- 1National Resources Center for Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing, China
- 2Institute of Food Science and Technology, Chinese Academy of Agriculture Sciences, Beijing, China
- 3Guangdong Provincial Key Laboratory of Plant Resources Biorefinery, School of Biomedical and Pharmaceutical Sciences, Guangdong University of Technology, Guangzhou, China
- 4National Center of China for Flowers Improvement, Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, Beijing, China
- 5Key Laboratory of Biology and Genetic Improvement of Flower Crops (North China), Ministry of Agriculture and Rural Affairs, Beijing, China
Essential oils have attracted wide attention in recent years due to their extensive applications in natural functional ingredients, pharmaceutical preparations, biomedical products, and the cosmetics industry. In this study, the chemical compositions and biological activities of essential oils extracted from six Lamiaceae herbs, including Pogostemon cablin (Blanco) Benth. (PCEO), Perilla frutescens (L.) Britton (PFEO), Salvia japonica Thunb. (SJEO), Rosmarinus officinalis L. (ROEO), Lavandula angustifolia Mill. (LAEO), and Agastache rugosa (Fisch. & C. A. Mey.) Kuntze (AREO), were determined and analyzed. A total of 167 components were identified from the six essential oils by GC-MS analysis, with 35, 24, 47, 46, 54, and 37 components in PCEO, PFEO, SJEO, ROEO, LAEO, and AREO, respectively. Hierarchical cluster analysis of chemical compositions showed that the composition of the six essential oils was significantly different in content, and they were clearly divided into six classes. However, all of these six essential oils exhibited promising anti-inflammatory activity by inhibiting the expression of interleukin-1, interleukin-6, tumor necrosis factor-α, and cyclooxygenase-2 in rats with adjuvant arthritis, among which PFEO had the best performance. In addition, the six essential oils displayed significant cytotoxicity on B16 (IC50 = 86.91–228.91 μg/mL) and LNCaP cell lines (IC50 = 116.4–189.63 μg/mL). Meanwhile, all of them presented satisfactory antioxidant activity (IC50 = 4.88–13.89 μg/mL) compared with Trolox C (IC50 = 13.83 μg/mL), and SJEO (IC50 = 7.93 μg/mL) served as an optimal candidate natural antioxidant by DPPH assay. Taken together, these results indicate that the six Lamiaceae essential oils manifest excellent and diverse biological activities, enabling them to be used as perfect natural functional ingredients in antioxidant, antitumor, or anti-arthritic drugs. This study provides more references for pharmaphylogeny research and drug discovery from folk medicinal plants.
Introduction
The Lamiaceae family contains 236 genera and about 7,173 species, almost cosmopolitan, except for the coldest regions of high latitude. Seven diversity centers were recognized: (1) Mediterranean and SW Central Asia; (2) Africa south of the Sahel and Madagascar; (3) China; (4) Australia; (5) South America; (6) Northern America and Mexico; (7) Indomalesian region (SE Asia). Based on their distribution, these species-rich genera fall generally into two groups: those mostly tropical in origin, including Vitex (Viticoideae), Clerodendrum (Ajugoideae), Callicarpa (Incertae sedis), Ocimum, Hyptis (Nepetoideae), and those which probably had a temperate origin, but often extend into the montane tropics, such as Ajuga, (Ajugoideae), Scutellaria (Scutellarioideae), Stachys (Lamioideae), Salvia, Clinopodium, Mentha, (Nepetoideae). In addition, Teucrium (Ajugoideae) has a distinct distribution in the southern hemisphere, but is most species-rich in the north, spreading down to the mountains of tropical Africa. The Lamiaceae plants contain many aromatic and medicinal plants that are widely used in traditional and modern medicine, food, and cosmetics industries (Vukovic et al., 2009; Nieto, 2017; Borges et al., 2019). In traditional and modern medicine, some Lamiaceae species, such as Perilla frutescens (L.) Britton, Pogostemon cablin (Blanco) Benth., Rosmarinus officinalis L., Lavandula angustifolia Mill., and Agastache rugosa (Fisch. & C. A. Mey.) Kuntze, are pervasively applied to dispel fever, expel superficial evils, eliminate stasis, induce diuresis, promote blood circulation, and reduce edema (Guo et al., 2016; Luo et al., 2019). R. officinalis. has been used as an analgesic, antispasmodic, and antidepressant to cure intercostal neuralgia, headaches, migraine, insomnia emotional upset, and depression in folk medicine (Ghasemzadeh Rahbardar and Hosseinzadeh, 2020). For a long time, the biological activities of extracts from these plant species have been studied, such as the antitumor, antioxidant, antimicrobial, and anti-inflammatory activities (Nieto, 2017; Guo et al., 2019; Karpinski, 2020).
Essential oil, an important category of plant extracts, has a multidirectional action mode and a variety of biological activities (Wojtunik-Kulesza et al., 2019). Essential oils can disrupt the cell and cell membrane via a permeabilization process. The lipophilic compounds of essential oils can pass through the cell wall; damage polysaccharides, fatty acids, and phospholipids; change the permeability for H+ and K+ cations to affect cellular pH; and damage organelles and disintegrate mitochondrial membrane (Karpinski, 2020). What is more, essential oils inhibit the biosynthesis of fungal DNA, RNA, proteins, and so on. They are widely applied in cosmetic additives, natural functional food, pharmaceutical preparations, and biomedical products (Nieto, 2017; Zhang et al., 2017b, 2020a). Specifically, essential oils from medicinal plants have attracted increasing attention in recent years for their multifaceted biological activities and diverse chemical compositions (Santos and Rao, 2000; El-Sayed et al., 2014; Xue, 2016). Lamiaceae family plants rich in essential oils have significant values in natural medicine, pharmacology, cosmetology, and aromatherapy. Some Lamiaceae species that are used in traditional medicine have been employed in the characterization of their essential oils, such as bioactivities and phytochemical composition. For example, essential oils of P. cablin (PCEO), P. frutescens (PFEO), R. officinalis (ROEO), and L. angustifolia (LAEO) are mainly composed of patchouli alcohol, linalool, α-terpineol, β-pinene, DL-menthol, and isobornyl acetate, which show strong anti-inflammatory activity by inhibiting the expression of interleukin 6 (IL-6), cyclooxygenase 2 (COX-2), tumor necrosis factor α (TNF-α), and nuclear factor-kappa B (NF-kB) in tetradecanoylphorbol acetate (TPA)-induced inflammation models (Luo et al., 2019; Zhang et al., 2020b). Moreover, these essential oils also demonstrate high antioxidant, antibacterial, and antifungal activities (Luo et al., 2019). The essential oil of A. rugosa (AREO), mainly composed of methyleugenol, estragole, and eugenol, exhibits strong pesticide activity against Meloidogyne incognita, with a LC50 value of 47.3 μg/mL (Li et al., 2013). ROEO also shows suppression of the lipopolysaccharide (LPS)-induced inflammation by inhibiting the expression of COX-2 and inducible nitric oxide synthase (iNOS) and blocking the production of TNF-α (Yu et al., 2013). Lavender essential oils have been used cosmetically and therapeutically for centuries, and their biological activities have been extensively studied (Cavanagh and Wilkinson, 2002). Borges et al. (2019) indicated that ROEO possesses strong anti-inflammatory activity and can be used as a remedy for inflammatory diseases (Borges et al., 2019). Though the essential oil extracted from Salvia japonica Thunb. (SJEO) has been widely used, little is known about its biological activity. Herb essential oils exert their diverse biological activities by acting on various pathways using different chemical components. The composition and bioactivity of essential oils extracted from Lamiaceae plants have been analyzed in many studies; however, their anti-inflammatory, antioxidant, antitumor, and anti-arthritis activities need to be systematically explored from multiple aspects (Nikolić et al., 2014; Vyry Wouatsa et al., 2014; Park et al., 2016; Mamadalieva et al., 2017, 2019; Mouahid et al., 2017; Borges et al., 2019; Karpinski, 2020).
The chemical composition of plant essential oil is influenced by numerous factors, such as the growing environment, harvest time, and plant organ used for essential oil extraction. Therefore, it is necessary to determine the phytochemical composition of the essential oil before carrying out further studies on their bioactivities. The current study aimed to elucidate the biological activity through the determination of the chemical composition of the essential oils extracted from six Lamiaceae plants with gas chromatography-mass spectrometry (GC-MS). The diversity of chemical components was analyzed by hierarchical cluster analysis in six essential oils from six Lamiaceae folk medicinal plants. Diverse biological activities, including anti-inflammatory, antitumor, and antioxidant activities, were evaluated through corresponding models. The model of complete Freund's adjuvant (CFA)-induced rheumatoid arthritis was used to evaluate the anti-inflammatory activity and related mechanisms of the six essential oils, and the LNCaP and B16 cell lines were used to estimate the antitumor activity. The 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical method was carried out to determine free radical scavenging capacity of these essential oils. Our study provides additional data to support the use of essential oils from Lamiaceae plants as a drug treatment.
Materials and methods
Plant materials and chemicals
The local name, storage locations, and collection dates of A. rugosa, L. angustifolia, P. frutescens, P. cablin, R. officinalis, and S. japonica samples are shown in Table 1. The leaves of A. rugosa, P. cablin, P. frutescens, R. officinalis were used for essential oil extraction, while the aerial parts of L. angustifolia and S. japonica were used for essential oils extractions. All plant samples were confirmed by Professor Nian Liu of Zhongkai University of Agriculture and Engineering (Guangzhou, China). All chemicals used in this study were purchased from Aladdin Reagent Inc. (Shanghai, China).
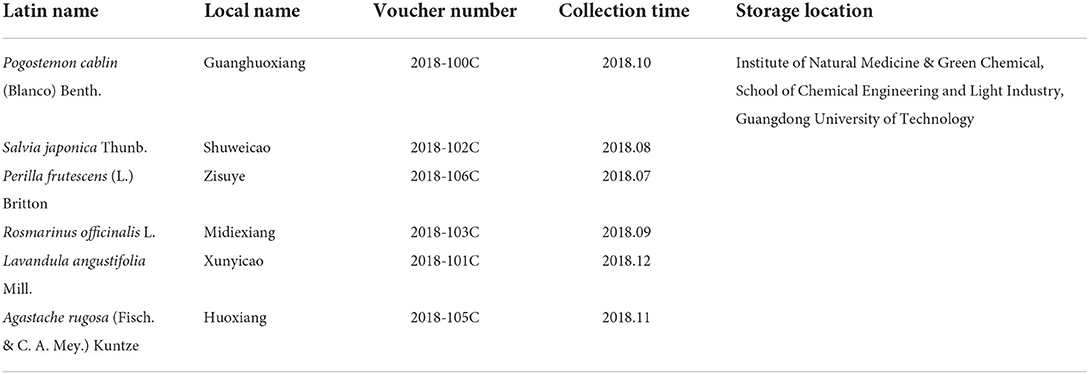
Table 1. Latin name, local name, voucher specimen number, and collection time of six Lamiaceae plants.
Essential oil extraction
The steam distillation method was used to extract essential oils from the six plant samples as previously described (Zhang et al., 2020b). The plants were cleaned, ground, passed through a 0.45-mm sieve, and distilled with a steam distillation device for 3.5 h (Zhang et al., 2017b). The isolated essential oils were dried as previously described in a former research and stored in individual brown glass bottles at 4 °C until use (Xiang et al., 2017).
GC-MS analysis
The phytochemical compositions of PCEO, PFEO, SJEO, ROEO, LAEO, and AREO were identified according to previous methods (Xiang et al., 2017; Zhang et al., 2017b) using a GC-MS system with a DB-5MS capillary column (0.25 mm × 30 m, i.d. 0.25 μm) (Agilent, Santa Clara, CA, USA). The carrier gas was Helium (He) at a flow rate of 1 mL/min. The initial temperature was set as 40°C for 1 min, then increased to 280°C by 3°C/min, and held at 280°C for 5 min. The split ratio was set as 100:1. For MS conditions, the ionization conditions were as follows: pressure of 50 kPa, electron energy of 70 eV, and ion source temperature of 200°C.
Each component of essential oils was determined based on the retention index (RI), which was calculated using a series of n-alkanes (C6-C40) (Xue et al., 2016). Additionally, the mass spectrum of each compound was searched against the NIST Standard Reference Database (NIST Chemistry WebBook, 2014, over 40,000 compounds in this database) and databases published elsewhere (Zhang et al., 2017a). The total ionization chromatography (TIC) was obtained and used for determining the contents of each compound (Supplementary Figure S1).
Animals
The animal experiments were carried out following ethical guidelines of the Laboratory Animal Center of Sun Yat-sen University. Male rats (6–8 weeks old, 210 ± 30 g body weight) were purchased from Sun Yat-sen University and raised under normal conditions (25 ± 2°C, 12/12 h light/dark cycle). Food and water were fed as required during the experiment.
Experimental treatments of adjuvant arthritis
According to our previous study, the rats were acclimatized for 7 days and then divided into 10 groups, with 10 rats in each group: (a) normal control (Con) group, (b) model (CFA) group, (c) negative control (NC) group, (d) positive control (PC) group, (e) PCEO group, (f) PFEO group, (g) ROEO group, (h) SJEO group, (i) LAEO group, and (j) AREO group (Zhang et al., 2020a).
Rats in the Con. group were not given any treatments. In the model group, CFA (0.1 mL) was subcutaneously injected into each rat after routine sterilization from Day 8 to Day 21 to induce arthritis. Then these rats were raised under normal conditions until use. Tween 80 was given to the rats in the NC group, while ibuprofen (100 mg/kg, dissolved in Tween 80) was given to those in the PC group from Day 8 to Day 20 (Khayyal et al., 2005). Rats in PCEO, PFEO, ROEO, SJEO, LAEO, and AREO groups were treated with corresponding essential oils (100 mg/kg, dissolved in Tween 80) from Day 8 till the end of the experiment. The arthritis score was recorded from Day 8 to Day 20 for all groups. The 5-point method was used to assess and grade the severity of the swelling, erythema, or stiffness in the paw: 0 = no signs of illness; 1 = mild swelling and erythema in the ankle/wrist; 2 = swelling and erythema in the ankle/wrist; 3 = severe swelling and erythema in the ankle/wrist; and 4 = severe illness in the paw or front leg. Both the hind feet were graded, and the total score was not allowed to exceed 8 (Funk et al., 2010). The rats were then sacrificed and their ankle joints were sampled and stored in 4% (v/v) paraformaldehyde for subsequent histological analysis.
Histological analysis and immunohistochemical staining
As described previously (Zhang et al., 2020a), the ankle joints were paraffin-embedded and the sections were observed using light microscopy (Olympus IX71, OLYMPUS, Japan). The IL-1, IL-6, COX-2, and TNF-α antibodies, all diluted at a 1:200 ratio, were used for immunohistochemical staining. The number of positive cells was counted with ImageJ using photos taken with a fluorescence microscope (NIH, Bethesda, MD, USA).
For immunohistochemical analysis, the sections were incubated overnight with IL-1β (dilution 1:200), IL-6 (dilution 1:200), COX-2 (dilution 1:200), and TNF-α (dilution 1:200) antibodies at 4°C and then treated with a secondary antibody and alkaline phosphatase-labeled streptavidin (1:200) at 25°C for up to 1 h. Sections were developed with 3,30-diaminobenzidine (DAB) solution. Image analysis software (Image-Pro Plus) was used to count the number of positive cells, and these were observed using a fluorescence microscope (NIH, Bethesda, MD) (Zhang et al., 2020b).
Determination of antitumor activity
Anittumor activity of essential oil was investigated via human prostate cancer cell model LNCap and mouse B16 melanoma cell lines in vitro. In PCEO, PFEO, ROEO, SJEO, LAEO, and AREO groups, the cytotoxicity of essential oils on LNCaP and B16 cells treated with essential oils was assessed using MTT [3 - (4,5 - dimethyl - 2 - thiazolyl) - 2,5 - diphenyltetrazolium bromide] values described in Zhang et al. (2020a). The B16 and LNCaP cells were cultured in DMEM and RPMI1640, respectively, supplemented with 10% FBS, 2 mM glutamine, 100 mg/mL streptomycin, and 100 U/mL penicillin. The cells were maintained in a humidified 5% CO2 incubator at 37 °C and were subcultured every 3–4 days to maintain logarithmic growth and allowed to grow for 24 h before the treatments were applied. The cells were then treated with different concentrations of the essential oil (Table 3), and then the absorbance at 570 nm was read on a microplate reader. The IC50 value of MTT assays is defined as essential oils concentration resulting in a 50% reduction of absorbance (Kanipandian and Thirumurugan, 2014). Hydroquinone and Paclitaxel were used as positive controls for B16 and LNCaP cells, respectively (Zhang et al., 2017c; Rodboon et al., 2020).
Determination of antioxidant activity
The antioxidant activity of the six essential oils was evaluated by DPPH free radical scavenging capacity (Xiang et al., 2017; Zhang et al., 2020a). DPPH solution (67 μg/mL) was mixed with each of the essential oils at various concentrations and incubated at 25°C for 30 min in the dark. The absorbance was then read at 517 nm. The scavenging percentage was calculated as follows:
Statistical analysis
One-way analysis of variance (ANOVA) was used to test statistical significance, and the result was considered significant at P ≤ 0.05. Data were presented as mean ± standard deviation (SD). Based on the content of each component, hierarchical cluster analysis (HCA) was performed for chemical compositions in the six essential oils using pheatmap package (version 1.0.12).
Results and discussion
Phytochemical compositions of the six essential oils
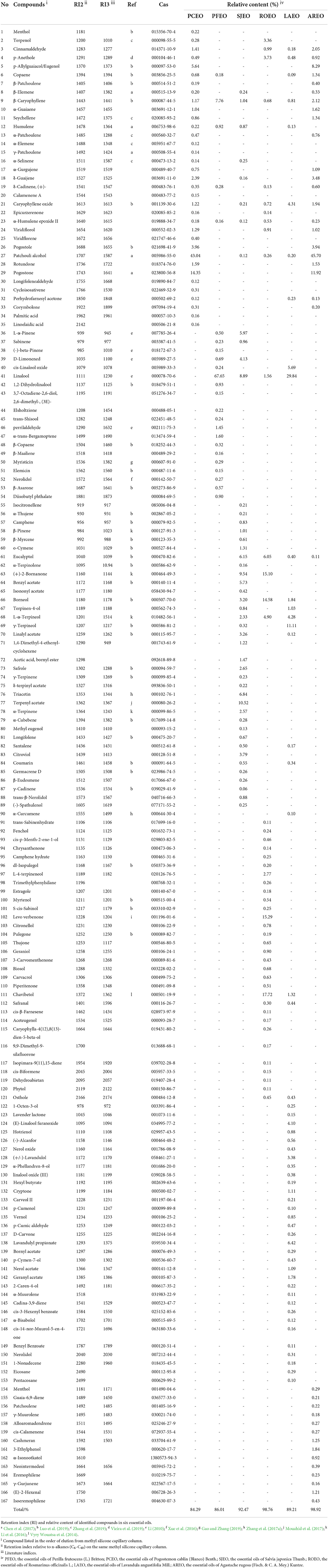
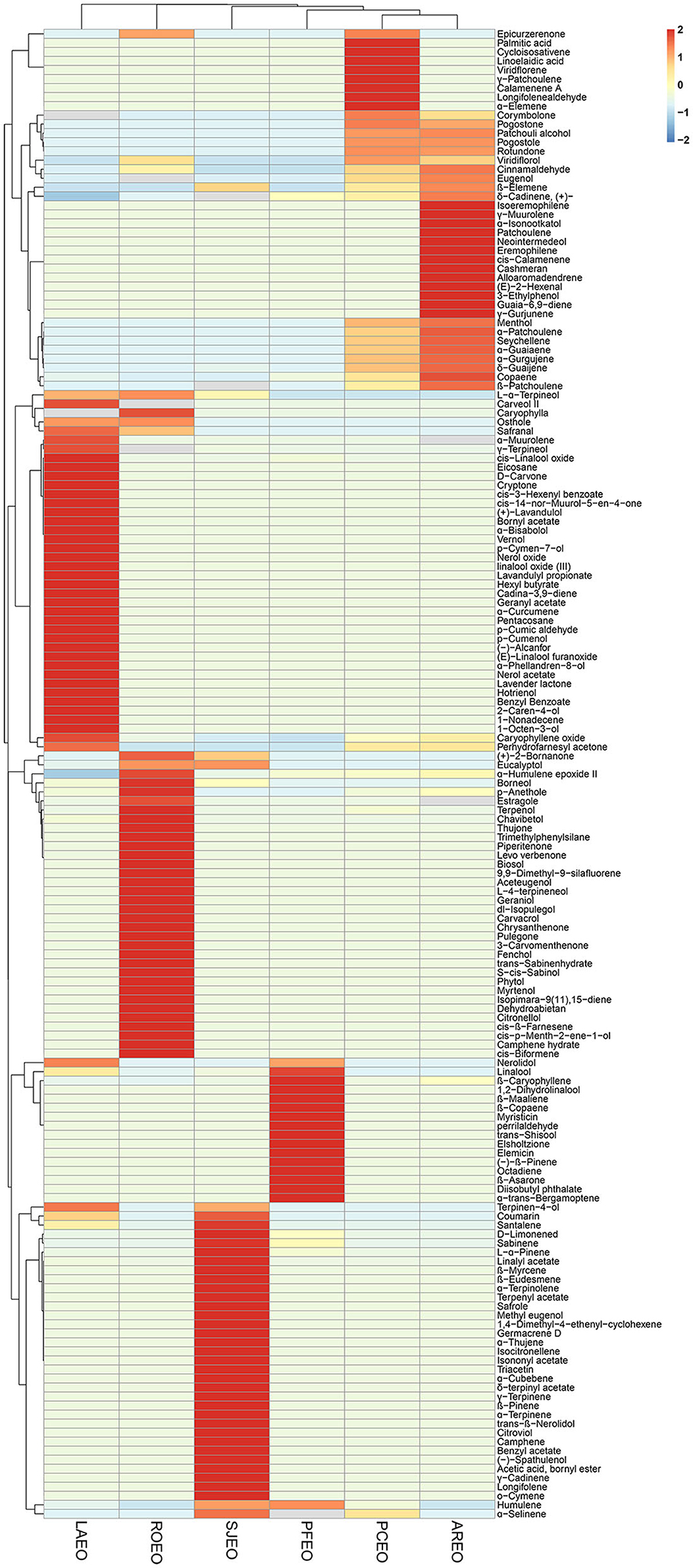
A total of 167 components were identified from six essential oils (Table 2) using GC-MS. Based on the species and quantity of compositions in each essential oil, the relationship of the six Lamiaceae folk medicinal plants was analyzed via hierarchical cluster. The results showed that the six essential oils were clearly divided into six classes, and the six essential oils have their unique principal components (Figure 1). The proportions of corymbolone, pogostone, patchouli alcohol, pogostole, rotundone, menthol, α-patchoulene, seychellene, α-guaiaene, α-gurgujene, and δ-guaijene showed similarity in PCEO and AREO. The proportions of L-α-terpineol, osthole, and safranal showed similarity in LAEO and ROEO. The proportions of nerolidol and linalool showed similarity in LAEO and PFEO. The proportion of terpinen-4-ol showed similarity in LAEO and SJEO. The proportions of (+)-2-bornanone and eucalyptol showed similarity in SJEO and ROEO. The proportion of humulene showed similarity in SJEO and PFEO. The proportion of α-selinene showed similarity in SJEO and PCEO. The proportion of epicurzerenone showed similarity trend in PCEO and ROEO (Figure 1). β-Caryophyllene was present in all six essential oils, while patchouli alcohol and caryophyllene oxide were found in all essential oils except PFEO. In summary, PCEO has the latest relationship with AREO, followed by PFEO, SJEO, ROEO, and LAEO. This relationship was also partly proved by the biological activities of six essential oils in the following results.
To further investigate the difference of the six essential oils, the major compounds of essential oils were compared in the analysis. The total relative contents of PCEO, PFEO, ROEO, SJEO, LAEO, and AREO were 84.29, 86.01, 98.76, 92.47, 89.21, and 98.92%, respectively. The dominant components of PCEO were patchouli alcohol (43.04%), pogostone (14.35%), and p-allylguaiacol/eugenol (5.638%). The major components of PFEO were linalool (67.65%) and β-caryophyllene (7.7564%). The main components of SJEO were terpenyl acetate (10.52%), (+)-2-bornanone (9.54%), camphor (9.54%), linalool (8.89%), L-α-pinene (5.97%), triacetin (6.84%), eucalyptol (6.15%), plastolin I (5.73%), and D-limonened (4.13%). The principal components of ROEO were chavibetol (17.72%), verbenone (15.29%), camphor (15.10%), borneol (14.58%), eucalyptol (6.05%), and α-terpineol (4.90%). The key components of LAEO were linalool (29.84%), γ-terpineol (11.11%), caryophyllene oxide (4.31%), cis-linalool oxide (5.69%), L-α-terpineol (4.28%), (E)-linalool furanoxide (4.10%), and lavandulyl propionate (6.42%). The primary components of AREO were patchouli alcohol (45.70%), pogostone (11.92%), and eugenol (8.29%). Linalool was found in LAEO, PFEO, ROEO, and SJEO, exhibiting the highest relative content in PFEO (67.66%), followed by LAEO (29.84%), SJEO (8.89%), and ROEO (1.56%). ROEO and AREO shared many common components, including patchouli alcohol, pogostone, and eugenol. In POEO and AREO, the relative contents of patchouli alcohol, pogostone, and eugenol were 43.04, 14.35, and 5.64%; and 45.70, 11.92, and 8.29%, respectively (Table 2). Meanwhile, the chemical structures of 15 components are shown in Figure 2 to provide more reference for later researchers, including patchouli alcohol, eugenol,β-caryophyllene, pogostone, L-α-pinene, cis-linalool oxide, linalool, eucalyptol, (+)-2-bornanone, benzyl acetate, (+)-borneol, L-α-terpineol, γ-terpineol, triacetin, terpenyl acetate, levo verbenone, chavibetol, (E)-linalool furanoxide, and lavandulyl propionate. All 15 components were picked up according to their content, the and content was more than 4 %.
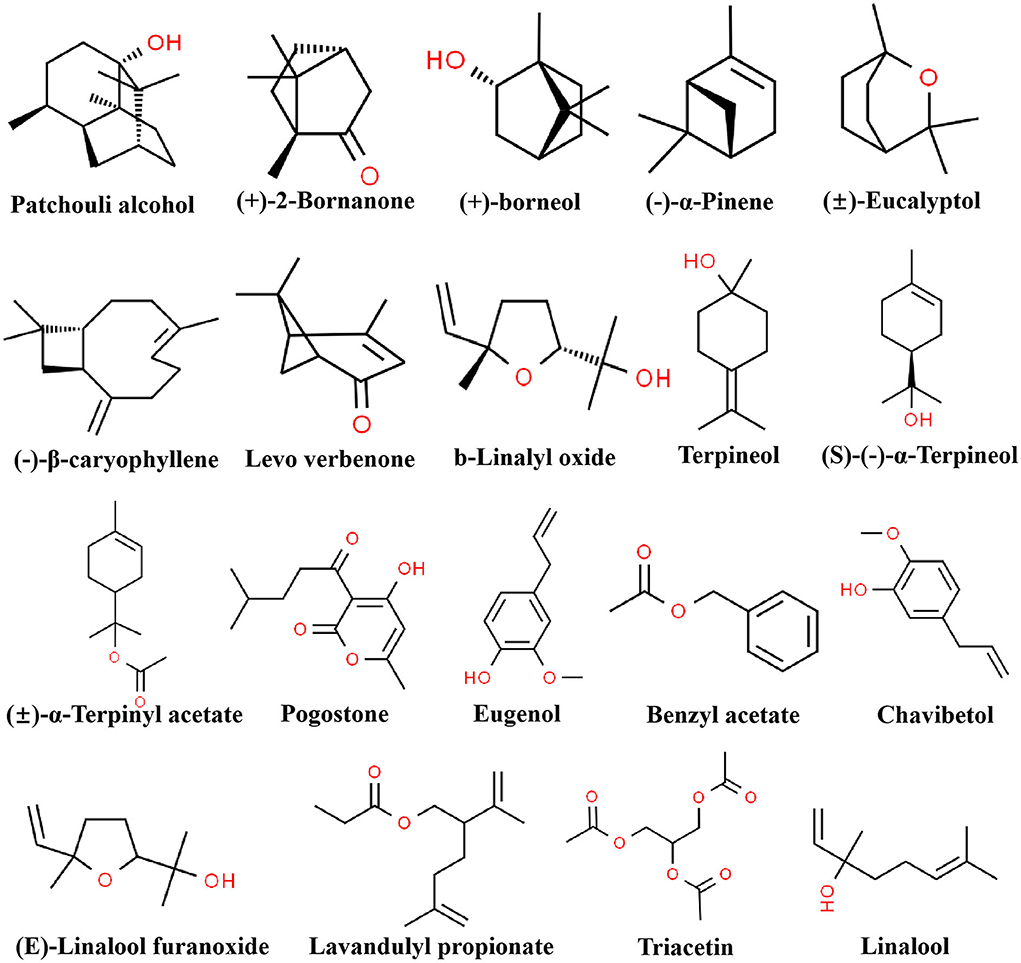
Figure 2. The chemical structure of 15 components in six essential oils. The relative content of each component was more than 4%.
The chemical composition of essential oil is influenced by various factors. Some components have been previously reported in the essential oils of Lamiaceae species. For example, PFEO is proposed to mainly contain linalool (46.55%) and 2-hexanoylfuran (30.79%); ROEO is composed of α-pinene (45.35%) and D-limonene (18.42%); PCEO mainly contains patchouli alcohol (28.27%), α-bulnesene (18.29%), and α-guaiene (14.53%); LAEO mainly includes isononyl acetate (22.52%), α-pinene (11.47%), and benzyl acetone (10.93%); and SJEO mainly contains o-cymene (41.20%), (Z, E)-α-farnesene (10.82%), and γ-muurolene (9.89%) (Luo et al., 2019).
Our results indicated that the six Lamiaceae essential oils have diverse chemical compositions, and they could serve as good sources of eugenol, patchouli alcohol, linalool, eucalyptol, β-caryophyllene, terpenyl acetate, chavibetol, camphor, γ-terpineol, borneol, and α-pinene. Some of these essential oils have been reported to demonstrate promising antioxidant, anti-nociceptive, anti-cardiotoxicity, anti-cancer, and anti-inflammatory activities (Sharma et al., 1994; Santos and Rao, 2000; Khan et al., 2014; Vyry Wouatsa et al., 2014; Fidyt et al., 2016; Mamadalieva et al., 2017; Nieto, 2017; Lian et al., 2018; Liu et al., 2018; de Souza et al., 2019; Luo et al., 2019; Oner et al., 2019).
Anti-arthritis activity of the six essential oils
As shown in Figure 3, all the six Lamiaceae essential oils displayed inhibitory effects on adjuvant arthritis in rats. Compared to the model group, PCEO, PFEO, ROEO, SJEO, LAEO, and AREO at a concentration of 100 mg/kg exhibited inhibitory effects on arthritis, among which PFEO manifested the highest anti-inflammatory activity, while PCEO showed the lowest. This result was consistent with that obtained from the PC group (ibuprofen treatment), which is effective for alleviating joint swelling in the rat models of arthritis.
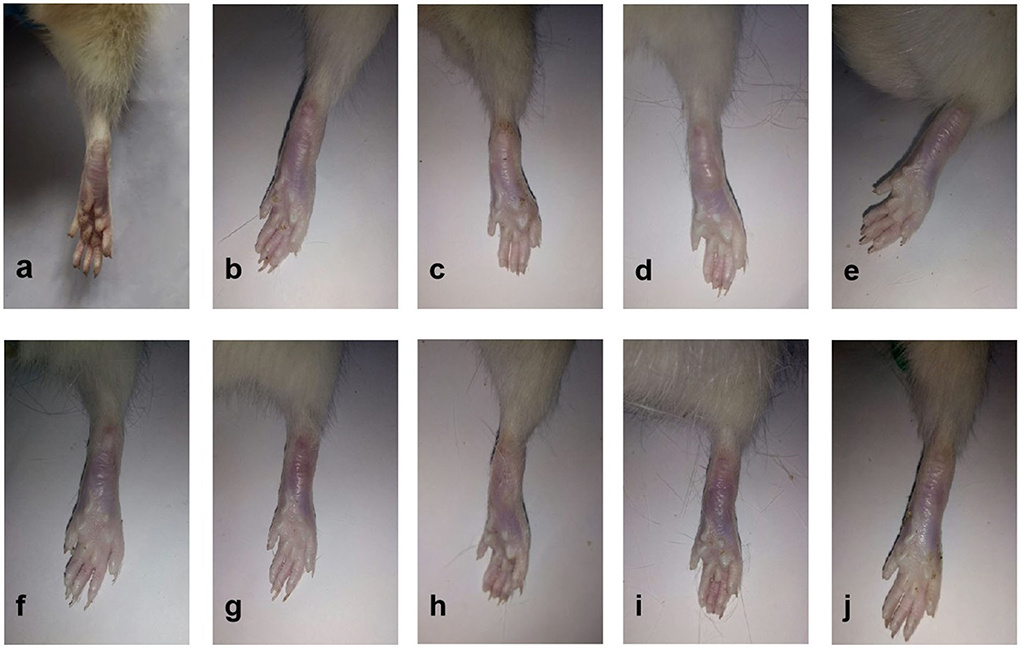
Figure 3. The appearance of rats with CFA-induced adjuvant arthritis. (a) Normal control (Con) group; (b) model (CFA) group; (c) negative control (NC) group; (d) positive control (PC) group; (e) PCEO group; (f) PFEO group; (g) ROEO group; (h) SJEO group; (i) LAEO group; and (j) AREO group.
The ankle joints of rats in the model group were significantly swollen compared with those of the control (Figure 3). In addition, the arthritis score of the positive control group (ibuprofen treatment) was significantly lower than that of the model group (Figure 4), which implied the success of CFA-induced arthritis. The arthritis scores of six essential oil treatment groups were lower than that of the model group, with PL displaying the lowest score, which indicated that PFEO might possess the strongest anti-arthritis capacity (Figure 4).
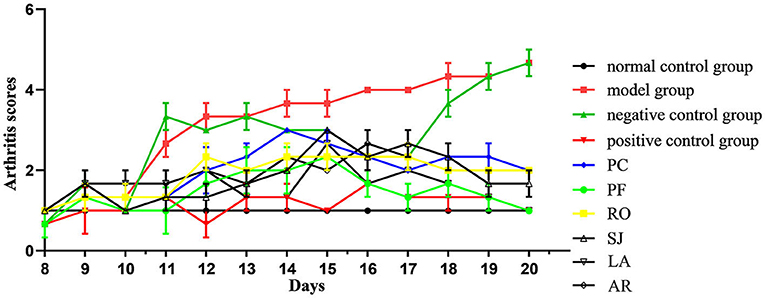
Figure 4. Arthritis scores of rats with CFA-induced adjuvant arthritis. The scores of normal control group, model (CFA) group, negative control (NC) group, positive control group, PCEO group (PC), PFEO group (PF), ROEO group (RO), SJEO group (SJ), LAEO group (LA), AREO group (AR) are shown with different color lines.
To obtain further insight into the anti-arthritis effect of six essential oils, histological and immunohistochemical characterizations were conducted using articular tissues. Severe cartilage damage, capillary hyperplasia, synovial proliferation, and lymphocyte infiltration were observed in the model group, while lymphocyte infiltration and cartilage damage were significantly inhibited in the PC group (ibuprofen treatment) (Figure 5). PCEO, PFEO, ROEO, SJEO, LAEO, and AREO exhibited similar effects relative to ibuprofen, which may largely decrease the damage.
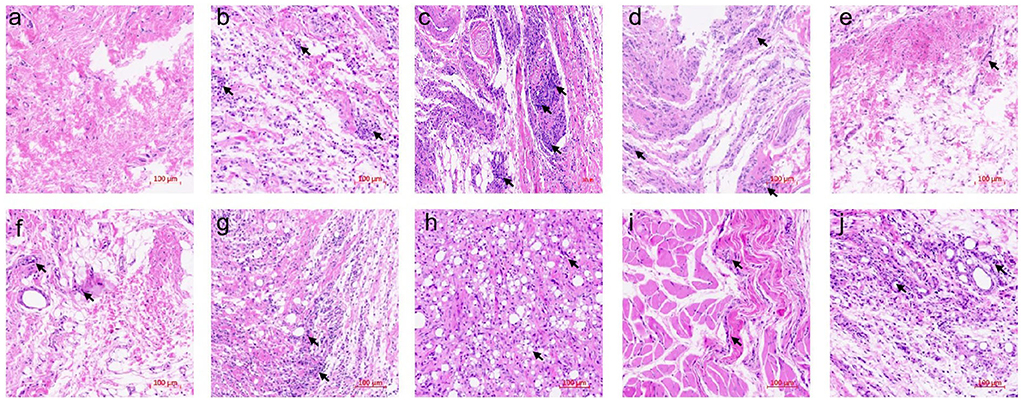
Figure 5. Histological sections of rat articular tissues showing severe cartilage damage, capillary hyperplasia, synovial proliferation, and lymphocyte infiltration (200× magnification). (a) Normal control (Con) group; (b) model (CFA) group; (c) negative control (NC) group; (d) positive control (PC) group; (e) PCEO group; (f) PFEO group; (g) ROEO group; (h) SJEO group; (i) LAEO group; (j) AREO group. Arrows indicate the lesion sites.
Complete Freund's adjuvant can induce numerous inflammatory responses of cytokines, including COX-2, iNOS, IL-1, and IL-6 (Zhang et al., 2020c). To further understand the anti-inflammatory mechanism of these Lamiaceae essential oils, the spatial-temporal expression profiles of inflammatory cytokines in rat articular tissues were investigated (Figure 6). In the model group, CFA treatment greatly induced the expression of COX-2, TNF-α, IL-1, and IL-6 in rat articular tissues, while the essential oil treatments notably reduced that of TNF-α, IL-1, and IL-6 compared to the Con group. Nevertheless, COX-2 expression was slightly decreased after ibuprofen treatment. Caryophyllene was a common component shared by the six essential oils, which was reported to inhibit the expression of TNF, IL-1β, and COX-2 in APP/PS1 mice (Alzheimer-like phenotype) through CB2 receptor activation and the PPARγ pathway (Cheng et al., 2014). In our study, PFEO exhibited the greatest anti-inflammatory capacity inhibiting adjuvant arthritis and largely reduced the expression of inflammatory cytokines including TNF-α, IL-1, and IL-6. Linalool, a dominant component in PFEO, exhibited notably anti-inflammatory potential by reducing the expression of IL-1β and TNF-α in BV2 microglia cell lines (Li et al., 2015). Our results are consistent with those of previous studies, indicating that linalool may be a potential anti-inflammatory compound in those essential oils. In all, our results demonstrated that essential oils of Lamiaceae herbs play a key role in alleviating inflammation by inhibiting inflammatory cytokine expression.
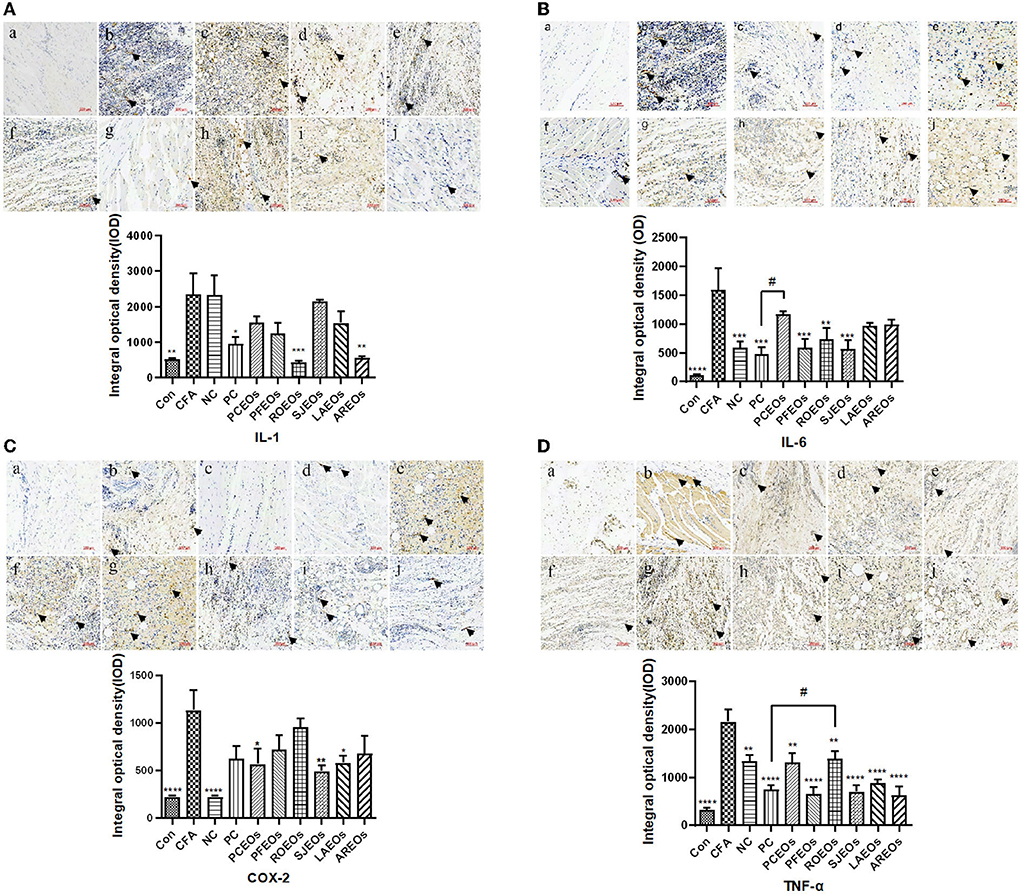
Figure 6. Immunohistochemical staining of rat articular tissues for COX-2 (A) IL-1 (B), IL-6 (C), and TNF-α (D) (200 × magnification). (a) normal control (Con) group, (b) model (CFA) group, (c) negative control (NC) group, (d) positive control (PC) group, (e) PCEO group, (f) PFEO group, (g) ROEO group, (h) SJEO group, (i) LAEO group, and (j) AREO group. Statistical significance relative to the model (CFA) group is indicated, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001, significantly different from the model (CFA) group. Arrows indicate the lesion sites. Statistical significance relative to the positive control (PC) group is indicated, #p ≤ 0.05.
Antitumor activity of the six essential oils
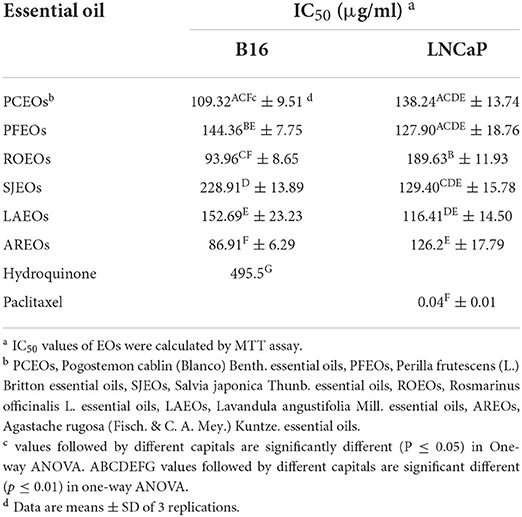
The antitumor activity was evaluated by examining in vitro inhibitory effects of these essential oils on LNCaP and B16 cells, and the results are shown in Table 3. The IC50 values of the six essential oils on LNCaP cells were between 116.41 and 189.63 μg/mL; LAEO (116.41 μg/mL) showed the strongest inhibitory effect, followed by AREO (126.20 μg/mL), PFEO (127.90 μg/mL), SJEO (129.40 μg/mL), PCEO (138.24 μg/mL), and ROEO (189.63 μg/mL). The IC50 values of these essential oils on B16 cells ranged from 86.91 to 228.91 μg/mL; AREO (86.91 μg/mL) exhibited the highest inhibitory effect, followed by ROEO (93.96 μg/mL), PCEO (109.32 μg/mL), PFEO (144.36 μg/mL), LAEO (152.69 μg/mL), and SJEO (189.63 μg/mL).
Previous studies have shown that patchouli alcohol, linalool, caryophyllene, borneol, and camphor achieve anticancer effects by inhibiting the expression of inflammatory factors (Santos and Rao, 2000; de Lima et al., 2014; Fidyt et al., 2016; Lian et al., 2018; Oner et al., 2019). Our results showed that the dominant components of six essential oils had different degrees of anticancer effects on B16 and LNCaP cells, consistent with previous studies. PCEO and AREO had better performance in inhibiting B16 and LNCaP cells. Patchouli alcohol, a dominant component of PCEO (43.04%) and AREO (45.70%), maybe a key anticancer ingredient in these two essential oils. Linalool was a dominant component in LAEO (29.84%) and PFEO (66.65%) and may be a key component that plays an important role in their anticancer activity. Borneol (14.59%) and camphor (15.10%) were the major components of ROEO, which were likely the key antitumor component in our in vitro cell experiment. Similarly, linalool (8.89%) and camphor (9.54%) may be principal components participating in the anticancer activity of SJEO.
Antioxidant activity of the six essential oils
The DPPH method, which is stable, simple, and fast, was employed to assess free radical-scavenging activity of the six essential oils (Luo et al., 2019; Zhang et al., 2020a,b). In this study, our results showed that the IC50 values of the six essential oils were between 7.93 and 13.83 μg/mL (Figure 7). SJEO (4.88 μg/mL) showed the highest free radical scavenging capacity, while PCEO had minimal capacity (13.89 μg/mL). The antioxidant activity of AREO (8.79 μg/mL), ROEO (8.98 μg/mL), LAEO (9.80 μg/mL), and PFEO (10.87 μg/mL) was superior to that of Trolox C (13.80 μg/mL).
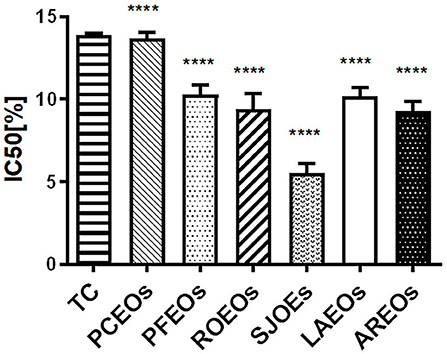
Figure 7. Measurement of DPPH free radical scavenging capacity of the six Lamiaceae essential oils using Trolox C (TC) as the positive control. Statistical significance relative to the Trolox C (TC) group is indicated, ****p ≤ 0.0001, significantly different from the positive control group.
The antioxidant activity of some Lamiaceae essential oils had been assessed using the DPPH method in the previous study (Spiridon et al., 2011; Luo et al., 2019). Luo et al. (2019) demonstrated that LAEO (IC50 = 1.07%) possesses higher antioxidant activity than PCEO (IC50 = 1.60%), ROEO (IC50, 2.80%), and PFEO (IC50, 18.77%). Spiridon et al. (2011) showed that LAEO (IC50 = 96.67 μg/mL) can effectively scavenge free radicals. In this study, SJEO (IC50 = 4.88 μg/mL) showed the highest activity on scavenging free radicals, followed by AREO, ROEO, LAEO, and PFEO.
The antioxidant activity of essential oils may be largely associated with their major components, among which eucalyptol, α-pinene, and linalool have been reported to demonstrate antioxidant activity (Khan et al., 2014; Oner et al., 2019; Zhang et al., 2020c). For example, α-pinene, with an IC50 of 310 μg/mL, was reported to possess antioxidant activity in DPPH assays (Bouzenna et al., 2017). In this study, terpenyl acetate (10.52%), camphor (9.54%), α-pinene (5.97%), linalool (5.97%), and benzyl acetate (5.73%) were the principal components of SJEO, which may account for its high antioxidant activity. Chavibetol (17.72%), camphor (15.10%), verbenone (15.29%), borneol (14.58%), and eucalyptol (6.04%) were the dominant components of ROEO, which may lead to high antioxidant activity. Taken together, our results indicate that some essential oils, as well as their major components, may serve as potent natural antioxidants.
Conclusion
The demand for essential oils is growing due to their potential applications in pharmacology and industry. The phytochemical composition of essential oils takes charge of their bio-activities and their pharmaceutical effects. However, the chemical composition of plant essential oils is affected by many factors, such as a growing environment and identification methods. In this study, we used modified methods to identify different chemical component profiles of essential oils extracted from six folk medicinal plants. A total of 167 components were identified and analyzed by GC-MS, among which the dominant components were patchouli alcohol, pogostone, linalool, chavibetol, β-caryophyllene, terpenyl acetate, camphor, chavibetol, verbenone, borneol, and γ-terpineol (Table 2). HCA of chemical compositions was first analyzed in six essential oils, which provides more reference for pharmaphylogeny research (Figure 1). Meanwhile, the chemical structure of 15 major compounds was exhibited to provide more reference for later research. Our results were remarkably different from the former reported and provide a new insight into the phytochemical composition of the six essential oils.
The six Lamiaceae essential oils exhibited diverse anti-inflammatory activities on CFA-induced adjuvant arthritis in rats. PFEO, with a high linalool concentration (67.65%), showed higher anti-inflammatory activity relative to the rest of the essential oils and ibuprofen. Anti-inflammation was achieved by inhibiting the expression of COX-2, IL-1, IL-6, and TNF-α. All six essential oils also demonstrated different DPPH radical scavenging capacities except PCEO. SJEO, with high concentrations of terpenyl acetate (10.52%) and camphor (9.54%), showed the highest antioxidant capacity. The six essential oils also exhibited significantly different antitumor activities on LNCaP and B16 cells. AREO, with a high proportion of patchouli alcohol (45.70%), showed the highest antitumor capacity by inhibiting B16 cells with the lowest concentration of 86.91 μg/mL. LAEO, high in linalool (29.84%), showed promising antitumor capacity by inhibiting LNCaP cells at the lowest dosage of 116.5 μg/mL. Collectively, these six Lamiaceae essential oils, possessing varied chemical compositions and biological activities, exhibit potential for serving as bio-functional additives in biomedical products, such as anti-inflammatory and antitumor drugs.
Five of the six medicinal plants belong to the subfamily Nepetoideae (Dumort.) Burnett, except for P. cablin, which belongs to the subfamily Lamioideae Harley, indicating that species with similar bio-activities and pharmaceutical effects are clustered in their phylogenetic relationships. More specifically, based on our study, PCEO showed the lowest effects on anti-inflammatory and antioxidant activities, which is consistent with the phylogeny (Supplementary Figure S2). In the views of pharmaphylogeny, species that are closely related are not only similar in physiological characteristics, but at the same time, it is also reflected in the similarity of phytochemical components. Our results showed that the principal components of the six essential oils were greatly distinct. Perhaps because the genes expressing these chemical compositions have suffered different selection pressures during evolution, or they have been of independent origin and have undergone different evolutionary pathways. However, we can still find chemical compositions that match the phylogenetic tree. For example, L. angustifolia and P. frutescens are sister groups, and the proportion of nerolidol and linalool showed similarity in LAEO and PFEO; R. officinalis and S. japonica are closely related, and the content of (+)-2-bornanone and eucalyptol showed similarity in SJEO and ROEO.
Although these six Lamiaceae plants are widely used and cultivated in China, only A. rugosa, P. frutescens and S. japonica are native to China. L. angustifolia and R. officinalis are native to Mediterranean, and P. cablin is distributed around the equator in Southeast Asia. This research provided more references for pharmaphylogeny and drug discovery from folk medicinal plants, and more studies need to be done for further exploring the drugs' function.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by the animal experiments were carried out following Ethical Guidelines of the Laboratory Animal Center of Sun Yat-sen University.
Author contributions
YK: data curation, writing–original draft, and writing–review and editing. LG: conceptualization and supervision. JS: data curation and software. PS: software and visualization. CK: data curation. LZ: conceptualization and methodology. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Key Research and Development Program of China (No. 2017YFC1700701), CACMS Innovation Fund (CI2021A03909), and the Key Laboratory of Biology and Genetic Improvement of Horticultural Crops, the Ministry of Agriculture and Rural Affairs, China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.919294/full#supplementary-material
Abbreviations
AREO, the essential oil of Agastache rugosa (Fisch. & C. A. Mey.) Kuntze; LAEO, the essential oil of Lavandula angustifolia Mill.; PCEO, the essential oil of Pogostemon cablin (Blanco) Benth.; PFEO, the essential oil of Perilla frutescens (L.) Britton; ROEO, the essential oil of Rosmarinus officinalis L.; SJEO, the essential oil of Salvia japonica Thunb.
References
Borges, R. S., Ortiz, B. L. S., Pereira, A. C. M., Keita, H., and Carvalho, J. C. T. (2019). Rosmarinus officinalis essential oil: A review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J. Ethnopharmacol. 229, 29–45. doi: 10.1016/j.jep.2018.09.038
Bouzenna, H., Hfaiedh, N., Giroux-Metges, M. A., Elfeki, A., and Talarmin, H. (2017). Potential protective effects of alpha-pinene against cytotoxicity caused by aspirin in the IEC-6 cells. Biomed. Pharmacother. 93, 961–968. doi: 10.1016/j.biopha.2017.06.031
Cavanagh, H. M., and Wilkinson, J. M. (2002). Biological activities of lavender essential oil. Phytother. Res. 16, 301–308. doi: 10.1002/ptr.1103
Chen, H., Zhang, L., Zhang, S., and Luo, Y. (2017). GC-MS Analysis of Volatile Oil Constiuents in Herbal Pair Pogostemonis Herba-Eupatorii Herba and the Single Herbs. Tradi. Chine. Drug Rese. Clinil Pharm. 28, 781–785. (Chinese).
Cheng, Y., Dong, Z., and Liu, S. (2014). beta-Caryophyllene ameliorates the Alzheimer-like phenotype in APP/PS1 Mice through CB2 receptor activation and the PPARgamma pathway. Pharmacology 94, 1–12. doi: 10.1159/000362689
de Lima, V. T., Vieira, M. C., Kassuya, C. A., Cardoso, C. A., Alves, J. M., Foglio, M. A., et al. (2014). Chemical composition and free radical-scavenging, anticancer and anti-inflammatory activities of the essential oil from Ocimum kilimandscharicum. Phytomedicine 21, 1298–1302. doi: 10.1016/j.phymed.2014.07.004
de Souza, W. F. M., Mariano, X. M., Isnard, J. L., de Souza, G. S., de Souza Gomes, A. L., de Carvalho, R. J. T., et al. (2019). Evaluation of the volatile composition, toxicological and antioxidant potentials of the essential oils and teas of commercial Chilean boldo samples. Food Res. Int. 124, 27–33. doi: 10.1016/j.foodres.2018.12.059
El-Sayed, R. M., Moustafa, Y. M., and El-Azab, M. F. (2014). Evening primrose oil and celecoxib inhibited pathological angiogenesis, inflammation, and oxidative stress in adjuvant-induced arthritis: novel role of angiopoietin-1. Inflammopharmacology 22, 305–317. doi: 10.1007/s10787-014-0200-5
Fidyt, K., Fiedorowicz, A., Strzadala, L., and Szumny, A. (2016). beta-caryophyllene and beta-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 5, 3007–3017. doi: 10.1002/cam4.816
Funk, J. L., Frye, J. B., Oyarzo, J. N., Zhang, H., and Timmermann, B. N. (2010). Anti-arthritic effects and toxicity of the essential oils of turmeric (Curcuma longa L.). J. Agric. Food Chem. 58, 842–849. doi: 10.1021/jf9027206
Gao, Y., and Zhang, Z. X. (2019). Extraction and component analysis of essential oil from perilla leaf. Guangzhou Chem. Indus 47, 112–114. (Chinese).
Ghasemzadeh Rahbardar, M., and Hosseinzadeh, H. (2020). Therapeutic effects of rosemary (Rosmarinus officinalis L.) and its active constituents on nervous system disorders. Iran J. Basic Med. Sci. 23, 1100–1112. doi: 10.22038/ijbms.2020.45269.10541
Guo, S., Geng, Z., Zhang, W., Liang, J., Wang, C., Deng, Z., et al. (2016). The chemical composition of essential oils from cinnamomum camphora and their insecticidal activity against the stored product pests. Int. J. Mol. Sci. 17, 1836. doi: 10.3390/ijms17111836
Guo, S. S., Wang, Y., Pang, X., Geng, Z. F., Cao, J. Q., and Du, S. S. (2019). Seven herbs against the stored product insect: Toxicity evidence and the active sesquiterpenes from Atractylodes lancea. Ecotoxicol. Environ. Saf. 169, 807–813. doi: 10.1016/j.ecoenv.2018.11.095
Kanipandian, N., and Thirumurugan, R. (2014). A feasible approach to phyto-mediated synthesis of silver nanoparticles using industrial crop Gossypium hirsutum (cotton) extract as stabilizing agent and assessment of its in vitro biomedical potential. Ind. Crop. Prod. 55, 1–10. doi: 10.1016/j.indcrop.2014.01.042
Karpinski, T. M.. (2020). Essential oils of lamiaceae family plants as antifungals. Biomolecules 10, 103. doi: 10.3390/biom10010103
Khan, A., Vaibhav, K., Javed, H., Tabassum, R., Ahmed, M. E., Khan, M. M., et al. (2014). 1,8-cineole (eucalyptol) mitigates inflammation in amyloid Beta toxicated PC12 cells: relevance to Alzheimer's disease. Neurochem. Res. 39, 344–352. doi: 10.1007/s11064-013-1231-9
Khayyal, M. T., El-Ghazaly, M. A., Abdallah, D. M., Okpanyi, S. N., Kelber, O., and Weiser, D. (2005). Mechanisms involved in the anti-inflammatory effect of a Standized Willow Bark Extract. Arzneimittelforschung 55, 11. doi: 10.1055/s-0031-1296917
Li, H. Q., Liu, Q. Z., Liu, Z. L., Du, S. S., and Deng, Z. W. (2013). Chemical composition and nematicidal activity of essential oil of Agastache rugosa against Meloidogyne incognita. Molecules 18, 4170–4180. doi: 10.3390/molecules18044170
Li, W. S.. (2010). Qualitative analysis of volatile oil from Perilla leaf by GC-MS. Henan Sci. Tech. 14,168–169. (Chinese).
Li, Y., Duan, Y. Q., Wang, H. X., and Xia, J. J. (2016). Analysis of aroma components in sage oil by GC combined with retention index. Cereals Oils 29, 65–68. (Chinese).
Li, Y., Lv, O., Zhou, F., Li, Q., Wu, Z., and Zheng, Y. (2015). Linalool Inhibits LPS-Induced Inflammation in BV2 Microglia Cells by Activating Nrf2. Neurochem. Res. 40, 1520–1525. doi: 10.1007/s11064-015-1629-7
Lian, D. W., Xu, Y. F., Ren, W. K., Fu, L. J., Chen, F. J., Tang, L. Y., et al. (2018). Unraveling the novel protective effect of patchouli alcohol against helicobacter pylori-induced gastritis: insights into the molecular mechanism in vitro and in vivo. Front. Pharmacol. 9, 1347. doi: 10.3389/fphar.2018.01347
Liu, W. J., Yin, Y. B., Sun, J. Y., Feng, S., Ma, J. K., Fu, X. Y., et al. (2018). Natural borneol is a novel chemosensitizer that enhances temozolomide-induced anticancer efficiency against human glioma by triggering mitochondrial dysfunction and reactive oxide species-mediated oxidative damage. Onco. Targets Ther. 11, 5429–5439. doi: 10.2147/OTT.S174498
Luo, W., Du, Z., Zheng, Y., Liang, X., Huang, G., Zhang, Q., et al. (2019). Phytochemical composition and bioactivities of essential oils from six Lamiaceae species. Ind. Crop. Prod. 133, 357–364. doi: 10.1016/j.indcrop.2019.03.025
Mamadalieva, N. Z., Sharopov, F., Satyal, P., Azimova, S. S., and Wink, M. (2017). Composition of the essential oils of three Uzbek Scutellaria species (Lamiaceae) and their antioxidant activities. Nat. Prod. Res. 31, 1172–1176. doi: 10.1080/14786419.2016.1222383
Mamadalieva, N. Z., Youssef, F. S., Ashour, M. L., Sasmakov, S. A., Tiezzi, A., and Azimova, S. S. (2019). Chemical composition, antimicrobial and antioxidant activities of the essential oils of three Uzbek Lamiaceae species. Nat. Prod. Res. 33, 2394–2397. doi: 10.1080/14786419.2018.1443088
Mouahid, A., Dufour, C., and Badens, E. (2017). Supercritical CO2 extraction from endemic Corsican plants; comparison of oil composition and extraction yield with hydrodistillation method. J. CO2 Util. 20, 263–273. doi: 10.1016/j.jcou.2017.06.003
Nieto, G.. (2017). Biological activities of three essential oils of the lamiaceae family. Medicines (Basel) 4, 63. doi: 10.3390/medicines4030063
Nikolić, M., Jovanović, K. K., Marković, T., Marković, D., Gligorijević, N., Radulović, S., et al. (2014). Chemical composition, antimicrobial, and cytotoxic properties of five Lamiaceae essential oils. Ind. Crop. Prod. 61, 225–232. doi: 10.1016/j.indcrop.2014.07.011
Oner, Z., Altinoz, E., Elbe, H., and Ekinci, N. (2019). The protective and therapeutic effects of linalool against doxorubicin-induced cardiotoxicity in Wistar albino rats. Hum. Exp. Toxicol. 38, 803–813. doi: 10.1177/0960327119842634
Park, C. G., Jang, M., Yoon, K. A., and Kim, J. (2016). Insecticidal and acetylcholinesterase inhibitory activities of Lamiaceae plant essential oils and their major components against Drosophila suzukii (Diptera: Drosophilidae). Ind. Crop. Prod. 89, 507–513. doi: 10.1016/j.indcrop.2016.06.008
Rodboon, T., Okada, S., and Suwannalert, P. (2020). Germinated 2020 riceberry rice enhanced protocatechuic acid and vanillic acid to suppress melanogenesis through cellular oxidant-related tyrosinase activity in B16 cells. Antioxidants (Basel). 9, 247. doi: 10.3390/antiox9030247
Santos, F. A., and Rao, V. S.. (2000). Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother Research 14, 240–4. doi: 10.1002/1099-1573(200006)14:4<240::aid-ptr573>3.0.co;2-x
Sharma, J. N., Srivastava, K. C., and Gan, E. K. (1994). Suppressive effects of eugenol and ginger oil on arthritic rats. Pharmacology., 49, 5. doi: 10.1159/000139248
Spiridon, I., Colceru, S., Anghel, N., Teaca, C. A., Bodirlau, R., and Armatu, A. (2011). Antioxidant capacity and total phenolic contents of oregano (Origanum vulgare), lavender (Lavandula angustifolia) and lemon balm (Melissa officinalis) from Romania. Nat. Prod. Res. 25, 1657–1661. doi: 10.1080/14786419.2010.521502
Vieira, J. N., Gonçalves, C. L., Villarreal, J., Goncalves, V., Lund, R., Freitag, R., et al. (2019). Chemical composition of essential oils from the apiaceae family, cytotoxicity, and their anti-fungal activity in vitro against candida species from oral cavity. Braz. J. Biol. 79, 432–437. doi: 10.1590/1519-6984.182206
Vukovic, N., Sukdolak, S., Solujic, S., and Niciforovic, N. (2009). Antimicrobial activity of the essential oil obtained from roots and chemical composition of the volatile constituents from the roots, stems, and leaves of Ballota nigra from Serbia. J. Med. Food 12, 435–441. doi: 10.1089/jmf.2008.0164
Vyry Wouatsa, N. A., Misra, L., and Venkatesh Kumar, R. (2014). Antibacterial activity of essential oils of edible spices, Ocimum canum and Xylopia aethiopica. J. Food Sci. 79, M972–M977. doi: 10.1111/1750-3841.12457
Wojtunik-Kulesza, K. A., Kasprzak, K., Oniszczuk, T., and Oniszczuk, A. (2019). Natural monoterpenes: Much more thanonly a scent. Chem. Biodiv. 16, e19004. doi: 10.1002/cbdv.201900434
Xiang, H., Zhang, L., Yang, Z., Chen, F., Zheng, X., and Liu, X. (2017). Chemical compositions, antioxidative, antimicrobial, anti-inflammatory and antitumor activities of Curcuma aromatica Salisb. essential oils. Ind. Crop. Prod. 108, 6–16. doi: 10.1016/j.indcrop.2017.05.058
Xue, H. J., Wei, J. N., Magalhães, S., Zhang, B., Song, K. Q., Liu, J., et al. (2016). Contact pheromones of 2 sympatric beetles are modified by the host plant and affect mate choice. Behav. Ecol. 27, 895–902. doi: 10.1093/beheco/arv238
Xue, S.. (2016). Research of the composition and antioxidant activity of essential oil from Folium Perillaes extracted by different methods. Sci. Tech. Food. Indus., 37, 67–74. (Chinese).
Yu, M. H., Choi, J. H., Chae, I. G., Im, H. G., Yang, S. A., More, K., et al. (2013). Suppression of LPS-induced inflammatory activities by Rosmarinus officinalis L. Food Chem. 136, 047–1054. doi: 10.1016/j.foodchem.2012.08.085
Zhang, L., Liang, X., Ou, Z., Ye, M., Shi, Y., Chen, Y., et al. (2020a). Screening of chemical composition, anti-arthritis, antitumor and antioxidant capacities of essential oils from four Zingiberaceae herbs. Ind. Crop. Prod. 149, 112342. doi: 10.1016/j.indcrop.2020.112342
Zhang, L., Liang, X., Wang, B., Lin, Z., Ye, M., Ma, R., et al. (2020b). Six herbs essential oils suppressing inflammatory responses via inhibiting COX-2/TNF-α/IL-6/NF-κB activation. Microchem. J. 156, 104769. doi: 10.1016/j.microc.2020.104769
Zhang, L., Pan, C., Ou, Z., Liang, X., Shi, Y., Chi, L., et al. (2020c). Chemical profiling and bioactivity of essential oils from Alpinia officinarum Hance from ten localities in China. Ind. Crop. Prod. 153, 112583. doi: 10.1016/j.indcrop.2020.112583
Zhang, L., Yang, Z., Chen, D., Huang, Z., Li, Y., Lan, X., et al. (2017c). Variation on composition and bioactivity of essential oils of four common Curcuma Herbs. Chem Biodivers. 14, e1700280. doi: 10.1002/cbdv.201700280
Zhang, L., Yang, Z., Wei, J., Su, P., Chen, D., Pan, W., et al. (2017a). Contrastive analysis of chemical composition of essential oil from twelve Curcuma species distributed in China. Ind. Crop. Prod. 108, 17–25. doi: 10.1016/j.indcrop.2017.06.005
Zhang, L., Yang, Z., Wei, J., Su, P., Pan, W., Zheng, X., et al. (2017b). Essential oil composition and bioactivity variation in wild-growing populations of Curcuma phaeocaulis Valeton collected from China. Ind. Crop. Prod. 103, 274–282. doi: 10.1016/j.indcrop.2017.04.019
Keywords: Lamiaceae herb, essential oil, chemical composition, folk medicinal plants, biological activities
Citation: Sun J, Sun P, Kang C, Zhang L, Guo L and Kou Y (2022) Chemical composition and biological activities of essential oils from six lamiaceae folk medicinal plants. Front. Plant Sci. 13:919294. doi: 10.3389/fpls.2022.919294
Received: 13 April 2022; Accepted: 04 July 2022;
Published: 01 August 2022.
Edited by:
Chunnian He, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Minhui Li, Baotou Medical College, ChinaJiushi Liu, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2022 Sun, Sun, Kang, Zhang, Guo and Kou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaping Kou, kouyaping@caas.cn; Lanping Guo, glp01@126.com; Lanyue Zhang, zhanglanyue@gdut.edu.cn
 Jiahui Sun
Jiahui Sun Peipei Sun2
Peipei Sun2 Chuanzhi Kang
Chuanzhi Kang Lanyue Zhang
Lanyue Zhang Lanping Guo
Lanping Guo Yaping Kou
Yaping Kou