- 1Department of Paediatrics and Adolescent Medicine, Turku University Hospital, University of Turku, Turku, Finland
- 2Department of Paediatric Neurology, Turku University Hospital, University of Turku, Turku, Finland
- 3Department of Obstetrics and Gynaecology, Turku University Hospital, University of Turku, Turku, Finland
- 4Department of Human Development and Family Studies, Purdue University, West Lafayette, IN, United States
- 5Department of General Practice, Turku University Hospital, Turku University, Turku, Finland
Background: Preterm infants are still at an increased risk for suboptimal neurodevelopmental outcomes when compared with term born infants. The development of a child born preterm can be jeopardized by suboptimal conditions during pregnancy, in addition to the suboptimal growth environment postnatally compared to the normal in utero environment. This review summarizes the literature on the role of chorioamnionitis, placental insufficiency, and maternal smoking on the developmental outcomes of preterm infants.
Methods: A systematic database search was performed to identify all original articles published on or before September 12, 2018 that evaluated the impact of clinical or histological chorioamnionitis, abnormal prenatal fetal and placental blood flow, and prenatal smoking exposure on the neuropsychological and cognitive outcomes of preterm infants. We identified a total of 54 studies. Thirty five original articles evaluated the effects of clinical or histological chorioamnionitis; 15 studies evaluated the effects of abnormal blood flow patterns; and four studies evaluated the effects of maternal smoking during pregnancy.
Results: The studies on prenatal risk factors showed conflicting results about the impact on the neurodevelopment of preterm infants. The majority of the studies did not show that chorioamnionitis poses a direct risk to the development of preterm infants. The role of abnormal prenatal placental and fetal blood flow on the development of preterm infants remained inconclusive because the sample sizes were often small and methodological problems complicated the interpretation of the data. Maternal smoking during pregnancy was assessed only in one cohort which showed that maternal smoking is a risk for suboptimal cognitive and neuropsychological development in preterm infants.
Conclusions: This review summarizes the data on several prenatal risk factors which play a role in the developmental outcomes of preterm infants. To optimize the developmental outcomes, we need to first optimize the fetal wellbeing before birth. More research that extends from the fetal life to long-term developmental outcomes is needed.
Introduction
Although the perinatal and neonatal care of preterm infants is constantly improving, preterm infants are still at increased risk for suboptimal cognitive and neuropsychological outcomes when compared with term infants. Preterm infants will inevitably have a different developmental environment compared to their physiological in utero environment, which poses a risk for the developing brain. This risk increases further with decreasing gestational age (Munck et al., 2010; Lind et al., 2011; Cheong et al., 2017; Hirvonen et al., 2017; Luu et al., 2017; Twilhaar et al., 2018). In addition to medical risk factors associated with preterm birth and intensive care needed in these situations, the premature babies are exposed to other risk factors associated with poor neurodevelopmental outcome, such as parental separation, stress, anxiety, and depression. Also the ability to succesfully breast feed the baby is often compromised in a case on very preterm delivery, which is an additional risk factor for mother-infant interaction and neurodevelopment (Flacking et al., 2012). Further more, there are several prenatal factors related to prematurity which have been suggested to increase the risk of developmental deficits in preterm infants. This review summarizes the research of prevalent prematurity associated prenatal risk factors with accumulating new research on later cognitive and neuropsychological outcomes of preterm infants.
Choriamnionitis is an important cause of preterm delivery. The incidence of chorioamnionitis increases with decreasing gestational age, with nearly all spontaneous preterm deliveries occurring around 24 weeks of gestation being associated with chorioamnionitis (Andrews et al., 2000; Goldenberg et al., 2000, 2008; Goldenberg, 2002). Although some studies have shown that histological and/or clinical chorioamnionitis are associated with suboptimal neurodevelopment in preterm infants, the findings are inconsistent as shown in previous reviews (Ylijoki et al., 2012; van Vliet et al., 2013; Maisonneuve et al., 2017). Whether chorioamnionitis leads to impaired outcomes compared to non-exposed preterm infants born at similar gestational age remains unclear. As inflammation may enhance maturation, chorioamnionitis may also have beneficial effects for preterm infants.
An abnormal placental and fetal blood flow pattern has been shown to occur in about 20% of all very preterm births (Leppänen et al., 2009). It is well known that increased impedance in the umbilical artery flow is associated with an increased perinatal mortality and morbidity, especially in growth restricted fetuses (Karsdorp et al., 1994). To cope with placental insufficiency, the fetus increases blood flow to the brain. This so called “brain sparing” is reflected as an increased ratio between umbilical artery (UA) and middle cerebral artery (MCA) pulsatile indices. Brain sparing has been associated with decreased total brain volume, cortical gray matter volume, and cerebral volumes (Tolsa et al., 2004; Maunu et al., 2007), as well as increased incidence of brain pathology (Leppänen et al., 2009). It has been hypothesized that impaired fetal brain growth may lead to an impaired neurocognitive outcome. However, the data are inconclusive, and there are no previous reviews highlighting the matter.
Maternal smoking during pregnancy is the most common preventable factor causing adverse effects on fetal development. It is associated with an increased risk of preterm birth and low birth weight (Andres and Day, 2000). The risk of sudden infant death syndrome has also been associated with maternal smoking during pregnancy (Mitchell and Milerad, 2006). In addition, maternal smoking has been associated with adverse effects on fetal brain development in term and preterm infants (Ekblad et al., 2010, 2015), and with long term adverse effects in exposed infants, such as psychiatric morbidity (Ekblad et al., 2010) and neurodevelopmental problems (Clifford et al., 2012; Polanska et al., 2015). Study populations have mainly been full-term children. In preterm children, the association of maternal smoking during pregnancy with cognitive and neuropsychological outcomes is less studied, and there are no previous reviews concerning the matter.
In this review, we aim to evaluate the significance of selected prenatal risk factors related to prematurity, such as smoking during pregnancy, abnormal prenatal blood flow patterns, and chorioamnionitis, on the neurodevelopment of preterm children. This information is important for the professionals involved in the follow-up of pregnant women, as well as the professionals, such as psychologists, speech therapists, pediatricians, child neurologists and teachers who are involved in the follow up, therapy and education of preterm children.
Methods
We performed a systematic electronic database search in the PubMed database (including MeSH search) to identify all original articles published on or before September 12, 2018 that evaluated the impact of clinical or histological chorioamnionitis, prenatal smoking exposure, and abnormal prenatal fetal and placental blood flow on neuropsychological and cognitive outcomes in preterm infants. With chorioamnionitis, the search terms used for the search were Chorioamnionitis combined with Development. Articles published before October 5, 2011 were part of a previously published review article (Ylijoki et al., 2012). We chose to use the same search terms to be able to combine the search results and update the previous findings with recent publications. With prenatal blood flow (Doppler velocimetry), the search terms used were doppler combined with cognitive outcome and placental doppler combined with outcome. With maternal smoking the search terms used for the search were Smoking, Pregnancy and Neurodevelopment or Cognitive Development. In addition, we performed a manual search of the reference lists of all included articles from the database search.
Study Selection
In the first phase, the publications were selected based on titles and abstracts to exclude irrelevant publications. Only publications written in English were included. Based on the full text articles, publication were excluded if they did not provide answer to the question of interest. Figures 1–3 show the flow chart of the literature search.
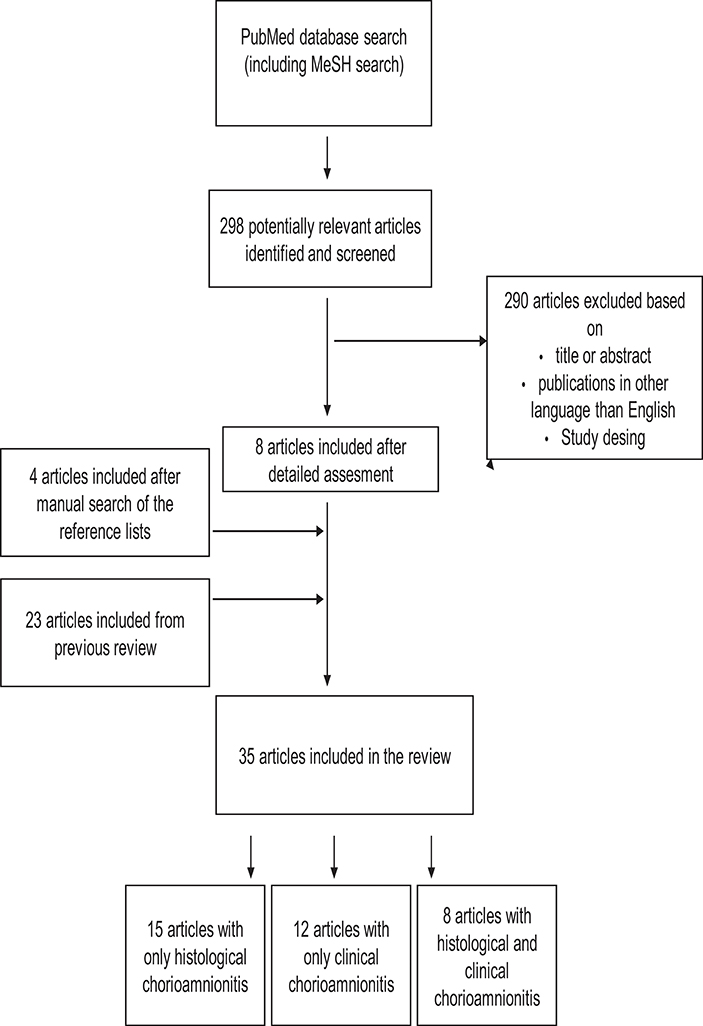
Figure 1. Study selection process for articles about the association between chorioamnionitis and cognitive and neuropsychological outcome in preterm infants. The search was performed for articles published between October 5, 2011and September 12, 2018. Articles published before October 5, 2011 was identified earlier as a part of our previous review.
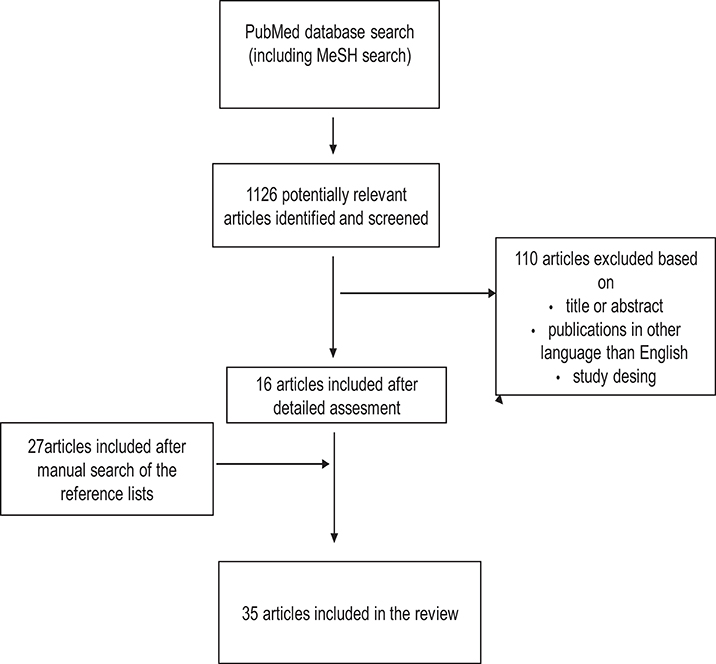
Figure 2. Study selection process for articles about the association between abnormal placental and fetal blood flow and cognitive and neuropsychological outcome in preterm infants.
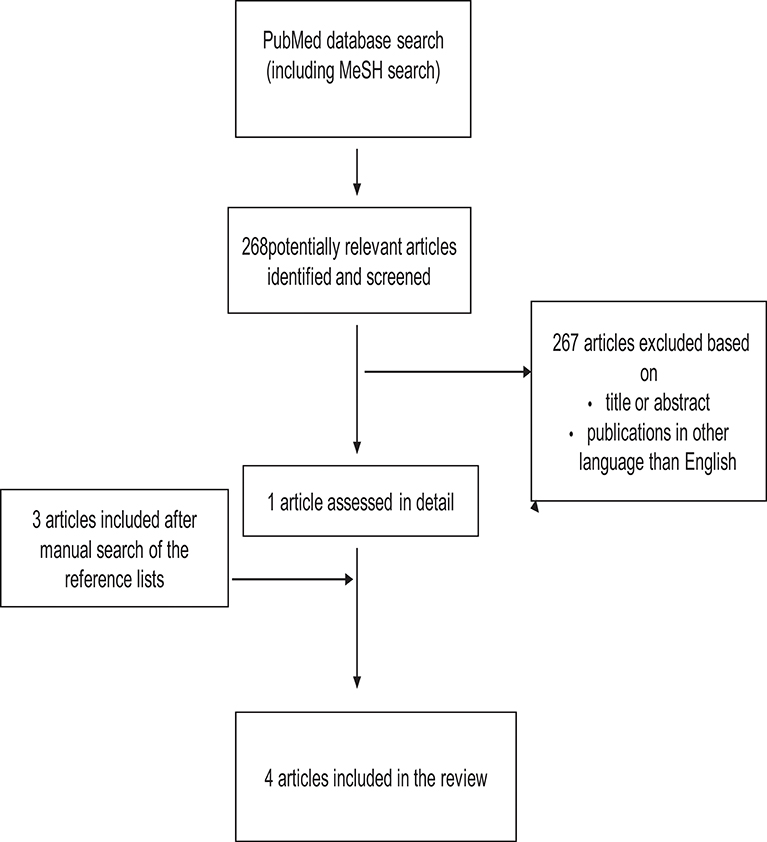
Figure 3. Study selection process for articles about the association between smoking during pregnancy and cognitive and neuropsychological outcome in preterm infants.
We only included articles with preterm infants (born before 37 weeks of gestation). The developmental outcomes in the included articles were neuropsychological and cognitive development. The methods of evaluation varied greatly among the different studies.
Chorioamnionitis (i.e., inflammation of the fetal membranes) can be classified as either histological or clinical chorioamnionitis. Clinical chorioamniotis is usually diagnosed based on a combination of clinical signs (ruptured membranes, maternal or fetal tachycardia, maternal leukocytosis, foul-smelling amnionitic fluid), but the criteria of the diagnosis in the articles was variable. Histological chorioamnionitis is based on the appearance of neutrophils in the placental tissue. Histological chorioamnionitis can be further classified as maternal or fetal chorioamnionitis (also called funisitis).
Studies using doppler ultrasound to assess fetoplacental blood flow from the umbilical artery, fetal median cerebral artery, and/or the aortic isthmus were included in this review.
All of the articles about the effects of smoking were about maternal smoking during pregnancy.
Results
We identified 54 studies about prenatal risk factors related to prematurity and their impact on cognitive and neuropsychological outcome. Thirty-five original articles evaluated the effects of clinical or histological chorioamnionitis; 15 studies evaluated the effects of abnormal prenatal Doppler velocimetry; and four studies evaluated the effects of maternal smoking during pregnancy.
Chorioamnionitis
The majority of the studies found no independent effect of chorioamnionitis on abnormal neurodevelopment (Morales, 1987; Dexter et al., 1999, 2000; Ambalavanan et al., 2000; Kosuge et al., 2000; Vermeulen et al., 2001; Dammann et al., 2003; Kent et al., 2005; Polam et al., 2005; Mu et al., 2007; Redline et al., 2007; Andrews et al., 2008; Helderman et al., 2012; Nasef et al., 2013; Soraisham et al., 2013; Manuck et al., 2014; Pappas et al., 2014; Källén et al., 2015; Miyazaki et al., 2016; Vander Haar and Gyamfi-Bannerman, 2016; Bierstone et al., 2018). There was a similar proportion of studies without association in the groups of histological and clinical chorioamnionitis. However, all studies with associations showed that chorioamninitis was a risk factor, not a protective factor, for later development. The results of the studies about chorioamnionitis are summarized in Table 1.
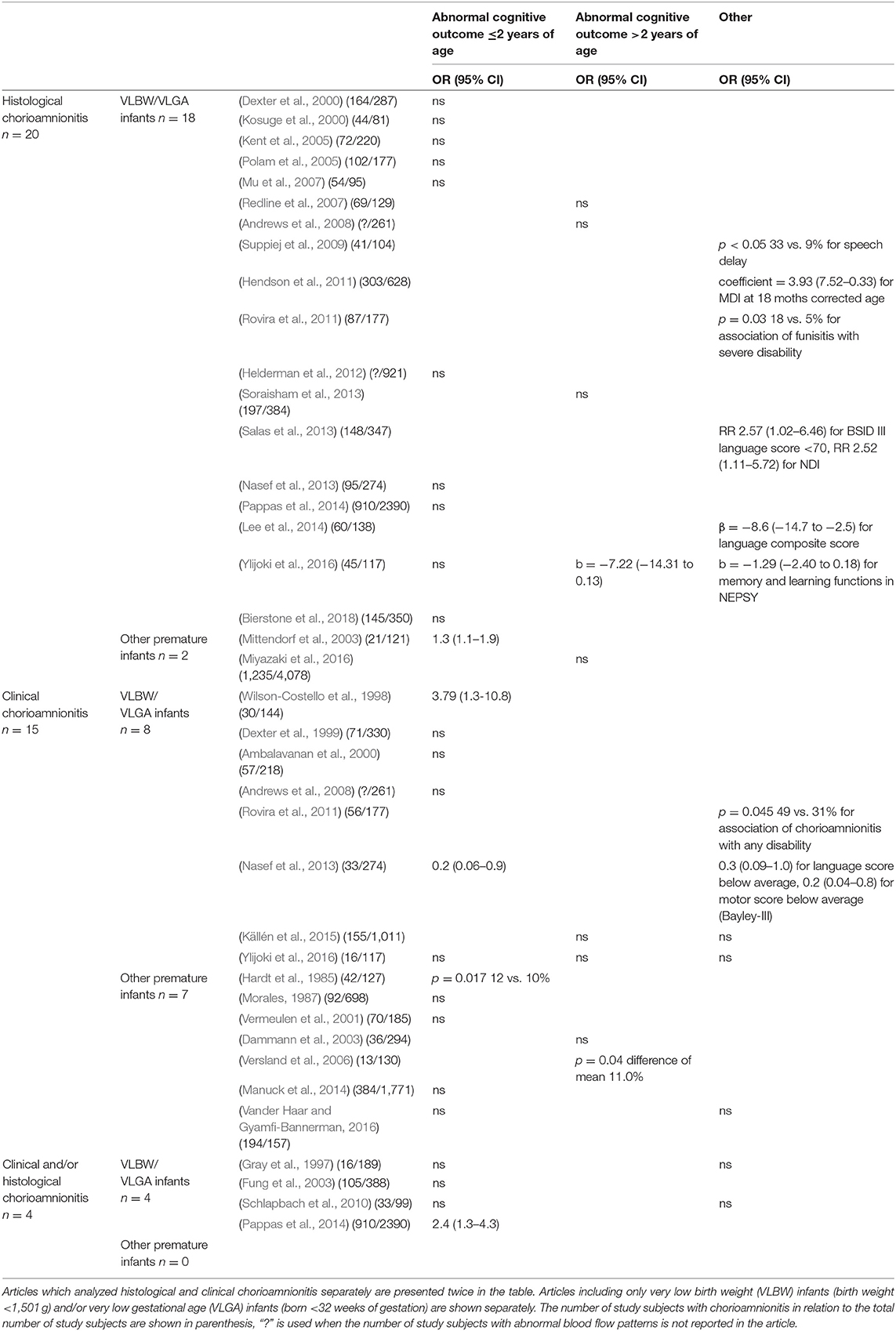
Table 1. The outcomes of the 35 included articles divided according to the definition of chorioamnionitis (clinical, histological or clinical, and/or histological) and the association with developmental outcomes (abnormal cognitive outcome ≤/>2 years of age or other).
Only seven of the 20 studies about histological chorioamnionitis demonstrated that histological chorioamnionitis was a risk factor for suboptimal development. Two studies found an association between histological chorioamnionitis and speech delay (Suppiej et al., 2009) and lower mental developmental index (Hendson et al., 2011) at 18 months of corrected age in very low birth weight and very low gestational age infants, but multivariate analyses were not performed in one of them (Suppiej et al., 2009). Rovira et al. (2011) found an association with severe disability in very low birth weight infants at 2 years of age in logistic regression analyses but gestational age was not included in the analyses. Two studies (Salas et al., 2013; Lee et al., 2014) found that histological chorioamnionitis and funisitis were associated with weaker language performance at 18–24 months of corrected age, but one of them (Salas et al., 2013) did not include gestational age in multivariate analyses. Mittendorf et al. (2003) found that funisitis predicted impaired neurodevelopment at 18 months of corrected age. Ylijoki et al. (2016) found that histological chorioamnionitis, but not funisitis associated with slightly weaker memory and learning functions as well as weaker cognitive performance at 5 years of age. Nine articles of histological chorioamnionitis (all of them discussed above) separately evaluated the effects of funisitis on the neurodevelopment of preterm born children. Four of these found that funisitis independently increased the risk for the developmental problems (Mittendorf et al., 2003; Redline et al., 2007; Rovira et al., 2011; Salas et al., 2013), while five studies did not find any associations (Redline et al., 2000; Helderman et al., 2012; Soraisham et al., 2013; Lee et al., 2014; Ylijoki et al., 2016).
Only five of 15 studies found an association between clinical chorioamnionitis and neurodevelopmental impairments in preterm born children. Hardt et al. (1985) found lower cognitive scores in children born after preterm rupture of membranes with chorioamnionitis compared to those without chorioamnionitis at 12 months corrected age. Wilson-Costello et al. (1998), Rovira et al. (2011), and Nasef et al. (2013) have reported an association between clinical chorioamnionitis and cognitive, verbal, and motor performance, as well as with neurological disability at 18–24 months of corrected age. Versland et al. (2006) had similar findings about increased risk for cognitive impairment up to 11 years of age.
In addition, there were four studies in which clinical and histological chorioamnionitis was not separated (Gray et al., 1997; Fung et al., 2003; Schlapbach et al., 2010; Pappas et al., 2014). One of these studies found an association between chorioamnionitis and lower cognitive scores at 18–22 months of corrected age (Pappas et al., 2014), while the others did not.
Placental and Fetal Blood Flow
Eleven studies reported outcomes in association with umbilical artery blood flow. Seven of these did not find an association between abnormal flow in the umbilical artery and neurocognitive outcome (Valcamonico et al., 2004, 2007; Kirsten et al., 2007; Leppänen et al., 2009; Shand et al., 2009; Torrance et al., 2010; Eger et al., 2013). Male fetuses with intrauterine growth restriction and absent or reversed end diastolic flow in the umbilical artery have performed worse on cognitive tests than those with appropriate growth for gestational age (Morsing et al., 2011). Similarly, preterm neonates with absent or reversed end diastolic flow have been shown to have more cognitive, mental, and motor disabilities than appropriately grown controls (Vossbeck et al., 2001). Unfortunately, in both of these studies, blood flow patterns were not assessed in the groups of appropriately grown fetuses, which is a serious methodological limitation. Pathological flow in the umbilical artery has also been associated with moderate or severe neurological impairment in children with intrauterine growth restriction, but not in those with normal prenatal growth (Spinillo et al., 2005). Growth restricted preterm children with suboptimal neurological outcomes at 1 year of age had higher pulsatility index in the umbilical artery flow than those with normal neurodevelopmental outcome in univariate analyses (Kaukola et al., 2005).
An increased placenta-cerebral ratio (UA/MCA-ratio) reflecting brain sparing in a fetus has been associated with adverse cognitive performance in very low birthweight children (Leppänen et al., 2009). An increased UA/MCA ratio was associated with impaired cognitive outcomes at 5 years of age, but not with neurodevelopmental outcomes at 3 years of age in the same patient population (Scherjon et al., 1998, 2000).
Some studies have suggested that retrograde (Fouron et al., 2001, 2005) or abnormal blood flow in the aortic isthmus (Leppänen et al., 2009) is associated with non-optimal neurodevelopment. However, retrograde flow did not associate with abnormal outcomes in one study with six patients with retrograde flow (Kaukola et al., 2005). The results of the articles about placental and fetal blood flow are summarised in Table 2.
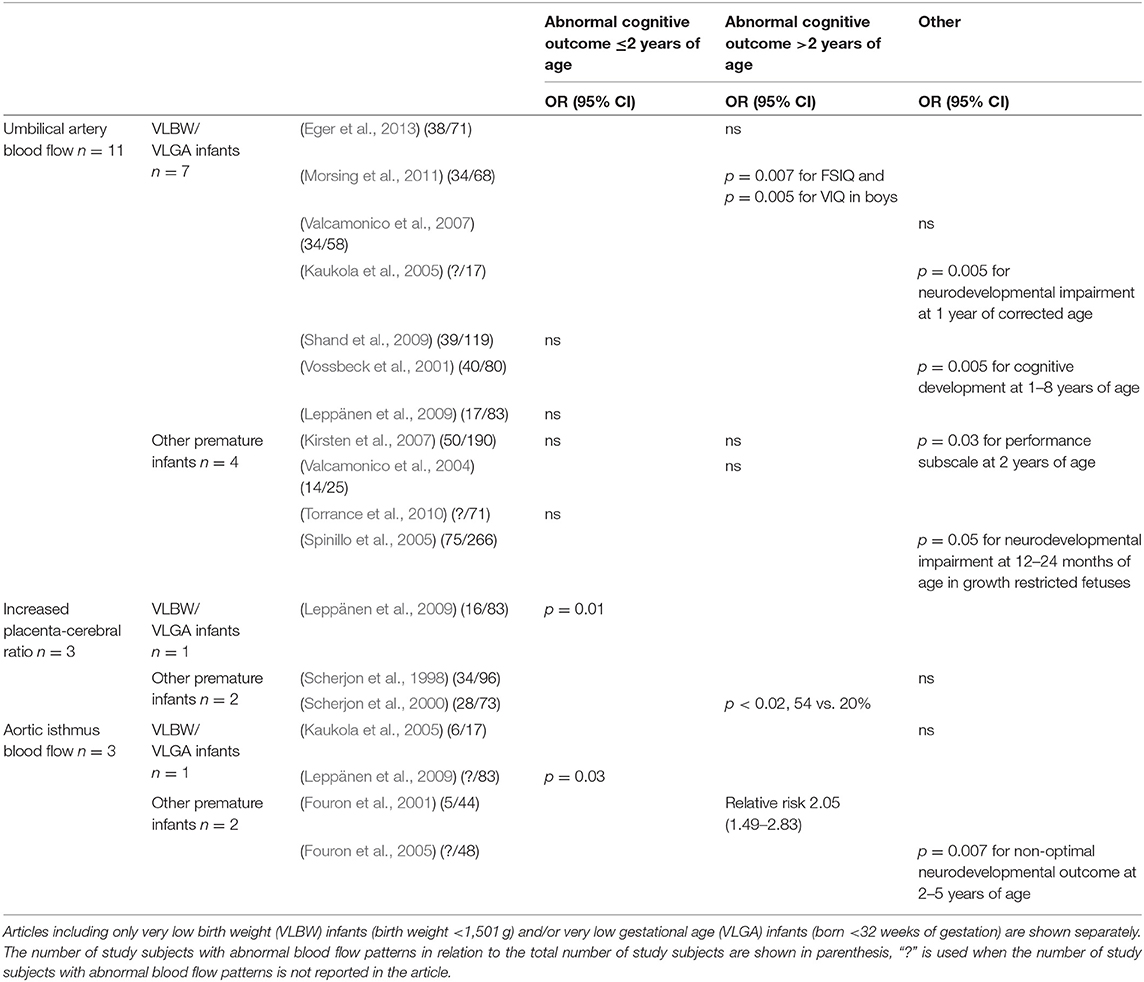
Table 2. The outcomes of the 15 included articles divided according to the placental or fetal blood flow measurements used (umbilical artery blood flow, increased placenta-cerebral ratio, retrograde flow in the aortic isthmus) and developmental outcomes (abnormal cognitive outcome ≤/>2 years of age or other).
Maternal Smoking During Pregnancy
The four articles assessing the association between prenatal smoking exposure and cognitive and neurodevelopmental outcomes in preterm infants were based on the same cohort studied in Austria at 12 months of corrected age (Kiechl-Kohlendorfer et al., 2009), 24 months of corrected age (Kiechl-Kohlendorfer et al., 2010), and 5 years of corrected age (Kiechl-Kohlendorfer et al., 2013; Gnigler et al., 2015). Smoking information was based on maternal self-report after birth. The mothers who refused to report their smoking status were classified as smokers. These articles showed associations between prenatal smoking exposure and adverse developmental outcomes at 12 and 24 months of corrected age (Kiechl-Kohlendorfer et al., 2009, 2010), as well as an association with numerical skills and processing speed at 5 years of corrected age (Kiechl-Kohlendorfer et al., 2013; Gnigler et al., 2015). The results of these articles are summarized in Table 3.
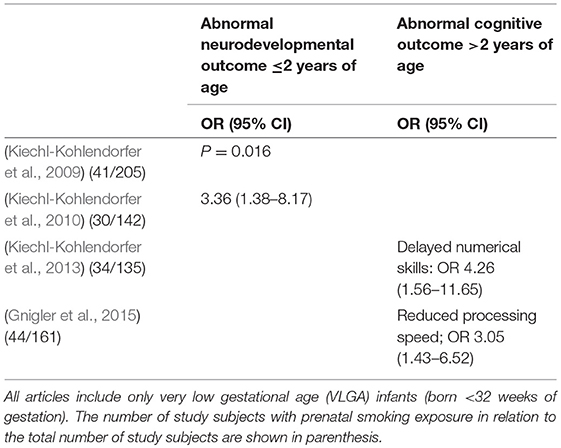
Table 3. The outcomes of the four included articles about maternal smoking during pregnancy divided according the developmental outcomes (abnormal cognitive outcome ≤/>2 years of age or other).
Discussion
There is conflicting data about the impact of prenatal risk factors on long-term development of preterm infants. It is challenging to do longitudinal studies extending from fetal risk factors or well-being to eventual long-term developmental outcomes, as so many of the studies have only small number of patients. Therefore, a summarizing, critical review of all the data is valuable.
A review of the effects of chorioamnionitis on the development of preterm infants helps clinicians get an overview of the heterogeneous studies with inconsistent results regarding this topic. Inconsistencies are partly due to small sample sizes, differences in patient populations, evaluation methods, and age points. This review classifies the studies according to the distinction between histological and clinical chorioamnionitis. Altogether, it seems that the majority of the publications do not support the belief that chorioamnionitis poses an independent risk for adverse development in preterm born children. This review does not give further support either to the hypothesis that clinical chorioamnionitis and funisitis are more deleterious to the developing central nervous system than histological chorioamnionitis.
One factor possibly explaining this complexity might be the maturation enhancing effects of chorioamnionitis on immature infants. Animal models have shown that chorioamnionitis significantly enhances lung maturation (Kramer et al., 2009), which might also be seen clinically in the lungs of preterm infants, although this comes with inflammatory consequences (Jobe, 2012). Therefore, the clinical effects of chorioamnionitis on the developmental outcome of preterm infants is complex. There are a few studies that show that histological chorioamniniotis has a protective effect on mortality rates (Hendson et al., 2011) and neurodevelopment of preterm infants when compared with placental underperfusion (van Vliet et al., 2012). Therefore, it seems that chorioamnionitis might have beneficial effects, in addition to the deleterious effects, on the developing preterm infant. Another factor modifying the effects of chorioamnionitis might be prenatal glucocorticoids. Most preterm infants are exposed to the anti-inflammatory effects of prenatal glucocorticoids. It is likely that this immunomodulation attenuates the effects of chorioamnionitis. Indeed, a meta-analysis has shown that prenatal steroid administration is associated with a reduced risk for brain lesions in clinical and histological chorioamnionitis (Been et al., 2011). One study found that histological chorioamnionitis was associated with CP only in those infants who had not been given two doses of prenatal corticosteroids (Kent et al., 2005). However, our review also includes recent publications with patients who had a high prenatal glucocorticoid administration rate where chorioamnionitis still seemed to be a significant risk factor for suboptimal development.
We can conclude that the available evidence does not suggest chorioamnionitis is a major independent risk factor for suboptimal cognitive and neuropsychological development in preterm born children. As there are no “healthy” preterm controls without other risk factors, we can only conclude that chorioamnionitis may not be a greater risk for the brain of a preterm infant than other underlying pathologies already present before preterm delivery.
Most of the data on the association of prenatal fetoplacental blood flow and later neurocognitive development is focused on umbilical artery blood flow. The pulsatility index of umbilical artery flow reflects the number of tertiary villous arterioles in the placenta (Acharya et al., 2004). Thus, pulsatility index of the umbilical artery flow is known to increase in placental insufficiency. Most of the studies did not find an association between increased pulsatility in the umbilical artery flow and later neurocognitive outcomes in preterm born infants. However, there are some data on abnormal umbilical blood flow and impaired neurodevelopment in fetuses suffering from placental insufficiency (Kaukola et al., 2005). However, the sample sizes were often small and methodological problems complicated the interpretation of the data.
The so called “brain sparing” effect has been considered protective to fetuses with growth restriction. Thus, it is interesting that an increased placenta-cerebral ratio has been associated with impaired cognitive outcomes in some (Scherjon et al., 2000; Leppänen et al., 2009), although not all studies (Scherjon et al., 1998). The data is too sparse to draw conclusions on the effect of aortic isthmic blood flow on infant neurocognitive development.
Prenatal smoking exposure has been associated with many adverse effects on fetal health as well as an increased risk for diseases in later life. The data show a negative association between prenatal smoking exposure and later cognition in preterm infants up to 5 years of age. However, it is challenging to prove a causal link between prenatal smoking exposure and cognitive outcomes in later life because genetic and familial factors are known confounders (Gilman et al., 2008; Knopik, 2009). Maternal education, which is one important familial factor, was adjusted for in the analyses of these studies with preterm infants (Kiechl-Kohlendorfer et al., 2009, 2010, 2013; Gnigler et al., 2015). The familial factors include also disadvantageous parenting and family functioning. Therefore, more precise measurements of genetic and familial influences should be taken into account (e.g., by sibling design). It has been shown in a sibling design study that the unexposed sibling was also at an increased risk of poor school performance at the age of 15 years Lambe et al. (2006). On the other hand, there is a large population study showing that the effects of prenatal smoking exposure remained also in a sibling design study (Ekblad et al., 2017). Individual genetic polymorphisms might also modify the effect of prenatal smoking exposure on cognitive functioning (Morales et al., 2009). Women who smoke during pregnancy may also be more likely to engage in other unhealthy behaviors, like alcohol drinking during pregnancy when smoking serves as an indicator for unhealthy lifestyle. A Finnish study showed that heavy smoking before pregnancy was associated with lower cognitive scores in children aged 56 months even if the mother did not smoke during pregnancy (Heinonen et al., 2011). The authors of the aforementioned study speculate that the association might be explained by maternal smoking-related health habits and status. In future studies, it would be interesting to also control also for cognitive abilities and executive functions of the parents.
The lack of reliable validation of smoking exposure is one significant limitation in most of the studies on the effects of smoking exposure. Smoking exposure can be verified by measuring cotinine, a metabolite of nicotine, in the saliva or hair of a mother during pregnancy (Shipton et al., 2009). Cotinine measurements would also reveal a significant environmental smoking exposure. In addition, there might be different effects of smoking exposure at different stages of pregnancy, and so evaluating changes in cotinine levels throughout pregnancy might be relevant.
One limitation of this review is the heterogeneous and rather small patient populations in the original articles. The inclusion criteria vary between the studies, and thus the comparison of the studies is difficult. The same applies to the outcome variables and time points. Also, some of the studies were done in the 1980s and 1990s, and it is well known that the treatments and outcomes of preterm infants have developed during the subsequent years.
This review summarizes the data on several prematurity related prenatal risk factors which play a role in the developmental outcomes of preterm infants. To optimize the developmental outcomes of this patient population we need to first optimize the fetal well-being before birth. More longitudinal research with large patient populations that extends from the fetal life to long-term developmental outcomes is needed. To draw definite conclusions about clinical practices such as the right timing of the delivery requires randomized controlled trials. It is also crucial to implement all practices which protect brain development and improve later neurodevelopmental outcomes of immature preterm infants.
Author Contributions
MY, EE, ME, and LL took part in the design of the study, the interpretation of data, and drafted the initial manuscript. All authors have approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants to MY from the C. G. Sundell foundation and to ME from the Foundation for Pediatric Research, the Orion Research Foundation sr, the Emil Aaltonen's Foundation, the Paulo Foundation, the Maud Kuistila Memorial Foundation, and the Turku University Hospital Research Foundation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We want to thank Fulbright Scholar, Ms. Sarah Holdren, BA, for language editing.
References
Acharya, G., Erkinaro, T., Mäkikallio, K., Lappalainen, T., and Rasanen, J. (2004). Relationships among doppler-derived umbilical artery absolute velocities, cardiac function, and placental volume blood flow and resistance in fetal sheep. Am. J. Physiol. Heart Circul. Physiol. 286, H1266–H1272. doi: 10.1152/ajpheart.00523.2003
Ambalavanan, N., Nelson, K. G., Alexander, G., Johnson, S. E., Biasini, F., and Carlo, W. A. (2000). Prediction of neurologic morbidity in extremely low birth weight infants. J. Perinatol. 20(8 Pt 1), 496–503. doi: 10.1038/sj.jp.7200419
Andres, R. L., and Day, M. C. (2000). Perinatal complications associated with maternal tobacco use. Semin. Neonatol. 5, 231–241. doi: 10.1053/siny.2000.0025
Andrews, W. W., Cliver, S. P., Biasini, F., Peralta-Carcelen, A. M., Rector, R., Alriksson-Schmidt, A. I., et al. (2008). Early preterm birth: association between in utero exposure to acute inflammation and severe neurodevelopmental disability at 6 years of age. Am. J. Obstet. Gynecol. 198, 466.e1–466.e11. doi: 10.1016/j.ajog.2007.12.031
Andrews, W. W., Hauth, J. C., and Goldenberg, R. L. (2000). Infection and preterm birth. Am. J. Perinatol. 17, 357–366. doi: 10.1055/s-2000-13448
Been, J., Degraeuwe, P. L., Kramer, B. W., and Zimmermann, L. J. (2011). Antenatal steroids and neonatal outcome after chorioamnionitis: a meta-analysis. BJOG 118, 113–122. doi: 10.1111/j.1471-0528.2010.02751.x
Bierstone, D., Wagenaar, N., Gano, D. L., Guo, T., Georgio, G., Groenendaal, F., et al. (2018). Association of histologic chorioamnionitis with perinatal brain injury and early childhood neurodevelopmental outcomes among preterm neonates. JAMA Pediatr. 172:534. doi: 10.1001/jamapediatrics.2018.0102
Cheong, J. L. Y., Anderson, P. J., Burnett, A. C., Roberts, G., Davis, N., Hickey, L., et al. (2017). Changing neurodevelopment at 8 years in children born extremely preterm since the 1990s. Pediatrics 139:e20164086. doi: 10.1542/peds.2016-4086
Clifford, A., Lang, L., and Chen, R. (2012). Effects of maternal cigarette smoking during pregnancy on cognitive parameters of children and young adults: a literature review. Neurotoxicol. Teratol. 34, 560–570. doi: 10.1016/j.ntt.2012.09.004
Dammann, O., Drescher, J., and Veelken, N. (2003). Maternal fever at birth and non-verbal intelligence at age 9 years in preterm infants. Dev. Med. Child Neurol. 45, 148–151. doi: 10.1111/j.1469-8749.2003.tb00922.x
Dexter, S. C., Malee, M. P., Pinar, H., Hogan, J. W., Carpenter, M. W., and Vohr, B. R. (1999). Influence of chorioamnionitis on developmental outcome in very low birth weight infants. Obstet. Gynecol. 94, 267–273.
Dexter, S. C., Pinar, H., Malee, M. P., Hogan, J., Carpenter, M. W., and Vohr, B. R. (2000). Outcome of very low birth weight infants with histopathologic chorioamnionitis. Obstet. Gynecol. 96, 172–177.
Eger, S. H. W., Sommerfelt, K., Kiserud, T., and Markestad, T. (2013). Foetal umbilical artery doppler in small preterms: (IQ) neurocognitive outcome at 5 years of age. Acta Paediatr. 102, 403–409. doi: 10.1111/apa.12164
Ekblad, M., Korkeila, J., and Lehtonen, L. (2015). Smoking during pregnancy affects foetal brain development. Acta Paediatr. 104, 12–18. doi: 10.1111/apa.12791
Ekblad, M., Korkeila, J., Parkkola, R., Lapinleimu, H., Haataja, L., Lehtonen, L., et al. (2010). Maternal smoking during pregnancy and regional brain volumes in preterm infants. J. Pediatr. 156, 185–190.e1. doi: 10.1016/j.jpeds.2009.07.061
Ekblad, M., Lehtonen, L., Korkeila, J., and Gissler, M. (2017). Maternal smoking during pregnancy and the risk of psychiatric morbidity in singleton sibling pairs. Nicotine Tob. Res. 19, 597–604. doi: 10.1093/ntr/ntx001
Flacking, R., Lehtonen, L., Thomson, G., Axelin, A., Ahlqvist, S., Moran, V. H., et al. (2012). Closeness and separation in neonatal intensive care. Acta Paediatr. 101, 1032–1037. doi: 10.1111/j.1651-2227.2012.02787.x
Fouron, J. C., Gosselin, J., Amiel-Tison, C., Infante-Rivard, C., Fouron, C., Skoll, A., et al. (2001). Correlation between prenatal velocity waveforms in the aortic isthmus and neurodevelopmental outcome between the ages of 2 and 4 years. Am. J. Obstet. Gynecol. 184, 630–636. doi: 10.1067/mob.2001.110696
Fouron, J. C., Gosselin, J., Raboisson, M. J., Lamoureux, J., Tison, C. A., Fouron, C., et al. (2005). The relationship between an aortic isthmus blood flow velocity index and the postnatal neurodevelopmental status of fetuses with placental circulatory insufficiency. Am. J. Obstet. Gynecol. 192, 497–503. doi: 10.1016/j.ajog.2004.08.026
Fung, G., Bawden, K., Chow, P., and Yu, V. (2003). Chorioamnionitis and outcome in extremely preterm infants. Ann. Acad. Med. Singapore 32, 305–310.
Gilman, S. E., Gardener, H., and Buka, S. L. (2008). Maternal smoking during pregnancy and children's cognitive and physical development: a causal risk factor? Am. J. Epidemiol. 168, 522–531. doi: 10.1093/aje/kwn175.
Gnigler, M., Neubauer, V., Griesmaier, E., Zotter, S., Kager, K., and Kiechl-Kohlendorfer, U. (2015). Very preterm children are at increased risk of reduced processing speed at 5 years of age, predicted by typical complications of prematurity and prenatal smoking. Acta Paediatr. 104, e124–e129. doi: 10.1111/apa.12859
Goldenberg, R. L., Culhane, J. F., Iams, J. D., and Romero, R. (2008). Epidemiology and causes of preterm birth. Lancet 371, 75–84. doi: 10.1016/S0140-6736(08)60074-4
Goldenberg, R. L., Hauth, J. C., and Andrews, W. W. (2000). Intrauterine infection and preterm delivery. N. Engl. J. Med. 342, 1500–1507. doi: 10.1056/NEJM200005183422007
Gray, P. H., Hurley, T. M., Rogers, Y. M., O'Callaghan, M. J., Tudehope, D. I., Burns, Y. R., et al. (1997). Survival and neonatal and neurodevelopmental outcome of 24-29 week gestation infants according to primary cause of preterm delivery. Aust. N. Z. J. Obstet. Gynaecol. 37, 161–168. doi: 10.1111/j.1479-828X.1997.tb02245.x
Hardt, N. S., Kostenbauder, M., Ogburn, M., Behnke, M., Resnick, M., and Cruz, A. (1985). Influence of chorioamnionitis on long-term prognosis in low birth weight infants. Obstet. Gynecol. 65, 5–10.
Heinonen, K., Räikkönen, K., Pesonen, A. K., Andersson, S., Kajantie, E., Eriksson, J. G., et al. (2011). Longitudinal study of smoking cessation before pregnancy and children's cognitive abilities at 56 months of age. Early Hum. Dev. 87, 353–359. doi: 10.1016/j.earlhumdev.2011.02.002
Helderman, J. B., O'Shea, T. M., Kuban, K. C., Allred, E. N., Hecht, J. L., Dammann, O., et al. (2012). Antenatal antecedents of cognitive impairment at 24 months in extremely low gestational age newborns. Pediatrics 129, 494–502. doi: 10.1542/peds.2011-1796.
Hendson, L., Russell, L., Robertson, C. M., Liang, Y., Chen, Y., Abdalla, A., et al. (2011). Neonatal and neurodevelopmental outcomes of very low birth weight infants with histologic chorioamnionitis. J. Pediatr. 158, 397–402. doi: 10.1016/j.jpeds.2010.09.010
Hirvonen, M., Ojala, R., Korhonen, P., Haataja, P., Eriksson, K., Rantanen, K., et al. (2017). Intellectual disability in children aged less than seven years born moderately and late preterm compared with very preterm and term-born children - a nationwide birth cohort study. J. Intellect. Disabil. Res. 61, 1034–1054. doi: 10.1111/jir.12394
Jobe, A. H. (2012). Effects of chorioamnionitis on the fetal lung. Clin. Perinatol. 39, 441–457. doi: 10.1016/j.clp.2012.06.010
Källén, K., Serenius, F., Westgren, M., Maršál, K. and EXPRESS Group (2015). Impact of obstetric factors on outcome of extremely preterm births in Sweden: prospective population-based observational study (EXPRESS). Acta Obstet. Gynecol. Scand. 94, 1203–1214. doi: 10.1111/aogs.12726
Karsdorp, V. H., van Vugt, J. M., van Geijn, H. P., Kostense, P. J., Arduini, D., Montenegro, N., et al. (1994). Clinical significance of absent or reversed end diastolic velocity waveforms in umbilical artery. Lancet 344, 1664–1668. doi: 10.1016/S0140-6736(94)90457-X
Kaukola, T., Räsänen, J., Herva, R., Patel, D. D., and Hallman, M. (2005). Suboptimal neurodevelopment in very preterm infants is related to fetal cardiovascular compromise in placental insufficiency. Am. J. Obstet. Gynecol. 193, 414–420. doi: 10.1016/j.ajog.2004.12.005
Kent, A., Lomas, F., Hurrion, E., and Dahlstrom, J. E. (2005). Antenatal steroids may reduce adverse neurological outcome following chorioamnionitis: neurodevelopmental outcome and chorioamnionitis in premature infants. J. Paediatr. Child Health 41, 186–190. doi: 10.1111/j.1440-1754.2005.00585.x
Kiechl-Kohlendorfer, U., Ralser, E., Pupp Peglow, U., Pehboeck-Walser, N., and Fussenegger, B. (2013). Early risk predictors for impaired numerical skills in 5-year-old children born before 32 weeks of gestation. Acta Paediatr. 102, 66–71. doi: 10.1111/apa.12036
Kiechl-Kohlendorfer, U., Ralser, E., Pupp Peglow, U., Reiter, G., Griesmaier, E., and Trawöger, R. (2010). Smoking in pregnancy: a risk factor for adverse neurodevelopmental outcome in preterm infants? Acta Paediatr. 99, 1016–1019. doi: 10.1111/j.1651-2227.2010.01749.x
Kiechl-Kohlendorfer, U., Ralser, E., Pupp Peglow, U., Reiter, G., and Trawöger, R. (2009). Adverse neurodevelopmental outcome in preterm infants: risk factor profiles for different gestational ages. Acta Paediatr. 98, 792–796. doi: 10.1111/j.1651-2227.2009.01219.x
Kirsten, G. F., Van Zyl, J. I., Van Zijl, F., Maritz, J. S., and Odendaal, H. J. (2007). Infants of women with severe early pre-eclampsia: the effect of absent end-diastolic umbilical artery doppler flow velocities on neurodevelopmental outcome. Acta Paediatr. 89, 566–570. doi: 10.1111/j.1651-2227.2000.tb00340.x
Knopik, V. S. (2009). Maternal smoking during pregnancy and child outcomes: real or spurious effect? Dev. Neuropsychol. 34, 1–36. doi: 10.1080/87565640802564366
Kosuge, S., Ohkuchi, A., Minakami, H., Matsubara, S., Uchida, A., Eguchi, Y., et al. (2000). Influence of chorioamnionitis on survival and morbidity in singletons live-born at <32 weeks of gestation. Acta obstet. Gynecol. Scand. 79, 861–865. doi: 10.1034/j.1600-0412.2000.079010861.x
Kramer, B. W., Kallapur, S., Newnham, J., and Jobe, A. H. (2009). Prenatal inflammation and lung development. Semin. Fetal Neonatal Med. 14, 2–7. doi: 10.1016/j.siny.2008.08.011
Lambe, M., Hultman, C., Torrång, A., Maccabe, J., and Cnattingius, S. (2006). Maternal smoking during pregnancy and school performance at age 15. Epidemiology 17, 524–530. doi: 10.1097/01.ede.0000231561.49208.be
Lee, I., Neil, J. J., Huettner, P. C., Smyser, C. D., Rogers, C. E., Shimony, J. S., et al. (2014). The impact of prenatal and neonatal infection on neurodevelopmental outcomes in very preterm infants. J. Perinatol. 34, 741–747. doi: 10.1038/jp.2014.79
Leppänen, M., Ekholm, E., Palo, P., Maunu, J., Munck, P., Parkkola, R., et al. (2009). Abnormal antenatal doppler velocimetry and cognitive outcome in very-low-birth-weight infants at 2 years of age. Ultrasound Obstet. Gynecol. 36, 178–185. doi: 10.1002/uog.7694
Lind, A., Korkman, M., Lehtonen, L., Lapinleimu, H., Parkkola, R., Matomäki, J., et al. (2011). Cognitive and neuropsychological outcomes at 5 years of age in preterm children born in the 2000s. Dev. Med. Child Neurol. 53, 256–262. doi: 10.1111/j.1469-8749.2010.03828.x
Luu, T. M., Rehman Mian, M. O., and Nuyt, A. M. (2017). Long-term impact of preterm birth. Clin. Perinatol. 44, 305–314. doi: 10.1016/j.clp.2017.01.003
Maisonneuve, E., Ancel, P. Y., Foix-L'Hélias, L., Marret, S., and Kayem, G. (2017). Impact of clinical and/or histological chorioamnionitis on neurodevelopmental outcomes in preterm infants: a literature review. J. Gynecol. Obstet. Hum. Reprod. 46, 307–316. doi: 10.1016/j.jogoh.2017.02.007
Manuck, T. A., Sheng, X., Yoder, B. A., and Varner, M. W. (2014). Correlation between initial neonatal and early childhood outcomes following preterm birth. Am. J. Obstet. Gynecol. 210, 426.e1–426.e9. doi: 10.1016/j.ajog.2014.01.046
Maunu, J., Ekholm, E., Parkkola, R., Palo, P., Rikalainen, H., Lapinleimu, H., et al. (2007). Antenatal doppler measurements and early brain injury in very low birth weight infants. J. Pediatr. 150, 51.e1–56.e1. doi: 10.1016/j.jpeds.2006.10.057
Mitchell, E. A., and Milerad, J. (2006). Smoking and the sudden infant death syndrome. Rev. Environ. Health 21, 81–103. doi: 10.1515/REVEH.2006.21.2.81
Mittendorf, R., Montag, A. G., MacMillan, W., Janeczek, S., Pryde, P. G., Besinger, R. E., et al. (2003). Components of the systemic fetal inflammatory response syndrome as predictors of impaired neurologic outcomes in children. Am. J. Obstet. Gynecol. 188, 1438–1434. discussion: 1444–1446. doi: 10.1067/mob.2003.380
Miyazaki, K., Furuhashi, M., Ishikawa, K., Tamakoshi, K., Hayashi, K., Kai, A., et al. (2016). Impact of chorioamnionitis on short- and long-term outcomes in very low birth weight preterm infants: The Neonatal Research Network Japan. J. Matern. Fetal. Neonatal. Med. 29, 331–337. doi: 10.3109/14767058.2014.1000852
Morales, E., Sunyer, J., Julvez, J., Castro-Giner, F., Estivill, X., Torrent, M., et al. (2009). GSTM1 polymorphisms modify the effect of maternal smoking during pregnancy on cognitive functioning in preschoolers. Int. J. Epidemiol. 38, 690–697. doi: 10.1093/ije/dyp141
Morales, W. J. (1987). The effect of chorioamnionitis on the developmental outcome of preterm infants at one year. Obstet. Gynecol. 70, 183–186.
Morsing, E., Asard, M., Ley, D., Stjernqvist, K., and Marsál, K. (2011). Cognitive function after intrauterine growth restriction and very preterm birth. Pediatrics 127, e874–e882. doi: 10.1542/peds.2010-1821
Mu, S.-C., Lin, C. H., Sung, T. C., Chen, Y. L., Lin, Y. C., Lee, C. C., et al. (2007). Neurodevelopmental outcome of very-low-birth-weight infants with chorioamnionitis. Acta Paediatr. Taiwan 48, 207–212.
Munck, P., Haataja, L., Maunu, J., Parkkola, R., Rikalainen, H., Lapinleimu, H., et al. (2010). Cognitive outcome at 2 years of age in Finnish infants with very low birth weight born between 2001 and 2006. Acta Paediatr. 99, 359–366. doi: 10.1111/j.1651-2227.2009.01589.x
Nasef, N., Shabaan, A. E., Schurr, P., Iaboni, D., Choudhury, J., Church, P., et al. (2013). Effect of clinical and histological chorioamnionitis on the outcome of preterm infants. Am. J. Perinatol. 30, 59–68. doi: 10.1055/s-0032-1321501
Pappas, A., Kendrick, D. E., Shankaran, S., Stoll, B. J., Bell, E. F., Laptook, A. R., et al. (2014). Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates. JAMA Pediatr. 168:137. doi: 10.1001/jamapediatrics.2013.4248
Polam, S., Koons, A., Anwar, M., Shen-Schwarz, S., and Hegyi, T. (2005). Effect of chorioamnionitis on neurodevelopmental outcome in preterm infants. Arch. Pediatr. Adolesc. Med. 159, 1032–1035. doi: 10.1001/archpedi.159.11.1032
Polanska, K., Jurewicz, J., and Hanke, W. (2015). Smoking and alcohol drinking during pregnancy as the risk factors for poor child neurodevelopment - a review of epidemiological studies. Int. J. Occup. Med. Environ. Health 28, 419–443. doi: 10.13075/ijomeh.1896.00424
Redline, R. W., Minich, N., Taylor, H. G., and Hack, M. (2007). Placental lesions as predictors of cerebral palsy and abnormal neurocognitive function at school age in extremely low birth weight infants (<1 kg). Pediatr. Dev. Pathol. 10, 282–292. doi: 10.2350/06-12-0203.1
Redline, R. W., Wilson-Costello, D., Borawski, E., Fanaroff, A. A., and Hack, M. (2000). The relationship between placental and other perinatal risk factors for neurologic impairment in very low birth weight children. Pediatr. Res. 47, 721–726. doi: 10.1203/00006450-200006000-00007
Rovira, N., Alarcon, A., Iriondo, M., Ibañez, M., Poo, P., Cusi, V., et al. (2011). Impact of histological chorioamnionitis, funisitis and clinical chorioamnionitis on neurodevelopmental outcome of preterm infants. Early Hum. Dev. 87, 253–257. doi: 10.1016/j.earlhumdev.2011.01.024
Salas, A. A., Faye-Petersen, O. M., Sims, B., Peralta-Carcelen, M., Reilly, S. D., McGwin, G., et al. (2013). Histological characteristics of the fetal inflammatory response associated with neurodevelopmental impairment and death in extremely preterm infants. J. Pediatr. 163, 652–657.e2. doi: 10.1016/j.jpeds.2013.03.081
Scherjon, S., Briët, J., Oosting, H., and Kok, J. (2000). The discrepancy between maturation of visual-evoked potentials and cognitive outcome at five years in very preterm infants with and without hemodynamic signs of fetal brain-sparing. Pediatrics 105, 385–391. doi: 10.1542/peds.105.2.385
Scherjon, S. A., Oosting, H., Smolders-DeHaas, H., Zondervan, H. A., and Kok, J. H. (1998). Neurodevelopmental outcome at three years of age after fetal “brain-sparing”. Early Hum. Dev. 52, 67–79.
Schlapbach, L. J., Ersch, J., Adams, M., Bernet, V., Bucher, H. U., and Latal, B. (2010). Impact of chorioamnionitis and preeclampsia on neurodevelopmental outcome in preterm infants below 32 weeks gestational age. Acta Paediatr. 99, 1504–1509. doi: 10.1111/j.1651-2227.2010.01861.x
Shand, A. W., Hornbuckle, J., Nathan, E., Dickinson, J. E., and French, N. P. (2009). Small for gestational age preterm infants and relationship of abnormal umbilical artery doppler blood flow to perinatal mortality and neurodevelopmental outcomes. Aust. N. Z. J. Obstet. Gynaecol. 49, 52–58. doi: 10.1111/j.1479-828X.2008.00941.x
Shipton, D., Tappin, D. M., Vadiveloo, T., Crossley, J. A., Aitken, D. A., and Chalmers, J. (2009). Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ 339:b4347. doi: 10.1136/bmj.b4347
Soraisham, A. S., Trevenen, C., Wood, S., Singhal, N., and Sauve, R. (2013). Histological chorioamnionitis and neurodevelopmental outcome in preterm infants. J. Perinatol. 33, 70–75. doi: 10.1038/jp.2012.49
Spinillo, A., Montanari, L., Bergante, C., Gaia, G., Chiara, A., and Fazzi, E. (2005). Prognostic value of umbilical artery doppler studies in unselected preterm deliveries. Obstet. Gynecol. 105, 613–620. doi: 10.1097/01.AOG.0000152382.13490.18
Suppiej, A., Franzoi, M., Vedovato, S., Marucco, A., Chiarelli, S., and Zanardo, V. (2009). Neurodevelopmental outcome in preterm histological chorioamnionitis. Early Hum. Dev. 85, 187–189. doi: 10.1016/j.earlhumdev.2008.09.410
Tolsa, C. B., Zimine, S., Warfield, S. K., Freschi, M., Sancho Rossignol, A., Lazeyras, F., et al. (2004). Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr. Res. 56, 132–138. doi: 10.1203/01.PDR.0000128983.54614.7E
Torrance, H. L., Bloemen, M. C., Mulder, E. J., Nikkels, P. G., Derks, J. B., de Vries, L. S., et al. (2010). Predictors of outcome at 2 years of age after early intrauterine growth restriction. Ultrasound Obstet. Gynecol. 36, 171–177. doi: 10.1002/uog.7627
Twilhaar, E. S., de Kieviet, J. F., Aarnoudse-Moens, C. S., van Elburg, R. M., and Oosterlaan, J. (2018). Academic performance of children born preterm: a meta-analysis and meta-regression. Arch. Dis. Child. Fetal Neonatal Ed. 103, F322–F330. doi: 10.1136/archdischild-2017-312916
Valcamonico, A., Accorsi, P., Battaglia, S., Soregaroli, M., Beretta, D., and Frusca, T. (2004). Absent or reverse end-diastolic flow in the umbilical artery: intellectual development at school age. Eur. J. Obstet. Gynecol. Reprod. Biol. 114, 23–28. doi: 10.1016/j.ejogrb.2003.09.033
Valcamonico, A., Accorsi, P., Sanzeni, C., Martelli, P., La Boria, P., Cavazza, A., et al. (2007). Mid- and long-term outcome of extremely low birth weight (ELBW) infants: an analysis of prognostic factors. J. Matern. Fetal Neonatal Med. 20, 465–471. doi: 10.1080/14767050701398413
van Vliet, E. O., de Kieviet, J. F., Oosterlaan, J., and van Elburg, R. M. (2013). Perinatal infections and neurodevelopmental outcome in very preterm and very low-birth-weight infants. JAMA Pediatr. 167:662. doi: 10.1001/jamapediatrics.2013.1199
van Vliet, E. O., de Kieviet, J. F., van der Voorn, J. P., Been, J. V., Oosterlaan, J., and van Elburg, R. M. (2012). Placental pathology and long-term neurodevelopment of very preterm infants. Am. J. Obstet. Gynecol. 206:489.e1–e489.e7. doi: 10.1016/j.ajog.2012.03.024
Vander Haar, E., and Gyamfi-Bannerman, C. (2016). Chorioamnionitis and neurocognitive development at age 2 years. Obstet. Gynecol. 127, 437–441. doi: 10.1097/AOG.0000000000001295
Vermeulen, G. M., Bruinse, H. W., and de Vries, L. S. (2001). Perinatal risk factors for adverse neurodevelopmental outcome after spontaneous preterm birth. Eur. J. Obstet. Gynecol. Reprod. Biol. 99, 207–212. doi: 10.1016/S0301-2115(01)00383-9
Versland, L. B., Sommerfelt, K., and Elgen, I. (2006). Maternal signs of chorioamnionitis: persistent cognitive impairment in low-birthweight children. Acta Paediatr. 95, 231–235. doi: 10.1080/08035250500352151
Vossbeck, S., de Camargo, O. K., Grab, D., Bode, H., and Pohlandt, F. (2001). Neonatal and neurodevelopmental outcome in infants born before 30 weeks of gestation with absent or reversed end-diastolic flow velocities in the umbilical artery. Eur. J. Pediatr. 160, 128–134. doi: 10.1007/s004310000680
Wilson-Costello, D., Borawski, E., Friedman, H., Redline, R., Fanaroff, A. A., and Hack, M. (1998). Perinatal correlates of cerebral palsy and other neurologic impairment among very low birth weight children. Pediatrics 102(2 Pt 1), 315–322. doi: 10.1542/peds.102.2.315
Ylijoki, M., Ekholm, E., Haataja, L., Lehtonen, L. and PIPARI Study Group (2012). Is chorioamnionitis harmful for the brain of preterm infants? A clinical overview. Acta Obstet. Gynecol. Scand. 91, 403–419. doi: 10.1111/j.1600-0412.2012.01349.x
Keywords: chorioamnionitis, smoking, doppler, preterm, development
Citation: Ylijoki MK, Ekholm E, Ekblad M and Lehtonen L (2019) Prenatal Risk Factors for Adverse Developmental Outcome in Preterm Infants—Systematic Review. Front. Psychol. 10:595. doi: 10.3389/fpsyg.2019.00595
Received: 29 November 2018; Accepted: 04 March 2019;
Published: 26 March 2019.
Edited by:
Livio Provenzi, Eugenio Medea (IRCCS), ItalyReviewed by:
Robert J. Ludwig, Columbia University Irving Medical Center, United StatesJeffrey Coldren, Youngstown State University, United States
Copyright © 2019 Ylijoki, Ekholm, Ekblad and Lehtonen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Milla K. Ylijoki, milla.reiman@utu.fi
 Milla K. Ylijoki
Milla K. Ylijoki Eeva Ekholm3
Eeva Ekholm3 Mikael Ekblad
Mikael Ekblad