of periodontal inflammation. Int. J. Mol. Sci. 18:440. doi: 10.3390/ijms18020440
Charles, J. M., and Key, L. L. (1998). Developmental spectrum of children with congenital osteopetrosis. J. Pediatr. 132, 371–374. doi: 10.1016/s0022-3476(98)70467-6
Chellaiah, M. A., and Ma, T. (2013). Membrane localization of membrane type 1 matrix metalloproteinase by CD44 regulates the activation of pro-matrix metalloproteinase 9 in osteoclasts. Biomed. Res. Int. 2013:302392. doi: 10.1155/2013/302392
Chen, D., Xie, R., Shu, B., Landay, A. L., Wei, C., Reiser, J., et al. (2019). Wnt signaling in bone, kidney, intestine, and adipose tissue and interorgan interaction in aging. Ann. N. Y. Acad. Sci. 1442, 48–60. doi: 10.1111/nyas.13945
Chen, M., Zhu, M., Awad, H., Li, T. F., Sheu, T. J., Boyce, B. F., et al. (2008). Inhibition of beta-catenin signaling causes defects in postnatal cartilage development. J. Cell Sci. 121, 1455–1465. doi: 10.1242/jcs.020362
Chen, X., Wang, L., Zhao, K., and Wang, H. (2018). Osteocytogenesis: roles of physicochemical factors, collagen cleavage, and exogenous molecules. Tissue Eng. Part B Rev. 24, 215–225. doi: 10.1089/ten.teb.2017.0378
Choi, Y. A., Kang, S. S., and Jin, E. J. (2009). BMP-2 treatment of C3H10T1/2 mesenchymal cells blocks MMP-9 activity during chondrocyte commitment. Cell Biol. Int. 33, 887–892. doi: 10.1016/j.cellbi.2009.04.020
Civitelli, R. (2008). Cell-cell communication in the osteoblast/osteocyte lineage. Arch. Biochem. Biophys. 473, 188–192. doi: 10.1016/j.abb.2008.04.005
Clark, I. M., Swingler, T. E., Sampieri, C. L., and Edwards, D. R. (2008). The regulation of matrix metalloproteinases and their inhibitors. Int. J. Biochem. Cell Biol. 40, 1362–1378. doi: 10.1016/j.biocel.2007.12.006
Coen, G., Ballanti, P., Silvestrini, G., Mantella, D., Manni, M., Di Giulio, S., et al. (2009). Immunohistochemical localization and mRNA expression of matrix Glaprotein and fetuin-A in bone biopsies of hemodialysis patients. Virchows Arch. 454, 263–271. doi: 10.1007/s00428-008-0724-4
Cohick, W. S., and Clemmons, D. R. (1993). The insulin-like growth factors. Annu. Rev. Physiol. 55, 131–153.
Cook, R., Sarker, H., and Fernandez-Patron, C. (2018). Pathologies of matrix metalloproteinase-2 underactivity: A perspective on a neglected condition. Can. J. Physiol. Pharmacol. 97, 1–7. doi: 10.1139/cjpp-2018-0525
Coussens, L. M., Fingleton, B., and Matrisian, L. M. (2002). Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295, 2387–2392. doi: 10.1126/science.1067100
Cox, J. H., Starr, A. E., Kappelhoff, R., Yan, R., Roberts, C. R., and Overall, C. M. (2010). Matrix metalloproteinase 8 deficiency in mice exacerbates inflammatory arthritis through delayed neutrophil apoptosis and reduced caspase 11 expression. Arthritis Rheum. 62, 3645–3655. doi: 10.1002/art.27757
Crotti, T. N., Flannery, M., Walsh, N. C., Fleming, J. D., Goldring, S. R., and McHugh, K. P. (2006). NFATc1 regulation of the human beta3 integrin promoter in osteoclast differentiation. Gene 372, 92–102. doi: 10.1016/j.gene.2005.12.012
Crotti, T. N., Sharma, S. M., Fleming, J. D., Flannery, M. R., Ostrowski, M. C., Goldring, S. R., et al. (2008). PU.1 and NFATc1 mediate osteoclastic induction of the mouse β3 integrin promoter. J. Cell Physiol. 215, 636–644. doi: 10.1002/jcp.21344
Cui, N., Hu, M., and Khalil, R. A. (2017). Biochemical and biological attributes of matrix metalloproteinases. Prog. Mol. Biol. Transl. Sci. 147, 1–73. doi: 10.1016/bs.pmbts.2017.02.005
Dai, J. C., He, P., Chen, X., and Greenfield, E. M. (2006). TNF alpha and PTH utilize distinct mechanisms to induce IL-6 and RANKL expression with markedly different kinetics. Bone 38, 509–520. doi: 10.1016/j.bone.2005.10.007
Dallas, S. L., Miyazono, K., Skerry, T. M., Mundy, G. R., and Bonewald, L. F. (1995). Dual role for the latent transforming growth factor-beta binding protein in storage of latent TGF-beta in the extracellular matrix and as a structural matrix protein. J. Cell. Biol. 131, 539–549. doi: 10.1083/jcb.131.2.539
Dallas, S. L., Prideaux, M., and Bonewald, L. F. (2013). The osteocyte: an endocrine cell and more. Endocr. Rev. 34, 658–690. doi: 10.1210/er.2012-1026
D’Angelo, M., Billings, P. C., Pacifici, M., Leboy, P. S., and Kirsch, T. (2001). Authentic matrix vesicles contain active metalloproteases (MMP): a role for matrix vesicle-associated MMP-13 in activation of transforming growth factor-beta. J. Biol. Chem. 276, 11347–11353. doi: 10.1074/jbc.m009725200
Datta, H. K., Ng, W. F., Walker, J. A., Tuck, S. P., and Varanasi, S. S. (2008). The cell biology of bone metabolism. J. Clin. Pathol. 61, 577–587. doi: 10.1136/jcp.2007.048868
Delaisse, J. M. (2014). The reversal phase of the bone-remodeling cycle: cellular prerequisites for coupling resorption and formation. Bonekey Rep. 3:561. doi: 10.1038/bonekey.2014.56
Delaisse, J. M., Andersen, T. L., Engsig, M. T., Henriksen, K., Troen, T., and Blavier, L. (2003). Matrix metalloproteinases (MMP) and cathepsin K contribute differently to osteoclastic activities. Microsc. Res. Tech. 61, 504–513. doi: 10.1002/jemt.10374
Delmas, P. D. (1995). “Biochemical markers for the assessment of bone turnover,” in Osteoporosis: Etiology, Diagnosis, and Management, 2nd Edn, eds B. Riggs and L. J. Melton, (Philadelphia: Lippincott-Raven), 319–333.
Dempster, D. W., Cosman, F., Parisien, M., Shen, V., and Lindsay, R. (1993). Anabolic actions of parathyroid hormone on bone. Endocr. Rev. 14, 690–709. doi: 10.1210/er.14.6.690
Ducy, P., Schinke, T., and Karsenty, G. (2000). The osteoblast: a sophisticated fibroblast under central surveillance. Science 289, 1501–1504. doi: 10.1126/science.289.5484.1501
Duerr, S., Stremme, S., Soeder, S., Bau, B., and Aigner, T. (2004). MMP-2/gelatinase A is a gene product of human adult articular chondrocytes and is increased in osteoarthritic cartilage. Clin. Exp. Rheumatol. 22, 603–608.
Durie, B. G. M., Katz, M., and Crowley, J. (2005). Osteonecrosis of the jaw and bisphosphonates. N. Engl. J. Med. 353, 99–102.
Egea, V., Zahler, S., Rieth, N., Neth, P., Popp, T., Kehe, K., et al. (2012). Tissue inhibitor of metalloproteinase-1 (TIMP-1) regulates mesenchymal stem cells through let-7f microRNA and Wnt/β-catenin signaling. Proc. Natl. Acad. Sci. U.S.A. 109, E309–E316. doi: 10.1073/pnas.1115083109
Eijken, M., Hewison, M., Cooper, M. S., de Jong, F. H., Chiba, H., Stewart, P. M., et al. (2005). 11beta-Hydroxysteroid dehydrogenase expression and glucocorticoid synthesis are directed by a molecular switch during osteoblast differentiation. Mol. Endocrinol. 19, 621–631. doi: 10.1210/me.2004-0212
Engsig, M. T., Chen, Q. J., Vu, T. H., Pedersen, A. C., Therkidsen, B., Lund, L. R., et al. (2000). Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J. Cell Biol. 151, 879–889.
Evans, B. R., Mosig, R. A., Lobl, M., Martignetti, C. R., Camacho, C., Grum-Tokars, V., et al. (2012). Mutation of membrane type-1 metalloproteinase, MT1-MMP, causes the multicentric osteolysis and arthritis disease winchester syndrome. Am. J. Hum. Genet. 91, 572–576. doi: 10.1016/j.ajhg.2012.07.022
Everts, V., de Vries, T. J., and Helfrich, M. H. (2009). Osteoclast heterogeneity: lessons from osteopetrosis and inflammatory conditions. Biochim. Biophys. Acta 1792, 757–765. doi: 10.1016/j.bbadis.2009.05.004
Everts, V., Delaissé, J. M., Korper, W., and Beertsen, W. (1998). Cysteine proteinases and matrix metalloproteinases play distinct roles in the subosteoclastic resorption zone. J. Bone Miner. Res. 13, 1420–1430. doi: 10.1359/jbmr.1998.13.9.1420
Everts, V., Delaissé, J. M., Korper, W., Jansen, D. C., Tigchelaar-Gutter, W., Saftig, P., et al. (2002). The bone lining cell: its role in cleaning Howship’s lacunae and initiating bone formation. J. Bone Miner. Res. 17, 77–90. doi: 10.1359/jbmr.2002.17.1.77
Everts, V., Delaisse, J. M., Korper, W., Niehof, A., Vaes, G., and Beertsen, W. (1992). Degradation of collagen in the bone-resorbing compartment underlying the osteoclast involves both cysteine-proteinases and matrix metalloproteinases. J. Cell Physiol. 150, 221–231. doi: 10.1002/jcp.1041500202
Everts, V., Korper, W., Hoeben, K. A., Jansen, I. D., Bromme, D., Cleutjens, K. B., et al. (2006). Osteoclastic bone degradation and the role of different cysteine proteinases and matrix metalloproteinases: differences between calvaria and long bone. J. Bone Miner. Res. 21, 1399–1408. doi: 10.1359/jbmr.060614
Everts, V., Korper, W., Jansen, D. C., Steinfort, J., Lammerse, I., Heera, S., et al. (1999). Functional heterogeneity of osteoclasts: matrix metalloproteinases participate in osteoclastic resorption of calvarial bone but not in resorption of long bone. FASEB J. 1999, 1219–1230. doi: 10.1096/fasebj.13.10.1219
Faccio, R., Teitelbaum, S. L., Fujikawa, K., Chappel, J., Zallone, A., Tybulewicz, V. L., et al. (2005). Vav3 regulates osteoclast function and bone mass. Nat. Med. 11, 284–290. doi: 10.1038/nm1194
Fanjul-Fernandez, M., Folgueras, A. R., Cabrera, S., and Lopez-Otin, C. (2010). Matrix metalloproteinases: evolution, gene regulation and functional analysis in mouse models. Biochim. Biophys. 1803, 3–19. doi: 10.1016/j.bbamcr.2009.07.004
Feng, J. Q., Ward, L. M., Liu, S., Lu, Y., Xie, Y., Yuan, B., et al. (2006). Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 38, 1310–1315. doi: 10.1038/ng1905
Fernandez-Patron, C., Kassiri, Z., and Leung, D. (2016). Modulation of systemic metabolism by MMP-2: from MMP-2 deficiency in mice to MMP-2 deficiency in patients. Compr. Physiol. 6, 1935–1949. doi: 10.1002/cphy.c160010
Ferreras, M., Felbor, U., Lenhard, T., Olsen, B. R., and Delaisse, J. M. (2000). Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett. 486, 247–251. doi: 10.1016/s0014-5793(00)02249-3
Fiedorczyk, M., Klimiuk, P. A., Sierakowski, S., Gindzienska-Sieskiewicz, E., and Chwiecko, J. (2006). Serum matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with early rheumatoid arthritis. J. Rheumatol. 33, 1523–1529.
Fields, G. B. (2015). New strategies for targeting matrix metalloproteinases. Matrix Biol. 4, 239–246. doi: 10.1016/j.matbio.2015.01.002
Filanti, C., Dickson, G. R., Di Martino, D., Ulivi, V., Sanguineti, C., Romano, P., et al. (2000). The expression of metalloproteinase-2, -9, and -14 and of tissue inhibitors-1 and -2 is developmentally modulated during osteogenesis in vitro, the mature osteoblastic phenotype expressing metalloproteinase-14. J. Bone Miner. Res. 15, 2154–2168. doi: 10.1359/jbmr.2000.15.11.2154
Fiore, E., Fusco, C., Romero, P., and Stamenkovic, I. (2002). Matrix metalloproteinase 9 (MMP-9/gelatinase B) proteolytically cleaves ICAM-1 and participates in tumor cell resistance to natural killer cell-mediated cytotoxicity. Oncogene 21, 5213–5223. doi: 10.1038/sj.onc.1205684
Francin, P. J., Abot, A., Guillaume, C., Moulin, D., Bianchi, A., Gegout-Pottie, P., et al. (2014). Association between adiponectin and cartilage degradation in human osteoarthritis. Osteoarthr. Cartil. 22, 519–526. doi: 10.1016/j.joca.2014.01.002
Franco, C., Patricia, H. R., Timo, S., Claudia, B., and Marcela, H. (2017). Matrix metalloproteinases as regulators of periodontal inflammation. Int. J. Mol. Sci. 18:E440. doi: 10.3390/ijms18020440
Gack, S., Vallon, R., Schmidt, J., Grigoriadis, A., Tuckermann, J., Schenkel, J., et al. (1995). Expression of interstitial collagenase during skeletal development of the mouse is restricted to osteoblast-like cells and hypertrophic chondrocytes. Cell Growth Differ. 6, 759–767.
Gallagher, S. K. (1997). Biochemical markers of bone metabolism as they relate to osteoporosis. MLO 29:50.
Gao, Y. Y., Liu, B. Q., Du, Z. X., Zhang, H. Y., Niu, X. F., and Wang, H. Q. (2010). Implication of oxygen regulated protein 150 (ORP150) in apoptosis induced by proteasome inhibitors in human thyroid cancer cells. J. Clin. Endocrinol. Metab. 95, E319–E326. doi: 10.1210/jc.2010-1043
Gearing, A. J. H., Beckett, P., Christodoulou, M., Churchill, M., Clements, J. M., Crimmin, M., et al. (1995). Matrix metalloproteinases and processing of pro-TNF-alpha. J. Leukoc. Biol. 57, 774–777.
Gelb, B. D., Shi, G. P., Chapman, H. A., and Desnick, R. J. (1996). Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science 273, 1236–1238. doi: 10.1126/science.273.5279.1236
Genovese, F., and Karsdal, M. A. (2016). Protein degradation fragments as diagnostic and prognostic biomarkers of connective tissue diseases: understanding the extracellular matrix message and implication for current and future serological biomarkers. Expert Rev. Proteomics 13, 213–225. doi: 10.1586/14789450.2016.1134327
Geoffroy, V., Marty-Morieux, C., Le Goupil, N., Clement-Lacroix, P., Terraz, C., Frain, M., et al. (2004). In vivo inhibition of osteoblastic metalloproteinases leads to increased trabecular bone mass. J. Bone Miner. Res. 19, 811–822. doi: 10.1359/jbmr.040119
Gong, Y., Slee, R. B., Fukai, N., Rawadi, G., Roman-Roman, S., Reginato, A. M., et al. (2001). LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107, 513–523.
Gonzalo, P., Guadamillas, M. C., Hernandez-Riquer, M. V., Pollan, A., Grande-García, A., Bartolome, R. A., et al. (2010). MT1-MMP is required for myeloid cell fusion via regulation of Rac1 signaling. Dev. Cell 18, 77–89. doi: 10.1016/j.devcel.2009.11.012
Gori, F., Hofbauer, L. C., Dunstan, C. R., Spelsberg, T. C., Khosla, S., and Riggs, B. L. (2000). The expression of osteoprotegerin and RANK ligand and the support of osteoclast formation by stromal-osteoblast lineage cells is developmentally regulated. Endocrinology 141, 4768–4776. doi: 10.1210/endo.141.12.7840
Greenlee, K. J., Werb, Z., and Kheradmand, F. (2007). Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol. Rev. 87, 69–98. doi: 10.1152/physrev.00022.2006
Gu, Y., Preston, M. R., El Haj, A. J., Howl, J. D., and Publicover, S. J. (2001). Three types of K+ currents in murine osteocyte-like cells (MLO-Y4). Bone 28, 29–37. doi: 10.1016/s8756-3282(00)00439-7
Guan, C. C., Yan, M., Jiang, X. Q., Zhang, P., Zhang, X. L., Li, J., et al. (2009). Sonic hedgehog alleviates the inhibitory effects of high glucose on the osteoblastic differentiation of bone marrow stromal cells. Bone 45, 1146–1152. doi: 10.1016/j.bone.2009.08.009
Hachemi, Y., Rapp, A. E., Picke, A. K., Weidinger, G., Ignatius, A., and Tuckermann, J. (2018). Molecular mechanisms of glucocorticoids on skeleton and bone regeneration after fracture. J. Mol. Endocrinol. 61, R75–R90. doi: 10.1530/JME-18-0024
Hadler-Olsen, E., Fadnes, B., Sylte, I., Uhlin-Hansen, L., and Winberg, J. O. (2011). Regulation of matrix metalloproteinase activity in health and disease. FEBS J. 278, 28–45. doi: 10.1111/j.1742-4658.2010.07920.x
Haeusler, G., Walter, I., Helmreich, M., and Egerbacher, M. (2005). Localization of matrix metalloproteinases, (MMPs) their tissue inhibitors, and vascular endothelial growth factor (VEGF) in growth plates of children and adolescents indicates a role for MMPs in human postnatal growth and skeletal maturation. Calcif. Tissue Int. 76, 326–335. doi: 10.1007/s00223-004-0161-6
Han, X., Boyd, P. J., Colgan, S., Madri, J. A., and Haas, T. L. (2003). Transcriptional up-regulation of endothelial cell matrix metalloproteinase-2 in response to extracellular cues involves GATA-2. J. Biol. Chem. 278, 47785–47791. doi: 10.1074/jbc.m309482200
Harris, S. E., Guo, D., Harris, M. A., Krishnaswamy, A., and Lichtler, A. (2003). Transcriptional regulation of BMP-2 activated genes in osteoblasts using gene expression microarray analysis: role of Dlx2 and Dlx5 transcription factors. Front. Biosci. 8:s1249–s1265.
Heino, T. J., Hentunen, T. A., and Vaananen, H. K. (2002). Osteocytes inhibit osteoclastic bone resorption through transforming growth factor-beta: enhancement by estrogen. J. Cell. Biochem. 85, 185–197. doi: 10.1002/jcb.10109
Hienz, S. A., Paliwal, S., and Ivanovski, S. (2015). Mechanisms of bone resorption in periodontitis. J. Immunol. Res. 2015:615486. doi: 10.1155/2015/615486
Hikita, A., Yana, I., Wakeyama, H., Nakamura, M., Kadono, Y., Oshima, Y., et al. (2006). Negative regulation of osteoclastogenesis by ectodomain shedding of receptor activator of NF-kappa B ligand. J. Biol. Chem. 281, 36846–36855. doi: 10.1074/jbc.m606656200
Hirao, M., Hashimoto, J., Yamasaki, N., Ando, W., Tsuboi, H., Myoui, A., et al. (2007). Oxygen tension is an important mediator of the transformation of osteoblasts to osteocytes. J. Bone Miner. Metab. 25, 266–276. doi: 10.1007/s00774-007-0765-9
Holliday, L. S., Welgus, H. G., Fliszar, C. J., Veith, G. M., Jeffrey, J. J., and Gluck, S. L. (1997). Initiation of osteoclast bone resorption by interstitial collagenase. J. Biol. Chem. 272, 22053–22058. doi: 10.1074/jbc.272.35.22053
Holmbeck, K., Bianco, P., Caterina, J., Yamada, S., Kromer, M., Kuznetsov, S. A., et al. (1999). MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 99, 81–92. doi: 10.1016/s0092-8674(00)80064-1
Holmbeck, K., Bianco, P., Chrysovergis, K., Yamada, S., and Birkedal-Hansen, H. J. (2003). MT1-MMP-dependent, apoptotic remodeling of unmineralized cartilage: a critical process in skeletal growth. Cell Biol. 163, 661–671. doi: 10.1083/jcb.200307061
Holmbeck, K., Bianco, P., Pidoux, I., Inoue, S., Billinghurst, R. C., Wu, W., et al. (2005). The metalloproteinase MT1-MMP is required for normal development and maintenance of osteocyte processes in bone. J. Cell Sci. 118, 147–156. doi: 10.1242/jcs.01581
Holmbeck, K., and Szabov, L. (2006). Aspects of extracellular matrix remodeling in development and disease. Birth Defects Res. C Embryo. Today 78, 11–23. doi: 10.1002/bdrc.20064
Holt, I., Davie, M. W., and Marshall, M. J. (1996). Osteoclasts are not the main source of interleukin-6 in mouse parietal bone. Bone 18, 221–226. doi: 10.1016/8756-3282(95)00482-3
Hou, P., Troen, T., Ovejero, M. C., Kirkegaard, T., Andersen, T. L., Byrjalsen, I., et al. (2004). Matrix metalloproteinase-12 (MMP-12) in osteoclasts: new lesson on the involvement of MMPs in bone resorption. Bone 34, 37–47. doi: 10.1016/j.bone.2003.08.011
Huang, W., Li, W. Q., Dehnade, F., and Zafarullah, M. (2002). Tissue inhibitor of metalloproteinases-4 (TIMP-4) gene expression is increased in human osteoarthritic femoral head cartilage. J. Cell. Biochem. 85, 295–303. doi: 10.1002/jcb.10138
Huang, W., Yang, S., Shao, J., and Li, Y. P. (2007). Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front. Biosci. 12:3068–3092.
Hughes, D. E., Salter, D. M., and Simpson, R. (1994). CD44 expression in human bone: a novel marker of osteocytic differentiation. J. Bone Miner. Res. 9, 39–44. doi: 10.1002/jbmr.5650090106
Ikeda, S., Morishita, Y., Tsutsumi, H., Ito, M., Shiraishi, A., Arita, S., et al. (2003). Reductions in bone turnover, mineral, and structure associated with mechanical properties of lumbar vertebra and femur in glucocorticoid-treated growing minipigs. Bone 33, 779–787. doi: 10.1016/s8756-3282(03)00263-1
Inada, M., Wang, Y., Byrne, M. H., Miyaura, C., and Krane, S. M. (2001). Mice with null mutation in collagenase-3 (Matrix Metalloproteinase [MMP]-13) exhibit altered bone remodeling and increased bone mass. J. Bone Miner. Res. 16:S149.
Inada, M., Wang, Y., Byrne, M. H., Miyaura, C., and Krane, S. M. (2002). Loss of function of matrix metalloproteinase-13 (MMP-13) affects collagen accumulation and bone formation. J. Bone Miner. Res. 16:S171.
Inada, M., Wang, Y., Byrne, M. H., Rahman, M. U., Miyaura, C., Lopez-Otin, C., et al. (2004). Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc. Natl. Acad. Sci. U.S.A. 101, 17192–17197. doi: 10.1073/pnas.0407788101
Inoue, K., Mikuni-Takagaki, Y., Oikawa, K., Itoh, T., Inada, M., Noguchi, T., et al. (2006). A crucial role for matrix metalloproteinase 2 in osteocytic canalicular formation and bone metabolism. J. Biol. Chem. 281, 33814–33824. doi: 10.1074/jbc.m607290200
Ishida, N., Hayashi, K., Hoshijima, M., Ogawa, T., Koga, S., Miyatake, Y., et al. (2002). Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J. Biol. Chem. 277, 41147–41156. doi: 10.1074/jbc.m205063200
Ishikawa, T., Nishigaki, F., Miyata, S., Hirayama, Y., Minoura, K., Imanishi, J., et al. (2005). Prevention of progressive joint destruction in collagen-induced arthritis in rats by a novel matrix metalloproteinase inhibitor, FR255031. Br. J. Pharmacol. 144, 133–143. doi: 10.1038/sj.bjp.0706054
Ito, A., Mukaiyama, A., Itoh, Y., Nagase, H., Thogersen, I. B., Enghild, J. J., et al. (1996). Degradation of interleukin 1 beta by matrix metalloproteinases. J. Biol. Chem. 271, 14657–14660.
Iyer, R. P., Patterson, N. L., Fields, G. B., and Lindsey, M. L. (2012). The history of matrix metalloproteinases: milestones, myths, and misperceptions. Am. J. Physiol. Heart Circ. Physiol. 303, H919–H930. doi: 10.1152/ajpheart.00577.2012
Jackson, M. T., Moradi, B., Smith, M. M., Jackson, C. J., and Little, C. B. (2014). Activation of matrix metalloproteinases 2, 9, and 13 by activated protein C in human osteoarthritic cartilage chondrocytes. Arthritis Rheumatol. 66, 1525–1536. doi: 10.1002/art.38401
Javaheri, B., Hopkinson, M., Poulet, B., Pollard, A. S., Shefelbine, S. J., Chang, Y. M., et al. (2016). Deficiency and also transgenic overexpression of TIMP-3 both lead to compromised bone mass and architecture in vivo. PLoS One 11:e0159657. doi: 10.1371/journal.pone.0159657
Jiang, Y. B., Zhao, J., Genant, H. K., Dequeker, J., and Geusens, P. (1997). Long-term changes in bone mineral and biomechanical properties of vertebrae and femur in aging, dietary calcium restricted and/or estrogen-deprived/-replaced rats. J. Bone Miner. Res. 12, 820–831. doi: 10.1359/jbmr.1997.12.5.820
Jimenez, M. J., Balbin, M., Lopez, J. M., Alvarez, J., Komori, T., and Lopez-Otın, C. (1999). Collagenase 3 is a target of Cbfa1, a transcription factor of the runt gene family involved in bone formation. Mol. Cell. Biol. 19, 4431–4442. doi: 10.1128/mcb.19.6.4431
Jing, R., Liu, Y., Guo, P., Ni, T., Gao, X., Mei, R., et al. (2018). Evaluation of common variants in matrix metalloproteinase-9 gene with lumbar disc herniation in han chinese population. Genet. Test Mol. Biomarkers 22, 622–629. doi: 10.1089/gtmb.2018.0080
Johansson, N., Saarialho-Kere, U., Airola, K., Herva, R., Nissinen, L., Westermarck, J., et al. (1997). Collagenase-3 (MMP-13) is expressed by hypertrophic chondrocytes, periosteal cells, and osteoblasts during human fetal bone development. Dev. Dyn. 208, 387–397. doi: 10.1002/(sici)1097-0177(199703)208:3<387::aid-aja9>3.0.co;2-e
Kajita, M., Itoh, Y., Chiba, T., Mori, H., Okada, A., Kinoh, H., et al. (2001). Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J. Cell Biol. 153, 893–904. doi: 10.1083/jcb.153.5.893
Kamioka, H., Honjo, T., and Takano-Yamamoto, T. (2001). A three-dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopy. Bone 28, 145–149. doi: 10.1016/s8756-3282(00)00421-x
Kamiya, N., Ye, L., Kobayashi, T., Mochida, Y., Yamauchi, M., Kronenberg, H. M., et al. (2008). BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development 135, 3801–3811. doi: 10.1242/dev.025825
Kapur, S., Baylink, D. J., and Lau, K. H. (2003). Fluid flow shear stress stimulates human osteoblast proliferation and differentiation through multiple interacting and competing signal transduction pathways. Bone 32, 241–251. doi: 10.1016/s8756-3282(02)00979-1
Karsdal, M. A., Andersen, T. A., Bonewald, L., and Christiansen, C. (2004). Matrix metalloproteinases (MMPs) safeguard osteoblasts from apoptosis during transdifferentiation into osteocytes: MT1-MMP maintains osteocyte viability. DNA Cell Biol. 23, 155–165. doi: 10.1089/104454904322964751
Karsdal, M. A., Fjording, M. S., Foged, N. T., Delaisse, J. M., and Lochter, A. (2001). Transforming growth factor-β-induced osteoblast elongation regulates osteoclastic bone resorption through a p38 mitogen-activated protein kinase- and matrix metalloproteinase-dependent pathway. J. Biol. Chem. 276, 39350–39358. doi: 10.1074/jbc.m008738200
Karsdal, M. A., Henriksen, K., Leeming, D. J., Woodworth, T., Vassiliadis, E., and Bay-Jensen, A. C. (2010). Novel combinations of Post-Translational Modification (PTM) neo-epitopes provide tissue-specific biochemical markers–are they the cause or the consequence of the disease? Clin. Biochem. 43, 793–804. doi: 10.1016/j.clinbiochem.2010.03.015
Karsdal, M. A., Larsen, L., Engsig, M. T., Lou, H., Ferreras, M., Lochter, A., et al. (2002). Matrix metalloproteinase-dependent activation of latent transforming growth factor-β controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J. Biol. Chem. 277, 44061–44067. doi: 10.1074/jbc.m207205200
Karsdal, M. A., Woodworth, T., Henriksen, K., Maksymowych, W. P., Genant, H., Vergnaud, P., et al. (2011). Biochemical markers of ongoing joint damage in rheumatoid arthritis - current and future applications, limitations and opportunities. Arthritis Res. Ther. 13:215. doi: 10.1186/ar3280
Kasper, G., Glaeser, J. D., Geissler, S., Ode, A., Tuischer, J., Matziolis, G., et al. (2007). Matrix metalloprotease activity is an essential link between mechanical stimulus and mesenchymal stem cell behavior. Stem Cells 25, 1985–1994. doi: 10.1634/stemcells.2006-0676
Kawaguchi, H., Pilbeam, C. C., and Raisz, L. G. (1994). Anabolic effects of 3,3’,5-triiodothyronine and triiodothyroacetic acid in cultured neonatal mouse parietal bones. Endocrinology 135, 971–976. doi: 10.1210/endo.135.3.7520864
Kawaguchi, H., Pilbean, C. C., Harrison, J. R., and Raisz, L. G. (1995). The role of prostaglandins in the regulation of bone metabolism. Clin. Orthop. 313, 36–46.
Kennedy, A. M., Inada, M., Krane, S. M., Christie, P. T., Harding, B., Lopez-Otin, C., et al. (2005). MMP13 mutation causes spondyloepimetaphyseal dysplasia. Missouri type (SEMDMO). J. Clin. Invest. 115, 2832–2842. doi: 10.1172/jci22900
Kerschan-Schindl, K., and Ebenbichler, G. (2012). “Osteoimmunological aspects of biomechanics,” in Principles of Osteoimmunology, Molecular Mechanisms and Clinical Applications, ed. P. Pietschmann, (New York, NY: SpringerWienNewYork), 97–107.
Kim, H. J., Zhao, H., Kitaura, H., Bhattacharyya, S., Brewer, J. A., Muglia, L. J., et al. (2006). Glucocorticoids suppress bone formation via the osteoclast. J. Clin. Invest. 116, 2152–2160. doi: 10.1172/jci28084
Kim, J. W., Simmer, J. P., Hart, T. C., Hart, P. S., Ramaswami, M. D., Bartlett, J. D., et al. (2005). MP-20 mutation in autosomal recessive pigmented hypomaturation amelogenesis imperfecta. J. Med. Genet. 42, 271–275. doi: 10.1136/jmg.2004.024505
Kim, M. H., Park, M., Baek, S. H., Kim, H. J., and Kim, S. H. (2011). Molecules and signaling pathways involved in the expression of OC-STAMP during osteoclastogenesis. Amino Acids 40, 1447–1459. doi: 10.1007/s00726-010-0755-4
Kim, Y., Sato, K., Asagiri, M., Morita, I., Soma, K., and Takayanagi, H. (2005). Contribution of nuclear factor of activated T cells c1 to the transcriptional control of immunoreceptor osteoclast-associated receptor but not triggering receptor expressed by myeloid cells-2 during osteoclastogenesis. J. Biol. Chem. 280, 32905–32913. doi: 10.1074/jbc.m505820200
Kini, U., and Nandeesh, B. N. (2012). “Physiology of bone formation, remodeling, and metabolism,” in Radionuclide and Hybrid Bone Imaging, eds I. Fogelman, G. Gnanasegaran, and H. Van der Wall, (Berlin: Springer-Verlag), 29–57. doi: 10.1007/978-3-642-02400-9_2
Klein, T., and Bischoff, R. (2011). Physiology and pathophysiology of matrix metalloproteases. Amino Acids 41, 271–290. doi: 10.1007/s00726-010-0689-x
Knothe-Tate, M. L., Adamson, J. R., Tami, A. E., and Bauer, T. W. (2004). The osteocyte. Int. J. Biochem. Cell Biol. 36, 1–8.
Koga, T., Inui, M., Inoue, K., Kim, S., Suematsu, A., Kobayashi, E., et al. (2004). Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428, 758–763. doi: 10.1038/nature02444
Koga, T., Matsui, Y., Asagiri, M., Kodama, T., de Crombrugghe, B., Nakashima, K., et al. (2005). NFAT and Osterix cooperatively regulate bone formation. Nat. Med. 11, 880–885. doi: 10.1038/nm1270
Kojima, T., Hasegawa, T., de Freitas, P. H., Yamamoto, T., Sasaki, M., Horiuchi, K., et al. (2013). Histochemical aspects of the vascular invasion at the erosion zone of the epiphyseal cartilage in MMP-9-deficient mice. Biomed. Res. 34, 119–128. doi: 10.2220/biomedres.34.119
Koolwijk, P., Sidenius, N., Peters, E., Sier, C. F., Hanemaaijer, R., Blasi, F., et al. (2001). Proteolysis of the urokinase-type plasminogen activator receptor by metalloproteinase-12: implication for angiogenesis in fibrin matrices. Blood 97, 3123–3131. doi: 10.1182/blood.v97.10.3123
Kosaki, N., Takaishi, H., Kamekura, S., Kimura, T., Okada, Y., Minqi, L., et al. (2007). Impaired bone fracture healing in matrix metalloproteinase-13 deficient mice. Biochem. Biophys. Res. Commun. 354, 846–851. doi: 10.1016/j.bbrc.2006.12.234
Koskinen, A., Vuolteenaho, K., Nieminen, R., Moilanen, T., and Moilanen, E. (2011). Leptin enhances MMP-1, MMP-3 and MMP-13 production in human osteoarthritic cartilage and correlates with MMP-1 and MMP-3 in synovial fluid from OA patients. Clin. Exp. Rheumatol. 29, 57–64.
Kotake, S., Udagawa, N., Takahashi, N., Matsuzaki, K., Itoh, K., Ishiyama, S., et al. (1999). IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest. 103, 1345–1352. doi: 10.1172/jci5703
Krane, S. M., and Inada, M. (2008). Matrix metalloproteinases and bone. Bone 43, 7–18. doi: 10.1016/j.bone.2008.03.020
Krishnan, V., Bryant, H. U., and Macdougald, O. A. (2006). Regulation of bone mass by Wnt signaling. J. Clin. Invest. 116, 1202–1209. doi: 10.1172/jci28551
Kusano, K., Miyaura, C., Inada, M., Tamura, T., Ito, A., Nagase, H., et al. (1998). Regulation of matrix metalloproteinases (MMP-2, -3, -9, and -13) by interleukin-1 and interleukin-6 in mouse calvaria: association of MMP induction with bone resorption. Endocrinology 139, 1338–1345. doi: 10.1210/endo.139.3.5818
Lacey, D. L., Timms, E., Tan, H. L., Kelley, M. J., Dunstan, C. R., Burgess, T., et al. (1998). Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176. doi: 10.1016/s0092-8674(00)81569-x
Lafleur, M. A., Mercuri, F. A., Ruangpanit, N., Seiki, M., Sato, H., and Thompson, E. W. (2006). Type I collagen abrogates the clathrin-mediated internalization of membrane type 1 matrix metalloproteinase (MT1-MMP) via the MT1-MMP hemopexin domain. J. Biol. Chem. 281, 6826–6840. doi: 10.1074/jbc.m513084200
Lam, J., Takeshita, S., Barker, J. E., Kanagawa, O., Ross, F. P., and Teitelbaum, S. L. (2000). TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Invest. 106, 1481–1488. doi: 10.1172/jci11176
Lanske, B., Amling, M., Neff, L., Guiducci, J., Baron, R., and Kronenberg, H. M. (1999). Ablation of the PTHrP gene or the PTH/PTHrP receptor gene leads to distinct abnormalities in bone development. J. Clin. Invest. 104, 399–407. doi: 10.1172/jci6629
Lassen, N. E., Andersen, T. L., Pløen, G. G., Søe, K., Hauge, E. M., Harving, S., et al. (2017). Coupling of bone resorption and formation in real time: new knowledge gained from human haversian BMUs. J. Bone Miner. Res. 32, 1395–1405. doi: 10.1002/jbmr.3091
Lazarus, S., Tseng, H. W., Lawrence, F., Woodruff, M. A., Duncan, E. L., and Pettit, A. R. (2017). Characterization of normal murine carpal bone development prompts re-evaluation of pathologic osteolysis as the cause of human carpal-tarsal osteolysis disorders. Am. J. Pathol. 187, 1923–1934. doi: 10.1016/j.ajpath.2017.05.007
Lee, H., Overall, C. M., McCulloch, C. A., and Sodek, J. (2006). A critical role for the membrane-type 1 matrix metalloproteinase in collagen phagocytosis. Mol. Biol. Cell. 17, 4812–4826. doi: 10.1091/mbc.e06-06-0486
Li, G., Liu, D., Zhang, Y., Qian, Y., Zhang, H., Guo, S., et al. (2013). Celastrol inhibits lipopolysaccharide-stimulated rheumatoid fibroblast-like synoviocyte invasion through suppression of TLR4/NF-κB-mediated matrix metalloproteinase-9 expression. PLoS One 8:e008905. doi: 10.1371/journal.pone.0068905
Li, N. G., Shi, Z. H., Tang, Y. P., Wang, Z. J., Song, S. L., Qian, L. H., et al. (2011). New hope for the treatment of osteoarthritis through selective inhibition of MMP-13. Curr. Med. Chem. 18, 977–1001. doi: 10.2174/092986711794940905
Li, P. B., Tang, W. J., Wang, K., Zou, K., and Che, B. (2017). Expressions of IL-1α and MMP-9 in degenerated lumbar disc tissues and their clinical significance. Eur. Rev. Med. Pharmacol. Sci. 21, 4007–4013.
Li, Y. P., Chen, W., Liang, Y., Li, E., and Stashenko, P. (1999). Atp6i-deficient mice exhibit severe osteopetrosis due to loss of osteoclast-mediated extracellular acidification. Nat. Genet. 23, 447–451. doi: 10.1038/70563
Liang, H. P. H., Xu, J., Xue, M., and Jackson, C. J. (2016). Matrix metalloproteinases in bone development and pathology: current knowledge and potential clinical utility. Metalloproteinases Med. 3, 93–102. doi: 10.2147/mnm.s92187
Liao, E. Y., Liao, H. J., Guo, L. J., Zhou, H. D., Wu, X. P., Dai, R. C., et al. (2004). Membrane-type matrix metalloproteinase-1 (MT1-MMP) is down-regulated in estrogen-deficient rat osteoblast in vivo. J. Endocrinol. Invest. 27, 1–5. doi: 10.1007/bf03350902
Lieu, S., Hansen, E., Dedini, R., Behonick, D., Werb, Z., Miclau, T., et al. (2011). Impaired remodeling phase of fracture repair in the absence of matrix metalloproteinase-2. Dis. Model Mech. 4, 203–211. doi: 10.1242/dmm.006304
Lindsey, M. L., Zouein, F. A., Tian, Y., Iyer, R. P., and De Castro Bras, L. E. (2015). Osteopontin is proteolytically processed by matrix metalloproteinase 9. Can. J. Physiol. Pharmacol. 293, 879–886. doi: 10.1139/cjpp-2015-0019
Liu, J., and Khalil, R. A. (2017). Matrix metalloproteinase inhibitors as investigational and therapeutic tools in unrestrained tissue remodeling and pathological disorders. Prog. Mol. Biol. Transl. Sci. 148, 355–420. doi: 10.1016/bs.pmbts.2017.04.003
Liu, S., Tang, W., Fang, J., Ren, J., Li, H., Xiao, Z., et al. (2009). Novel regulators of Fgf23 expression and mineralization in Hyp bone. Mol. Endocrinol. 23, 1505–1518. doi: 10.1210/me.2009-0085
Liu, S., Zhou, J., Tang, W., Jiang, X., Rowe, D. W., and Quarles, D. L. (2006). Pathogenic role of Fgf23 in Hyp mice. Am. J. Physiol. Endocrinol. Metab. 291, E38–E49. doi: 10.1210/me.2009-0085
Liu, Y. H., Tang, Z., Kundu, R. K., Wu, L., Luo, W., Zhu, D., et al. (1999). Msx2 gene dosage influences the number of proliferative osteogenic cells in growth centers of the developing murine skull: a possible mechanism for MSX2-mediated craniosynostosis in humans. Dev. Biol. 205, 260–274. doi: 10.1006/dbio.1998.9114
Loffek, S., Schilling, O., and Franzke, C. W. (2011). Series “matrix metalloproteinases in lung health and disease”: biological role of matrix metalloproteinases: a critical balance. Eur. Respir. J. 38, 191–208. doi: 10.1183/09031936.00146510
Logan, C. Y., and Nusse, R. (2004). The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810.
Lopez-Otin, C., Palavalli, L. H., and Samuels, Y. (2009). Protective roles of matrix metalloproteinases: from mouse models to human cancer. Cell Cycle 8, 3657–3662. doi: 10.4161/cc.8.22.9956
Lovibond, A. C., Haque, S. J., Chambers, T. J., and Fox, S. W. (2003). TGF-beta-induced SOCS3 expression augments TNF-alpha-induced osteoclast formation. Biochem. Biophys. Res. Commun. 309, 762–767. doi: 10.1016/j.bbrc.2003.08.068
Lozito, T. P., Jackson, W. M., Nesti, L. J., and Tuan, R. S. (2014). Human mesenchymal stem cells generate a distinct pericellular zone of MMP activities via binding of MMPs and secretion of high levels of TIMPs. Matrix Biol. 34, 132–143. doi: 10.1016/j.matbio.2013.10.003
Lozito, T. P., and Tuan, R. S. (2011). Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J. Cell Physiol. 226, 385–396. doi: 10.1002/jcp.22344
Lu, C., Li, X. Y., Hu, Y., Rowe, R. G., and Weiss, S. J. (2010). MT1-MMP controls human mesenchymal stem cell trafficking and differentiation. Blood 115, 221–229. doi: 10.1182/blood-2009-06-228494
Lu, P., Takai, K., Weaver, V. M., and Werb, Z. (2011). Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 3:a005058. doi: 10.1101/cshperspect.a005058
Luan, Z., Chase, A. J., and Newby, A. C. (2003). Statins inhibit secretion of metalloproteinases-1, -2, -3, and -9 from vascular smooth muscle cells and macrophages. Arterioscler. Thromb. Vasc. Biol. 23, 769–775. doi: 10.1161/01.atv.0000068646.76823.ae
Luo, J., Yang, Z., Ma, Y., Yue, Z., Lin, H., Qu, G., et al. (2016). LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat. Med. 22, 539–546. doi: 10.1038/nm.4076
Lv, C., Yang, S., Chen, X., Zhu, X., Lin, W., Wang, L., et al. (2017). MicroRNA-21 promotes bone mesenchymal stem cells migration in vitro by activating PI3K/Akt/MMPs pathway. J. Clin. Neurosci. 46, 156–162. doi: 10.1016/j.jocn.2017.07.040
Lynch, C. C. (2011). Matrix metalloproteinases as master regulators of the vicious cycle of bone metastasis. Bone 48, 44–53. doi: 10.1016/j.bone.2010.06.007
Lynch, C. C., Hikosaka, A., Acuff, H. B., Martin, M. D., Kawai, N., Singh, R. K., et al. (2005). MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell 7, 485–496. doi: 10.1016/j.ccr.2005.04.013
MacDonald, B. R. (1986). Parathyroid hormone, prostaglandins and bone resorption. World Rev. Nutr. Diet 47, 163–201. doi: 10.1159/000412334
Madsen, D. H., Jürgensen, H. J., Ingvarsen, S., Melander, M. C., Albrechtsen, R., Hald, A., et al. (2013). Differential actions of the endocytic collagen receptor uPARAP/Endo180 and the collagenase MMP-2 in bone homeostasis. PLoS One 8:e71261. doi: 10.1371/journal.pone.0071261
Maeda, Y., Nakamura, E., Nguyen, M. T., Suva, L. J., Swain, F. L., Razzaque, M. S., et al. (2007). Indian Hedgehog produced by postnatal chondrocytes is essential for maintaining a growth plate and trabecular bone. Proc. Natl. Acad. Sci. U.S.A. 104, 6382–6387. doi: 10.1073/pnas.0608449104
Maffioli, P., and Derosa, G. (2015). “Overview of biochemical markers of bone metabolism,” in Bone Disease, Biomarkers in Disease: Methods, Discoveries and Applications, ed. V. R. Preedy, (Dordrecht: Springer Science+Business Media), 1–19. doi: 10.1007/978-94-007-7745-3_24-1
Mahl, C., Egea, V., Megens, R. T. A., Pitsch, T., Santovito, D., Weber, C., et al. (2016). RECK (reversion-inducing cysteine-rich protein with Kazal motifs) regulates migration, differentiation and Wnt/β-catenin signaling in human mesenchymal stem cells. Cell Mol. Life Sci. 73, 1489–1501. doi: 10.1007/s00018-015-2054-4
Malaponte, G., Hafsi, S., Polesel, J., Castellano, G., Spessotto, P., Guarneri, C., et al. (2016). Tumor microenvironment in diffuse large B-cell lymphoma: matrix metalloproteinases activation is mediated by osteopontin overexpression. Biochim. Biophys. Acta 1863, 483–489. doi: 10.1016/j.bbamcr.2015.09.018
Mancini, A., and di Battista, J. A. (2006). Transcriptional regulation of matrix metalloprotease gene expression in health and disease. Front. Biosci. 11:423–446.
Manduca, P., Castagnino, A., Lombardini, D., Marchisio, S., Soldano, S., Ulivi, V., et al. (2009). Role of MT1-MMP in the osteogenic differentiation. Bone 44, 251–265. doi: 10.1016/j.bone.2008.10.046
Mannello, F., Tonti, G., and Papa, S. (2005). Matrix metalloproteinase inhibitors as anticancer therapeutics. Curr. Cancer Drug Targets 5, 285–298. doi: 10.2174/1568009054064615
Manolagas, S. C. (2000). Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 21, 115–137. doi: 10.1210/edrv.21.2.0395
Marie, P. J. (2003). Fibroblast growth factor signaling controlling osteoblast differentiation. Gene 316, 23–32. doi: 10.1016/s0378-1119(03)00748-0
Martignetti, J. A., Aqeel, A. A., Sewairi, W. A., Boumah, C. E., Kambouris, M., Mayouf, S. A., et al. (2001). Mutation of the matrix metalloproteinase 2 gene (MMP2) causes a multicentric osteolysis and arthritis syndrome. Nat. Genet. 28, 261–265. doi: 10.1038/90100
Massova, I., Kotra, L. P., Fridman, R., and Mobashery, S. (1998). Matrix metalloproteinases: structures, evolution, and diversification. FASEB J. 12, 1075–1095. doi: 10.1096/fasebj.12.12.1075
Matsuguchi, T., Chiba, N., Bandow, K., Kakimoto, K., Masuda, A., and Ohnishi, T. (2009). JNK activity is essential for Atf4 expression and late-stage osteoblast differentiation. J. Bone Miner. Res. 24, 398–410. doi: 10.1359/jbmr.081107
Matsumoto, M., Kogawa, M., Wada, S., Takayanagi, H., Tsujimoto, M., Katayama, S., et al. (2004). Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J. Biol. Chem. 279, 45969–45979. doi: 10.1074/jbc.m408795200
Matsuo, K., Galson, D. L., Zhao, C., Peng, L., Laplace, C., Wang, K. Z., et al. (2004). Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J. Biol. Chem. 279, 26475–26480. doi: 10.1074/jbc.m313973200
Mattot, V., Raes, M. B., Henriet, P., Eeckhout, Y., Stehelin, D., Vandenbunder, B., et al. (1995). Expression of interstitial collagenase is restricted to skeletal tissue during mouse embryogenesis. J. Cell Sci. 108, 529–535.
Mattsson, J. P., Schlesinger, P. H., Keeling, D. J., Teitelbaum, S. L., Stone, D. K., and Xie, X. S. (1994). Isolation and reconstitution of a vacuolar-type proton pump of osteoclast membranes. J. Biol. Chem. 269, 24979–24982.
Mauramo, M., Ramseier, A. M., Mauramo, E., Buser, A., Tervahartiala, T., Sorsa, T., et al. (2018). Associations of oral fluid MMP-8 with periodontitis in Swiss adult subjects. Oral Dis. 24, 449–455. doi: 10.1111/odi.12769
McGowan, P. M., and Duffy, M. J. (2008). Matrix metalloproteinase expression and outcome in patients with breast cancer: analysis of a published database. Ann. Oncol. 19, 1566–1572. doi: 10.1093/annonc/mdn180
Meikle, M. C., Bord, S., Hembry, R. M., Compston, J., Croucher, P. I., and Reynolds, J. J. (1992). Human osteoblasts in culture synthesize collagenase and other matrix metalloproteinases in response to osteotropic hormones and cytokines. J. Cell Sci. 103, 1093–1099.
Meng, F., Yang, H., Aitha, M., George, S., Tierney, D. L., and Crowder, M. W. (2016). Biochemical and spectroscopic characterization of the catalytic domain of MMP16 (cdMMP16). J. Biol. Inorg. Chem. 21, 523–535. doi: 10.1007/s00775-016-1362-y
Messaritou, G., East, L., Roghi, C., Isacke, C. M., and Yarwood, H. (2009). Membrane type-1 matrix metalloproteinase activity is regulated by the endocytic collagen receptor Endo180. J. Cell Sci. 122, 4042–4048. doi: 10.1242/jcs.044305
Mikuni-Takagaki, Y. (1999). Mechanical responses and signal transduction pathways in stretched osteocytes. J. Bone Miner. Metab. 17, 57–60. doi: 10.1007/s007740050065
Miller, B., Spevak, L., Lukashova, L., Javaheri, B., Pitsillides, A. A., Boskey, A., et al. (2017). Altered bone mechanics, architecture and composition in the skeleton of TIMP-3-deficient mice. Calcif. Tissue Int. 100, 631–640. doi: 10.1007/s00223-017-0248-5
Mills, B. G., and Frausto, A. (1997). Cytokines expressed in multinucleated cells: Paget’s disease and giant cell tumors versus normal bone. Calcif. Tissue Int. 61, 16–21. doi: 10.1007/s002239900285
Mimura, H., Cao, X., Ross, F. P., Chiba, M., and Teitelbaum, S. L. (1994). 1,25-Dihydroxyvitamin D3 transcriptionally activates the beta 3-integrin subunit gene in avian osteoclast precursors. Endocrinology 134, 1061–1066. doi: 10.1210/endo.134.3.8119143
Mittal, R., Patel, A. P., Debs, L. H., Nguyen, D., Patel, K., Grati, M., et al. (2016). Intricate functions of matrix metalloproteinases in physiological and pathological conditions. J. Cell Physiol. 231, 2599–2621. doi: 10.1002/jcp.25430
Miyamoto, T. (2006). The dendritic cell-specific transmembrane protein DC-STAMP is essential for osteoclast fusion and osteoclast bone-resorbing activity. Mod. Rheumatol. 16, 341–342. doi: 10.3109/s10165-006-0524-0
Miyauchi, A., Alvarez, J., Greenfield, E. M., Teti, A., Grano, M., Colucci, S., et al. (1991). Recognition of osteopontin and related peptides by an alpha v beta 3 integrin stimulates immediate cell signals in osteoclasts. J. Biol. Chem. 266, 20369–20374.
Miyauchi, A., Gotoh, M., Kamioka, H., Notoya, K., Sekiya, H., Takagi, Y., et al. (2006). AlphaVbeta3 integrin ligands enhance volume-sensitive calcium influx in mechanically stretched osteocytes. J Bone Miner. Metab. 24, 498–504. doi: 10.1007/s00774-006-0716-x
Miyauchi, A., Notoya, K., Mikuni-Takagaki, Y., Takagi, Y., Goto, M., Miki, Y., et al. (2000). Parathyroid hormone-activated volume-sensitive calcium influx pathways in mechanically located osteocytes. J. Biol. Chem. 275, 3335–3342. doi: 10.1074/jbc.275.5.3335
Mocsai, A., Humphrey, M. B., Van Ziffle, J. A., Hu, Y., Burghardt, A., Spusta, S. C., et al. (2004). The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc. Natl. Acad. Sci. U.S.A. 101, 6158–6163. doi: 10.1073/pnas.0401602101
Mohanakrishnan, V., Balasubramanian, A., Mahalingam, G., Partridge, N. C., Ramachandran, I., and Selvamurugan, N. (2018). Parathyroid hormone-induced down-regulation of miR-532-5p for matrix metalloproteinase-13 expression in rat osteoblasts. J. Cell Biochem. 119, 6181–6193. doi: 10.1002/jcb.26827
Mosekilde, L., Danielsen, D. D., and Knudsen, U. B. (1993). The effect of aging and ovariectomy on the vertebral bone mass and biomechanical properties of mature rats. Bone 14, 1–6. doi: 10.1016/8756-3282(93)90248-9
Mosig, R. A., Dowling, O., DiFeo, A., Ramirez, M. C., Parker, I. C., Abe, E., et al. (2007). Loss of MMP-2 disrupts skeletal and craniofacial development and results in decreased bone mineralization, joint erosion and defects in osteoblast and osteoclast growth. Hum. Mol. Genet. 16, 1113–1123. doi: 10.1093/hmg/ddm060
Mott, J. D., Thomas, C. L., Rosenbach, M. T., Takahara, K., Greenspan, D. S., and Banda, M. J. (2000). Post-translational proteolytic processing of procollagen C-terminal proteinase enhancer releases a metalloproteinase inhibitor. J. Biol. Chem. 275, 1384–1390. doi: 10.1074/jbc.275.2.1384
Mundy, G. (1993). Cytokines and growth factors in the regulation of bone remodeling. J. Bone Miner. Res. 8, S505–S510.
Murray, E. J. B., Tram, K. K.-T., Spencer, M. J., Tidball, J. G., Murray, S. S., and Lee, D. B. N. (1995). PTH mediated osteoblast retraction: possible participation of the calpain pathway. Miner. Electrolyte Metab. 21, 184–188.
Nagase, H., Visse, R., and Murphy, G. (2006). Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 69, 562–573. doi: 10.1016/j.cardiores.2005.12.002
Nakashima, A., and Tamura, M. (2006). Regulation of matrix metalloproteinase-13 and tissue inhibitor of matrix metalloproteinase-1 gene expression by WNT3A and bone morphogenetic protein-2 in osteoblastic differentiation. Front. Biosci. 11:1667–1678.
Nannuru, K. C., Futakuchi, M., Varney, M. L., Vincent, T. M., Marcusson, E. G., and Singh, R. K. (2010). Matrix metalloproteinase (MMP)-13 regulates mammary tumor-induced osteolysis by activating MMP9 and transforming growth factor-beta signaling at the tumor-bone interface. Cancer Res. 70, 3494–3504. doi: 10.1158/0008-5472.CAN-09-3251
Nash, T. J., Howlett, C. R., Martin, C., Steele, J., Johnson, K. A., and Hicklin, D. J. (1994). Effects of platelet-derived growth factor on tibial osteotomies in rabbits. Bone 15, 203–208. doi: 10.1016/8756-3282(94)90709-9
Neuhold, L. A., Killar, L., Zhao, W., Sung, M. L., Warner, L., Kulik, J., et al. (2001). Postnatal expression in hyaline cartilage of constitutively active human collagenase-13 (MMP-13) induces osteoarthritis in mice. J. Clin. Investig. 107, 35–44. doi: 10.1172/jci10564
Nguyen, M., Arkell, J., and Jackson, C. J. (2000). Activated protein C directly activates human endothelial gelatinase A. J. Biol. Chem. 275, 9095–9098. doi: 10.1074/jbc.275.13.9095
Nyman, J. S., Lynch, C. C., Perrien, D. S., Thiolloy, S., O’Quinn, E. C., Patil, C. A., et al. (2011). Differential effects between the loss of MMP-2 and MMP-9 on structural and tissue-level properties of bone. J. Bone. Miner. Res. 26, 1252–1260. doi: 10.1002/jbmr.326
Oh, J., Takahashi, R., Adachi, E., Kondo, S., Kuratomi, S., Noma, A., et al. (2004). Mutations in two matrix metalloproteinase genes, MMP-2 and MT1-MMP, are synthetic lethal in mice. Oncogene 23, 5041–5048. doi: 10.1038/sj.onc.1207688
Oh, J., Takahashi, R., Kondo, S., Mizoguchi, A., Adachi, E., Sasahara, R. M., et al. (2001). The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell 107, 789–800. doi: 10.1016/s0092-8674(01)00597-9
Okada, M., Shinmei, Y., Tanaka, O., Naka, K., Kimura, A., Nakanishi, I., et al. (1992). Localization of matrix metalloproteinase 3 (stromelysin) in osteoarthritic cartilage and synovium. Lab. Invest. 66, 680–690.
Ortega, N., Behonick, D., Dominique, S., and Werb, Z. (2003). How proteases regulate bone morphogenesis. Ann. N. Y. Acad. Sci. 995, 109–116. doi: 10.1111/j.1749-6632.2003.tb03214.x
Ortega, N., Behonick, D. J., Colnot, C., Cooper, D. N., and Werb, Z. (2005). Galectin-3 is a downstream regulator of matrix metalloproteinase-9 function during endochondral bone formation. Mol. Biol. Cell 16, 3028–3039. doi: 10.1091/mbc.e04-12-1119
Ortega, N., Wang, K., Ferrara, N., Werb, Z., and Vu, T. H. (2010). Complementary interplay between matrix metalloproteinase-9, vascular endothelial growth factor and osteoclast function drives endochondral bone formation. Dis. Model Mech. 3, 224–235. doi: 10.1242/dmm.004226
Overall, C. M., Wrana, J. L., and Sodek, J. (1991). Transcriptional and posttranscriptional regulation of 72-kDa gelatinase type-IV collagenase by transforming growth factor-beta-1 in human fibroblasts: comparisons with collagenase and tissue inhibitor of matrix metalloproteinase gene-expression. J. Biol. Chem. 266, 14064–14071.
Ozdemir, D., Hart, P. S., Ryul, O. H., Choi, S. J., Ozdemir-Karatas, M., Firatli, E., et al. (2005). MMP20 active-site mutation in hypomaturation amelogenesis imperfecta. J. Dent. Res. 84, 1031–1035. doi: 10.1177/154405910508401112
Page-McCaw, A., Ewald, A. J., and Werb, Z. (2007). Matrix metalloproteinases and the regulation of tissue remodeling. Nat. Rev. Mol. Cell Biol. 8, 221–233.
Paiva, K. B. S., and Granjeiro, J. M. (2014). Bone tissue remodeling and development: focus on matrix metalloproteinase functions. Arch. Biochem. Biophys. 561, 74–87. doi: 10.1016/j.abb.2014.07.034
Paiva, K. B. S., and Granjeiro, J. M. (2017). Matrix metalloproteinases in bone resorption, remodeling, and repair. Prog. Mol. Biol. Transl. Sci. 148, 203–303. doi: 10.1016/bs.pmbts.2017.05.001
Pap, T., and Korb-Pap, A. (2015). Cartilage damage in osteoarthritis and rheumatoid arthritis-two unequal siblings. Nat. Rev. Rheumatol. 11, 606–615. doi: 10.1038/nrrheum.2015.95
Pap, T., Shigeyama, Y., Kuchen, S., Fernihough, J. K., Simmen, B., Gay, R. E., et al. (2000). Differential expression pattern of membrane-type matrix metalloproteinases in rheumatoid arthritis. Arthritis Rheum. 43, 1226–1232. doi: 10.1002/1529-0131(200006)43:6<1226::aid-anr5>3.0.co;2-4
Papathanasiou, I., Malizos, K. N., and Tsezou, A. (2012). Bone morphogenetic protein-2-induced Wnt/beta-catenin signaling pathway activation through enhanced low-density-lipoprotein receptor-related protein 5 catabolic activity contributes to hypertrophy in osteoarthritic chondrocytes. Arthritis Res. Ther. 14:R82. doi: 10.1186/ar3805
Parra-Torres, A. Y., Valdés-Flores, M., Orozco, L., and Velázquez-Cruz, R. (2013). “Molecular aspects of bone remodeling,” in Topics in Osteoporosis, ed. M. V. Flores, (London: IntechOpen).
Pego, E. R., Fernandez, I., and Nuñez, M. J. (2018). Molecular basis of the effect of MMP-9 on the prostate bone metastasis: a review. Urol. Oncol. 36, 272–282. doi: 10.1016/j.urolonc.2018.03.009
Pei, D., Kang, T., and Qi, H. (2000). Cysteine array matrix metalloproteinase (CA-MMP)/MMP-23 is a type II transmembrane matrix metalloproteinase regulated by a single cleavage for both secretion and activation. J. Biol. Chem. 275, 33988–33997. doi: 10.1074/jbc.m006493200
Pei, D., and Weiss, S. J. (1995). Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature 375, 244–247. doi: 10.1038/375244a0
Pettit, A. R., Chang, M. K., Hume, D. A., and Raggatt, L. G. (2008). Osteal macrophages: a new twist on coupling during bone dynamics. Bone 43, 976–982. doi: 10.1016/j.bone.2008.08.128
Piccard, H., Van den Steen, P. E., and Opdenakker, G. (2007). Hemopexin domains as multifunctional liganding modules in matrix metalloproteinases and other proteins. J. Leukoc. Biol. 81, 870–892. doi: 10.1189/jlb.1006629
Pirapaharan, D. C., Olesen, J. B., Andersen, T. L., Christensen, S. B., Kjærsgaard-Andersen, P., Delaisse, J. M., et al. (2019). Catabolic activity of osteoblast lineage cells contributes to osteoclastic bone resorption in vitro. J. Cell Sci. 15, 132. doi: 10.1242/jcs.229351
Pivetta, E., Scapolan, M., Pecolo, M., Wassermann, B., Abu-Rumeileh, I., Balestreri, L., et al. (2011). MMP-13 stimulates osteoclast differentiation and activation in tumour breast bone metastases. Breast Cancer Res. 13:R105. doi: 10.1186/bcr3047
Plotkin, L. I., and Bruzzaniti, A. (2019). Molecular signaling in bone cells: regulation of cell differentiation and survival. Adv. Protein Chem. Struc. Biol. 116, 237–281. doi: 10.1016/bs.apcsb.2019.01.002
Plotkin, L. I., Lezcano, V., Thostenson, J., Weinstein, R. S., Manolagas, S. C., and Bellido, T. (2008). Connexin 43 is required for the anti-apoptotic effect of bisphosphonates on osteocytes and osteoblasts in vivo. J. Bone Miner. Res. 23, 1712–1721. doi: 10.1359/jbmr.080617
Plotkin, L. I., Manolagas, S. C., and Bellido, T. (2002). Transduction of cell survival signals by connexin-43 hemichannels. J. Biol. Chem. 277, 8648–8657. doi: 10.1074/jbc.m108625200
Plotkin, L. I., Mathov, I., Aguirre, J. I., Parfitt, A. M., Mangolas, S. C., and Bellido, T. (2005). Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am. J. Physiol. Cell Physiol. 289, C633–C643.
Poole, K. E., van Bezooijen, R. L., Loveridge, N., Hamersma, H., Papapoulos, S. E., Lowik, C. W., et al. (2005). Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 19, 1842–1844. doi: 10.1096/fj.05-4221fje
Porter, S., Clark, I. M., Kevorkian, L., and Edward, D. R. (2005). The ADAMTS metalloproteinases. Biochem. J. 386, 15–27. doi: 10.1042/bj20040424
Puliti, M., Momi, S., Falcinelli, E., Gresele, P., Bistoni, F., and Tissi, L. (2012). Contribution of matrix metalloproteinase 2 to joint destruction in group B Streptococcus-induced murine arthritis. Arthritis Rheum. 64, 1089–1097. doi: 10.1002/art.33450
Quinn, J. M. W., Neale, S., Fujikawa, Y., McGee, J. D., and Athanasou, N. A. (1998). Human osteoclast formation from blood monocytes, peritoneal macrophages, and bone marrow cells. Calcif. Tissue Int. 62, 527–531. doi: 10.1007/s002239900473
Qvist, P., Christiansen, C., Karsdal, M. A., Madsen, S. H., Sondergaard, B. C., and Bay-Jensen, A. C. (2010). Application of biochemical markers in development of drugs for treatment of osteoarthritis. Biomarkers 15, 1–19. doi: 10.3109/13547500903295873
Ragab, A. A., Nalepka, J. L., Bi, Y., and Greenfield, E. M. (2002). Cytokines synergistically induce osteoclast differentiation: support by immortalized or normal calvarial cells. Am. J. Physiol. 283, C679–C687.
Raisz, L. G. (1993). Bone cell biology: new approaches and unanswered questions. J. Bone Miner. Res. 8, 457–465.
Rajaram, S., Murawala, H., Buch, P., Patel, S., and Balakrishnan, S. (2016). Inhibition of BMP signaling reduces MMP-2 and MMP-9 expression and obstructs wound healing in regenerating fin of teleost fish Poecilia latipinna. Fish Physiol. Biochem. 42, 787–794. doi: 10.1007/s10695-015-0175-1
Rauner, M., Stein, N., and Hofbauer, L. C. (2012). “Basics of bone biology,” in Principles of Osteoimmunology, Molecular Mechanisms and Clinical Applications, ed. P. Pietschmann, (New York, NY: SpringerWienNewYork), 1–26. doi: 10.1007/978-3-7091-0520-7_1
Rawlinson, S. C., Pitsillides, A. A., and Lanyon, L. E. (1996). Involvement of different ion channels in osteoblasts’and osteocytes’ early responses to mechanical strain. Bone 19, 609–614. doi: 10.1016/s8756-3282(96)00260-8
Raynal, C. P., Delmas, D., and Chenu, C. (1996). Bone sialoprotein stimulates in vitro bone resorption. Endocrinology 137, 2347–2354. doi: 10.1210/endo.137.6.8641185
Roach, H. I. (1994). Why does bone matrix contain non-collagenous proteins? The possible roles of osteocalcin, osteonectin, osteopontin and bone sialoprotein in bone mineralisation and resorption. Cell Biol. Int. 18, 617–628. doi: 10.1006/cbir.1994.1088
Roodman, G. D. (1999). Cell biology of the osteoclast. Exp. Hematol. 27, 1229–1241. doi: 10.1016/s0301-472x(99)00061-2
Roodman, G. D., Kurihara, N., Ohsaki, Y., Kukita, A., Hosking, D., Demulder, A., et al. (1992). Interleukin-6: a potential autocrine/paracrine agent in Paget’s disease of bone. J. Clin. Invest. 89, 46–52. doi: 10.1172/jci115584
Rose, B. J., and Kooyman, D. L. (2016). A tale of two joints: the role of matrix metalloproteases in cartilage biology. Dis. Markers. 2016, 4895050. doi: 10.1155/2016/4895050
Rosen, C. J., and Donahue, L. R. (1998). Insulin-like growth factors and bone-the osteoporosis connection revisited. Proc. Soc. Exp. Biol. Med. 219, 1–7. doi: 10.3181/00379727-219-44310
Rouzier, C., Vanatka, R., Bannwarth, S., Philip, N., Coussement, A., Paquis-Flucklinger, V., et al. (2006). A novel homozygous MMP2 mutation in a family with Winchester syndrome. Clin. Genet. 69, 271–276. doi: 10.1111/j.1399-0004.2006.00584.x
Rowe, P. S. N., Kumagai, Y., Gutierrez, G., Garrett, I. R., Blacher, R., Rosen, D., et al. (2004). MEPE has the properties of an osteoblastic phosphatonin and minhibin. Bone 34, 303–319. doi: 10.1016/j.bone.2003.10.005
Rowe, R. G., and Weiss, S. (2009). Navigating ECM barriers at the invasive front: the cancer cell-stroma interface. Annu. Rev. Cell Dev. Biol. 25, 567–595. doi: 10.1146/annurev.cellbio.24.110707.175315
Ruan, M., Pederson, L., Bradley, E. W., Bamberger, A. M., and Oursler, M. J. (2010). Transforming growth factor-{beta} coordinately induces suppressor of cytokine signaling 3 and leukemia inhibitory factor to suppress osteoclast apoptosis. Endocrinology 151, 1713–1722. doi: 10.1210/en.2009-0813
Rubin, J., Rubin, C., and Jacobs, C. R. (2006). Molecular pathways mediating mechanical signalling in bone. Gene 367, 1–16. doi: 10.1016/j.gene.2005.10.028
Ruchon, A. F., Tenenhouse, H. S., Marcinkiewicz, M., Siegfried, G., Aubin, J. E., DesGroseillers, L., et al. (2000). Developmental expression and tissue distribution of Phex protein: effect of the Hyp mutation and relationship to bone markers. J. Bone Miner. Res. 15, 1440–1450. doi: 10.1359/jbmr.2000.15.8.1440
Rutter, J. L., Mitchell, T. I., Buttice, G., Meyers, J., Gusella, J. F., Ozelius, L. J., et al. (1998). A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter creates an Ets binding site and augments transcription. Cancer Res. 58, 5321–5325.
Sabbota, A. L., Kim, H. R., Zhe, X., Fridman, R., Bonfil, R. D., and Cher, M. L. (2010). Shedding of RANKL by tumor-associated MT1-MMP activates Src-dependent prostate cancer cell migration. Cancer Res 70, 5558–5566. doi: 10.1158/0008-5472.CAN-09-4416
Sabeh, F., Fox, D., and Weiss, S. J. (2010). Membrane-type I matrix metalloproteinase-dependent regulation of rheumatoid arthritis synoviocyte function. J. Immunol. 184, 6396–6406. doi: 10.4049/jimmunol.0904068
Saftig, P., Hunziker, E., Wehmeyer, O., Jones, S., Boyde, A., Rommerskirch, W., et al. (1998). Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 95, 13453–13458. doi: 10.1073/pnas.95.23.13453
Sage, E. H., Reed, M., Funk, S. E., Truong, T., Steadele, M., Puolakkainen, P., et al. (2003). Cleavage of the matricellular protein SPARC by matrix metalloproteinase 3 produces polypeptides that influence angiogenesis. J. Biol. Chem. 278, 37849–37857. doi: 10.1074/jbc.m302946200
Sahebjam, S., Khokha, R., and Mort, J. S. (2007). Increased collagen and aggrecan degradation with age in the joints of Timp3(-/-) mice. Arthritis Rheum. 56, 905–909. doi: 10.1002/art.22427
Sarker, H., Hardy, E., Haimour, A., Maksymowych, W. P., Botto, L. D., and Fernandez-Patron, C. (2019). Identification of fibrinogen as a natural inhibitor of MMP-2. Sci. Rep. 9:4340. doi: 10.1038/s41598-019-40983-y
Sasaki, T., Göhring, W., Mann, K., Maurer, P., Hohenester, E., Knäuper, V., et al. (1997). Limited cleavage of extracellular matrix protein BM-40 by matrix metalloproteinases increases its affinity for collagens. J. Biol. Chem. 272, 9237–9243. doi: 10.1074/jbc.272.14.9237
Satokata, I., Ma, L., Ohshima, H., Bei, M., Woo, I., Nishizawa, K., et al. (2000). Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat. Genet. 24, 391–395. doi: 10.1038/74231
Sawicki, G. (2013). Intracellular regulation of matrix metalloproteinase-2 activity: new strategies in treatment and protection of heart subjected to oxidative stress. Scientifica 2013:130451. doi: 10.1155/2013/130451
Schneider, G. B., Key, L. L., and Popoff, S. N. (1998). Osteopetrosis. Therapeutic strategies. Endocrinologist 8, 409–417. doi: 10.1097/00019616-199811000-00004
Semenov, M., Tamai, K., and He, X. (2005). SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J. Biol. Chem. 280, 26770–26775. doi: 10.1074/jbc.m504308200
Shah, M., Huang, D., Blick, T., Connor, A., Reiter, L. A., Hardink, J. R., et al. (2012). An MMP13-selective inhibitor delays primary tumor growth and the onset of tumor-associated osteolytic lesions in experimental models of breast cancer. PLoS One 7:e29615. doi: 10.1371/journal.pone.0029615
Shahnazari, M., Martin, B. R., Legette, L. L., Lachcik, P. J., Welch, J., and Weaver, C. M. (2009). Diet calcium levelbut not calcium supplement particle size affects bone density and mechanical properties in ovariectomized rats. J. Nutr. 139, 1308–1314. doi: 10.3945/jn.108.101071
Shen, Y., Winkler, I. G., Barbier, V., Sims, N. A., Hendy, J., and Levesque, J. P. (2010). Tissue inhibitor of metalloproteinase-3 (TIMP-3) regulates hematopoiesis and bone formation in vivo. PLoS One 5:e13086. doi: 10.1371/journal.pone.0013086
Sher, L. B., Woitge, H. W., Adams, D. J., Gronowicz, G. A., Krozowski, Z., Harrison, J. R., et al. (2004). Transgenic expression of 11beta-hydroxysteroid dehydrogenase type 2 in osteoblasts reveals an anabolic role for endogenous glucocorticoids in bone. Endocrinology 145, 922–929. doi: 10.1210/en.2003-0655
Shi, J., Son, M. Y., Yamada, S., Szabova, L., Kahan, S., Chrysovergis, K., et al. (2008). Membrane-type MMPs enable extracellular matrix permissiveness and mesenchymal cell proliferation during embryogenesis. Dev. Biol. 313, 196–209. doi: 10.1016/j.ydbio.2007.10.017
Shore, E. M., Xu, M., Feldman, G. J., Fenstermacher, D. A., Cho, T. J., Choi, I. H., et al. (2006). A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat. Genet. 38, 525–527. doi: 10.1038/ng1783
Silver, I. A., Murrills, R. J., and Etherington, D. J. (1988). Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp. Cell. Res. 175, 266–276. doi: 10.1016/0014-4827(88)90191-7
Sims, N. A., and Martin, T. J. (2014). Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep. 3:481. doi: 10.1038/bonekey.2013.215
Singh, A., Rajasekaran, N., Hartenstein, B., Szabowski, S., Gajda, M., Angel, P., et al. (2013). Collagenase-3 (MMP-13) deficiency protects C57BL/6 mice from antibody-induced arthritis. Arthritis Res. Ther. 15:R222. doi: 10.1186/ar4423
Sobue, T., Hakeda, Y., Kobayashi, Y., Hayakawa, H., Yamashita, K., Aoki, T., et al. (2001). Tissue inhibitor of metalloproteinases 1 and 2 directly stimulate the bone-resorbing activity of isolated mature osteoclasts. J. Bone Miner. Res. 16, 2205–2214. doi: 10.1359/jbmr.2001.16.12.2205
Song, I. W., Li, W. R., Chen, L. Y., Shen, L. F., Liu, K. M., Yen, J. J., et al. (2014). Palmitoyl acyltransferase, Zdhhc13, facilitates bone mass acquisition by regulating postnatal epiphyseal development and endochondral ossification: a mouse model. PLoS One 9:e92194. doi: 10.1371/journal.pone.0092194
Sottrup-Jensen, L. (1989). Alpha-macroglobulins: structure, shape, and mechanism of proteinase complex formation. J. Biol. Chem. 264, 11539–11542.
Springman, E. B., Angleton, E. L., Birkedal-Hansen, H., and Van Wart, H. E. (1990). Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active-site zinc complex in latency and a “cysteine switch” mechanism for activation. Proc. Natl. Acad. Sci. U.S.A. 87, 364–368. doi: 10.1073/pnas.87.1.364
Stahle-Backdahl, M., Sandstedt, B., Bruce, K., Lindahl, A., Jimenez, M. G., Vega, J. A., et al. (1997). Collagenase-3 (MMP-13) is expressed during human fetal ossification and re-expressed in postnatal bone remodeling and in rheumatoid arthritis. Lab. Invest. 76, 717–728.
Sternlicht, M. D., and Werb, Z. (2001). How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell. Dev. Biol. 17, 463–516. doi: 10.1146/annurev.cellbio.17.1.463
Stickens, D., Behonick, D. J., Ortega, N., Heyer, B., Hartenstein, B., Yu, Y., et al. (2004). Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development 131, 5883–5895. doi: 10.1242/dev.01461
Sugimoto, K., Nakamura, T., Tokunaga, T., Uehara, Y., Okada, T., Taniwaki, T., et al. (2018). Matrix metalloproteinase promotes elastic fiber degradation in ligamentum flavum degeneration. PLoS One 13:e0200872. doi: 10.1371/journal.pone.0200872
Suh, W. K., Wang, S. X., Jheon, A. H., Moreno, L., Yoshinaga, S. K., Ganss, B., et al. (2004). The immune regulatory protein B7-H3 promotes osteoblast differentiation and bone mineralization. Proc. Natl. Acad. Sci. U.S.A. 101, 12969–12973. doi: 10.1073/pnas.0405259101
Sulkala, M., Tervahartiala, T., Sorsa, T., Larmas, M., Salo, T., and Tjaderhane, L. (2007). Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch. Oral Biol. 52, 121–127. doi: 10.1016/j.archoralbio.2006.08.009
Sun, B., Sun, J., Han, X., Liu, H., Li, J., Du, J., et al. (2016). Immunolocalization of MMP 2, 9 and 13 in prednisolone induced osteoporosis in mice. Histol. Histopathol. 31, 647–656. doi: 10.14670/HH-11-702
Sundaram, K., Nishimura, R., Senn, J., Youssef, R. F., London, S. D., and Reddy, S. V. (2007). RANK ligand signaling modulates the matrix metalloproteinase-9 gene expression during osteoclast differentiation. Exp. Cell Res. 313, 168–178. doi: 10.1016/j.yexcr.2006.10.001
Takayanagi, H., Kim, S., Koga, T., Nishina, H., Isshiki, M., Yoshida, H., et al. (2002). Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 3, 889–901. doi: 10.1016/s1534-5807(02)00369-6
Takemura, Y., Moriyama, Y., Ayukawa, Y., Kurata, K., Rakhmatia, Y. D., and Koyano, K. (2019). Mechanical loading induced osteocyte apoptosis and connexin 43 expression in three-dimensional cell culture and dental implant model. J. Biomed. Mater. Res. A 107, 815–827. doi: 10.1002/jbm.a.36597
Tamamura, Y., Otani, T., Kanatani, N., Koyama, E., Kitagaki, J., Komori, T., et al. (2005). Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J. Biol. Chem. 280, 19185–19195. doi: 10.1074/jbc.m414275200
Tanaka, S., Takahashi, N., Udagawa, N., Tamura, T., Akatsu, T., Stanley, E. R., et al. (1993). Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J. Clin. Invest. 91, 257–263. doi: 10.1172/jci116179
Tanaka-Kamioka, K., Kamioka, H., Ris, H., and Lim, S. S. (1998). Osteocyte shape is dependent on actin filaments and osteocyte processes are unique actin-rich projections. J. Bone Miner. Res. 13, 1555–1568. doi: 10.1359/jbmr.1998.13.10.1555
Tang, S. Y., Herber, R. P., Ho, S. P., and Alliston, T. (2012). Matrix metalloproteinase-13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance. J. Bone Miner. Res. 27, 1936–1950. doi: 10.1002/jbmr.1646
Tang, Y., Rowe, R. G., Botvinick, E. L., Kurup, A., Putnam, A. J., Seiki, M., et al. (2013). MT1-MMP-dependent control of skeletal stem cell commitment via a β1-integrin/YAP/TAZ signaling axis. Dev. Cell 25, 402–416. doi: 10.1016/j.devcel.2013.04.011
Tang, Y., Wu, X., Lei, W., Pang, L., Wan, C., Shi, Z., et al. (2009). TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 15, 757–765. doi: 10.1038/nm.1979
Tauro, M., and Lynch, C. C. (2018). Cutting to the Chase: How matrix metalloproteinase- 2 activity controls breast-cancer-to-bone metastasis. Cancers 10:E185. doi: 10.3390/cancers10060185
Teitelbaum, S. L. (2000). Bone resorption by osteoclasts. Science 289, 1504–1508. doi: 10.1126/science.289.5484.1504
Teitelbaum, S. L. (2007). Osteoclasts: what do they do and how do they do it? Am. J. Pathol. 170, 427–435. doi: 10.2353/ajpath.2007.060834
Teitelbaum, S. L., Abu-Amer, Y., and Ross, F. P. (1995). Molecular mechanisms of bone resorption. J. Cell Biochem. 59, 1–10.
Teitelbaum, S. L., and Ross, F. P. (2003). Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4, 638–649. doi: 10.1038/nrg1122
Tester, A. M., Waltham, M., Oh, S. J., Bae, S. N., Bills, M. M., Walker, E. C., et al. (2004). Pro-matrix metalloproteinase-2 transfection increases orthotopic primary growth and experimental metastasis of MDA-MB-231 human breast cancer cells in nude mice. Cancer Res. 64, 652–658. doi: 10.1158/0008-5472.can-0384-2
Teti, A., Blair, H. C., Schlesinger, P., Grano, M., Zambonin-Zallone, A., Kahn, A. J., et al. (1989). Extracellular protons acidify osteoclasts, reduce cytosolic calcium, and promote expression of cell-matrix attachment structures. J. Clin. Invest. 84, 773–780. doi: 10.1172/jci114235
Thiolloy, S., Halpern, J., Holt, G. E., Schwartz, H. S., Mundy, G. R., Matrisian, L. M., et al. (2009). Osteoclast-derived matrix metalloproteinase-7, but not matrix metalloproteinase-9, contributes to tumor-induced osteolysis. Cancer Res. 69, 6747–6755. doi: 10.1158/0008-5472.CAN-08-3949
Thompson, P. D., Clarkson, P., and Karas, R. H. (2003). Statin-associated myopathy. JAMA 289, 1681–1690.
Tian, E., Zhan, F., Walker, R., Rasmussen, E., Ma, Y., Barlogie, B., et al. (2003). The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 349, 2483–2494. doi: 10.1056/nejmoa030847
Tokito, A., and Jougasaki, M. (2016). Matrix metalloproteinases in non-neoplastic disorders. Int. J. Mol. Sci. 17:1178. doi: 10.3390/ijms17071178
Tondravi, M. M., McKercher, S. R., Anderson, K., Erdmann, J. M., Quiroz, M., Maki, R., et al. (1997). Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. Nature 386, 81–84. doi: 10.1038/386081a0
Toyosawa, S., Oya, K., Sato, S., and Ishida, K. (2012). Osteocyte and DMP1. Clin. Calcium 22, 713–720.
Tozum, T. F., Oppenlander, M. E., Koh-Paige, A. J., Robins, D. M., and McCauley, L. K. (2004). Effects of sex steroid receptor specificity in the regulation of skeletal metabolism. Calcif. Tissue Int. 75, 60–70. doi: 10.1007/s00223-004-0119-8
Troeberg, L., and Nagase, H. (2012). Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim. Biophys. Acta 1824, 133–145. doi: 10.1016/j.bbapap.2011.06.020
Tuysuz, B., Mosig, R., Altun, G., Sancak, S., Glucksman, M. J., and Martignetti, J. A. (2009). A novel matrix metalloproteinase 2 (MMP2) terminal hemopexin domain mutation in a family with multicentric osteolysis with nodulosis and arthritis with cardiac defects. Eur. J. Hum. Genet. 17, 565–572. doi: 10.1038/ejhg.2008.204
Udagawa, N., Takahashi, N., Jimi, E., Matsuzaki, K., Tsurukai, T., Itoh, K., et al. (1999). Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: receptor activator of NF-kappa B ligand. Bone 25, 517–523. doi: 10.1016/s8756-3282(99)00210-0
Vaananen, H. K., and Horton, M. (1995). The osteoclast clear zone is a specialized cell-extracellular matrix adhesion structure. J. Cell Sci. 108, 2729–2732.
Van Wart, H. E., and Birkedal-Hansen, H. (1990). The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. U.S.A. 87, 5578–5582. doi: 10.1073/pnas.87.14.5578
Vandenbroucke, R. E., and Libert, C. (2014). Is there new hope for therapeutic matrix metalloproteinase inhibition? Nature Rev. Drug Discov. 13, 904–927. doi: 10.1038/nrd4390
Vandooren, J., Van Damme, J., and Opdenakker, G. (2014). On the structure and functions of gelatinase B/matrix metalloproteinase-9 in neuroinflammation. Prog. Brain Res. 214, 193–206. doi: 10.1016/B978-0-444-63486-3.00009-8
Veidal, S. S., Larsen, D. V., Chen, X., Sun, S., Zheng, Q., Bay-Jensen, A. C., et al. (2012). MMP mediated type V collagen degradation (C5M) is elevated in ankylosing spondylitis. Clin. Biochem. 45, 541–546. doi: 10.1016/j.clinbiochem.2012.02.007
Velasco, G., Pendas, A. M., Fueyo, A., Knäuper, V., Murphy, G., and Lopez-Otın, C. (1999). Cloning and characterization of human MMP-23, a new matrix metalloproteinase predominantly expressed in reproductive tissues and lacking conserved domains in other family members. J. Biol. Chem. 274, 4570–4576. doi: 10.1074/jbc.274.8.4570
Visse, R., and Nagase, H. (2003). Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 92, 827–839. doi: 10.1161/01.res.0000070112.80711.3d
Vivinus-Nebot, M., Rousselle, P., Breittmayer, J. P., Cenciarini, C., Berrih-Aknin, S., Spong, S., et al. (2004). Mature human thymocytes migrate on laminin-5 with activation of metalloproteinase-14 and cleavage of CD44. J. Immunol. 172, 1397–1406. doi: 10.4049/jimmunol.172.3.1397
Vu, T. H., Shipley, J. M., Bergers, G., Berger, J. E., Helms, J. A., Hanahan, D., et al. (1998). MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypetrophic chondrocytes. Cell 93, 411–422. doi: 10.1016/s0092-8674(00)81169-1
Wada, T., Nakashima, T., Oliveira-dos-Santos, A. J., Gasser, J., Hara, H., Schett, G., et al. (2005). The molecular scaffold Gab2 is a crucial component of RANK signaling and osteoclastogenesis. Nat Med. 11, 394–399. doi: 10.1038/nm1203
Wagenaar-Miller, R. A., Engelholm, L. H., Gavard, J., Yamada, S. S., Gutkind, J. S., Behrendt, N., et al. (2007). Complementary roles of intracellular and pericellular collagen degradation pathways in vivo. Mol. Cell. Biol. 27, 6309–6322. doi: 10.1128/mcb.00291-07
Westbroek, I., De Rooij, K. E., and Nijweide, P. J. (2002). Osteocyte-specific monoclonal antibody MAb OB7.3 is directed against Phex protein. J. Bone Miner. Res. 17, 845–853. doi: 10.1359/jbmr.2002.17.5.845
Wiebe, S. H., Hafezi, M., Sandhu, H. S., Sims, S. M., and Dixon, S. J. (1996). Osteoclast activation in inflammatory periodontal diseases. Oral Dis. 2, 167–180. doi: 10.1111/j.1601-0825.1996.tb00218.x
Wu, H., Du, J., and Zheng, Q. (2008). Expression of MMP-1 in cartilage and synovium of experimentally induced rabbit ACLT traumatic osteoarthritis: immunohistochemical study. Rheumatol. Int. 29, 31–36. doi: 10.1007/s00296-008-0636-2
Wutzl, A., Rauner, M., Seemann, R., Millesi, W., Krepler, P., Pietschmann, P., et al. (2010). Bone morphogenetic proteins 2, 5, and 6 in combination stimulate osteoblasts but not osteoclasts in vitro. J. Orthop. Res. 28, 1431–1439. doi: 10.1002/jor.21144
Xian, L., Wu, X., Pang, L., Lou, M., Rosen, C. J., Qiu, T., et al. (2012). Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat. Med. 18, 1095–1101. doi: 10.1038/nm.2793
Xue, M., March, L., Sambrook, P. N., and Jackson, C. J. (2007). Differential regulation of matrix metalloproteinase 2 and matrix metalloproteinase 9 by activated protein C: relevance to inflammation in rheumatoid arthritis. Arthritis Rheum. 56, 2864–2874. doi: 10.1002/art.22844
Xue, M., McKelvey, K., Shen, K., Minhas, N., March, L., Park, S. Y., et al. (2014). Endogenous MMP-9 and not MMP-2 promotes rheumatoid synovial fibroblast survival, inflammation and cartilage degradation. Rheumatology 53, 2270–2279. doi: 10.1093/rheumatology/keu254
Yamada, Y., Ando, F., Niino, N., and Shimokata, H. (2004). Association of a polymorphism of the matrix metalloproteinase-9 gene with bone mineral density in japanese men. Metabolism 53, 135–137. doi: 10.1016/j.metabol.2003.09.003
Yamagiwa, H., Tokunaga, K., Hayami, T., Hatano, H., Uchida, M., Endo, N., et al. (1999). Expression of metalloproteinase-13 (collagenase-3) is induced during fracture healing in mice. Bone 25, 197–203. doi: 10.1016/s8756-3282(99)00157-x
Yamaguchi, A., Komori, T., and Suda, T. (2000). Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr. Rev. 21, 393–411. doi: 10.1210/edrv.21.4.0403
Yee, C. S., Schurman, C. A., White, C. R., and Alliston, T. (2019). Investigating osteocytic perilacunar/canalicular remodeling. Curr. Osteoporos Rep. 17, 157–168. doi: 10.1007/s11914-019-00514-0
Zankl, A., Bonafe, L., Calcaterra, V., Di Rocco, M., and Superti-Furga, A. (2005). Winchester syndrome caused by a homozygous mutation affecting the active site of matrix metalloproteinase 2. Clin. Genet. 67, 261–266. doi: 10.1111/j.1399-0004.2004.00402.x
Zeng, Y., Rosborough, R. C., Li, Y., Gupta, A. R., and Bennett, J. (1998). Temporal and spatial regulation of gene expression mediated by the promoter for the human tissue inhibitor of metalloproteinases-3 (TIMP-3)-encoding gene. Dev. Dyn. 211, 228–237. doi: 10.1002/(sici)1097-0177(199803)211:3<228::aid-aja4>3.0.co;2-j
Zhang, B., Henney, A., Eriksson, P., Hamsten, A., Watkins, H., and Ye, S. (1999). Genetic variation at the matrix metalloproteinase-9 locus on chromosome 20q12.2-13.1. Hum. Genet. 105, 418–423. doi: 10.1007/s004399900167
Zhang, J. F., Wang, G. L., Zhou, Z. J., Fang, X. Q., Chen, S., and Fan, S. W. (2018). Expression of matrix metalloproteinases, tissue inhibitors of metalloproteinases, and interleukins in vertebral cartilage endplate. Orthop. Surg. 10, 306–311. doi: 10.1111/os.12409
Zhang, K., Barragan-Adjemian, C., Ye, L., Kotha, S., Dallas, M., Lu, Y., et al. (2006). E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol. Cell Biol. 26, 4539–4552. doi: 10.1128/mcb.02120-05
Zhang, W., Yang, N., and Shi, X. M. (2008). Regulation of mesenchymal stem cell osteogenic differentiation by glucocorticoid-induced leucine zipper (GILZ). J. Biol. Chem. 283, 4723–4729. doi: 10.1074/jbc.m704147200
Zhao, H., Cai, G., Du, J., Xia, Z., Wang, L., and Zhu, T. (1997). Expression of matrix metalloproteinase-9 mRNA in osteoporotic bone tissues. J. Tongji. Med. Univ. 17, 28–31. doi: 10.1007/bf02887998
Zhao, S., Zhang, Y. K., Harris, S., Ahuja, S. S., and Bonewald, L. F. (2002). MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J. Bone Miner. Res. 17, 2068–2079. doi: 10.1359/jbmr.2002.17.11.2068
Zheng, H., Liu, J., Tycksen, E., Nunley, R., and McAlinden, A. (2019). MicroRNA-181a/b-1 over-expression enhances osteogenesis by modulating PTEN/PI3K/AKT signaling and mitochondrial metabolism. Bone 123, 92–102. doi: 10.1016/j.bone.2019.03.020
Zhou, Z., Apte, S. S., Soininen, R., Cao, R., Baaklini, G. Y., Rauser, R. W., et al. (2000). Impaired endochondral ossification and angiogenesis in mice deficient in membrane type matrix metalloproteinase I. Proc. Natl. Acad. Sci. U.S.A. 97, 4052–4057. doi: 10.1073/pnas.060037197
Keywords: bone, remodeling, metabolism, matrix metalloproteinase, deficiency, underactivity
Citation: Hardy E and Fernandez-Patron C (2020) Destroy to Rebuild: The Connection Between Bone Tissue Remodeling and Matrix Metalloproteinases. Front. Physiol. 11:47. doi: 10.3389/fphys.2020.00047
Received: 24 September 2019; Accepted: 21 January 2020;
Published: 05 February 2020.
Edited by:
Yoshimi Nakagawa, University of Tsukuba, JapanReviewed by:
David M. Findlay, The University of Adelaide, AustraliaVincent Everts, VU University Amsterdam, Netherlands
Copyright © 2020 Hardy and Fernandez-Patron. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eugenio Hardy, eugenio@cim.sld.cu; Carlos Fernandez-Patron, cf2@ualberta.ca
 Eugenio Hardy
Eugenio Hardy Carlos Fernandez-Patron
Carlos Fernandez-Patron