- 1Key Laboratory of Cultivation and Protection for Non-Wood Forest Trees, Ministry of Education, Central South University of Forestry and Technology, Changsha, China
- 2College of Food Science, Central South University of Forestry and Technology, Changsha, China
The tung tree is an important woody oil tree species. Tung oil extracted from the tung fruit seeds is used in the manufacture of environmentally friendly paint. This study investigated the effects of the application of brassinolide (BR) under different temperature conditions on the chlorophyll content, photosynthesis, chlorophyll fluorescence, leaf structure, and chloroplast ultrastructure in Vernicia fordii and Vernicia montana. The conditions used were 8°C-Control (low temperature and no BR), 8°C-BR (low temperature and BR application), 28°C-Control (normal temperature and no BR), and 28°C-BR (normal temperature and BR application), and effects were monitored from 5 to 15 days after the treatments (DAT). The results showed that the low temperature treatment (8°C-Control) significantly reduced the net photosynthetic rate (Pn), stomatal conductance (Gs), maximum fluorescence (Fm), maximum photochemical efficiency (Fv/Fm), and actual photochemical and quantum efficiency (ΦPSII) compared to the control condition (28°C-Control). However, the external application of BR alleviated the negative effects of low-temperature stress to some degree for all the above parameters for both species tested, except for Pn and Gs at 15 DAT. There were no significant differences in most of the parameters in either species between the 28°C-Control and 28°C-BR treatments. At 10 and 15 DAT of low-temperature stress, the 8°C-Control treatment significantly reduced leaf cell tense ratio (CTR) and increased spongy ratio (SR) compared to the 28°C-Control, whereas BR application alleviated the adverse effects. Moreover, the 8°C-Control treatment significantly destroyed the chloroplast structure, loosening the thylakoids until they disintegrated, while exogenous spraying of BR protected the chloroplast structure and enabled it to function properly in both species. Our results suggested that long-term low temperatures significantly reduced the photosynthetic efficiency of tung tree seedlings, affecting the formation of the internal structure of plant leaves and destroying the integrity and function of the chloroplast. To prevent this, external application of BR to tung tree seedlings could enhance the photosynthetic potential of tung trees by maintaining the stability of the leaf structure, morphology, and function, and alleviating the damage caused by cold injury. The results also showed that V. fordii seedlings are more resistant to low temperatures than V. montana seedlings.
Introduction
The tung tree is a heliophile that grows quickly, yielding fruits within three years due to its high photosynthetic efficiency (Shockey et al., 2006; Chen et al., 2019). Vernicia fordii is a deciduous tree belonging to the Euphorbiaceae. Native to China, it is one of the four woody oil tree species in China with a long history of cultivation and high economic value (Gu et al., 2019; Liu et al., 2019; Zhang et al., 2019). Vernicia montana is also a deciduous tree belonging to the Euphorbiaceae. It is tall and strong and has excellent disease resistance and a longer fruiting period than V. fordii (Li et al., 2017a; Liu et al., 2019a). Tung oil is a widely used material in environmental protection coatings. It has excellent adhesion and glossiness, is fast-drying, and is resistant to heat, acids, alkalis, and cracking as a result of frost. Additionally, tung oil used in environmental paints is harmless to humans and therefore has a broad range of applications (Cao and Shockey, 2012; Li et al., 2017a; Li et al., 2019). Previous research has shown that the tung tree is one of the most promising biomass energy tree species (Tan et al., 2011; Lin et al., 2016).
Temperature is a major environmental factor that limits the distribution, productivity, and survivability of plants (Theocharis et al., 2012). Wu et al. (2015) confirmed that the expansion of tea plant (Camellia sinensis) cultivation is restricted due to temperature. Low-temperature conditions can damage the apple epidermis, causing serious losses in productivity (Rudell et al., 2011). In recent years, along with global climate changes, the frequency, duration, and severity of low-temperature episodes have increased in many regions of the world (Kodra et al., 2011; Sanghera et al., 2011). Plants experiencing low-temperature and freeze injury often exhibit serious physiological and morphological responses, including damage to various cellular structures and decreased chlorophyll content, photosynthetic rate, stomatal conductance, transpiration rate, and electron transport rate (ETR) (Moon et al., 1995; Saxby et al., 2003; Ogweno et al., 2009; Schreiber et al., 2013). However, plants can use different strategies and undergo physiological reactions to adapt to low-temperature conditions. For example, endogenous ABA and ascorbic acid concentrations in the leaves and shoots increase in response to the low-temperature damage (Dallaire et al., 1994; Yoshikawa et al., 2007; Stevens et al., 2008). Furthermore, low temperature conditions cause a decrease in the chlorophyll content, superoxide dismutase activity, and maximal quantum yield of PSII photochemistry but an increase in dark respiration and abscisic acid content (Huner et al., 1993; Moon et al., 1995; Yoshikawa et al., 2007; Ogweno et al., 2009).
The primary problem of freezing injury is reductions in yield due to the killing of tung trees in the north subtropical zone (Chen, 1987; He and Wu, 1991). He and Wu (1991) revealed that the low-temperature condition seriously affected the germination and growth of the tung tree. More recently, in China, cold snaps have occurred more frequently in spring, leading to serious damage to the growth and development of tung trees and resulting in large economic losses (Zhang et al., 2018). In addition, a low-temperature environment causes a reduced seed number and seed oil content in Jatropha curcas L., which belongs to Euphorbiaceae and has growth habits similar to those of the tung tree (Xiong et al., 2011; Wang et al., 2013). To date, no reports exist on the effects on photosynthesis and chloroplast ultrastructure of tung trees under different temperature treatments. It is vital to investigate cold resistance in tung trees and to find an effective means to alleviate the damage caused by cold weather.
Brassinolide (BR) is an important plant stress hormone that regulates physiological processes and can promote plant resistance to low-temperature environments (Shakirova et al., 2016; Wani et al., 2017; Vardhini, 2017; Chen et al., 2018). By analyzing the expression of a subset of cold-stress marker genes, Kagale et al. (2006) confirmed that BR treatment enhanced the tolerance of Arabidopsis thaliana and Brassica napus to cold stress. Moreover, BR application in cases of cold stress increased the chlorophyll content of Cucumis sativus L., enhanced photosystem II and antioxidant enzymes activity, and protected the photosynthetic membrane system from oxidative damage (Fariduddin et al., 2011). Fujii and Saka (2001) confirmed that BR promoted cell elongation in young rice seedlings under low-temperature stress. Vardhini (2017) reported that anatomical characteristics of plants could be ameliorated and protected by BR application under various abiotic stress conditions. Therefore, it is important to explore the mechanisms of BR to improve the low-temperature tolerance of tung tree seedlings, especially in terms of photosynthesis, anatomical structure, and chloroplast ultrastructure.
We studied the effects of exogenous BR on the photosynthetic characteristics, leaf anatomical structure, and chloroplast ultrastructure of two species of tung tree seedlings under different temperature conditions. Our objective was to explore the mechanisms underlying the BR-induced alleviation of long-term low-temperature stress on the photosynthetic processes of the two tung trees species. The chlorophyll content, photosynthesis, and chlorophyll fluorescence of the tung tree species were measured, and the leaf anatomical structure and chloroplast ultrastructure were investigated.
Materials and Methods
Plant Materials and Treatments
Seedlings of V. fordii and V. montana were obtained from the national conservation bank of tung tree germplasm resources (Xiangxi Region, Hunan Province, China). On January 15, 2018, seeds were sterilized in 0.5% KMnO4 for 20 min. The sterilized seeds were stored in wet sand for 3 months, and germinated seeds were selected and transplanted on March 30, 2018 to rounded plastic containers (inner diameter, 25 cm; height, 20 cm) containing red soil, perlite, and vermiculite at a ratio of 1:1:1 (v/v/v). The plants were grown under natural conditions at the Central South University of Forestry and Technology, Changsha, China (28°10' N; 113°23' E).
From June 8, 2018 to June 10, 2018, BR (0.05 mg·L-1, 15 mL per pot) was sprayed on the tung tree seedlings at 7:00 h, and the same dose of pure water was sprayed on other seedlings as a control. In the morning of June 11, 2018, all plant materials were transferred to a controlled growth chamber at day/night low temperatures of 8/5°C and day/night normal temperatures of 28/25°C, with 70% relative humidity and a 12h d-1 photoperiod. The lamp parameters were 16W, 220V, 50Hz, 9×, and a photosynthetic photon flux density (PPFD) of 200 µmol·m-2·s-1. Each treatment contained 18 pots of seedlings, and the whole experiment comprised 144 pots of seedlings.
The soil water content was maintained at an 80 ± 5% field capacity (FC) level by watering each day during the experiment. The photosynthetic pigment content, photosynthetic efficiency, and chlorophyll fluorescence parameters of the tung tree seedlings were measured on June 15 (5 days after treatment [5 DAT]), June 20 (10 DAT), and June 25 (15 DAT). Samples were also taken for observing leaf anatomy structure on these three days, and samples for observing chloroplast ultrastructure were taken on 15 DAT.
Chlorophyll Content
Leaves at a similar position were selected and cut into very thin filaments. The filaments were soaked in an acetone-ethanol mixture (1:1, v/v) for 24 h in 4°C and darkness until they turned white. The filaments were shaken evenly every 2 h during the experiment. The absorbance at 663 and 645 nm in the solution was measured with a spectrophotometer (UV-1100, Mapada, China), and the chlorophyll a (Chl a) and b (Chl b) concentrations were calculated: C (Chl a) (mg·dm-2) = (12.72× A663)–(2.59× A645), C (Chl b) (mg·dm-2) = (22.88× A645)–(4.68× A663), C (Chla+b)(mg·dm-2) = C (Chl a) + C (Chl b). All chlorophyll content data in this work were presented as average ± standard error (SE) of three biological replicates.
Photosynthetic Physiological Parameters
Photosynthetic physiological parameters were measured between 9:00 and 11:00 h (one leaf per plant; three plants per replicate) using a LI-6400XT Portable Photosynthesis System (LI-COR, Lincoln, NE, USA). The net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci), and transpiration rate (Tr) were measured at a PPFD source of 1,000 µmol·m-2·s-1 provided by LEDs emitting blue- and red-light sources. The leaf measurement area was 6 cm2 and the air flow rate was 500 µmol·s-1. The instantaneous water-use efficiency (WUE) was calculated as WUE = Pn/ Tr (Qu et al., 2019). CO2 was supplied at a concentration of 400 µmol·mol-1 using small cylinders. The temperature in the leaf cuvette under normal/low-temperature conditions was 28/8°C, and the relative humidity was 70%. All photosynthetic data in this work were presented as average ± SE of three biological replicates.
Chlorophyll Fluorescence Parameters
Complete portable fluorescent-photosynthetic gas exchange assay system (LI-COR, USA) was connected by the 6400-40 fluorescent leaf chamber and the LI-6400XT analyzer. The system was used to measure chlorophyll fluorescence parameters. Mature leaves were selected and marked before the first measurements were taken. The chlorophyll fluorescence parameters of the labelled leaves were determined after a 2-h dark treatment between 21:00 and 23:00 and after 60 min of photo activation between 9:00 and 10:00. The maximum fluorescence (Fm), maximum photochemical efficiency (Fv/Fm), actual photochemistry quantum efficiency (ΦPSII), and photochemistry quenching coefficient (qP) were measured. The air flow rate was 300 µmol·s-1, and the leaf measurement area was 2 cm2. All chlorophyll fluorescence data in this work were presented as average ± SE of three biological replicates.
Leaf Anatomical Structure
The leaf anatomical structure was analyzed by imaging leaf sections using an optical microscope. The third functionally mature leaf below the shoot tip of seedlings was selected, and 5- × 4-mm pieces were cut from the middle of the leaf near the vein. Three samples from each treatment group were chosen. The samples were fixed in formaldehyde alcohol acetic acid solution (FAA, 1:1 [v/v]) for 24 h and then placed in 70% ethanol and stored at 4°C. The samples were dehydrated in a series of graded ethanol solutions (70%, 85%, 90%, 95%, and 100%), infiltrated with paraffin wax for polymerization, sectioned (three pieces per sample) using a microtome (Leica, GER), dyed (leaves of V. fordii were dyed with saffron and Fast Green, and leaves of V. montana were dyed with saffron), and imaged under a microscope (Leica Gre, Heidelberg, Germany). The best and most representative image of each treatment group was selected to reveal (Figures 6 and 7). The palisade tissue, spongy tissue, and total thickness of leaves were measured. The leaf cell tense ratio (CTR) was calculated as CTR = palisade tissue/total leaf thickness. The leaf spongy ratio (SR) was calculated as SR = sponge tissue/total leaf thickness. All measured data were presented as average ± SD of three biological replicates.
Chloroplast Ultrastructure Observation
The middle portion of each leaf blade was cut into 1-mm2 strips and fixed in 2.5% glutaraldehyde solution (prepared with 0.1 mol L–1 sodium phosphate, pH 7.3) for 24 h at 4°C. After washing three times (30 min each), the tissue was fixed in 1% osmium tetroxide for 2 h at room temperature and dehydrated in a series of graded ethanol solutions (Gao et al., 2015). The leaf samples were then embedded with epoxy resin, placed in an ion sputter coater, and gilded for 20 min. Semi-thin sections (0.5 µm) were cut with a diamond knife using an ultramicrotome (EM UC7, Leica) and mounted on copper grids. Imaging was performed using a transmission electron microscope (HT7700; Hitachi, Tokyo, Japan). We selected two representative images from all of our obtained images of each treatment group (Figures 8 and 9).
Statistical Analysis
Microsoft Office Excel 2013 was used to process the data. Origin 9.0 was used to create the plots, and SPSS 19.0 software was used to analyze the variance to test for differences. Treatment means were compared using one-way analysis of variance (ANOVA) and Duncan's multiple range test with a probability of p ≤ 0.05.
Results
Chlorophyll Content
With prolonged low-temperature stress, the leaves of both species gradually became dehydrated, withered, and yellow (Figure 1). Compared with the 28°C-Control, the Chl a, Chl b, and Chl a+b contents of both species were significantly reduced under low temperature at 10 and 15 DAT, but the 8°C-Control treatment resulted in increased Chl a, Chl b, and Chl a+b contents at 5 DAT (Table 1). At the normal temperature of 28°C, exogenous spraying of BR resulted in increased Chl a, Chl b, and Chl contents in both species compared to the 28°C-Control. Similar results were achieved for both species with BR spraying at the low temperature, though with an exception at 15 DAT (Table 1).
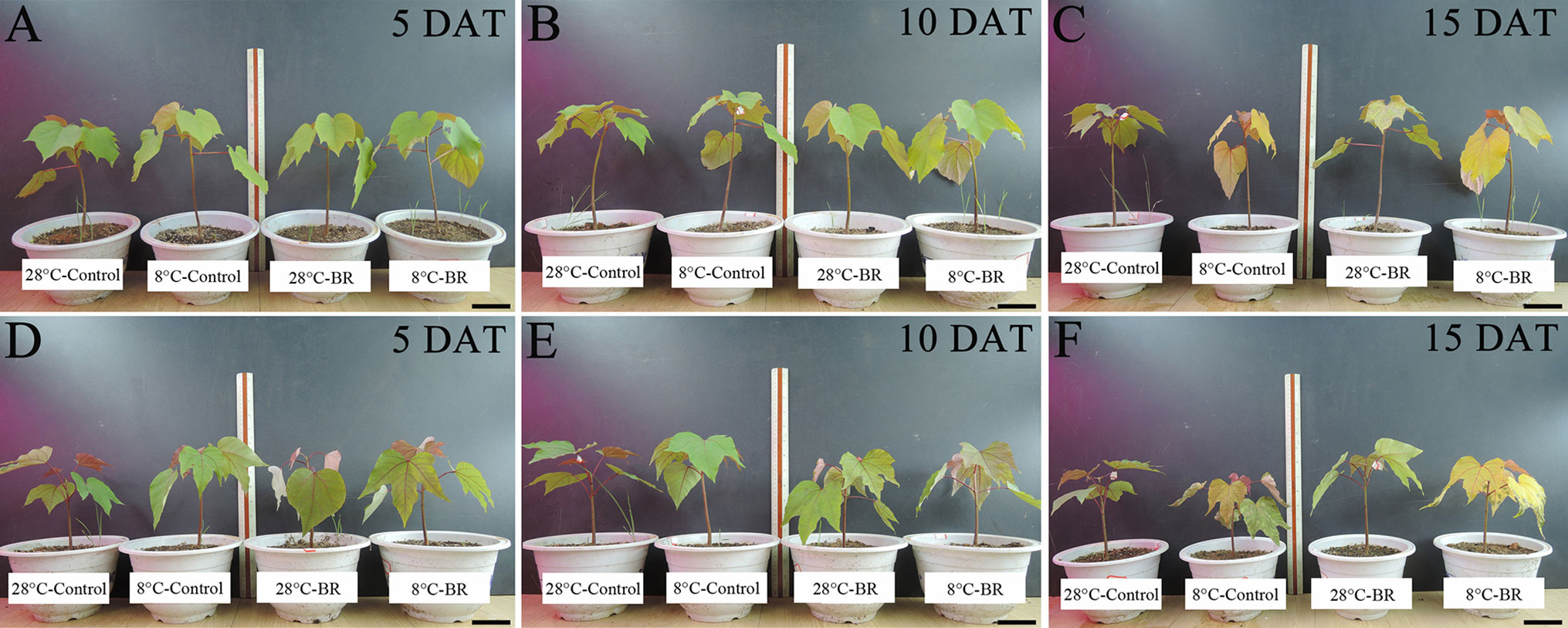
Figure 1 Effects of BR on the growth of two tung tree species under normal and low-temperature conditions. Treatments were (1) 28°C-Control, normal temperature and no BR; (2) 28°C-BR, normal temperature and BR application; (3) 8°C-Control, low temperature and no BR; and (4) 8°C-BR, low temperature and BR application. Species were (1) Vernicia fordii-(A–C) (2) Vernicia montana-(D–F) Scale bars are 10 cm.
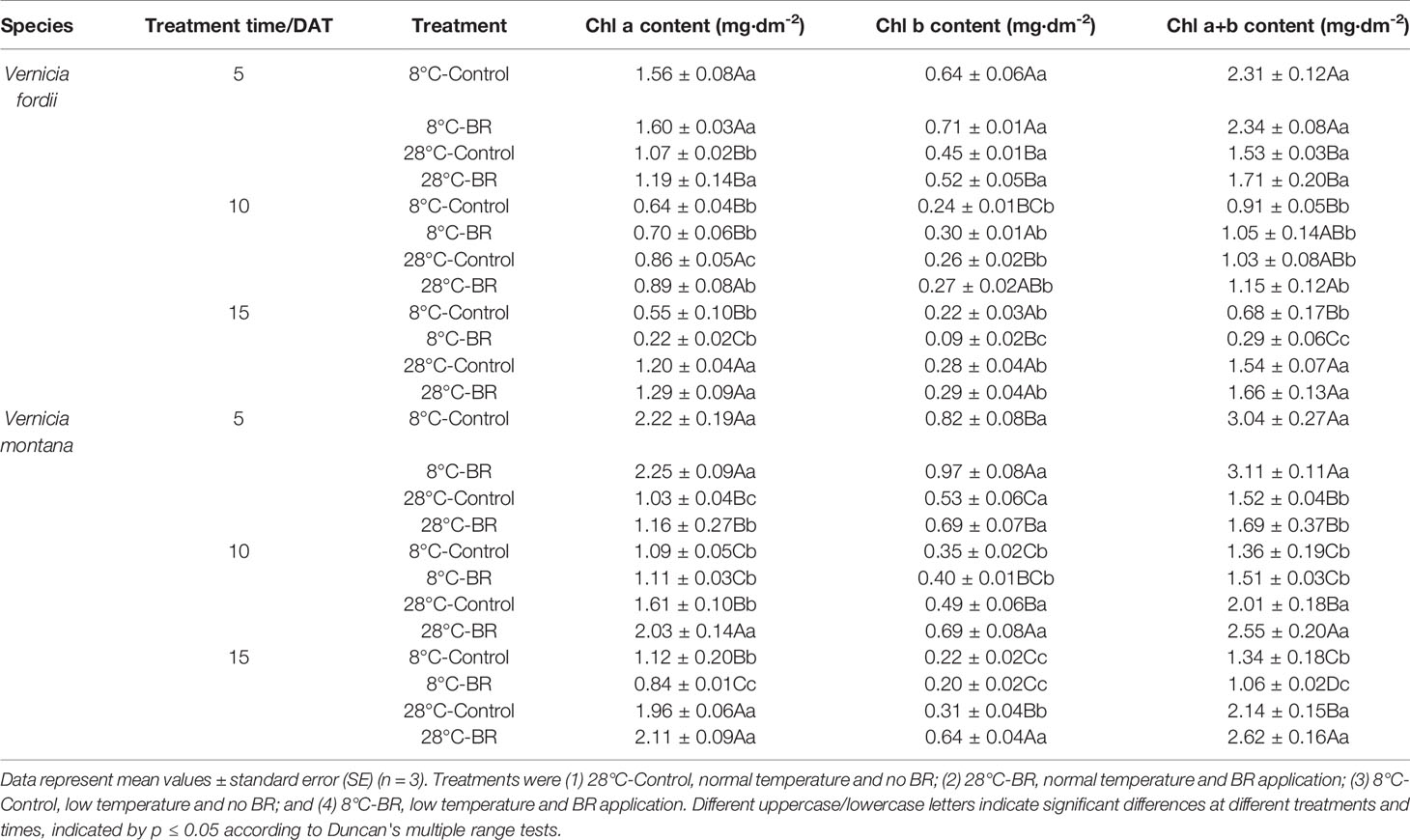
Table 1 Effects of BR on chlorophyll content of two tung tree species under normal and low-temperature conditions.
The Chl content of both species under low-temperature stress at 10 and 15 DAT was significantly lower than that of the control at 5 DAT (Table 1). Exogenous spraying of BR appeared to ameliorate the reduction in Chl content at 5 and 10 DAT (Table 1).
Photosynthetic Parameters
The Pn, Gs, and WUE of V. fordii significantly decreased as the low-temperature stress continued, whereas the Ci significantly increased (Figures 2A–C). However, the Pn and Gs of V. fordii always increased under normal-temperature conditions, but the WUE always decreased (Figures 2A–B, D). The Pn and WUE of V. montana significantly decreased as the low-temperature stress continued (Figures 3A, D), and the Ci increased significantly (Figure 3C). Under low-temperature conditions, the Pn and Gs of V. montana leaves were significantly decreased compared to those in normal-temperature conditions (Figures 3A, B). The Ci was higher under low-temperature conditions, except at 5 DAT, and the WUE was lower at 10 and 15 DAT (Figures 3C, D).
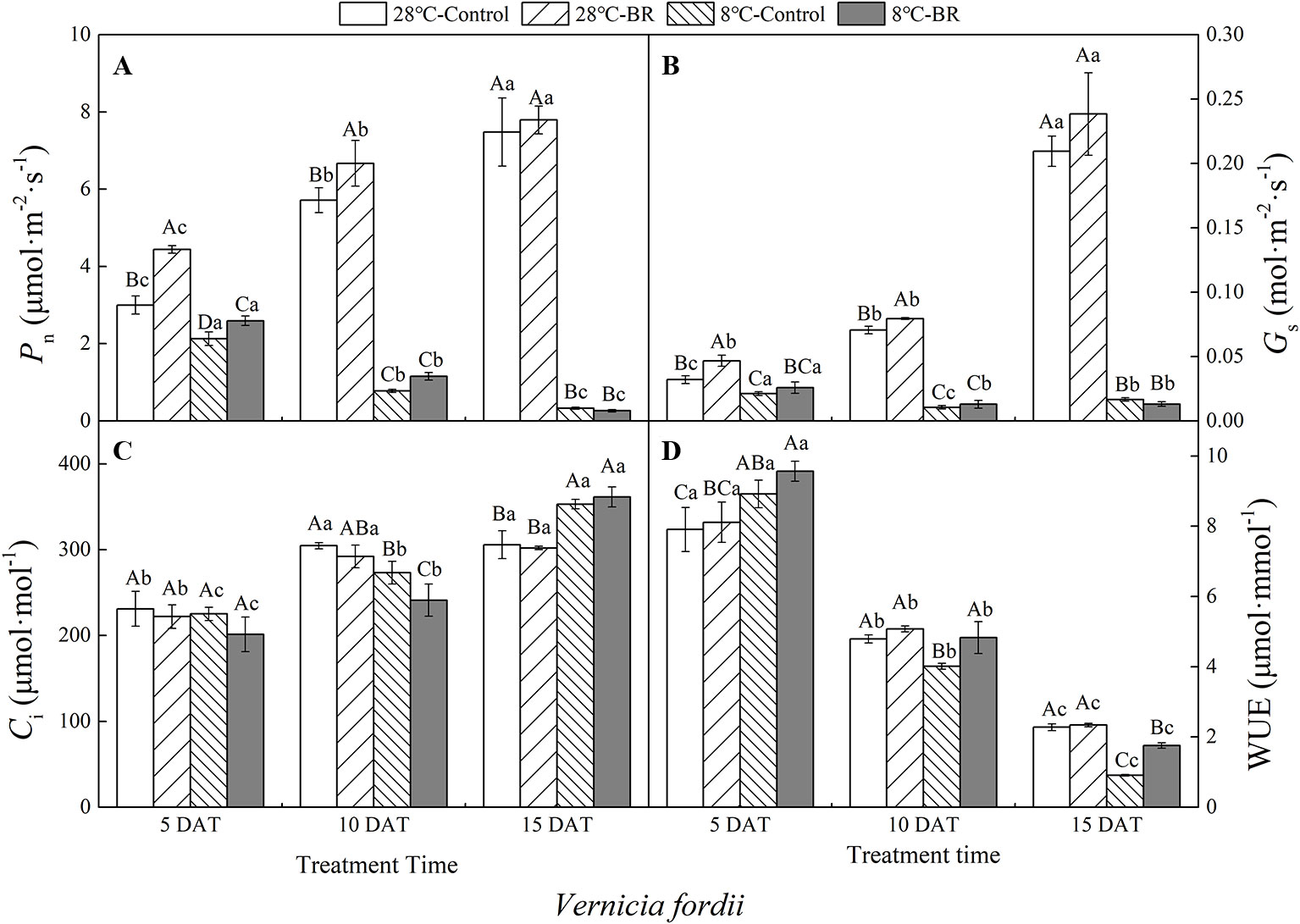
Figure 2 Effects of BR on photosynthetic parameters of Vernicia fordii under normal and low-temperature conditions. Treatments were (A) 28°C-Control, normal temperature and no BR; (B) 28°C-BR, normal temperature and BR application; (C) 8°C-Control, low temperature and no BR; and (D) 8°C-BR, low temperature and BR application. Different uppercase/lowercase letters indicate significant differences at different treatments and times, indicated by p ≤ 0.05 according to Duncan's multiple range tests, and vertical bars indicate ± SE of mean (n = 3).
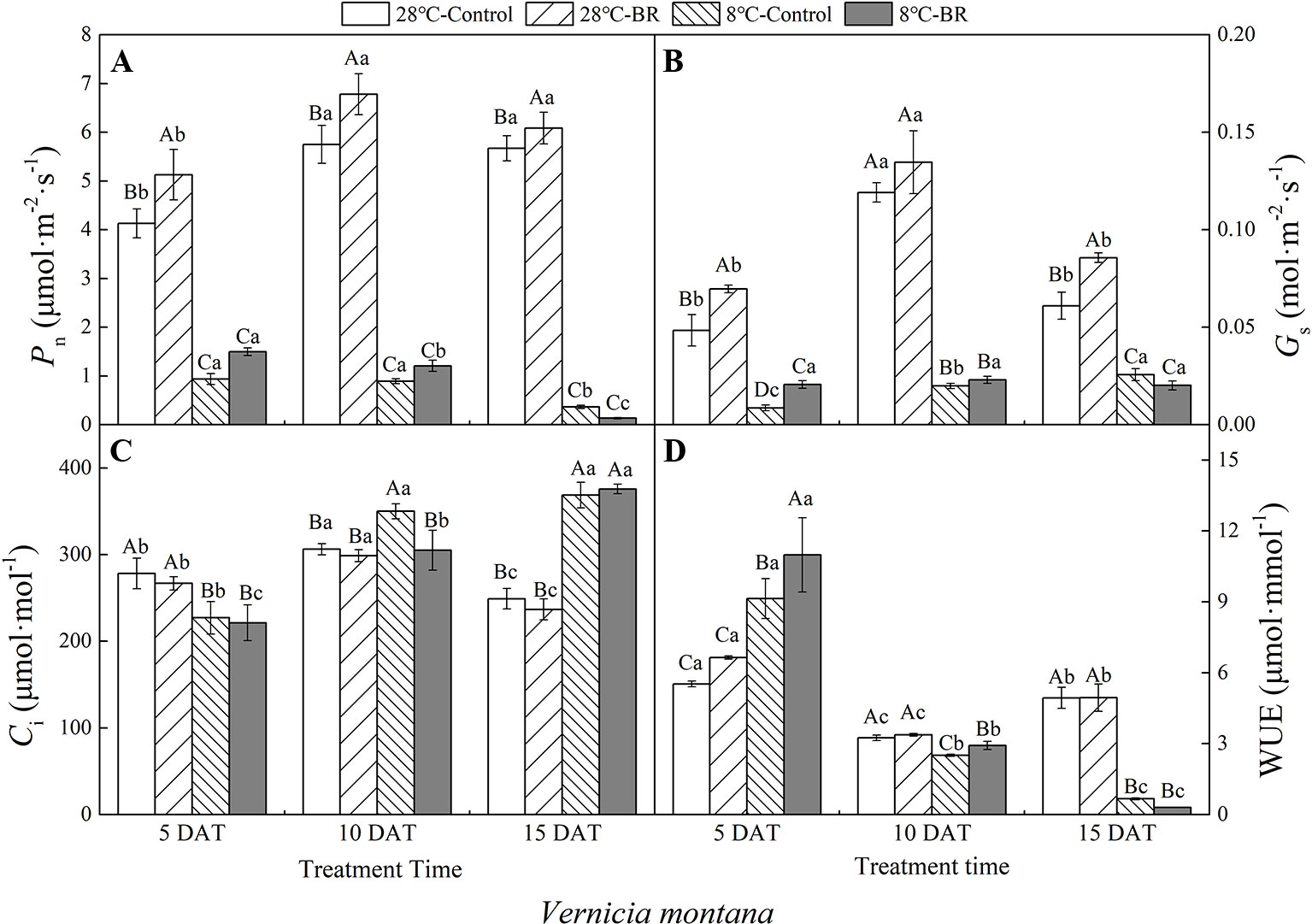
Figure 3 Effects of BR on photosynthetic parameters of Vernicia montana under normal and low-temperature conditions. Treatments were (A) 28°C-Control, normal temperature and no BR; (B) 28°C-BR, normal temperature and BR application; (C) 8°C-Control, low temperature and no BR; and (D) 8°C-BR, low temperature and BR application. Different uppercase/lowercase letters indicate significant differences at different treatments and times, indicated by p ≤ 0.05 according to Duncan's multiple range tests, and vertical bars indicate ± SE of mean (n = 3).
Exogenous spraying of BR increased the Pn, Gs, and WUE of both species compared to the control 28°C-Control group. Similar results were observed for both species under low-temperature stress, except for at 15 DAT (Figures 2 and 3). Exogenous spraying of BR did not alter this trend but resulted in higher values for each parameter than in non-BR-treated plants at 5 and 10 DAT (Figures 2 and 3).
Chlorophyll Fluorescence Parameters
Compared with the control 28°C-Control, the Fm, Fv/Fm, ΦPSII, and qP of both species were significantly reduced under low-temperature stress, indicating that a low-temperature environment significantly affects the activity of the photosynthetic apparatus in tung tree seedlings (Figures 4 and 5). Exogenous spraying of BR increased the Fm, Fv/Fm, ΦPSII, and qP in both species at 28°C except for the Fv/Fm in V. montana at 15 DAT. These results indicate that the addition of BR did not significantly promote the activity of the photosynthetic apparatus in tung trees under normal-temperature conditions. However, in the 8°C-Control treatment, exogenous spraying of BR resulted in increased Fm in both tung tree species during the entire experimental period, except for 15 DAT in V. fordii, and the Fv/Fm values were also increased, except for 5 DAT in V. fordii (Figures 4A–B and 5A–B). These results matched our expectations.
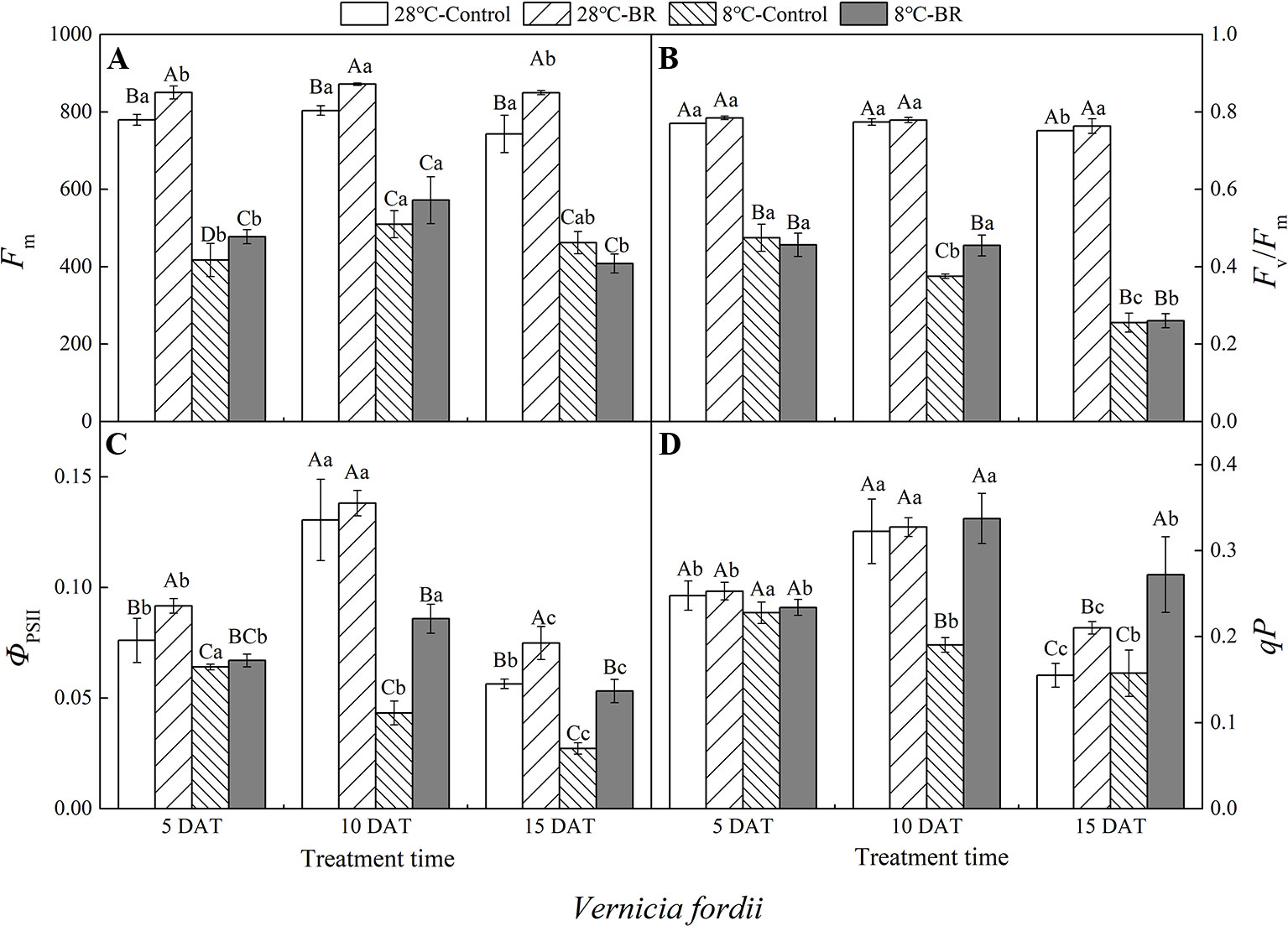
Figure 4 Effects of BR on chlorophyll fluorescence parameters of Vernicia fordii under normal and low-temperature conditions. Treatments were (A) 28°C-Control, normal temperature and no BR; (B) 28°C-BR, normal temperature and BR application; (C) 8°C-Control, low temperature and no BR; and (D) 8°C-BR, low temperature and BR application. Different uppercase/lowercase letters indicate significant differences at different treatments and times, indicated by p ≤ 0.05 according to Duncan's multiple range tests, and vertical bars indicate ± SE of mean (n = 3).
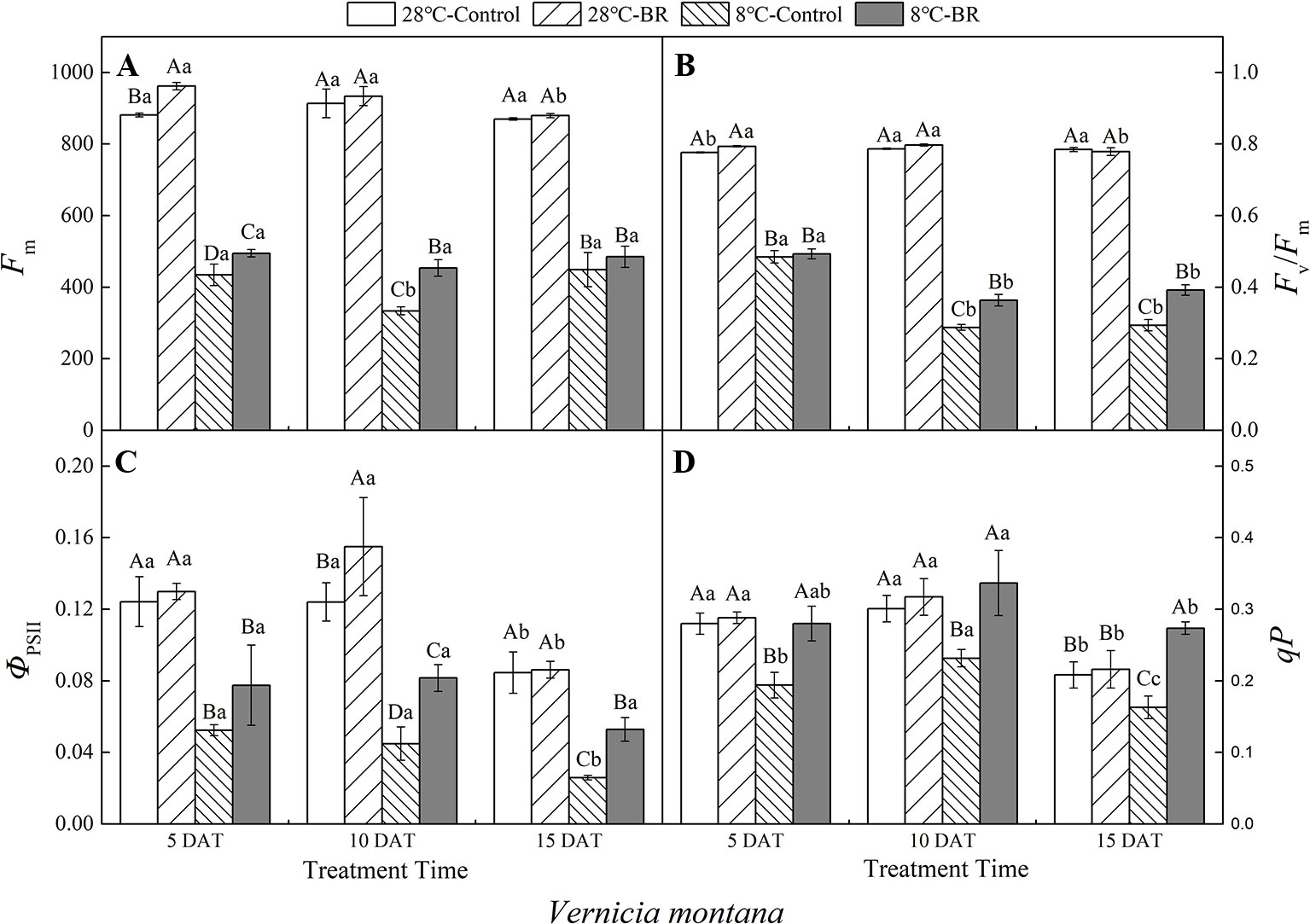
Figure 5 Effects of BR on chlorophyll fluorescence parameters of Vernicia montana under normal and low-temperature conditions. Treatments were (A) 28°C-Control, normal temperature and no BR; (B) 28°C-BR, normal temperature and BR application; (C) 8°C-Control, low temperature and no BR; and (D) 8°C-BR, low temperature and BR application. Different uppercase/lowercase letters indicate significant differences at different treatments and times, indicated by p ≤ 0.05 according to Duncan's multiple range tests, and vertical bars indicate ± SE of mean (n = 3).
Under the long-term low-temperature stress of the 8°C-Control treatment, the Fv/Fm, ΦPSII, and qP showed a continuous downward trend (p < 0.05) in V. fordii (Figures 4B–D). Application of BR had no significant effect on the ΦPSII or qP at 5 DAT, whereas they increased by 98.41 and 77.22%, respectively, at 10 DAT compared to the 8°C-Control. The ΦPSII and qP increased by 95.77 and 72.74%, respectively, compared to the control 8°C-Control at 15 DAT, indicating that BR treatment could improve the photosynthetic activity of the tung tree under long-term low-temperature stress conditions (Figures 4C, D).
Under long-term low-temperature stress, the qP in the 8°C-Control and BR treatment groups increased initially and then decreased (p < 0.05) over all three time periods in V. montana (Figure 5D). The ΦPSII in the 8°C-Control treatment showed a significant and continuous downward trend as the low-temperature stress persisted (p < 0.05) (Figure 5C). However, compared with the 8°C-Control, the ΦPSII in the 8°C-BR treatment group improved by 47.99, 81.89, and 104.35% at 5, 10, and 15 DAT, respectively (Figure 5C). In addition, the Fv/Fm under both treatments showed a downward trend as the low-temperature stress persisted, whereas, compared with the control 8°C-Control, the Fv/Fm of V. montana pre-treated with BR increased by 26.48 and 33.55% (p < 0.05) at 10 and 15 DAT, respectively (Figure 5B).
Leaf Anatomical Structure
Under low-temperature treatment, the palisade tissue thickness, spongy tissue thickness, and leaf thickness of both species pre-treated with BR were all significantly thicker than those of the control 8°C-Control during the entire experiment, expect for V. montana at 15 DAT (Table 2). Compared with the 8°C-Control treatment, the leaf thickness of V. fordii pre-treated with BR increased by 49.50, 11.56, and 25.58% (p < 0.05) at 5, 10, and 15 DAT, respectively. Compared with the 8°C-Control treatment, the CTR of both species pre-treated with BR was higher at 10 and 15 DAT, whereas the SR was lower during the entire experiment (Table 2). Compared with the 28°C-Control treatment, the CTR of the 28°C-BR treatment was higher in both species at all time points (Table 2), which showed that exogenous spraying of BR increased the proportion of palisade tissue in tung tree leaves. Moreover, exogenous spraying of BR on both species increased the palisade tissue thickness under normal temperatures, consistent with our findings under low-temperature stress. Interestingly, even though exogenous application of BR did not increase the leaf thickness of either species under normal temperatures, external application of BR did increase the leaf thickness under low-temperature stress at 5 and 10 DAT (Table 2).
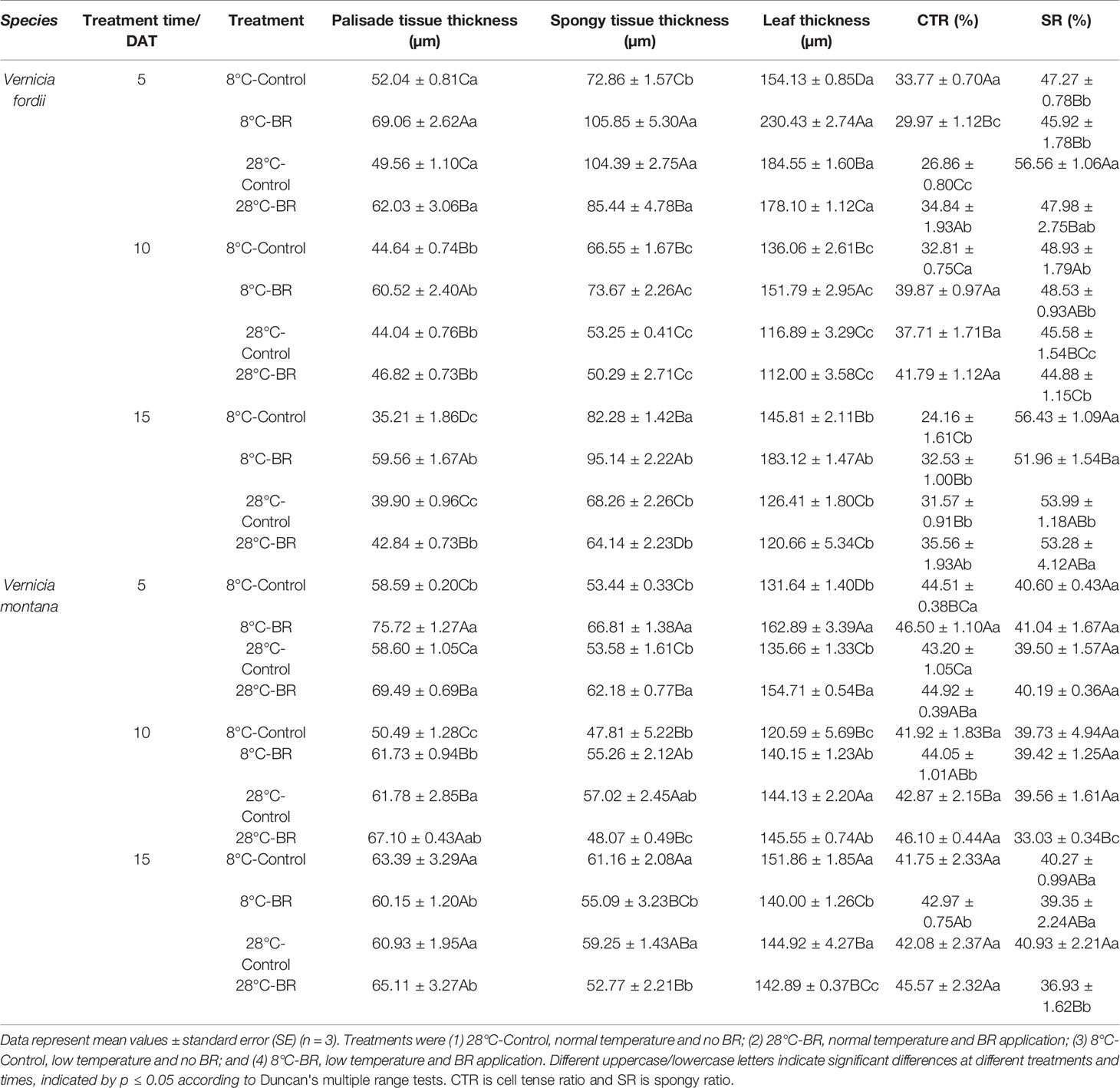
Table 2 Effects of BR on leaf anatomical structure parameters of two tung tree species under normal and low-temperature conditions.
Tung tree leaves are composed of the upper epidermis, lower epidermis, and mesophyll. The epidermis consists of irregular oblong monolayers of varying sized cells, and the mesophyll consists of a layer of palisade tissue cells and multiple layers of spongy tissue cells (Figures 6 and 7). Under normal-temperature conditions, exogenous spraying of BR did not significantly alter the leaf anatomical structure of either species compared with the control 28°C-Control (Figures 6a1–c1, 6a2–c2, 7a1–c1, and 7a2–c2). Compared with the 28°C-Control treatment, the palisade tissue of V. fordii and the spongy tissue of V. montana were loosened and had increased cell spacing under low-temperature stress at 5 DAT (Figures 6a3 and 7a3). The palisade tissue of both species and the spongy tissue of V. montana were loosened and the cell spacing larger at 15 DAT (Figures 6c3 and 7c3), but exogenous application of BR prevented this effect (Figures 6–7a4 and 6–7c4).
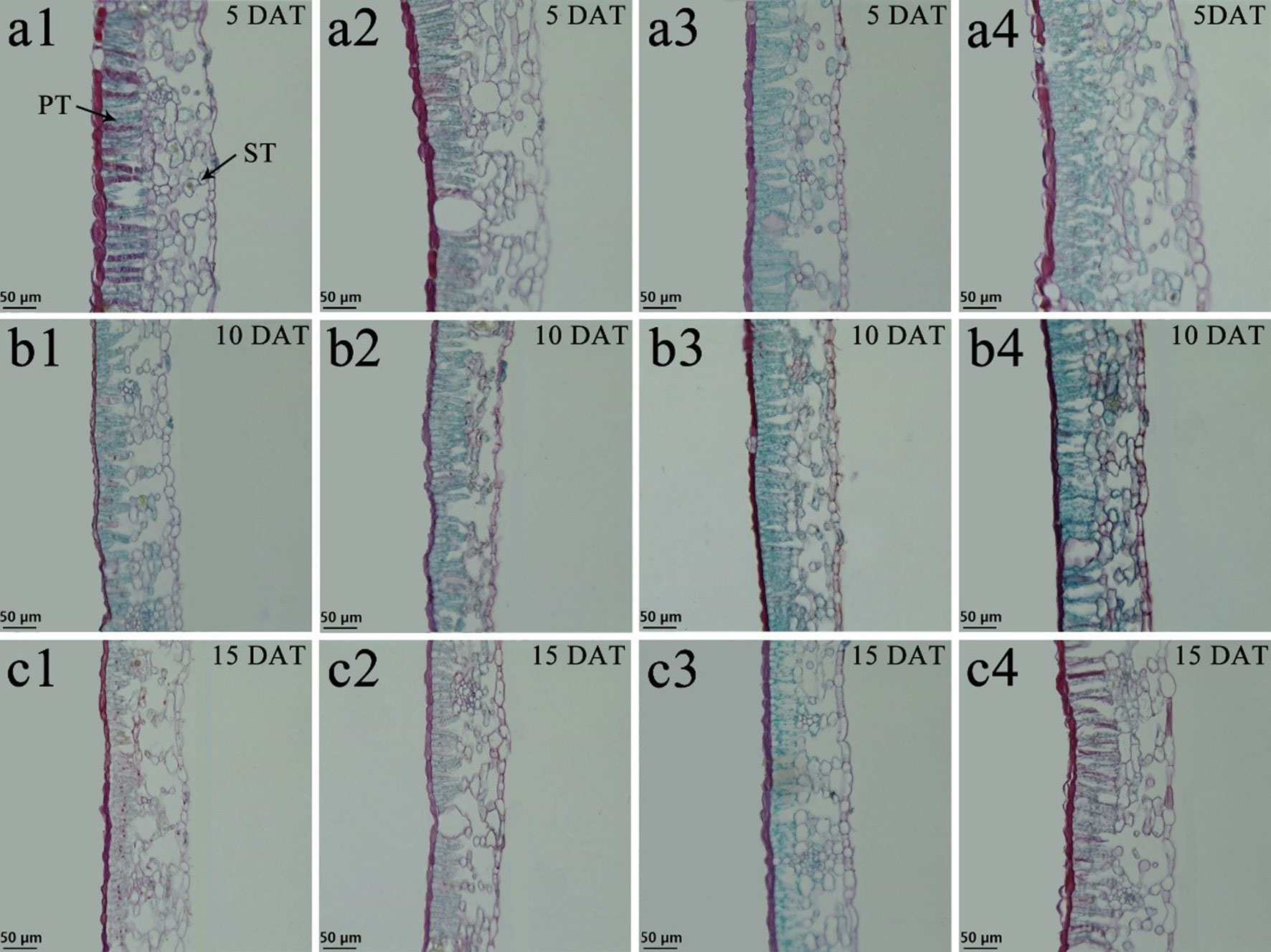
Figure 6 Effects of BR on leaf anatomical structure of Vernicia fordii under normal and low-temperature conditions. Treatments were (1) 28°C-Control, normal temperature and no BR, a1-c1; (2) 28°C-BR, normal temperature and BR application, a2–c2; (3) 8°C-Control, low temperature and no BR, a3–c3; and (4) 8°C-BR, low temperature and BR application, a4-c4. All the microscopic multiples were 20× ×DAT, days after treatment; PT, palisade tissue; ST, spongy tissue. Bars, 50 μm.
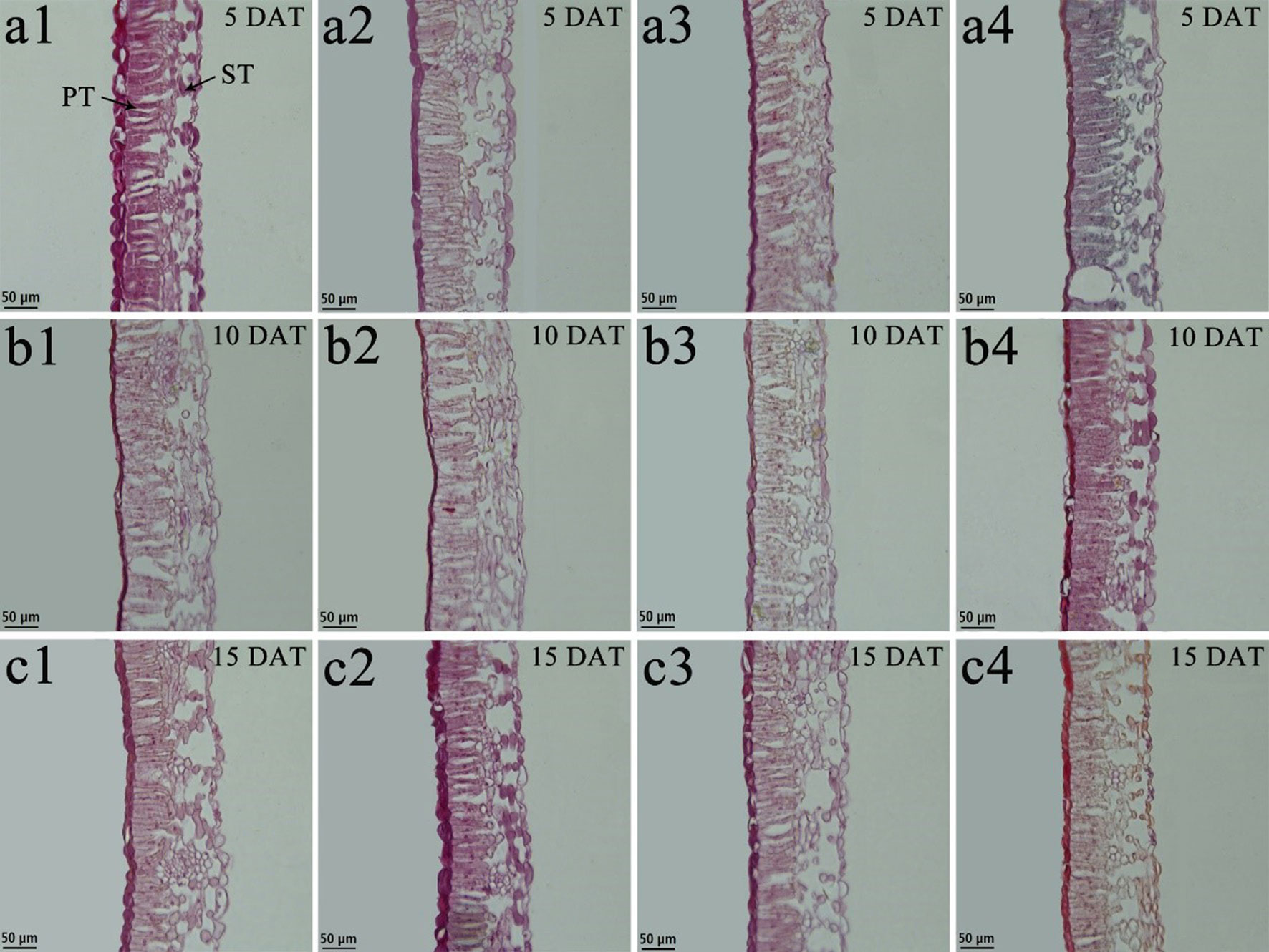
Figure 7 Effects of BR on leaf anatomical structure of Vernicia montana under normal and low-temperature conditions. Treatments were (1) 28°C-Control, normal temperature and no BR, a1-c1; (2) 28°C-BR, normal temperature and BR application, a2–c2; (3) 8°C-Control, low temperature and no BR, a3–c3; and (4) 8°C-BR, low temperature and BR application, a4-c4. All the microscopic multiples were 20× ×. DAT, days after treatment; PT, palisade tissue; ST, spongy tissue. Bars, 50 μm.
Chloroplast Ultrastructure Observation
The chloroplast ultrastructure of both species changed under the different temperature and BR treatments, as shown in Figures 8 and 9. Chloroplasts from samples grown under normal temperature conditions were almost adjacent to the cell wall, and the cell shapes were mainly regular and integral (Figures 8a1, b1, a2, b2, 9a1, b1, a2, b2). Moreover, few starch granules were observed in most chloroplasts, and the stroma lamellae were neatly arranged (Figures 8A–B and 9A–B). In contrast, the stroma lamellae of the 28°C-BR V. fordii were slightly expanded (Figure 8B). Few chloroplasts of the 8°C-Control treatment in V. fordii had a normal appearance, and some of their structures were destroyed. In addition, most of the chloroplast structure in V. montana was destroyed and the thylakoid structure was loosened and disintegrated (Figure 9C). Moreover, the number of liposomes in the chloroplasts was increased in V. montana (Figures 8C, 9C). However, the chloroplasts in both species pre-treated with BR remained intact (Figures 8D and 9D). In both species, the chloroplasts contained a small amount of starch, and the stroma lamellae were loosely arranged, with slight swelling and expansion (Figures 8D and 9D). Also, the number of liposomes in V. montana chloroplasts was increased (Figure 9D).
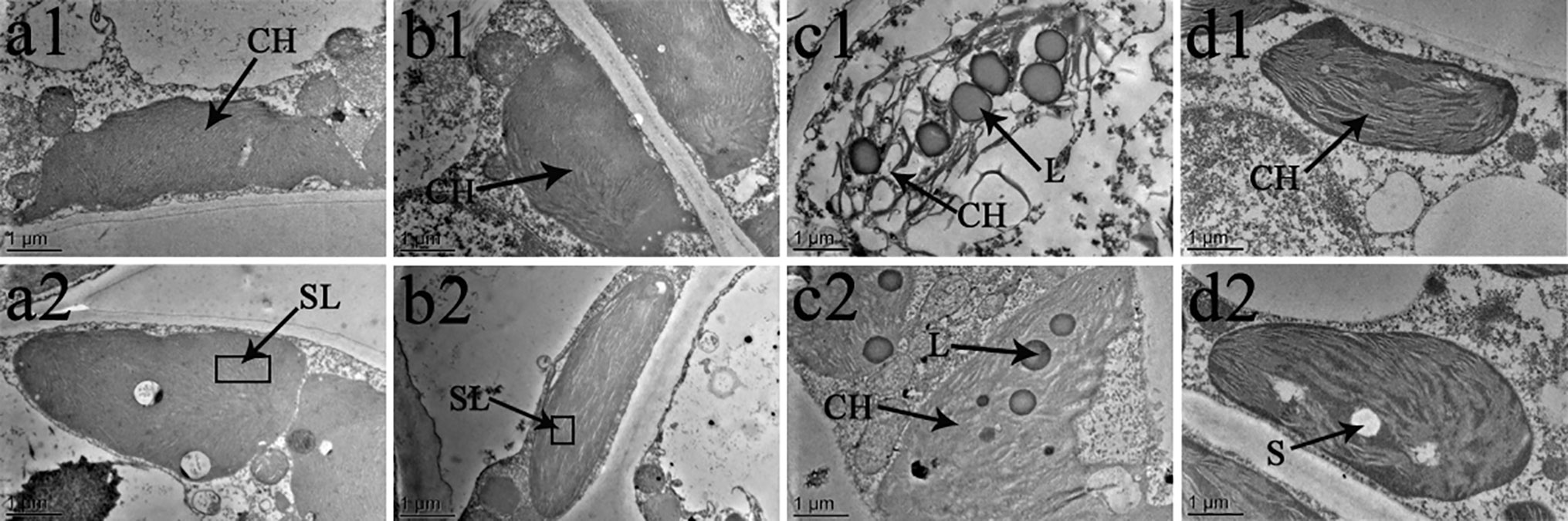
Figure 8 effects of br on chloroplast ultrastructure of vernicia fordii under normal and low-temperature conditions. Treatments were (1) 28°C-Control, normal temperature and no BR, a1–2; (2) 28°C-BR, normal temperature and BR application, b1–2; (3) 8°C-Control, low temperature and no BR, c1–2; and (4) 8°C-BR, low temperature and BR application, d1-2. CH, chloroplast; SL, stroma lamella; L, liposomes; S, starch. Images were photographed with TEM.
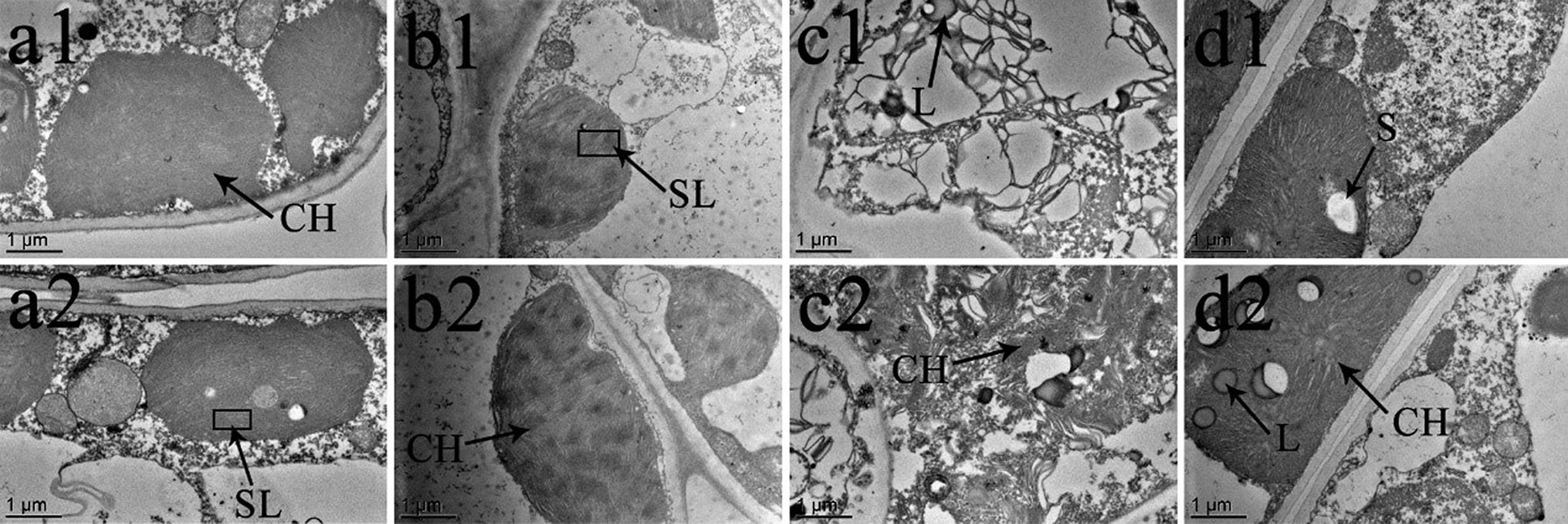
Figure 9 Effects of BR on chloroplast ultrastructure of Vernicia montana under normal and low-temperature conditions. Treatments were (1) 28°C-Control, normal temperature and no BR, a1–2; (2) 28°C-BR, normal temperature and BR application, b1–2; (3) 8°C-Control, low temperature and no BR, c1–2; and (4) 8°C-BR, low temperature and BR application, d1–2. Abbreviations: CH, chloroplast; SL, stroma lamella; L, liposomes; S, starch. Images were photographed with TEM.
Discussion
Plant photosynthetic processes are highly sensitive to environmental changes and depend on aspects such as chlorophyll content, photosynthetic parameters, and chlorophyll fluorescence parameters (Stirbet and Govindje, 2011). Low-temperature stress increases reactive oxygen species (ROS) in plant metabolic pathways, reduces tolerance to antioxidant enzymes, damages protein and DNA, accelerates chlorophyll decomposition in leaves, slows down plant metabolism, reduces the availability of chlorophyll synthesis substrate, and, thus, results in a reduced leaf chlorophyll content (Yang et al., 2016; Liu et al., 2019b). In our study, with prolonged low-temperature stress, the Chl a, Chl b, and Chla+b contents of both species continued to decrease and were lower than those of the plants maintained under normal temperature conditions, as reported previously. However, our chlorophyll content results at 5 DAT under low-temperature conditions were higher than those under normal temperatures, in contrast to previous findings (Yang et al., 2016). This might be due to the changes in endogenous hormone levels, information transmission, and the regulation of plant physiological responses in both species under short-term low-temperature stress (Kurepin et al., 2015). Plants are able to construct a low-temperature defense system that actively increases their chlorophyll contents and prevents decreases in photosynthesis and energy production caused by a gradual decrease in the chlorophyll content. In contrast, plants maintained at the correct temperature do not need to increase their rate of photosynthesis and energy production by increasing their chlorophyll content. Exogenous application of BR to cotton seedlings under low-temperature stress increased the Chl a, Chl b, and Chla+b contents and protected chloroplasts (Li et al., 2018), which is similar to our results. However, the Chl a, Chl b, and Chl a+b contents in the control-treated plants were higher than those in the BR-treated plants at 15 DAT under low-temperature conditions. It is possible that an appropriate concentration of BR promotes chlorophyll synthesis, whereas a high concentration of BR inhibits the activities of chlorophyll enzymes (Hayat et al., 2010). In addition, exogenous hormone application has been shown to promote the production of endogenous hormones in plants (Muhammad et al., 2006). Therefore, under long-term low-temperature stress, high levels of endogenous BR may accumulate, leading to an inhibition of the activities of chlorophyll enzymes and reduction chlorophyll synthesis in the leaves of both species.
Photosynthesis requires the balancing of the light energy absorbed and the energy consumed by the plant's metabolic sinks and is therefore very sensitive to environmental changes. Low temperature exacerbates the imbalance between the energy source and the metabolic sink, causing significant changes in photosynthesis (Ensminger et al., 2006). In this study, the Pn of both species of tung tree seedlings grown in low-temperature conditions decreased significantly and continued to decline as the period of stress prolonged, seriously affecting the normal growth of the plants. Studies have shown that the decreased photosynthesis caused by the low-temperature stress is due to both stomatal and non-stomatal factors (Allen and Ort, 2001; Yamori et al., 2012; Cao et al., 2017). When Ci and Gs decrease simultaneously, the decrease in Pn is mainly caused by stomatal factors. When Pn decreases along with an increase in Ci, the main limiting factors of photosynthesis are non-stomatal factors (Farquhar and Sharkey, 1982). In this study, the decrease in Pn in both species was accompanied by a simultaneous decrease in Ci and Gs, indicating that stomatal factors were the main limiting factors for photosynthesis at 5 DAT. At 10 DAT, the Pn of V. fordii decreased with a simultaneous decrease in Ci and Gs. Compared with the initial stages of stress, the Gs was lower, while the Ci began to increase. The Pn of V. montana decreased with the increase in Ci, whereas the Gs was slightly higher than that at the initial stage of stress. This indicates that the limiting factors for photosynthesis in both species during the middle period of stress included both stomatal and non-stomatal factors. The stomatal factors were the dominant limiting factors for photosynthesis in V. fordii, but non-stomatal factors were the dominant limiting factors for V. montana. As the stress period lengthened, the Pn of both species continued to decrease, whereas the Ci and Gs kept increasing. This indicates that non-stomatal factors were the main limiting factors for photosynthesis at 15 DAT. Previous studies have shown that exogenous BR treatment improved the apparent quantum efficiency, dark respiration rate, and carboxylation efficiency of photosynthetic enzymes, thereby increasing the net photosynthetic rate of plants and increasing the accumulation of organic matter (Yu et al., 2002; Anuradha and Rao, 2009; Li et al., 2017b). Similarly, in our study, the Pn with BR treatment was higher than that in the control for both species under normal temperatures and at the early and middle stages of cold stress, indicating that exogenous BR treatment increases the photosynthetic rate and cold resistance and alleviates the decrease in Pn caused by cold. However, the Pn with BR treatment under cold conditions was lower than that in the control at 15 DAT, which may be due to the increases in the endogenous BR synthesis induced by long-term low-temperature stress. Excessive accumulation of endogenous BR leads to a decrease in the Pn (Muhammad et al., 2006; Hayat et al., 2010). However, the reduced Pn may lead to an increased WUE, thus protecting the activity of the photosynthetic apparatus.
Chlorophyll fluorescence parameters could be used to evaluate the function of the photosynthetic apparatus and the effects of low-temperature stress on plants. These parameters could reflect the photosynthetic potential of plants, the ability of plants to convert light energy into chemical energy, and the photosynthetic activity level (Schreiber et al., 1986; Demmig-Adams, 1990; Olaf and Jan, 1990; Wolfgang and Olle, 1990). This study showed that low-temperature stress reduced the Fm in both tung tree species. The lower Fm means that the non-photochemical processes of the photosynthetic organs increased, and the photoelectron transfer efficiency and potential decreased (Li et al., 2017b). Moreover, the Fv/ Fm, ΦPSII, and qP decreased in both species during low-temperature stress, indicating that the photosynthetic potential, photosynthetic activity, the ability to convert light energy into chemical energy, and the ability to dissipate excess light energy into heat decreased to varying degrees. Thus, cold conditions increased the degree of leaf inhibition. Exogenous application of BR increased the Fm, maintained the activity of the PSII reaction center, shortened the non-photochemical processes of the photosynthetic organs, and enhanced the efficiency and potential of photoelectron transfer in both species during the low-temperature stress. At the same time, the decreases in Fv/ Fm and ΦPSII were accompanied by decreases in Pn, Gs, and Tr, indicating that the non-stomatal factors responsible for the decreased photosynthetic rate in both species during the middle and late stages of low-temperature stress were due to the reduced activity of the photosynthetic apparatus. As the stress time extended, the Fv/Fm and ΦPSII of V. fordii and V. montana subjected to control treatment continued to decrease, indicating that the degree of damage to the photosynthetic organs increased with the longer stress time. The Fv/Fm, ΦPSII, and qP of the BR-treated plants were always higher than that in control-treated plants, indicating that exogenous BR spraying alleviated the damage to the photosynthetic organs of the tung tree seedlings during low-temperature stress and promoted photosynthesis. Clearly, our results from the present study are consistent with the other early reports (Anuradha and Rao, 2009; Yu et al., 2015).
Anatomical observation of the plant tissues is important to clarify any internal changes. Leaves are sensitive to low-temperature stress as they are the main organs for photosynthesis and transpiration. Therefore, changes in the leaf anatomical structure are the basis of the plant response and adaptation to environmental changes (Schreiber et al., 2013; Kaur et al., 2019). Leaf structural parameters were affected by the environment but did not accurately indicate the cold resistance of the plants. CTR and SR could be used to measure the cold resistance of plants, as they combine the differences in palisade tissue thickness, spongy tissue thickness, and total leaf thickness. A higher CTR and lower SR indicate increased cold resistance (Cao et al., 2014). The CTRs of the two tung tree species under low-temperature conditions were lower than that under normal temperatures, and the SRs were higher than that under normal temperatures during the middle and late stages of stress, suggesting that a long term low temperature treatments can affect the leaf structure and compactness. However, the results are in contrast to those at the early stage of the low temperature stress. This may be due to the fact that the plants that were exposed to cold conditions, in order to prevent the damage to the structure of leaves caused by a low-temperature environment, improved their CTR and enhanced leaf tightness. Moreover, in this study, with the prolongation of low-temperature stress, the CTR in V. fordii continued to decline, but the SR continued to increase, indicating that the long-term low-temperature stress seriously affected the internal structure of leaves, weakened the cold resistance potential of plants, and aggravated the damage to the plant. However, the changes in the CTR and SR in V. montana were less apparent than that in V. fordii. This might be due to the V. montana leaf tissues which are capable of adapting to low temperature conditions by increasing or decreasing the thickness of the palisade tissue and spongy tissue at the same time, so that the tightness and looseness of the leaf tissue were relatively consistent (Teng et al., 2018). At the low temperatures conditions, the CTR following BR treatment was always higher than that following the control (except for V. fordii at 5 DAT), and the SR following BR treatment (except for V. montana at 5 DAT) was lower than that following control, suggesting that the exogenous application of BR improved the cold resistance of plants and promoted the stability of the leaf structure.
As the photosynthetic organs, chloroplasts are vulnerable to stress-induced damages. Early structural changes occur in the chloroplasts when the leaves suffer from abiotic stresses (Li et al., 2018). Therefore, examination of the chloroplast ultrastructure is important to investigate photosynthesis and the effects of abiotic stress on the photosynthetic apparatus (Hu et al., 2015). In this study, we compared the changes in the chloroplast ultrastructure of two tung tree species at 15 DAT. We found that a low-temperature treatment significantly damaged the chloroplast structure, loosening the thylakoids until they were disintegrated and increasing the number of liposomes in the chloroplasts, indicating that the photosynthetic apparatus was destroyed in both species during the late stage of the low-temperature stress. Similar observations have also been made by other workers (Taylor and Craig, 1971; Marzanna et al., 2002). Thus, we can conclude that the decrease in the rate of photosynthesis during the late stage of low-temperature stress was mostly due to non-stomatal factors that damaged the photosynthetic mechanism by analysing results above. Furthermore, most of the chloroplasts in V. montana leaves had disintegrated, and the damage was even more severe than that in V. fordii. In contrast to the control, the chloroplasts of plants sprayed with BR were normal and intact, and only the stroma lamellae were loosely arranged and slightly swollen and expanded. In addition, a small number of starch granules were present in the chloroplasts, and the number of liposomes in the V. montana chloroplasts was increased. This finding is consistent with those of Yuan et al. (2012) and Gill et al. (2017) and suggests that exogenous application of BR can protect the chloroplasts of both tung tree species, alleviating the damage to the photosynthetic apparatus caused by long-term low-temperature treatments, maintaining photosynthetic efficiency, and ensuring the basic survival needs of the plants. This supported the above conclusion that long-term low temperature conditions could limit the tung tree growth by damaging the chloroplasts structure and reducing the photosynthetic product production, while exogenous application of BR could increase the activity of photosynthetic apparatus, which might be a potential mechanism for tung trees to tolerate low-temperature stress through investing more energy into protecting the plant rather than having the energy produced by chloroplasts be consumed on the chloroplasts when there is no externally applied BR. Therefore, we could conclude that exogenous application of BR could be a potential way to prevent tung tree to suffer from the damage of low temperature stress.
Comparison of the photosynthetic characteristics between V. fordii and V. montana under the low-temperature conditions has revealed that damage to the photosynthetic mechanism in V. montana is the main factor affecting photosynthesis in the middle period of stress, whereas the damage to V. fordii does not become a dominant factor until the late period of stress. In addition, based on observations of the chloroplast ultrastructure, we have found that the low temperature treatment causes less damage to the chloroplasts in V. fordii than in V. montana. Therefore, it can be concluded that V. fordii has greater cold resistance than V. montana.
Conclusions
Our results clearly showed that low temperature caused decreases in the Pn and WUE, slowed growth, reduced photosynthesis, altered the internal leaf structure, and destroyed the chloroplast structure thus negatively affecting the normal growth and development of V. fordii and V. montana seedlings. Exogenous spraying of BR enhanced the photosynthetic potential, shortened the non-photochemical processes of the photosynthetic organs, increased the efficiency and potential of photoelectron transmission, maintained the activity of the photosynthetic organs, promoted the stability of the leaf structure and morphology, and effectively alleviated the damage caused by the cold stress. Moreover, both stomatal and non-stomatal factors played an important role in the reduction of the photosynthetic efficiency of tung tree seedlings caused by the cold stress. The decrease in the photosynthetic efficiency caused by the short-term cold-induced injury was mainly due to stomatal factors, while the decrease in photosynthetic efficiency caused by the long-term cold-induced injury was mainly caused by non-stomatal factors that damaged the photosynthetic mechanism. Finally, V. fordii exhibited a stronger cold resistance to cold condition than V. montana.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Author Contributions
FZ analyzed the results. KL and YG prepared plant materials and collected the samples. LZ and WL prepared Figures 6–9. FZ, KL, and ZL wrote the main manuscript text. All authors reviewed the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Key R&D Program of China (2017YFD0600703) and the Key Research Program of the Education Bureau of Hunan Province, China (18A161). The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/VHGGg6
References
Allen, D. J., Ort, D. R. (2001). Impacts of chilling temperature on photosynthesis in warm-climate plants. Trends Plant Sci. 6, 36–42. doi: 10.1016/s1360-1385(00)01808-2
Anuradha, S., Rao, S. S. R. (2009). Effect of 24-epibrassinolide on the photosynthetic activity of radish plants under cadmium stress. Photosynthetica 47, 317–320. doi: 10.1007/s11099-009-0050-3
Cao, H. P., Shockey, J. M. (2012). Comparison of Taqman and SYBR green qPCR methods for quantitative gene expression in Tung tree tissues. J. Agric. Food Chem. 60 (50), 12296–12303. doi: 10.1021/jf304690e
Cao, H. X., Huang, H. J., Lei, X. T., Zhang, D. P., Zhang, R. L., Sun, C. X. (2014). The effect of different low temperature treatment on the anatomical structure of oil palm leaves. Chin. J. Trop. Crop 35 (3), 454–459. doi: 10.3969/j.issn.1000-2561.2014.03.007
Cao, X. C., Zhong, C., Zhu, L. F., Zhang, J. H., Sajid, H., Wu, L. H., et al. (2017). Glycine increases cold tolerance in rice via the regulation of N uptake, physiological characteristics, and photosynthesis. Plant Physiol. Biochem. 112, 251–260. doi: 10.1016/j.plaphy.2017.01.008
Chen, Z. F., Wang, Z., Yang, Y. G., Li, M., Xu, B. C. (2018). Abscisic acid and brassinolide combined application synergistically enhances drought tolerance and photosynthesis of tall fescue under water stress. Sci. Hortic. 228, 1–9. doi: 10.1016/j.scienta.2017.10.004
Chen, J., Liu, W. J., Fan, Y. R., Zhou, X., Tang, X. W., Zhang, L. (2019). Identification and analysis of tRNA genes provide new insights into oil biosynthesis in tung tree (Vernicia fordii Hemsl.). Ind. Crop Prod. 137, 74–80. doi: 10.1016/j.indcrop.2019.05.016
Chen, B. Z. (1987). Preliminary report on the damage of tung tree. Forest. Sci. Tech. 10, 6–8. doi: 10.13456/j.cnki.lykt.1987.04.003
Dallaire, S., Houde, M., Yves, G., Saini, H. S., Boileau, S., Chevrier, N., et al. (1994). ABA and low temperature induce freezing tolerance via distinct regulatory pathways in wheat. Plant Cell Physiol. 35 (1), 1–9. doi: 10.1093/oxfordjournals.pcp.a078559
Demmig-Adams, B. (1990). Carotenoids and photo protection in plants: a role for the xanthophyll zeaxanthin. BBA-Bioenergetics 1020 (1), 1–24. doi: 10.1016/0005-2728(90)90088-L
Ensminger, I., Busch, F., Huner, N. P. A. (2006). Photostasis and cold acclimation: sensing low temperature through photosynthesis. Physiol. Plant 126, 28–44. doi: 10.1111/j.1399-3054.2006.00627.x
Fariduddin, Q., Yusuf, M., Chalkoo, S., Hayat, S., Ahmad, A. (2011). 28-homobrassinolide improves growth and photosynthesis in Cucumis sativus L. through an enhanced antioxidant system in the presence of chilling stress. Photosynthetica 49 (1), 55–64. doi: 10.1007/s11099-011-0022-2
Farquhar, G. D., Sharkey, T. D. (1982). Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 33 (1), 74–79. doi: 10.1146/annurev.pp.33.060182.001533
Fujii, S., Saka, H. (2001). The promotive effect of brassinolide on lamina joint-cell elongation, germination and seedling growth under low-temperature stress in rice (Oryza sativa L.). Plant Prod. Sci. 4, 210–214. doi: 10.1626/pps.4.210
Gao, C., Yuan, D. Y., Yang, Y., Wang, B. F., Liu, D. M., Zou, F. (2015). Pollen tube growth and double fertilization in Camellia oleifera. J. AMER. Soc Hortic. Sci. 140 (1), 12–18. doi: 10.21273/JASHS.140.1.12
Gill, M. B., Cai, K. F., Zhang, G. P., Zeng, F. R. (2017). Brassinolide alleviates the drought-induced adverse effects in barley by modulation of enzymatic antioxidants and ultrastructure. Plant Growth Regul. 82 (3), 447–455. doi: 10.1007/s10725-017-0271-6
Gu, Y. Y., Zhang, F. H., Zeng, Y. L., Zhang, L., Tan, X. F., Cao, H. P., et al. (2019). Physiological responses of tung tree (Vernicia fordii) saplings to different red, white and blue light-emitting diodes. Int. J. Agric. Biol. 22, 569–577. doi: 10.17957/IJAB/15.1101
Hayat, S., Hasan, S. A., Yusuf, M., Hayat, Q., Ahmad, A. (2010). Effect of 28-homobrasino-lide on photosynthesis, fluorescence and antioxidant system in the presence or absence of salinity and temperature in Vigna radiata. Environ. Exp. Bot. 69 (2), 105–112. doi: 10.1016/j.envexpbot.2010.03.004
He, F., Wu, J. J. (1991). Mechanism of the affecting low temperature stress on seed germination and seedling growth of tung oil trees. Nonwood For. Res. 9 (1), 1–10. doi: 10.14067/j.cnki.1003-8981.1991.01.001
Hu, L. L., Yu, J. H., Liao, W. B., Zhang, G. B., Xie, J. M., Lv, J., et al. (2015). Moderate ammonium: nitrate alleviates low light intensity stress in mini Chinese cabbage seedling by regulating root architecture and photosynthesis. Sci. Hortic. 186, 143–153. doi: 10.1016/j.scienta.2015.02.020
Huner, N. P. A., Quist, G., Hurry, V. M., Krol, M., Falk, S., Griffith, M. (1993). Photosynthesis, photo inhibition and low temperature acclimation in cold tolerant plants. Photosynth. Res. 37 (1), 19–39. doi: 10.1007/bf02185436
Kagale, S., Divi, U. K., Krochko, J. E., Keller, W. A., Krishna, P. (2007). Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napusto a range of abiotic stresses. Planta 225 (2), 353–364. doi: 10.2307/23389554
Kaur, R., Kaur, N., Singh, H. (2019). Pericarp and pedicel anatomy in relation to fruit cracking in lemon (Citrus limon L Burm.). Sci. Hortic. 246, 462–468. doi: 10.1016/j.scienta.2018.11.040
Kodra, E., Steinhaeuser, K., Ganguly, A. R. (2011). Persisting cold extremes under 21st-century warming scenarios. Geophys. Res. Lett. 38, 1–5. doi: 10.1029/2011GL047103
Kurepin, L. V., Ivanov, A. G., Zaman, M., Pharis, R. P., Allakhverdiev, S. I., Hurry, V., et al. (2015). Stress-related hormones and glycinebetaine interplay in protection of photosynthesis under abiotic stress conditions. Photosynth. Res. 126 (2/3), 221–235. doi: 10.1007/s11120-015-0125-x
Li, Z., Long, H. X., Zhang, L., Liu, Z. M., Cao, H. P., Shi, M. W., et al. (2017a). The complete chloroplast genome sequence of tung tree (Vernicia fordii): organization and phylogenetic relationships with other angiosperms. Sci. Rep. 7, 1869. doi: 10.1038/s41598-017-02076-6
Li, Z., Tan, X. F., Lu, K., Liu, L. M., Wu, L. L. (2017b). The effect of CaCl2 on calcium content, photosynthesis, and chlorophyll fluorescence of tung tree seedlings under drought conditions. Photosynthetica 55 (3), 553–560. doi: 10.1007/s11099-016-0676-x
Li, Q. C., Wang, H. B., Wang, H. J., Zheng, W., Wu, D. M., Wang, Z. Z. (2018). Effects of kinetin on plant growth and chloroplast ultrastructure of two Pteris species under arsenate stress. Ecotox. Environ. Safe. 158, 37–43. doi: 10.1016/j.ecoenv.2018.04.009
Li, Z., Shi, K., Zhang, F. H., Zhang, L., Long, H. X., Zeng, Y. L., et al. (2019). Growth, physiological, and biochemical responses of tung tree (Vernicia fordii) seedlings to different light intensities. Hortscience 54 (8), 1361–1369. doi: 10.21273/HORTSCI14035-19
Lin, Q., Li, Z., Zhang, L., Tan, X. F., Long, H. X., Wu, L. L. (2016). High-efficiency regeneration of seedlings from hypocotyl explants of tung tree (Vernicia fordii). Int. J. Agric. Biol. 18, 370–376. doi: 10.17957/IJAB/15.0097
Liu, K., Zhang, F. H., Li, Z., Liang, W. H., Lan, J. X. (2019). Effects of different magnesium levels on light-response curves and physiological characteristics of tung tree. J. Cent. S. U. For. Tech. 12, 40–45. doi: 10.14067/j.cnki.1673-923x.2019.12.006
Liu, M. L., Long, H. X., Li, W. Y., Shi, M. W., Cao, H. P., Zhang, L., et al. (2019a). Boosting C16 fatty acid biosynthesis of Escherichia coli, yeast and tobacco by tung tree (Vernicia fordii Hemsl.) beta-hydroxyacyl-acyl carrier protein dehydratase gene. Ind. Crop Prod. 127, 46–54. doi: 10.1016/j.indcrop.2018.10.067
Liu, Y. S., Geng, J. C., Sha, X. Y., Zhao, Y. X., Hu, T. M., Yang, P. Z. (2019b). Effect of rhizobium symbiosis on low-temperature tolerance and antioxidant response in alfalfa (Medicago sativa L.). Front. Plant Sci. 10, 538. doi: 10.3389/fpls.2019.00538
Marzanna, S., Mieczyslaw, K., Alina, K. (2002). Low temperature- induced modfications in cell ultrastructure and localization of phenolics in winter oilseed rape (Brassica napus L. var. oleifera L.) leaves. Ann. Bot. 90 (5), 637–645. doi: 10.1007/BF00484418
Moon, B. Y., Higashi, S. I., Murata, G. N. (1995). Unsaturation of the membrane lipids of chloroplasts stabilizes the photosynthetic machinery against low-temperature photo inhibition in transgenic tobacco plants. Proc. Nat. Acad. Sci. 92, 6219–6223. doi: 10.2307/2367814
Muhammad, I., Muhammad, A., Shafiq, U. R., Eui, S. R. (2006). Does polyamine seed pretreatment modulate growth and levels of some plant growth regulators in hexaploid wheat (Triticum aestivum L.) plants under salt stress? Bot. Stud. 47, 239–250.
Ogweno, J. O., Song, X. S., Hu, W. H., Shi, K., Zhou, Y. H., Yu, J. Q. (2009). Detached leaves of tomato differ in their photosynthetic physiological response to moderate high and low temperature stress. Sci. Hortic. 123 (1), 17–22. doi: 10.1016/j.scienta.2009.07.011
Olaf, K., Jan, F. H. S. (1990). The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 25 (3), 147–150. doi: 10.1007/BF00033156
Qu, X. J., Wang, H., Chen, M., Liao, J., Yuan, J., Niu, G. H. (2019). Drought stress–induced physiological and metabolic changes in leaves of two oil tea cultivars. J. AMER. Soc Hortic. Sci. 144 (n), 1–9. doi: 10.21273/JASHS04775-19
Rudell, D. R., Buchanan, D. A., Leisso, R. S., Whitakeer, B. D., Mattheis, J. P., Zhu, Y. M., et al. (2011). Ripening, storage temperature, ethylene action, and oxidative stress alter apple peel phytosterol metabolism. Phytochemistry 72 (11-12), 1328–1340. doi: 10.1016/j.phytochem.2011.04.018
Sanghera, G. S., Wani S., H., Hussain, W., Singh N, B. (2011). Engineering cold stress tolerance in crop plants. Curr. Genomics 12 (1), 30–43. doi: 10.2174/138920211794520178
Saxby, T., Dennison, W. C., Hoegh-Guldberh, O. (2003). Photosynthetic responses of the coral Montipora digitata to cold temperature stress. Mar. Ecol. Prog. Ser. 248, 85–97. doi: 10.3354/meps248085
Schreiber, U., Schliwa, U., Bilger, W. (1986). Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 10 (1-2), 51–62. doi: 10.1007/bf00024185
Schreiber, S. G., Hamann, A., Hacke, U. G., Thomas, B. R. (2013). Sixteen years of winter stress: an assessment of cold hardiness, growth performance and survival of hybrid poplar clones at a boreal planting site. Plant Cell Environ. 36, 419–428. doi: 10.1111/j.1365-3040.2012.02583.x
Shakirova, F., Allagulova, C., Maslennikova, D., Fedorova, K., Yuldashev, R., Lubyanova, A., et al. (2016). Involvement of dehydrins in 24-epibrassinolide-induced protection of wheat plants against drought stress. Plant Physiol. Bioch. 108, 539–548. doi: 10.1016/j.plaphy.2016.07.013
Shockey, J. M., Gidda, S. K., Chapital, D. C., Kuan, J. C., Dhanoa, P. K., Bland, J. M., et al. (2006). Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell. 18, 2294–2313. doi: 10.1105/tpc.106.043695
Stevens, R., Page, D., Gouble, B., Garchery, C., Zamir, D., Causse, M. (2008). Tomato fruit ascorbic acid content is linked with monodehydroascorbate reductase activity and tolerance to chilling stress. Plant Cell Environ. 31 (8), 1086–1096. doi: 10.1111/j.1365-3040.2008.01824.x
Stirbet, A., Govindje, E. (2011). On the relation between the Kautsky effect (chlorophylla a fluorescence induction) and Photosystem II: basics and applications of the OJIP fluorescence transient. J. Photoch. Photobio. B. 104 (1-2), 236–257. doi: 10.1016/j.jphotobiol.2010.12.010
Tan, X. F., Jiang, G. X., Tan, F. Y. (2011). Research report on industrialization development strategy of Vernicia fordii in China. Nonwood For. Res. 29, 1–5. doi: 10.14067/j.cnki.1003-8981.2011.03.001
Taylor, A. O., Craig, A. S. (1971). Plant under climatic stress II. Low temperature, high light effects on chloroplast ultrastructure. Plant Physiol. 47, 719–725. doi: 10.1104/pp.47.5.719
Teng, Y., Li, A. D., Hao, Z. Y., Zhang, H. L., Zhang, L. M., Cai, G. J. (2018). Anatomical structure of Passiflora caerulea L. and relationship between leaf structure and cold resistance under low temperature stress. Acta Agric. Zhejiangensis. 30 (11), 1849–1858. doi: 10.3969/j.issn.1004-1524.2018.11.07
Theocharis, A., Christophe, C., Barka, E. A. (2012). Physiological and molecular changes in plants grown at low temperatures. Planta 235 (6), 1091–1105. doi: 10.1007/s00425-012-1641-y
Vardhini, B. V. (2017). Modifications of morphological and anatomical characteristics of plants by application of brassinosteroids under various abiotic stress conditions. Plant Gene. 11, 70–89. doi: 10.1016/j.plgene.2017.06.005
Wang, L. X., Jiang, D. F., Gu, X. P., Bian, C. J., Li, Q. (2013). Analysis of jatropha's wintering security based on low-temperature damage index in Guizhou Province. Southwest China J. Agric. Sci. 26 (06), 2350–2355. doi: 10.16213/j.cnki.scjas.2013.06.084
Wani, A. S., Tahir, I., Ahmad, S. S., Dar, R. A., Nisar, S. (2017). Efficacy of 24-epibrassinolidein improving the nitrogen metabolism and antioxidant system in chickpea cultivars under cadmium and/or NaCl stress. Sci. Hortic. 225, 48–55. doi: 10.1016/j.scienta.2017.06.063
Wolfgang, B., Olle, B. (1990). Role of the xanthophyll cycle in photo protection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 25 (3), 173–185. doi: 10.1007/bf00033159
Wu, Z. J., Li, X. H., Liu, Z. W., Li, H., Wang, Y. X., Zhuang, J. (2015). Transcriptome-based discovery of AP2/ERF transcription factors related to temperature stress in tea plant (Camellia sinensis). Funct. Integr. Genomic. 15 (6), 741–752. doi: 10.1007/s10142-015-0457-9
Xiong, H., Gu, X. P., Yu, F., Lou, Y. P. (2011). The impact of the weather condition on the Jatropha curcas quality. Chin. J. Agrometeorol. 32 (S1), 124–129. [in Chinese]
Yamori, W., Masumoto, C., Fukayama, H., Makino, A. (2012). Rubisco activase is a key regulator of non-steady-state photosynthesis at any leaf temperature and, to a lesser extent, of steady-state photosynthesis at high temperature. Plant J. 71, 871–880. doi: 10.1111/j.1365-313x.2012.05041.x
Yang, Z. Q., Han, D., Wang, L., Jin, Z. F. (2016). Changes in photosynthetic parameters and antioxidant enzymatic activity of four tea varieties during a cold wave. Acta Ecol. Sin. 36 (3), 629–641. doi: 10.5846/stxb201405130981. [in Chinese].
Yoshikawa, H., Honda, C., Kondo, S. (2007). Effect of low-temperature stress on abscisic acid, jasmonates, and polyamines in apples. Plant Growth Regul. 52 (3), 199–206. doi: 10.1007/s10725-007-9190-2
Yu, J. Q., Zhou, Y. H., Ye, S. F., Huang, L. F. (2002). 24-epibrassinolide and abscisic acid protect cucumber seedlings from chilling injury. J. Hortic. Sci. Biotechnol. 77, 470–473. doi: 10.1080/14620316.2002.11511524
Yuan, L. Y., Shu, S., Sun, J., Guo, S. R., Takafumi, T. (2012). Effects of 24-epibrassinolide on the photosynthetic characteristics, antioxidant system, and chloroplast ultrastructure in Cucumis sativus L.under Ca(NO3)2 stress. Photosynth. Res. 112 (3), 205–214. doi: 10.1007/s11120-012-9774-1
Zhang, F. H., Li, Z., Tan, X. F., Yang, C. C., Li, W. Y., Li, Y. L. (2018). Effects of BR application on the growth and physiological characteristics of Tung tree seedlings at different temperatures. Nonwood For. Res. 36 (3), 17–24. doi: 10.14067/j.cnki.1003-8981.2018.03.004
Keywords: Vernicia fordii, Vernicia montana, low-temperature stress, brassinolide, chloroplast ultrastructure, chlorophyll fluorescence
Citation: Zhang F, Lu K, Gu Y, Zhang L, Li W and Li Z (2020) Effects of Low-Temperature Stress and Brassinolide Application on the Photosynthesis and Leaf Structure of Tung Tree Seedlings. Front. Plant Sci. 10:1767. doi: 10.3389/fpls.2019.01767
Received: 11 September 2019; Accepted: 17 December 2019;
Published: 31 January 2020.
Edited by:
Shifeng Cao, Zhejiang Wanli University, ChinaReviewed by:
Xiaofeng Tan, Columbia University, United StatesAndrzej Bajguz, University of Białystok, Poland
Suriyan Cha-um, National Science and Technology Development Agency (NSTDA), Thailand
Copyright © 2020 Zhang, Lu, Gu, Zhang, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ze Li, lize1853@163.com
†These authors have contributed equally to this work
 Fanhang Zhang
Fanhang Zhang Kun Lu
Kun Lu Yiyang Gu
Yiyang Gu Lin Zhang
Lin Zhang Wenying Li
Wenying Li Ze Li
Ze Li