- Ministry of Education Key Laboratory of Molecular and Cellular Biology, Hebei Collaboration Innovation Center for Cell Signaling, Hebei Key Laboratory of Molecular and Cellular Biology, College of Life Sciences, Hebei Normal University, Shijiazhuang, China
Brassinosteroids (BRs) are essential plant growth- and development-regulating phytohormones. When applied exogenously, BRs ameliorate heat shock (HS)-induced cell damage and enhance plant thermotolerance; however, the molecular mechanism by which BRs regulate plant thermotolerance is unknown. In this study, by analyzing the thermotolerance of a series of BR signaling mutants and plants that overexpressed different BR signaling components, we obtained comprehensive data showing that BRASSINOSTEROID INSENSITIVE 2 (BIN2) plays a major role in mediating the crosstalk between BR signaling and plant HS responses. By RNA-Seq, 608 HS- and BIN2-regulated genes were identified. An analysis of the 1-kb promoter sequences of these genes showed enrichment of an abscisic acid (ABA) INSENSITIVE 5 (ABI5)-binding cis-element. Physiological studies showed that thermotolerance was reduced in bin2-1 mutant and ABI5-OX plants but increased in the abi5 mutant, and that the abi5 mutation could recover the thermotolerance of bin2-1 plants to a wild-type level, suggesting that ABI5 functions downstream of BIN2 in regulating plant thermotolerance. Further, HS treatment increased the cellular abundance of BIN2. Both bin2-1 mutant and BIN2-OX plants showed early flowering, while the BIN2 loss-of-function mutant bin2-3 bil1 bil2 flowered late. Given these findings, we propose that under HS conditions plants increase BIN2 activity to promote early flowering and ensure species survival; however, this reduces the thermotolerance and survivability of individual plants partially by activating ABI5.
Introduction
With the increasing impact of the greenhouse effect, it is estimated that the global surface temperature has increased by approximately 1.09°C compared with that observed in the pre-industrial era1. Because of global warming, the appearance of seasonally extreme heat has become increasingly frequent. This makes heat stress one of the most important environmental factors limiting plant growth and productivity. Heat stress has a deleterious influence on almost every aspect of plant growth and development; it can change the thylakoid structure in chloroplasts, inactivate Rubisco, reduce the number of photosynthetic pigments, and destroy photosystem II to quickly inhibit photosynthesis (Danilova et al., 2018; Dogra et al., 2019; Hu et al., 2020). Heat stress also inhibits plant development, promotes early flowering, causes tissue and organ senescence, inhibits pollen formation and fertilization, reduces seed vigor, and suppresses seed germination and grain filling (Ma et al., 2019; Jung et al., 2020; Li et al., 2021a; Poidevin et al., 2021; Ren et al., 2021). One study showed that from 1964 to 2007, drought and extreme heat significantly reduced global cereal production by 9–10% (Lesk et al., 2016). Therefore, uncovering the mechanism by which plants respond to heat stress and using that knowledge to promote the breeding of heat stress-tolerant crops will protect future global agricultural productivity.
When they encounter high temperatures, plants quickly reprogram their cellular transcriptome and metabolism to increase the production and accumulation of heat shock (HS) proteins (HSPs), antioxidants, and osmolytes to enhance thermotolerance (Li et al., 2019; Ali et al., 2020). HS also regulates the expression of phytohormone biosynthesis and hormone response-related genes (Li et al., 2019, 2021b). Phytohormones such as abscisic acid (ABA), cytokinin, salicylic acid (SA), and jasmonic acid (JA) could all enhance the HS tolerance of plants (Li et al., 2021b); however, it is unclear whether these phytohormones function independently or interdependently to ameliorate HS-induced damage and increase plant heat tolerance.
Brassinosteroids (BRs) are a group of plant steroid hormones. Since their discovery in the 1970s, BRs have been shown to play essential roles in regulating diverse growth and developmental processes in plants (Yang et al., 2011). Genetic, biochemical, and molecular biological studies have revealed the signaling pathway by which BRs regulate plant growth and development. Extracellular BRs are perceived by the plasma membrane-localized receptor BRASSINOSTEROID INSENSITIVE 1 (BRI1) and its co-receptor, BRI1 ASSOCIATED RECEPTOR KINASE 1 (BAK1) (Li and Chory, 1997; Li et al., 2002; Nam and Li, 2002). BR binding promotes transphosphorylation between BRI1 and BAK1, and it activates downstream signaling components, including BR SIGNALING KINASEs (BSKs), CONSTITUTIVE DIFFERENTIAL GROWTH 1 (CDG1), and BRI1-SUPPRESSOR 1 (BSU1), via sequential protein phosphorylation (Mora-Garcia et al., 2004; Tang et al., 2008; Kim et al., 2009, 2011). Upon being activated by BR signaling, the protein phosphatase BSU1 dephosphorylates and inactivates downstream BRASSINOSTEROID INSENSITIVE 2 (BIN2) family protein kinases, and it promotes BIN2 degradation via the KINK SUPPRESSED in bzr1-1D (KIB1)-mediated proteasome pathway (Peng et al., 2008; Kim et al., 2009; Zhu et al., 2017). BIN2 is a negative regulator of BR signaling. When the extracellular BR level is low, BIN2 phosphorylation inactivates two homologous transcription factors, BRASSINAZOLE RESISTANT 1 (BZR1) and BRI1-EMS-SUPPRESSOR 1 (BES1), resulting in the accumulation of BZR1/BES1 in the cytoplasm (Vert and Chory, 2006; Gampala et al., 2007). BR signaling inactivates BIN2 and promotes the translocation of phosphorylated BZR1 from the cytoplasm to the nucleus where it is dephosphorylated by the protein phosphatase PP2A; it then binds to the promoters of downstream target genes, regulating their expression (Sun et al., 2010; Tang et al., 2011; Wang et al., 2021).
Besides its role in regulating plant growth and development, BR signaling has been found to regulate plant adaptations to various environmental abiotic stresses such as high or low temperatures, drought, and salt (Planas-Riverola et al., 2019). When applied exogenously, BRs increase the thermotolerance of a variety of plant species, including Arabidopsis, rice, tomato, barley, eggplant, Cucumis melo, and Brassica napus (Kagale et al., 2007; Sadura and Janeczko, 2018; Yin et al., 2018). Consistent with these results, mutants deficient in BR biosynthesis or signaling are more susceptible to HS treatment (Yin et al., 2018; Fang et al., 2020), while plants overexpressing BR biosynthesis- or signaling-related genes show increased HS tolerance (Sahni et al., 2016; Yin et al., 2018). Meanwhile, reports indicate that BR biosynthesis- or signaling-deficient mutants have greater HS tolerance than wild-type plants (Mazorra et al., 2011; Sadura et al., 2019). The contradictory HS-related phenotypes observed in these studies suggest that BRs regulate HS responses in plants in a tissue/organ-specific manner and via multiple mechanisms. Therefore, identifying the components that mediate the crosstalk between BR signaling and plant HS responses is necessary to uncover the mechanism by which BRs regulate plant thermotolerance.
In this study, we systematically analyzed the thermotolerance of several BR biosynthesis/signaling-deficient or hyperactive Arabidopsis mutants and transgenic plants. Our data show that BIN2 plays an essential role in mediating the regulation of plant HS responses by BR signaling. HS induced the accumulation of BIN2, which accelerated the life cycle of Arabidopsis plants under HS conditions by promoting early flowering, while decreasing their thermotolerance. These results reveal a novel mechanism by which plants promote early flowering to adapt to HS under natural conditions, and they explain why BR treatment increases the thermotolerance of Arabidopsis plants.
Materials and Methods
Plant Materials and Growth Conditions
Most of the Arabidopsis mutants and transgenic plants used in this study were reported previously (Wang et al., 2002; Mora-Garcia et al., 2004; Tang et al., 2008; Yan et al., 2009; Hu and Yu, 2014; Zhou et al., 2015; Yang et al., 2016; Chen et al., 2019). Seeds (30 per genotype) were surface-sterilized and sown on glass plates containing 30 ml of half-strength Murashige and Skoog (1/2 MS) agar medium (0.6% Phytagel, 0.5 × MS basal salt mixture, and 1% sucrose, with the pH adjusted to 5.7–5.8 using KOH). For BR treatment, Petri dishes with two compartments were used; each compartment contained 15 ml of 1/2 MS agar medium supplied with or without 0.1 μM epibrassinolide (eBL). The seeds were stratified at 4°C for 2–3 days then placed in a growth chamber (Percival Scientific, Perry, IA, United States) under long-day (LD; 16 h of light/8 h of dark) conditions at 22°C for 7 days before HS treatment, material harvesting, or transplantation to soil with subsequent growth in a greenhouse under the same growth conditions in order to examine flowering-related phenotypes.
Thermotolerance Assays
For the basal thermotolerance (BT) assay, plates containing 7-day-old seedlings were transferred to a new growth chamber (Percival Scientific) with the inside temperature preset to 45°C (HS treatment) or kept in the same growth chamber (22°C; control) and left for 60–75 min. For the acclimated thermotolerance (AT) assay, plates containing 7-day-old seedlings were first exposed to 37°C for 1 h. The plants were then allowed to recover at 22°C for 2 h before being subjected to 45°C or 22°C in a growth chamber (Percival Scientific) for 3 h. In both the BT and AT assays, after HS treatment the plants were allowed to continue to grow at 22°C under LD conditions for 3–4 days before being photographed and examined for survival. Each HS treated glass plate is considered as one biological replicate. In general, at least 30 biological replicates collected from four to five independent experiments were used for survival rate quantification, unless otherwise indicated.
Ion Leakage Assay
The electrical conductivity of deionized water was set as C0. Immediately after HS treatment, plants were incubated in deionized water (10 ml per 30 seedlings) and shaken mildly at room temperature for 1 h after which the conductivity of the solution was measured (C1). The seedlings were then boiled in the same deionized water for 1 h and the conductivity of the solution was measured again after cooling to room temperature (C2). Ion leakage (%) was calculated as the ratio of (C1 − C0)/(C2 − C0) (Li et al., 2017).
RNA Sequencing and Bioinformatic Analysis
Seven-day-old seedlings grown on 1/2 MS agar medium were transferred to a 37°C growth chamber (Percival Scientific) or kept in the same 22°C growth chamber (Percival Scientific) and left for 1 h before tissue samples were harvested for total RNA extraction using an RNeasy Plant Mini Kit (Qiagen, Germantown, MD, United States). The 2 μg of RNA per sample was sent for Oxford Nanopore Technologies-based single-molecule real-time sequencing following a standard protocol. Super-long reads, which contain sequence information about a single complete transcript, were identified and aligned to the Arabidopsis reference genome (TAIR11) using bioinformatics analysis tools (Minimap2) on the BMKCloud platform2. Three biological replicates were used, and differentially expressed genes (DEGs) were identified using the edgeR package (version 3.8.6) with the following parameters: fold change (FC) ≥ 2 and p-value < 0.05.
Gene Ontology (GO) analysis was performed online using AgriGO3. For cis-element motif enrichment analysis, promoter and 5′-untranslated sequences (−1000 to −1) of the target genes were downloaded from the Arabidopsis Resource Center4 (TAIR11) and loaded onto the MEME Suite webtool (version 5.4.1) for motif-based sequence analysis5.
Gene Expression Analysis
One-week-old seedlings grown on 1/2 MS agar medium were treated at 37°C or 22°C in growth chambers for the indicated time. Harvested plant tissue was ground in liquid nitrogen to a fine powder, and total RNA was extracted from 100 mg of tissue using TRIzol Reagent (CWBio, Taizhou, Jiangsu, China) according to the manual provided by the manufacturer. First-strand cDNA was synthesized from 1 μg of total RNA using HiScript II Q Select RT SuperMix for qPCR (+gDNA wiper) (Vazyme Biotech Co., Nanjing, Jiangsu, China). Quantitative PCR (qPCR) was performed using ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech Co.) on a C1000 Touch Thermal Cycler CFX384 (Bio-Rad Hercules, CA, United States). The relative abundance of each transcript was determined by the comparative threshold cycle method (Schmittgen and Livak, 2008), using UBC30 as an equal loading control. The gene-specific primers used are listed in Supplementary Table 4.
Immunoblotting
Plant tissues were ground to a fine powder in liquid nitrogen. In a heat block, SDS sample buffer (0.125 M Tris-HCl, pH 6.8, 4% SDS, 20% glycerol, 2% β-mercaptoethanol, bromophenol blue, and 1× protease inhibitor cocktail) was preheated to 95°C and then added to the tissue powder at a ratio of 20 μl per 10 mg of tissue powder. After vigorous vortexing, the homogenate was heated at 95°C for 5 min and then centrifuged at 12,000 × g for 10 min at 4°C. The supernatant was used for SDS-PAGE and immunoblotting with anti-GFP (Roche, Basel, Switzerland) or anti-tubulin (Sigma-Aldrich, St. Louis, MO, United States) antibodies.
Results
Brassinosteroids Regulate Basal Thermotolerance and Acclimated Thermotolerance in Plants
To determine the role of BRs in regulating plant HS tolerance, we first evaluated whether altering the endogenous BR biosynthesis level would alter the thermotolerance of Arabidopsis plants. Compared with wild-type plants, the BT and AT were significantly reduced in the BR biosynthesis-deficient mutant det2, but increased in transgenic plants overexpressing the rate-limiting BR biosynthesis gene DWF4 (DWF4-OX) (Supplementary Figure 1A and Figures 1A,B). Electrolyte leakage is an indicator of HS-induced plasma membrane damage. Corresponding with our thermotolerance result, after HS treatment electrolyte leakage was significantly increased in the det2 mutant but reduced in DWF4-OX plants (Figure 1B).
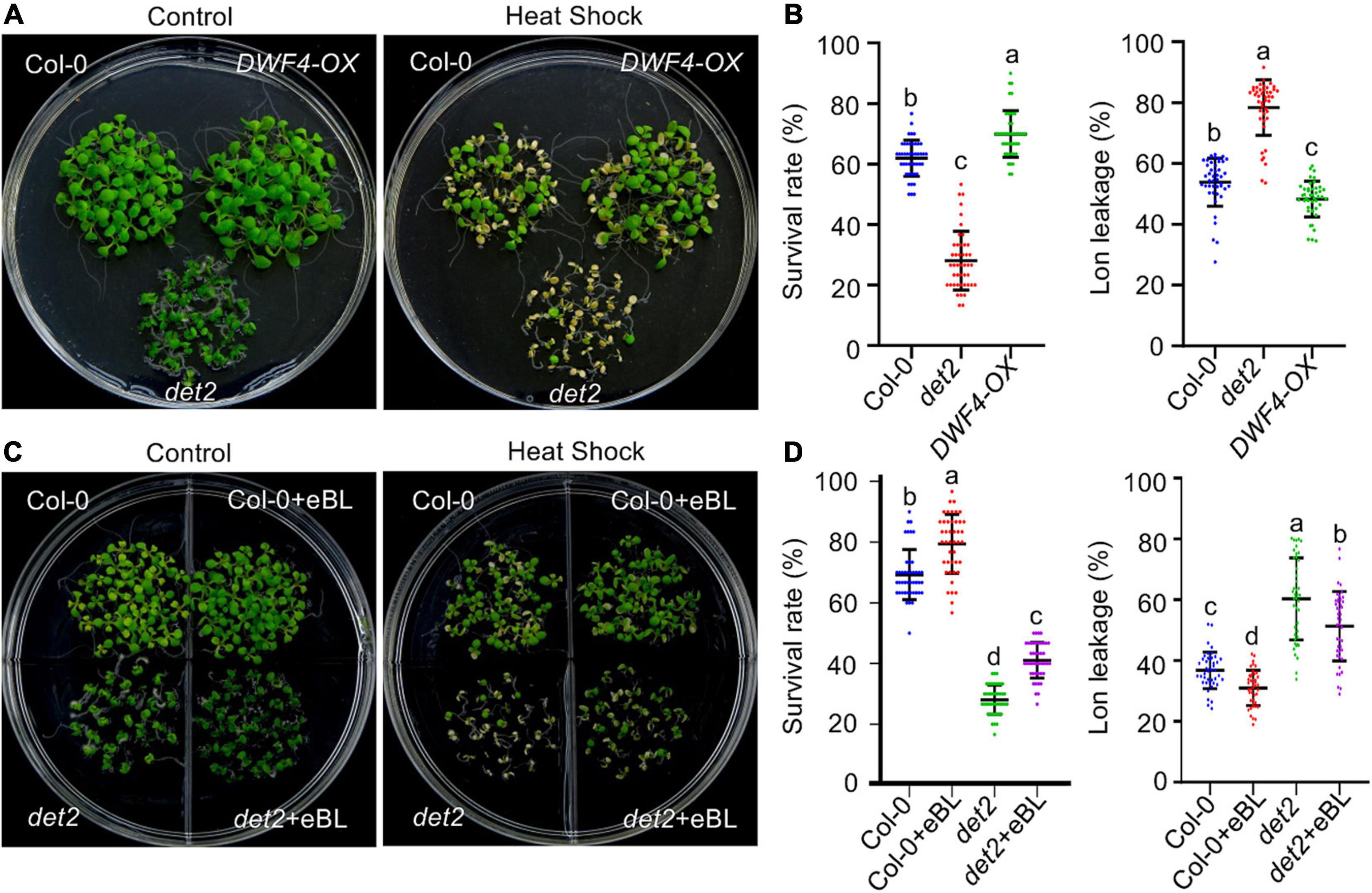
Figure 1. Brassinosteroid regulates acquired thermotolerance in Arabidopsis. (A,C) The phenotypes of HS-treated and untreated plants. One-week-old seedlings grown on 1/2 MS agar medium supplemented with or without 0.1 μM eBL under LD conditions at 22°C were exposed to 37°C for 1 h. After recovering at 22°C for 2 h, the seedlings were exposed to 22°C (control) or 45°C (HS) for 3 h and then allowed to recover at 22°C for 3 days before being imaged. (B,D) Quantification of the survival rates of and ion leakage from the plants shown in panels (A,C). Error bars indicate the mean ± standard deviation (SD). Statistically significant differences are indicated by different lowercase letters (p < 0.05, one-way ANOVA).
Next, we tested whether exogenously applied BRs would recover the reduced thermotolerance observed in the det2 mutant. Both wild-type and det2 mutant plants were grown in two compartment plates containing 1/2 MS agar medium supplied with or without 0.1 μM eBL for 1 week before high temperature treatment. The addition of eBL to the growth medium significantly improved the BT and AT, and reduced electrolyte leakage, in wild-type and det2 plants (Supplementary Figure 1B and Figures 1C,D). Nevertheless, det2 mutants grown in a eBL-containing medium were much more sensitive to HS compared with wild-type seedlings grown in the absence of BR. These results show that although exogenously applied eBL could enhance plant thermotolerance, they did not fully complement the reduced thermotolerance of the det2 mutant. Thus, the spatial-temporal activation of BR signaling in vivo plays a critical role in regulating plant responses to high temperatures.
BRASSINOSTEROID INSENSITIVE 1, BRASSINOSTEROID SIGNALING KINASE 3, and BRASSINOSTEROID INSENSITIVE 1-SUPPRESSOR 1 Contribute to Brassinosteroid-Regulated Thermotolerance in Plants
To investigate which BR signaling component plays a major role in mediating BR-enhanced HS tolerance in plants, the survival rates of BR signaling mutants and transgenic plants overexpressing BR signaling components were systematically assessed following HS treatment. Because strong BR signaling mutants show extreme dwarfism, weak BR signaling mutants were used to avoid potential artifacts caused by the severe vegetative growth retardation observed in strong BR signaling mutants. Both the BT and AT were significantly reduced in the BR receptor (BRI1)-deficient mutants bri1-301 and bri1-5 (Figures 2A,B,E,F and Supplementary Figures 1C,E), as well as in the mutant bsk3 (Figures 2C,D and Supplementary Figure 1D), but increased in transgenic plants overexpressing BRI1 (Figures 2A,B and Supplementary Figure 1C) and BSK3 (Figures 2C,D and Supplementary Figure 1D). The reduced thermotolerance of the bsk3 mutant is caused by the knock down of the expression of BSK3 (Tang et al., 2008); introducing the BSK3 genomic sequence fused with a C-terminal YFP tag into the bsk3 mutant (BSK3-com) fully recovered the thermotolerance of the bsk3 mutant to a wild-type level (Figures 2C,D and Supplementary Figure 1D). bsu1-1D is a gain-of-function activation-tagged mutant that overexpresses BSU1. Similar to its ability to recover the vegetative growth of the bri1-5 mutant (Mora-Garcia et al., 2004), bsu1-1D partially restored the reduced thermotolerance of bri1-5 plants, even though the BT and AT of bsu1-1D were similar to those of the wild-type control (Figures 2E,F and Supplementary Figure 1E). An analysis of electrolyte leakage in these HS-treated plants supported our altered thermotolerance data (Figures 2B,D,F). Together, these results suggest that BRI1-, BSK3-, and BSU1-mediated BR signaling contributes to the enhanced thermotolerance of BR-treated plants.
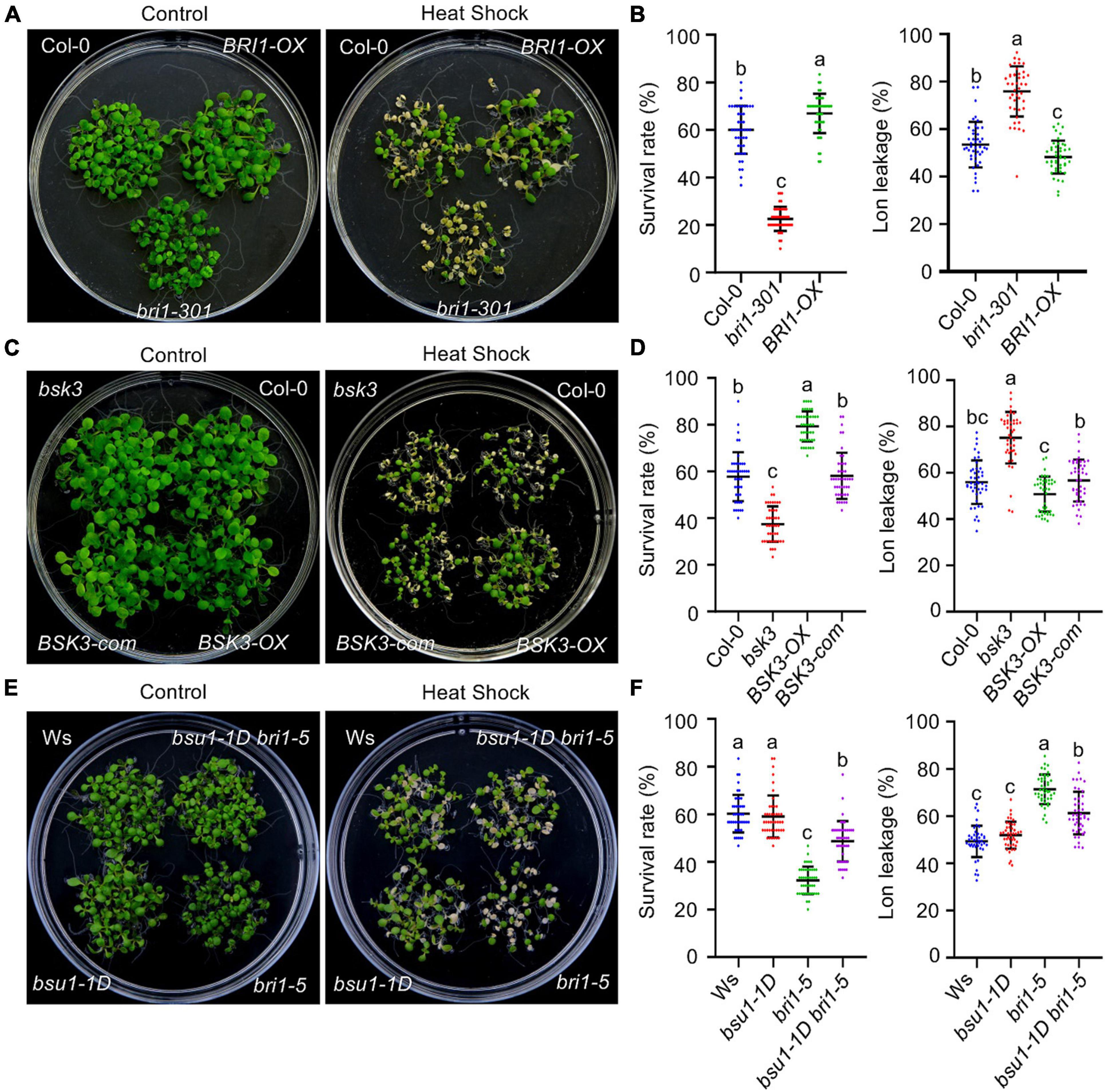
Figure 2. The BR signaling components BRI1, BSK3, and BSU1 regulate acquired thermotolerance in Arabidopsis. (A,C,E) Photographs of HS-treated and untreated plants after 3 days of recovery at 22°C. (B,D,F) Quantification of the survival rates of and ion leakage from the plants shown in panels (A,C,E). Error bars indicate the mean ± SD. Statistically significant differences are indicated by different lowercase letters (p < 0.05, one-way ANOVA).
BRASSINOSTEROID INSENSITIVE 2 Mediates Crosstalk Between the Brassinosteroid Signaling Pathway and Plant Thermotolerance
BRASSINOSTEROID INSENSITIVE 2 is the downstream target of BSU1 in BR signaling. BIN2 and its homologs BIN2-LIKE1 (BIL1) and BIL2 function redundantly in regulating BR signaling (Yan et al., 2009). To explore the relationship between BIN2 and BR-regulated plant HS responses, the thermotolerance of the loss-of-function mutant bin2-3 bil1 bil2 (bin2-t) and 35Spro:BIN2-myc (BIN2-OX) plants were examined. When exposed to HS, both the BT and AT were significantly increased in the bin2-t mutant but decreased in BIN2-OX plants (Figures 3A,B and Supplementary Figure 1F). Correspondingly, electrolyte leakage was increased in the BIN2-OX plants (Figure 3B).
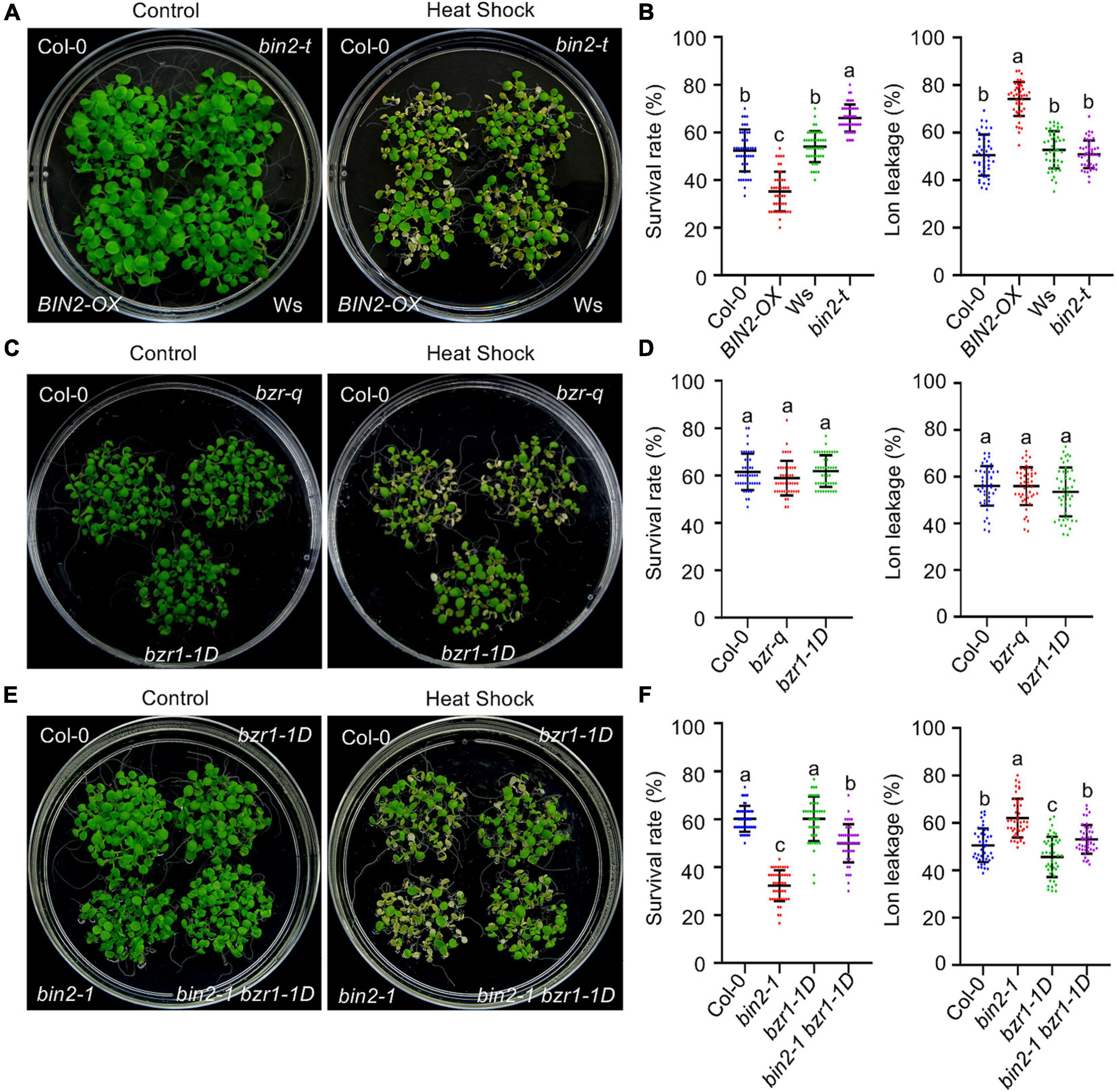
Figure 3. The BR signaling component BIN2 (but not BZRs) plays a major role in regulating acquired thermotolerance in Arabidopsis. (A,C,E) Photographs of HS-treated and untreated plants after 3 days of recovery at 22°C. bin2-t represents the bin2-3 bil1 bil2 triple mutant and bzr-q is a bes1 beh1 beh2 beh3 quadruple mutant. (B,D,F) Quantification of the survival rates of and ion leakage from the plants shown in panels (A,C,E). Error bars indicate the mean ± SD. Different lowercase letters indicate statistically significant differences (p < 0.05, one-way ANOVA).
BRASSINAZOLE RESISTANT 1/BRI1-EMS-SUPPRESSOR 1 play an essential role in BR-regulated gene expression, and the activity of BZR1/BES1 in BR signaling is tightly controlled by BIN2 phosphorylation (Vert and Chory, 2006). Besides BZR1 and BES1, there are four additional BZR1/BES1 HOMOLOG (BEH) genes encoded in the Arabidopsis genome. These BZR1/BES1 family transcription factors (BZRs) play redundant regulatory roles in BR signaling (Chen et al., 2019). We therefore tested whether there is a correlation between cellular BZR activity and plant thermotolerance. Upon HS treatment, bes1 beh1 beh2 beh3 quadruple mutant (bzr-q) plants (Chen et al., 2019) and bzr1-1D, a gain-of-function bzr1 mutant with constitutively activated expression of downstream BR target genes even in the absence of BRs (Wang et al., 2002), exhibited similar survival rates and electrolyte leakage compared with wild-type plants under both non-acclimated and acclimated conditions (Figures 3C,D and Supplementary Figure 1G), indicating BZRs do not play a major role in regulating plant thermotolerance.
To obtain additional information on the role of BZRs in regulating plant HS responses, the thermotolerance of bin2-1, a gain-of-function mutant that stabilizes BIN2 (Li and Nam, 2002), and bin2-1 bzr1-1D mutant plants was examined. Similar to BIN2-OX plants, the BT and AT were significantly decreased and electrolyte leakage after HS was increased in the bin2-1 mutant. Introducing the bzr1-1D mutation into bin2-1 mutant plants increased the survival rate from 37.7 ± 6.3% to 52.7 ± 6.2% under non-acclimated conditions, and from 32.3 ± 6.4% to 49.9 ± 8% under acclimated conditions. However, when compared with the survival rate of wild-type control plants (68.3 ± 7.4% under BT conditions and 60.1 ± 5.4% under AT conditions), the bin2-1 bzr1-1D double mutant was still hypersensitive to HS (Figures 3E,F and Supplementary Figure 1H). Consistent with these survival data, plant electrolyte leakage after HS was significantly increased in the bin2-1 mutant, and partially recovered but still lower than in the wild-type control in the bin2-1 bzr1-1D double mutant (Figure 3F). Together, these results suggest that BIN2 plays a dominant role in mediating crosstalk between the BR signaling pathway and plant HS responses. However, the enhanced thermotolerance observed in the bin2-1 bzr1-1D mutant (compared with bin2-1) suggests that BIN2 and BZR1 oppositely regulate the activity of a subset of common downstream targets to influence plant thermotolerance.
Heat Shock Increases BRASSINOSTEROID INSENSITIVE 2 Protein Abundance and the Nuclear Accumulation of BRASSINAZOLE RESISTANT 1
The above results suggested that BR signaling inactivates BIN2 to increase plant thermotolerance. The question is why plants would need to activate BR signaling to defend themselves against HS damage under natural conditions. Would HS alter the activity and/or subcellular localization of BIN2 and BZRs? By real-time quantitative PCR (qPCR), we found that treating plants at 37°C for 1 or 2 h significantly inhibited the expression of BIN2 and BZR1, respectively, and that the transcript levels of BIN2 and BZR1 continued to be suppressed by treatment at 37°C for up to 24 h (Figure 4A). We also examined whether HS affected the protein abundance of BIN2 and BZR1 using BIN2pro:BIN2-YFP and BZR1pro:BZR1-YFP transgenic plants. In contrast to our transcription data, treatment at 37°C for 1 h increased the BIN2-YFP protein level, and the abundance of BIN2-YFP continued to increase as the HS treatment period was increased (Figure 4B). In comparison, the protein abundance of phosphorylated BZR1-YFP was not obviously altered, while the level of dephosphorylated BZR1 was significantly reduced at 1 h after HS treatment (Figure 4B).
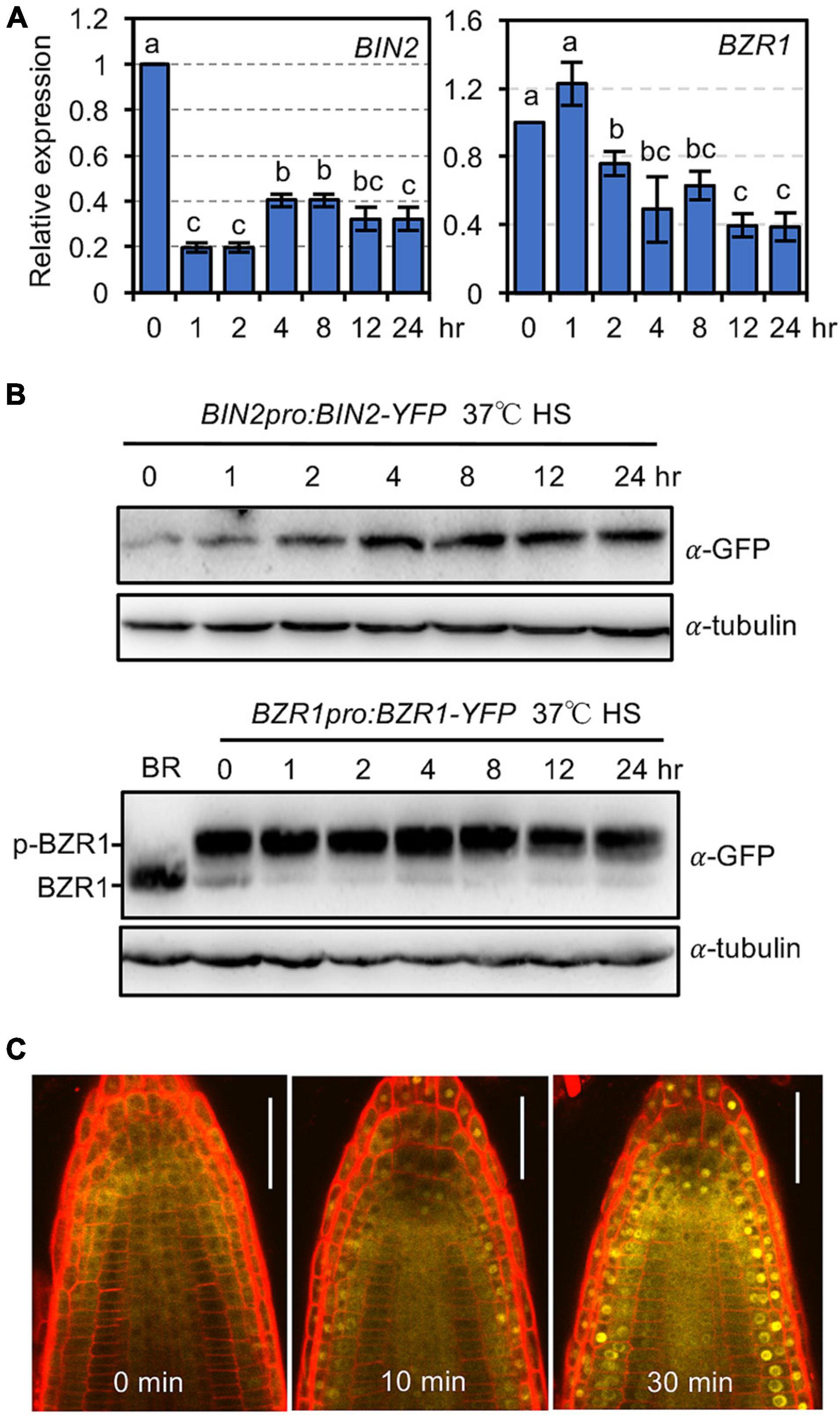
Figure 4. Heat shock induces BIN2 protein accumulation and promotes the nuclear localization of BZR1. (A) The relative expression of BIN2 and BZR1 transcripts in 1-week-old Col-0 seedlings grown at 37°C for the indicated time. Error bars indicate the mean ± SD (n = 3). Statistically significant differences are indicated by different lowercase letters (p < 0.05, one-way ANOVA). (B) Immunoblotting revealed the abundance of BIN2-YFP and BZR1-YFP in 1-week-old seedlings treated at 37°C for the indicated time. For BR treatment, seedlings were soaked in ddH2O containing 1 μM eBL for 2 h. (C) Confocal microscopic examination of the subcellular localization of BZR1-YFP in the roots of 5-day-old plants grown on 1/2 MS agar medium containing 0.5 μM PCZ. The plates were treated at 37°C for the indicated time, and then the roots were mounted in a solution containing 0.5 μM PCZ and 1 mg/mL of propidium iodide and observed. Scale bars, 20 μm.
Brassinosteroid signaling promotes the nuclear accumulation and dephosphorylation of BZR1 to activate downstream target genes. Previously, it was shown that ambient high temperature treatment induced the nuclear localization of BZR1 (Ibañez et al., 2018). To investigate whether HS also promotes the nuclear accumulation of BZR1, the subcellular localization of BZR1-YFP was examined in 1-week-old root cells from BZR1pro:BZR1-YFP-expressing plants by confocal microscopy. Nuclear localization of BZR1-YFP was observed after 10 min of treatment at 37°C, even in the presence of 0.5 μM propiconazole (PCZ), a BR biosynthesis inhibitor (Figure 4C). The nuclear localization of BZR1-YFP became increasingly obvious when the HS treatment period was extended to 30 min (Figure 4C). Considering that HS increased the cellular BIN2 protein level but decreased the dephosphorylated BZR1-YFP level, it is likely that the HS-induced accumulation of BIN2 prevented nuclear-localized BZR1 from being dephosphorylated by PP2A.
The Identification of BRASSINOSTEROID INSENSITIVE 2 and Heat Shock Coregulated Genes
To gain insight into BIN2-mediated crosstalk between the BR signaling pathway and plant HS responses, RNA-Seq was employed to identify BIN2 and HS coregulated genes at the genome-wide level using the following parameters as cutoffs: FC ≥ 2 and P < 0.05. In total, 3998 DEGs (1417 upregulated and 2581 downregulated) whose expression level was affected by treatment for 1 h at 37°C were identified (Figure 5A and Supplementary Table 1). Meanwhile, 1515 DEGs (533 upregulated and 982 downregulated) whose expression was differently regulated in 1-week-old bin2-1 and Col-0 plants grown at 22°C under LD conditions were identified (Figure 5A and Supplementary Table 2). An examination of the RNA-Seq data revealed several genes among the 3998 HS-regulated DEGs whose expression was previously demonstrated to be upregulated by HS treatment, including HSFA2, HSFA7a, HSFB2a, HSP90.1, HSP70, HSP60, HSP22, HSP21, and HSP18.2 (Li et al., 2019). Additionally, genes known to be downregulated by BR signaling, including BR6OX2, CYP90D1, ROT3, and DWF4, were found to be upregulated in the bin2-1 mutant. Together, these results indicate that our HS treatment and RNA-Seq experiments were successful.
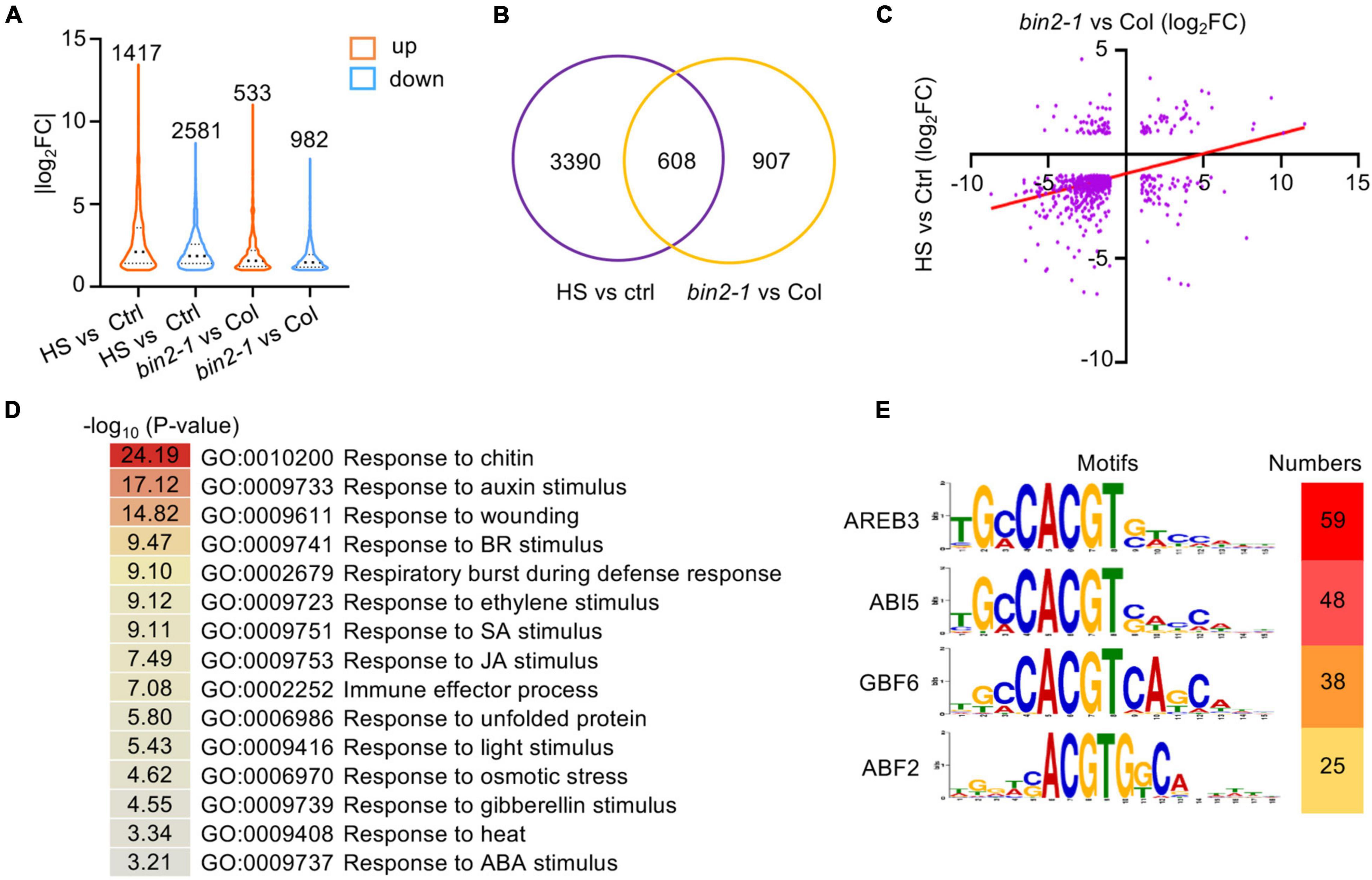
Figure 5. Transcriptome analysis of HS- and BIN2-regulated genes. (A) The numbers and distribution of HS- and BIN2-regulated genes. One-week-old seedlings grown on 1/2 MS agar medium were treated with (Col-0) or without (bin2-1 and Col-0) HS at 37°C for 1 h. (B) A Venn diagram showing the number of genes that were differentially regulated by HS treatment or the bin2-1 mutation. (C) The relative expression levels of the 608 HS and BIN2 coregulated genes shown in panel (B). X-axis: the relative expression levels determined by comparing HS-treated and untreated Col-0 seedlings. Y-axis: the relative expression levels determined by comparing bin2-1 and Col-0 plants grown at 22°C. (D) GO classification of the 608 HS and BIN2 coregulated genes. (E) The top four enriched cis-elements in the 608 HS and BIN2 coregulated genes are shown.
Among the DEGs identified in the bin2-1 mutant, 608 (40.13%) were regulated by HS treatment (Figure 5B). These genes were designated HS and BIN2 coregulated DEGs. Interestingly, most of these HS and BIN2 coregulated DEGs (445, 73.19%) were downregulated by HS treatment and in the bin2-1 mutant (Figure 5C and Supplementary Table 3). GO enrichment analysis showed that genes involved in plant hormone responses (e.g., to auxin, BR, ethylene, SA, JA, gibberellin, and ABA), defense responses (e.g., to chitin, wounding, the respiratory burst during defensive responses, and the immune effector process), and abiotic stress responses (e.g., to unfolded protein, light stimulus, osmotic stress, and heat stress) were enriched in 608 HS- and BIN2-coregulated DEGs (Figure 5D).
BRASSINOSTEROID INSENSITIVE 2 Suppresses Plant Thermotolerance Partially Through ABSCISIC ACID INSENSITIVE 5
To uncover the transcription factors that mediate the expression of our HS- and BIN2-regulated DEGs, 1000 bp of genomic sequence, including the promoter and 5′-untranslated region located upstream of the ATG, from the 608 HS and BIN2 coregulated DEGs were subjected to local motif enrichment analysis using the MEME Suite webtool. Cis-elements that were recognized by AREB3, ABI5, GBF6, and/or ABF2 were the top-scoring motifs (Figure 5E). As the sequences of these enriched motifs are very similar and all contain an ACGT core sequence, and given that AREB3, ABI5, and ABF2 are important transcription factors in ABA signaling, HS and BIN2 likely regulate the expression of a subset of ABA-responsive genes to influence plant thermotolerance.
Previously, it was reported that bin2-1 mutant plants were hypersensitive to ABA (Yang et al., 2016), and that BIN2 phosphorylates and stabilizes ABI5 to enhance the expression of ABI5-targeted genes (Hu and Yu, 2014). The enrichment of ABI5-recognizing cis-elements in our HS and BIN2 coregulated DEGs prompted us to test whether ABI5 participates in BIN2 regulated plant HS responses. We first examined if high temperature would regulate ABI5 transcript or protein abundance. qPCR showed that 37°C treatment for 1 h could significantly increase the expression of ABI5. As HS treatment continues, the transcript level of ABI5 starts to decrease and remain low or slightly lower than HS untreated plants (Supplementary Figure 2A). In comparison, ABI5-YFP protein from ABI5pro:ABI5-YFP transgenic plant remains unchanged when plants were subjected to 37°C for 2 h, but starts to accumulate continuously when HS treatment time increased from 4 h to 24 h (Supplementary Figure 2B). Next, we tested whether the expression level of ABI5 would affect plant tolerance to HS treatment. Plants were exposed to 45°C for 60 min (non-acclimation conditions) or 3 h (acclimation conditions), and then allowed to recover at 22°C for 4 days. Quantification of the seedling survival rate showed that both the BT and AT were increased in the abi5 mutant, which had no detectable ABI5 protein (Zhou et al., 2015), but decreased in transgenic plants overexpressing ABI5 (ABI5-OX) (Hu and Yu, 2014). This enhanced thermotolerance seemed to be specifically regulated by the reduced expression of ABI5 in the abi5 mutant, as the transfer of the genomic ABI5 sequence back into abi5 (ABI5 com) plants restored the BT of abi5 to a wild-type level, while it reduced the AT of abi5 to a level that was lower than in the wild-type control (Figures 6A,B and Supplementary Figure 1I). Consistent with this, HS-induced electrolyte leakage was significantly lower in the abi5 mutant, higher in ABI5-OX plants, and at a similar level in the ABI5-com plants compared with that in the wild-type control (Figure 6B). These results demonstrate that ABI5 is a negative regulator of plant HS responses.
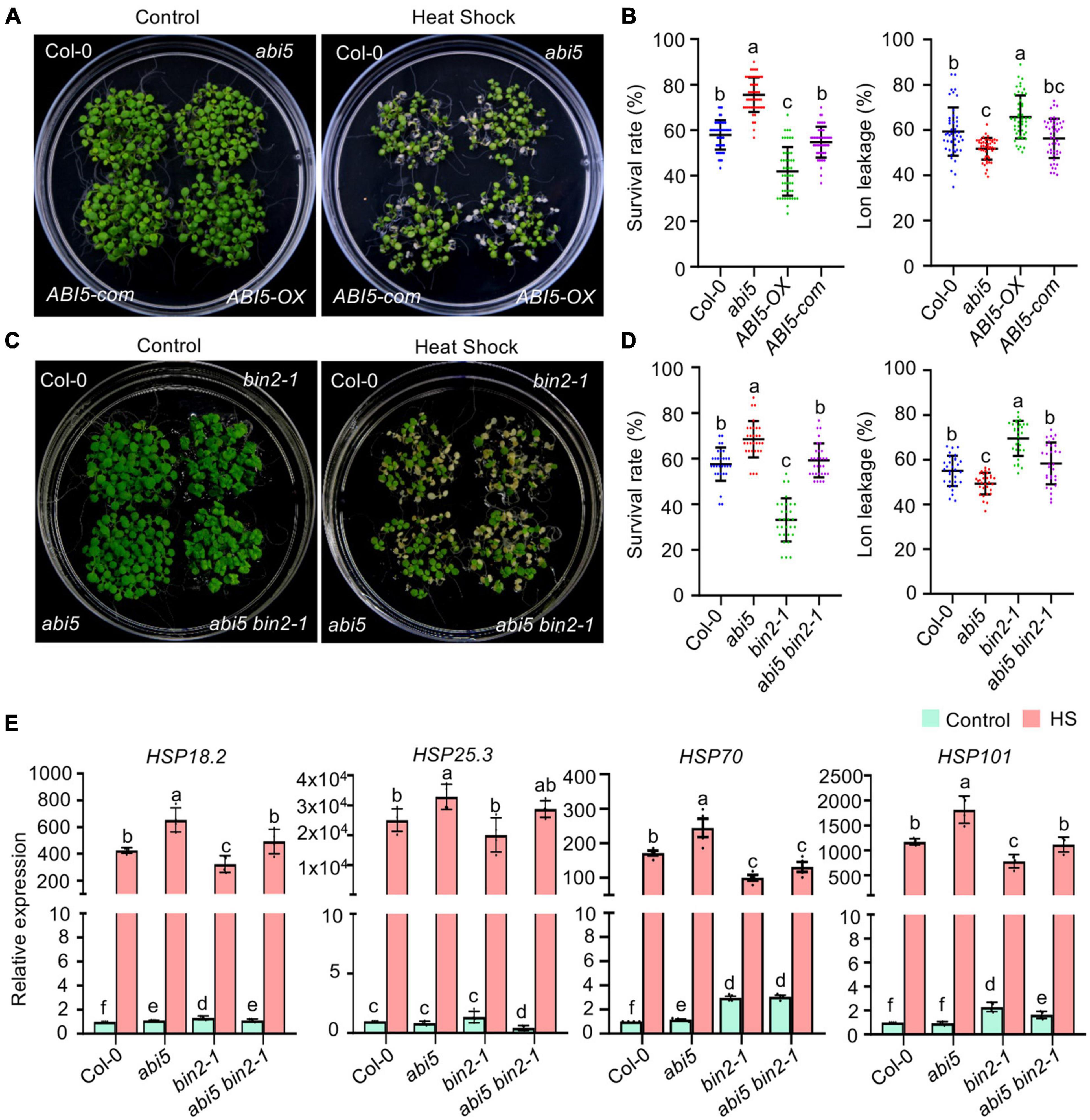
Figure 6. Abscisic acid (ABA) INSENSITIVE 5 (ABI5) functions downstream of BIN2 to regulate acquired thermotolerance in Arabidopsis. (A,C) Photographs of HS-treated and untreated plants after 3 days of recovery at 22°C. ABI5-com is an ABI5 complementation line in the abi5 background. ABI5-OX was produced in the Col-0 background. (B,D) Quantification of the survival rates of and ion leakage from the plants shown in panels (A,C). Error bars indicate the mean ± SD. Statistically significant differences are indicated by different lowercase letters (p < 0.05, one-way ANOVA). (E) Quantitation of HSPs transcript levels in one-week-old plants that were treated with 22°C (control) or 37°C (HS) for 1 h. Error bars indicate the mean ± SD (n = 4). Statistically significant differences are indicated by different lowercase letters (p < 0.05, Student’s t-test).
Because both bin2-1 mutant and ABI5-OX plants were hypersensitive to HS treatment while BIN2 phosphorylation increased cellular ABI5 activity, we wondered whether increased ABI5 activity was responsible for the reduced thermotolerance of the bin2-1 mutant. To test this hypothesis, we generated an abi5 bin2-1 double mutant by genetic crossing and assessed its thermotolerance. Similar to the above results, both the BT and AT were significantly increased in the abi5 mutant and decreased in bin2-1 mutant plants. Introduction of the abi5 T-DNA insertion mutation into a bin2-1 mutant background increased the BT and AT of bin2-1 plants to wild-type levels (Figures 6C,D and Supplementary Figure 1J). Together with our HS-induced electrolyte leakage data (Figure 6D), these results suggest that bin2-1-activated ABI5 partially contributes to the reduced thermotolerance observed in bin2-1 plants.
Under stressful conditions, plants generally induce the expression of HSPs to prevent stress-induced protein aggregation and help ameliorate stress-induced cellular damage (Haq et al., 2019). To investigate whether BIN2 and ABI5 regulate a common set of downstream target genes to influence plant thermotolerance, the expression of HSP18.2, HSP25.3, HSP70, and HSP101 was examined by qPCR in bin2-1 and abi5 plants. Treatment at 37°C for 1 h dramatically increased the expression of all four genes. In accordance with the thermotolerance of the mutants (Figures 6C,D and Supplementary Figure 1J), the expression of these HSPs was significantly increased in the abi5 mutant, lower in the bin2-1 mutant, and at a similar level for HSP18.2, HSP25.3, and HSP101 in the abi5 bin2-1 double mutant compared with that in Col-0 (control) plants (Figure 6E).
Increased BRASSINOSTEROID INSENSITIVE 2 Activity Promotes Early Flowering
The above results showed that HS induced the accumulation of BIN2, leading to ABI5 activation and reduced plant thermotolerance. We next considered how plants benefit from the HS-induced accumulation of BIN2. When bin2-1 mutant, bin2-t mutant, and BIN2-OX plants were grown at 22°C under LD conditions, both the bin2-1 mutant and BIN2-OX plants flowered 3–5 days earlier than Col-0 control plants (Figure 7 and Supplementary Figure 3), while the bin2-t mutant flowered about 5 days later than Ws control plants (Figure 7). Quantitation of the rosette leaves also showed that at the onset of flowering, the bin2-1 mutant and BIN2-OX plants had fewer leaves than the Col-0 control (Figure 7 and Supplementary Figure 3), while the bin2-t mutant had more leaves than did the Ws control (Figure 7), indicating that increased BIN2 activity promotes early flowering in Arabidopsis.
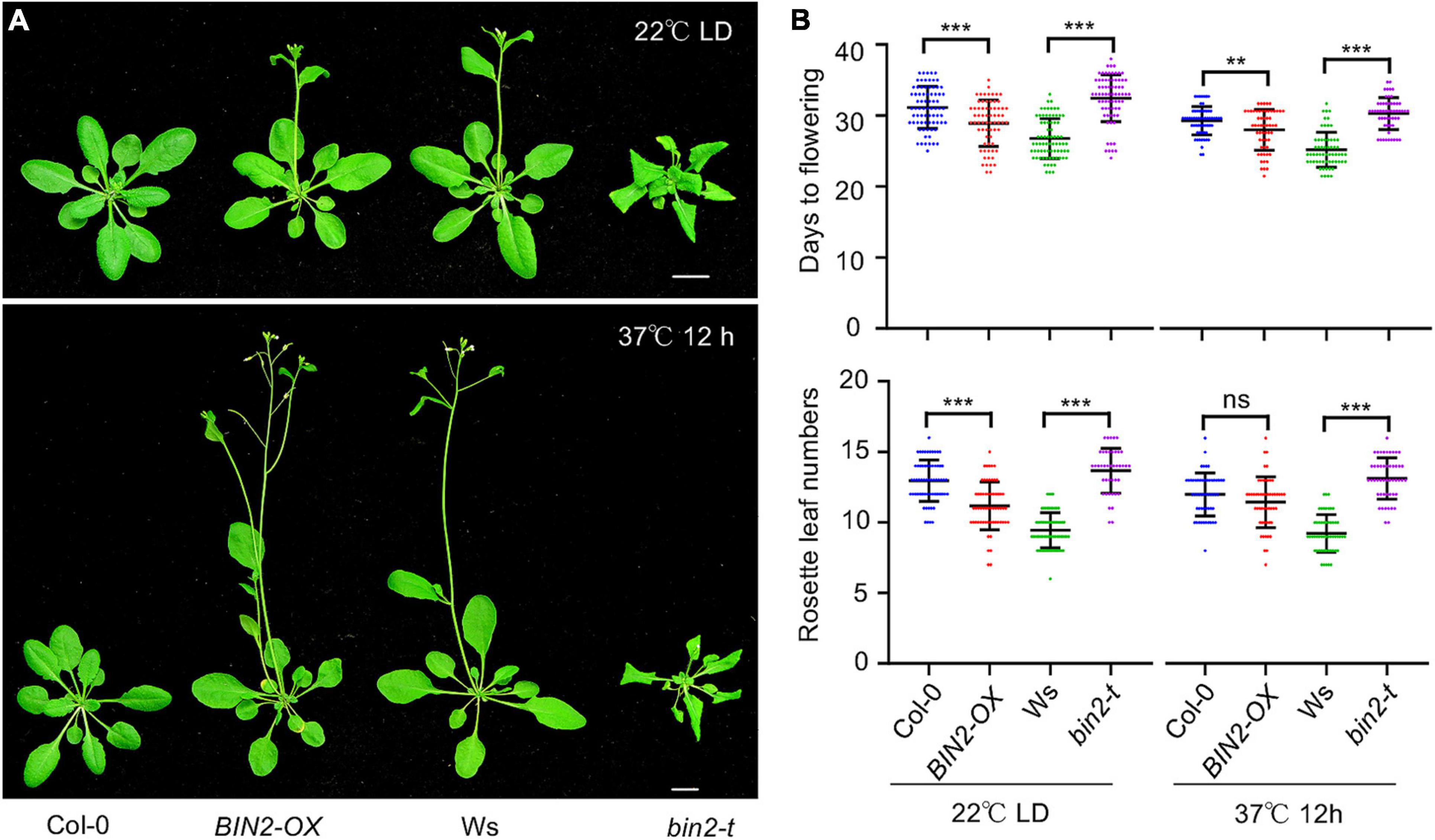
Figure 7. BRASSINOSTEROID INSENSITIVE 2 (BIN2) positively regulates flowering time in Arabidopsis. (A) Photographs of Col-0, BIN2-OX, Ws, and bin2-3 bil1 bil2 (bin2-t) plants. Two-week-old plants grown in soil under LD conditions at 22°C were either exposed to 37°C for 12 h or kept at 22°C. All plants were then allowed to continue growing at 22°C until the first flower opened. (B) Quantitation of the days to flowering and the rosette leaf numbers for the flowering plants shown in panel (A). Error bars indicate the mean ± SD (n ≥ 3). Statistical significance is indicated by asterisks (**p < 0.01; ***p < 0.001; ns, not significant, Student’s t-test).
One strategy that plants use to cope high temperatures is early flowering. If high temperatures really increase intracellular BIN2 activity to promote early flowering, the differences in flowering time between wild-type and bin2-1 mutant or BIN2-OX plants should be smaller under high-temperature conditions. Accordingly, we heat-shocked 2-week-old plants (grown at 22°C under LD conditions) at 37°C for 12 h. The plants were then allowed to continue growing at 22°C under LD conditions until flowering. HS treatment promoted flowering in Col-0 (from 31 ± 3 days to 29 ± 2 days) and BIN2-OX (from 28 ± 3 days to 27 ± 3 days) plants such that it occurred 2 days and 1 day earlier, respectively. In addition, upon HS treatment, Col-0 had one less leaf (from 13 ± 1 to 12 ± 2) while BIN2-OX had the same number of leaves (from 11 ± 2 to 11 ± 2) when the plants started flowering. In comparison, following HS treatment, both the Ws (from 27 ± 3 days to 25 ± 2 days) and bin2-t mutant (32 ± 3 days to 30 ± 2 days) plants flowered 2 days earlier, and the Ws plants had the same number of rosette leaves (9 ± 1) while the bin2-t mutant (from 14 ± 2 to 13 ± 1) had one less leaf when the plants started flowering (Figure 7). Together, these results suggest that increased BIN2 activity contributes to early flowering under HS conditions.
Discussion
Under high temperature stress, plants quickly adjust their cellular metabolism and gene expression patterns to cope with the changed environment. The exogenous application of plant phytohormones such as ABA, cytokinin, BRs, SA, or JA can mitigate heat-induced damage and increase the thermotolerance of plants (Li et al., 2021b). However, the mechanisms by which phytohormone signaling regulates plant thermotolerance are not well understood. In this study, by sequentially examining the thermotolerance of different BR signaling mutants and plants overexpressing major BR signaling components, we obtained comprehensive genetic evidence showing that BIN2 mediates the crosstalk between BR signaling and plant HS responses.
Several lines of evidence from our study suggest that HS can increase cellular BIN2 activity. First, HS induced the accumulation of BIN2 protein, while the BIN2 transcript level was reduced. HS likely increases the de novo biosynthesis of BIN2 or enhances the protein’s stability, thereby increasing the cellular abundance of BIN2. Second, we recently showed that BZR1 phosphorylation by BIN2 and dephosphorylation by PP2A all happen in the nucleus (Wang et al., 2021). Mobilizing BZR1 into the nucleus by MG132 treatment increased the chance of an interaction between BZR1 and nuclear-localized PP2A and promoted BZR1 dephosphorylation, even in the absence of BRs (Wang et al., 2021). If the same principle applies to the HS-induced translocation of BZR1 to the nucleus, we should be able to observe an increased level of dephosphorylated BZR1 after HS treatment. The discovery that HS treatment could induce the nuclear localization of BZR1 while also decreasing the dephosphorylation level of BZR1 suggests that HS exposure increases BIN2 activity and prevents nuclear-localized BZR1 from being dephosphorylated by PP2A. Third, our transcriptomic analysis showed that HS exposure and the bin2-1 mutation downregulate a similar group of genes that are involved in regulating plant responses to hormones and abiotic/biotic stresses. Taken together, these results suggest that HS suppresses plant thermotolerance by increasing BIN2 activity. Exogenously applied BRs promotes BIN2 degradation, thereby increasing plant thermotolerance.
Interestingly, 10 min of exposure to heat stress (37°C) quickly induced the nuclear translocation of BZR1, even in the absence of BRs. The HS-induced nuclear localization of BZR1 might be mediated by sumoylation because HS is known to induce massive protein sumoylation (Rytz et al., 2018) and the sumoylation of BZR1 promotes its nuclear localization (Srivastava et al., 2020). By RNA-Seq analysis, we found that BR metabolism- or biosynthesis-related genes were significantly downregulated by short-term (up to 30 min) exposure to 37°C. Also, a BZR1-recognizing cis-element was enriched in DEGs regulated by 10 min of HS treatment, suggesting that BZR1 helps regulate early plant responses to high temperature exposure (Li et al., 2019). It would be interesting to examine whether the accumulation of phosphorylated BZRs in the nucleus regulates the expression of downstream target genes via protein–protein interactions with other transcription factors.
As a protein kinase, BIN2 plays a major role in regulating plant growth and development and in plant responses to biotic/abiotic stresses via the phosphorylation of a variety of substrates (Mao et al., 2021). By motif analysis, we found that an ABI5-targeting cis-element was enriched in the 1-kb promoter sequences of HS and bin2-1 coregulated DEGs. Similar to BIN2, HS treatment could also induce the accumulation of ABI5 protein. As ABA is a positive regulator of plant thermotolerance that increases the accumulation and activity of ABI5, it is surprising that the thermotolerance was increased in abi5 but decreased in ABI5-OX plants. However, these data support findings showing that ABI5 is a BIN2 substrate and that BIN2 phosphorylation stabilizes and increases the activity of ABI5 (Hu and Yu, 2014). The increased thermotolerance of the abi5 bin2-1 double mutant (compared with the bin2-1 mutant) suggests that the HS-induced accumulation of BIN2 activates ABI5 to suppress plant thermotolerance. This conclusion can also explain the fact that the thermotolerance of the bzr1-1D mutant was similar to that of wild type, while the bzr1-1D mutation increased the thermotolerance of the bin2-1 mutant. Previously, we showed that ABI5 is a direct target of BZR1 and that bzr1-1D reduced the expression of ABI5 in bin2-1 mutant plants (Yang et al., 2016). This may help explain the partial reversal of the reduced thermotolerance in our bin2-1 plants by bzr1-1D mutation. As both bzr1-1D and abi5 could only partially recover the reduced thermotolerance of the bin2-1 mutant, additional BIN2 substrates are probably also involved in regulating plant thermotolerance.
Early flowering is an adaptive trait that plants use to cope with biotic and abiotic stresses (Kazan and Lyons, 2016). In doing so, plants switch from vegetative to reproductive growth to ensure that the species can survive in an unfavorable environment. Though studies indicate that ambient high temperatures can promote early flowering by suppressing the expression of floral repressor genes such as FLOWERING LOCUS C (FLC), FLOWERING LOCUS M (FLM), and SHORT VEGETATIVE PHASE (SVP) through PIF4, FCA, and SPL3 (Capovilla et al., 2015), it is unknown whether a similar mechanism applies to heat-shocked plants. In this study, 12 h of treatment at 37°C during the day promoted early flowering in Arabidopsis. Increased BIN2 activity caused by the overexpression of BIN2 or the bin2-1 mutation promoted early flowering both at room temperature and after HS treatment. Conversely, the flowering time in the bin2-t mutant was significantly delayed, indicating that BIN2 is a positive regulator of flowering. However, when grown under LD conditions, the abi5 mutant also flowered earlier, while ABI5-OX plants had a late-flowering phenotype (Wang et al., 2013), in contrast to the early flowering phenotypes of bin2-1 and our BIN2-OX plants. Therefore, despite the fact that BIN2 suppresses plant thermotolerance by activating ABI5, ABI5 is not responsible for the early flowering observed in our BIN2-OX and bin2-1 plants. BIN2 likely suppresses plant thermotolerance and promotes flowering via different downstream target proteins.
It is surprising that the bin2-1 mutant and BIN2-OX plants flowered early because BR biosynthesis- and BR signaling-deficient mutants such as det2, cpd, dwf4, and bri1 flower late (Azpiroz et al., 1998; Domagalska et al., 2007; Izawa, 2021). In contrast, the overexpression of BRI1 promotes early flowering (Singh et al., 2016). In the present study, under our growth conditions, BIN2-OX and bin2-1 plants exhibited early flowering regardless of whether we counted the number of days to flowering or the number of rosette leaves at flowering. In the bin2-1 mutant, the upstream BR signaling pathway is still intact while downstream BZRs are inactive. This could cause feedback-based upregulation of BR biosynthesis genes. Therefore, upstream BR signaling components should be hyperactive in the bin2-1 mutant. Could upstream BR signaling components such as BRI1, BAK1, BSKs, and BSU1 promote flowering directly through a BIN2- and BZR-independent mechanism? This hypothesis could explain the severe late flowering phenotype of the bzr1-1D mutant; BR biosynthesis-related genes are downregulated in this mutant, and this would inactivate upstream BR signaling components. However, this hypothesis cannot explain why BES1-OX plants flowered early while BES1-RNAi plants flowered late (Wang et al., 2019). It is possible that BR signaling components regulate flowering time via multiple mechanisms.
In conclusion, by analyzing plants with mutations in or that overexpressed different BR signaling components, we found that BR signaling regulates plant thermotolerance by promoting BIN2 degradation. Additionally, our data suggest that to cope with HS exposure plants increase the abundance of BIN2 to promote flowering but at a cost of reducing plant thermotolerance. Together, our findings provide an excellent example of how plants sacrifice the survivability of individuals at high temperatures to ensure the survival of the species.
Data Availability Statement
The original contributions presented in the study are publicly available. This data can be found here: The RNAseq datasets generated and analyzed for this study have been deposited in China National Center for Bioinformation (https://www.cncb.ac.cn/), with the project number: PRJCA007585.
Author Contributions
WT and DS designed the research. HR, KG, and XW did the experiments with YW’s assistant and under the supervision of DS and WT. HR and WZ analyzed the RNA-seq data. KG and WT wrote the manuscript together. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the Education Department of Hebei Province (GCC2014002), the Science and Technology Department of Hebei Province (C2020205028), and National Natural Science Foundation of China (32070293).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Diqiu Yu (Yunnan University) for providing the ABI5-com and ABI5-OX seeds. We would also like to thank Dr. Peng Xu (University of California at San Francisco) for his pioneer investigation of the involvement of BSK3 in regulating plant thermotolerance, which inspired this research.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.838062/full#supplementary-material
Supplementary Figure 1 | Brassinosteroids regulate plant basal thermotolerance by suppressing a BIN2-ABI5 module in Arabidopsis. (A–J) Survival rate of various BR biosyntheis or signaling mutants, and plants that overexpressing different BR signaling components. One-week-old seedlings grown on 1/2 MS agar medium supplied with or without 0.1 μM eBL under LD conditions at 22°C were grown at 45°C for 1 h. The survival rates of the plants were determined after 3 days of recovery at 22°C. Error bars indicate the mean ± SD. Statistically significant differences are indicated by different lowercase letters (p < 0.05, Student’s t-test).
Supplementary Figure 2 | The regulation of ABI5 transcript and protein abundance by HS. (A) The relative expression of ABI5 transcripts in 1-week-old Col-0 seedlings grown at 37°C for the indicated time. Error bars indicate the mean ± SD (n = 3). Statistically significant differences are indicated by different lowercase letters (p < 0.05, one-way ANOVA). (B) Immunoblotting revealed the abundance of ABI5-YFP in 1-week-old ABI5pro:ABI5-YFP expressing seedlings treated with 37°C for the indicated time.
Supplementary Figure 3 | Increased cellular BIN2 activity or protein levels promote early flowering in Arabidopsis. (A) Phenotypes of Col-0, bin2-1, and BIN2-OX plants grown in soil at 22°C under LD conditions until the first flower opened. (B) Quantitation of the days to flowering and rosette leaf numbers for the flowering plants shown in panel (A). Error bars indicate the mean ± SD. Different lowercase letters indicate statistically significant differences (p < 0.05, Student’s t-test).
Footnotes
- ^ https://www.ipcc.ch
- ^ www.biocloud.net
- ^ http://systemsbiology.cau.edu.cn/agriGOv2/
- ^ www.arabidopsis.org
- ^ https://meme-suite.org/meme/doc/centrimo.html?man_type=web
References
Ali, S., Rizwan, M., Arif, M. S., Ahmad, R., Hasanuzzaman, M., Ali, B., et al. (2020). Approaches in enhancing thermotolerance in plants: an updated review. J. Plant Growth Regul. 39, 456–480. doi: 10.1007/s00344-019-09994-x
Azpiroz, R., Wu, Y., LoCascio, J. C., and Feldmann, K. A. (1998). An Arabidopsis brassinosteroid-dependent mutant is blocked in cell elongation. Plant Cell 10, 219–230. doi: 10.1105/tpc.10.2.219
Capovilla, G., Schmid, M., and Pose, D. (2015). Control of flowering by ambient temperature. J. Exp. Bot. 66, 59–69. doi: 10.1093/jxb/eru416
Chen, L. G., Gao, Z., Zhao, Z., Liu, X., Li, Y., Zhang, Y., et al. (2019). BZR1 family transcription factors function redundantly and indispensably in BR signaling but exhibit BRI1-independent function in regulating anther development in Arabidopsis. Mol. Plant 12, 1408–1415. doi: 10.1016/j.molp.2019.06.006
Danilova, M. N., Kudryakova, N. V., Andreeva, A. A., Doroshenko, A. S., Pojidaeva, E. S., and Kusnetsov, V. V. (2018). Differential impact of heat stress on the expression of chloroplast-encoded genes. Plant Physiol. Biochem. 129, 90–100. doi: 10.1016/j.plaphy.2018.05.023
Dogra, V., Duan, J., Lee, K. P., and Kim, C. (2019). Impaired PSII proteostasis triggers a UPR-like response in the var2 mutant of Arabidopsis. J. Exp. Bot. 70, 3075–3088. doi: 10.1093/jxb/erz151
Domagalska, M. A., Schomburg, F. M., Amasino, R. M., Vierstra, R. D., Nagy, F., and Davis, S. J. (2007). Attenuation of brassinosteroid signaling enhances FLC expression and delays flowering. Development 134, 2841–2850. doi: 10.1242/dev.02866
Fang, J., Zhu, W., and Tong, Y. (2020). Knock-down the expression of brassinosteroid receptor TaBRI1 reduces photosynthesis, tolerance to high light and high temperature stresses and grain yield in wheat. Plants (Basel) 9:840. doi: 10.3390/plants9070840
Gampala, S. S., Kim, T. W., He, J. X., Tang, W., Deng, Z., Bai, M. Y., et al. (2007). An essential role for 14-3-3 proteins in brassinosteroid signal transduction in Arabidopsis. Dev. Cell 13, 177–189. doi: 10.1016/j.devcel.2007.06.009
Haq, S. U., Khan, A., Ali, M., Khattak, A. M., Gai, W. X., Zhang, H. X., et al. (2019). Heat shock proteins: dynamic biomolecules to counter plant biotic and abiotic stresses. Int. J. Mol. Sci. 20:5321. doi: 10.3390/ijms20215321
Hu, S., Ding, Y., and Zhu, C. (2020). Sensitivity and responses of chloroplasts to heat stress in plants. Front. Plant Sci. 11:375. doi: 10.3389/fpls.2020.00375
Hu, Y., and Yu, D. (2014). BRASSINOSTEROID INSENSITIVE2 interacts with ABSCISIC ACID INSENSITIVE5 to mediate the antagonism of brassinosteroids to abscisic acid during seed germination in Arabidopsis. Plant Cell 26, 4394–4408. doi: 10.1105/tpc.114.130849
Ibañez, C., Delker, C., Martinez, C., Bürstenbinder, K., Janitza, P., Lippmann, R., et al. (2018). Brassinosteroids dominate hormonal regulation of plant thermomorphogenesis via BZR1. Curr. Biol. 28, 303–310. doi: 10.1016/j.cub.2017.11.077
Izawa, T. (2021). What is going on with the hormonal control of flowering in plants? Plant J. 105, 431–445. doi: 10.1111/tpj.15036
Jung, J. H., Barbosa, A. D., Hutin, S., Kumita, J. R., Gao, M. J., Derwort, D., et al. (2020). A prion-like domain in ELF3 functions as a thermosensor in Arabidopsis. Nature 585, 256–260. doi: 10.1038/s41586-020-2644
Kagale, S., Divi, U. K., Krochko, J. E., Keller, W. A., and Krishna, P. (2007). Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 225, 353–364. doi: 10.1007/s00425-006-0361-6
Kazan, K., and Lyons, R. (2016). The link between flowering time and stress tolerance. J. Exp. Bot. 67, 47–60. doi: 10.1093/jxb/erv441
Kim, T. W., Guan, S., Burlingame, A. L., and Wang, Z. Y. (2011). The CDG1 kinase mediates brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2. Mol. Cell 43, 561–571. doi: 10.1016/j.molcel.2011.05.037
Kim, T. W., Guan, S., Sun, Y., Deng, Z., Tang, W., Shang, J. X., et al. (2009). Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat. Cell Biol. 11, 1254–1260. doi: 10.1038/ncb1970
Lesk, C., Rowhani, P., and Ramankutty, N. (2016). Influence of extreme weather disasters on global crop production. Nature 529, 84–87. doi: 10.1038/nature16467
Li, B. J., Gao, Z. H., Liu, X. Y., Sun, D. Y., and Tang, W. Q. (2019). Transcriptional profiling reveals a time-of-day-specific role of REVEILLE 4/8 in regulating the first wave of heat shock-induced gene expression in Arabidopsis. Plant Cell 31, 2353–2369. doi: 10.1105/tpc.19.00519
Li, H., Ye, K., Shi, Y., Cheng, J., Zhang, X., and Yang, S. (2017). BZR1 positively regulates freezing tolerance via CBF-Dependent and CBF-Independent pathways in Arabidopsis. Mol. Plant 10, 545–559. doi: 10.1016/j.molp.2017.01.004
Li, J., and Chory, J. (1997). A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90, 929–938. doi: 10.1016/s0092-8674(00)8357-8
Li, J., and Nam, K. H. (2002). Regulation of brassinosteroid signaling by a GSK3/SHAGGY-Like kinase. Science 295, 1299–1301. doi: 10.1126/science.1065769
Li, J., Wen, J. Q., Lease, K. A., Doke, J. T., Tax, F. E., and Walker, J. C. (2002). BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110, 213–222. doi: 10.1016/s0092-8674(02)00812-7
Li, N., Bo, C., Zhang, Y., and Wang, L. (2021a). Phytochrome interacting factors PIF4 and PIF5 promote heat stress induced leaf senescence in Arabidopsis. J. Exp. Bot. 72, 4577–4589. doi: 10.1093/jxb/erab158
Li, N., Euring, D., Cha, J. Y., Lin, Z., Lu, M., Huang, L. J., et al. (2021b). Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front. Plant Sci. 11:627969. doi: 10.3389/fpls.2020.627969
Ma, W., Guan, X. Y., Li, J., Pan, R. H., Wang, L. Y., Liu, F. J., et al. (2019). Mitochondrial small heat shock protein mediates seed germination via thermal sensing. Proc. Natl. Acad. Sci. U.S.A. 116, 4716–4721. doi: 10.1073/pnas.1815790116
Mao, J., Li, W., Liu, J., and Li, J. (2021). Versatile physiological functions of plant GSK3-like kinases. Genes 12:697. doi: 10.3390/genes12050697
Mazorra, L. M., Holton, N., Bishop, G. J., and Nunez, M. (2011). Heat shock response in tomato brassinosteroid mutants indicates that thermotolerance is independent of brassinosteroid homeostasis. Plant Physiol. Biochem. 49, 1420–1428. doi: 10.1016/j.plaphy.2011.09.005
Mora-Garcia, S., Vert, G., Yin, Y., Cano-Delgado, A., Cheong, H., and Chory, J. (2004). Nuclear protein phosphoatases with kelch-repeat domains modulate the response to brassinosteroids in Arabidopsis. Genes Dev. 18, 448–460. doi: 10.1101/gad.1174204
Nam, K. H., and Li, J. M. (2002). BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110, 203–212. doi: 10.1016/s0092-8674(02)00814-0
Peng, P., Yan, Z., Zhu, Y., and Li, J. (2008). Regulation of the Arabidopsis GSK3-like kinase BRASSINOSTEROID-INSENSITIVE 2 through proteasome mediated protein degradation. Mol. Plant 1, 338–346. doi: 10.1093/mp/ssn001
Planas-Riverola, A., Gupta, A., Betegón-Putze, I., Bosch, N., Ibañes, M., and Caño-Delgado, A. I. (2019). Brassinosteroid signaling in plant development and adaptation to stress. Development 146:dev151894. doi: 10.1242/dev.151894
Poidevin, L., Forment, J., Unal, D., and Ferrando, A. (2021). Transcriptome and translatome changes in germinated pollen under heat stress uncover roles of transporter genes involved in pollen tube growth. Plant Cell Environ. 44, 2167–2184. doi: 10.1111/pce.13972
Ren, Y., Huang, Z., Jiang, H., Wang, Z., Wu, F., Xiong, Y., et al. (2021). A heat stress responsive NAC transcription factor heterodimer plays key roles in rice grain filling. J. Exp. Bot. 72, 2947–2964. doi: 10.1093/jxb/erab027
Rytz, T. C., Miller, M. J., Mcloughlin, F., Augustine, R. C., Marshall, R. S., Juan, Y. T., et al. (2018). SUMOylome profiling reveals a diverse array of nuclear targets modified by the SUMO ligase SIZ1 during heat stress. Plant Cell 20, 1077–1099. doi: 10.1105/tpc.17.00993
Sadura, I., and Janeczko, A. (2018). Physiological and molecular mechanisms of brassinosteroid-induced tolerance to high and low temperature in plants. Biol. Plant 62, 601–616. doi: 10.1007/s10535-018-0805-4
Sadura, I., Pociecha, E., Dziurka, M., Oklestkova, J., Novak, O., Gruszka, D., et al. (2019). Mutations in the HvDWARF, HvCPD and HvBRI1 genes-involved in brassinosteroid biosynthesis/signalling: altered photosynthetic efficiency, hormonal homeostasis and tolerance to high/low temperatures in barley. J. Plant Growth Reg. 38, 1062–1081. doi: 10.1007/s00344-019-09914-z
Sahni, S., Prasad, B. D., Liu, Q., Grbic, V., Sharpe, A., Singh, S. P., et al. (2016). Overexpression of the brassinosteroid biosynthetic gene DWF4 in Brassinca napus simultaneously increases seed yield and stress tolerance. Sci. Rep. 6:28298. doi: 10.1038/srep28298
Schmittgen, T. D., and Livak, K. J. (2008). Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108. doi: 10.1038/nprot.2008.73
Singh, A., Breja, P., Khurana, J. P., and Khurana, P. (2016). Wheat Brassinosteroid-Insensitive1 (TaBRI1) Interacts with members of TaSERK gene family and cause early flowering and seed yield enhancement in Arabidopsis. PLoS One 11:e0153273. doi: 10.1371/journal.pone.0153273
Srivastava, M., Srivastava, A. K., Orosa-puente, B., Campanaro, A., Zhang, C., and Sadanandom, A. (2020). SUMO conjugation to BZR1 enable brassinosteroid signaling to integrate environmental cues to shape plant growth. Curr. Biol. 30, 1410–1423. doi: 10.1016/j.cub.2020.01.089
Sun, Y., Fan, X., Cao, D. M., Tang, W., He, K., Zhu, J. Y., et al. (2010). Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev. Cell 19, 765–777. doi: 10.1016/j.devcel.2010.10.010
Tang, W., Kim, T. W., Oses-Prieto, J. A., Sun, Y., Deng, Z., Zhu, S., et al. (2008). BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science 321, 557–560. doi: 10.1126/science.1156973
Tang, W., Yuan, M., Wang, R., Yang, Y., Wang, C., Oses-Prieto, J. A., et al. (2011). PP2A activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating BZR1. Nat. Cell Biol. 13, 124–131. doi: 10.1038/ncb2151
Vert, G., and Chory, J. (2006). Downstream nuclear events in brassinosteroid signaling. Nature 441, 96–100. doi: 10.1038/nature04681
Wang, F., Gao, Y. S., Liu, Y. W., Zhang, X., Gu, X. X., Ma, D. B., et al. (2019). BES1-regulated BEE1 controls photoperiodic flowering downstream of blue light signaling pathway in Arabidopsis. New Phytol. 223, 1407–1419. doi: 10.1111/nph.15866
Wang, R., Wang, R., Liu, M., Yuan, W., Zhao, Z., Liu, X., et al. (2021). Nucleocytoplasmic trafficking and turnover mechanisms of BRASSINAZOLE RESISTANT 1 in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 118:e2101838118. doi: 10.1073/pnas.2101838118
Wang, Y., Li, L., Ye, T., Lu, Y., Chen, X., and Wu, Y. (2013). The inhibitory effect of ABA on floral transition is mediated by ABI5 in Arabidopsis. J. Exp. Bot. 64, 675–684. doi: 10.1093/jxb/ers361
Wang, Z. Y., Nakano, T., Gendron, J., He, J., Chen, M., Vafeados, D., et al. (2002). Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell 2, 505–513. doi: 10.1016/s1534-5807(02)00153-3
Yan, Z., Zhao, J., Peng, P., Chihara, R. K., and Li, J. (2009). BIN2 functions redundantly with other Arabidopsis GSK3 like kinases to regulate brassinosteroid signaling. Plant Physiol. 150, 710–721. doi: 10.1104/pp.109.138099
Yang, C. J., Zhang, C., Lu, Y. N., Jin, J. Q., and Wang, X. L. (2011). The mechanisms of brassinosteroids action: from signal transduction to plant development. Mol. Plant 4, 588–600. doi: 10.1093/mp/ssr020
Yang, X., Bai, Y., Shang, J., Xin, R., and Tang, W. (2016). The antagonistic regulation of abscisic acid-inhibited root growth by brassinosteroids is partially mediated via direct suppression of ABSCISIC ACID INSENSITIVE 5 expression by BRASSINAZOLE RESISTANT 1. Plant Cell Environ. 39, 1994–2003. doi: 10.1111/pce.12763
Yin, Y., Qin, K., Song, X., Zhang, Q., Zhou, Y., Xia, X., et al. (2018). BZR1 transcription factor regulates heat stress tolerance through FERONIA receptor-like kinase mediated reactive oxygen species signaling in tomato. Plant Cell Physiol. 59, 2239–2254. doi: 10.1093/pcp/pcy146
Zhou, X., Hao, H., Zhang, Y., Bai, Y., Zhu, W., and Qin, Y. (2015). SOS2-LIKE PROTEIN KINASE5, an SNF1-RELATED PROTEIN KINASE3-type protein kinase, is important for abscisic acid responses in arabidopsis through phosphorylation of ABSCISIC ACID-INSENSITIVE5. Plant Physiol. 168, 659–676. doi: 10.1104/pp.114.255455
Keywords: heat shock, brassinosteroid, BIN2, ABI5, thermotolerance, flowering
Citation: Ren H, Wu X, Zhao W, Wang Y, Sun D, Gao K and Tang W (2022) Heat Shock-Induced Accumulation of the Glycogen Synthase Kinase 3-Like Kinase BRASSINOSTEROID INSENSITIVE 2 Promotes Early Flowering but Reduces Thermotolerance in Arabidopsis. Front. Plant Sci. 13:838062. doi: 10.3389/fpls.2022.838062
Received: 17 December 2021; Accepted: 04 January 2022;
Published: 27 January 2022.
Edited by:
Dawei Zhang, Sichuan University, ChinaReviewed by:
Wen-hui Lin, Shanghai Jiao Tong University, ChinaSusheng Song, Capital Normal University, China
Copyright © 2022 Ren, Wu, Zhao, Wang, Sun, Gao and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kang Gao, gk2684@mail.hzau.edu.cn; Wenqiang Tang, tangwq@mail.hebtu.edu.cn
†These authors have contributed equally to this work
 Huimin Ren
Huimin Ren Xuedan Wu†
Xuedan Wu† Weishuang Zhao
Weishuang Zhao Yuetian Wang
Yuetian Wang Kang Gao
Kang Gao Wenqiang Tang
Wenqiang Tang