- 1Department of Psychology, University of California, Riverside, Riverside, CA, United States
- 2Graduate Neuroscience Program, University of California, Riverside, Riverside, CA, United States
- 3Division of Biomedical Sciences and Graduate Biomedical Sciences Program, School of Medicine, University of California, Riverside, Riverside, CA, United States
The mechanisms underlying the common association between autism spectrum disorders (ASD) and sensory processing disorders (SPD) are unclear, and treatment options to reduce atypical sensory processing are limited. Fragile X Syndrome (FXS) is a leading genetic cause of intellectual disability and ASD behaviors. As in most children with ASD, atypical sensory processing is a common symptom in FXS, frequently manifesting as sensory hypersensitivity. Auditory hypersensitivity is a highly debilitating condition in FXS that may lead to language delays, social anxiety and ritualized repetitive behaviors. Animal models of FXS, including Fmr1 knock out (KO) mouse, also show auditory hypersensitivity, providing a translation relevant platform to study underlying pathophysiological mechanisms. The focus of this review is to summarize recent studies in the Fmr1 KO mouse that identified neural correlates of auditory hypersensitivity. We review results of electroencephalography (EEG) recordings in the Fmr1 KO mice and highlight EEG phenotypes that are remarkably similar to EEG findings in humans with FXS. The EEG phenotypes associated with the loss of FMRP include enhanced resting EEG gamma band power, reduced cross frequency coupling, reduced sound-evoked synchrony of neural responses at gamma band frequencies, increased event-related potential amplitudes, reduced habituation of neural responses and increased non-phase locked power. In addition, we highlight the postnatal period when the EEG phenotypes develop and show a strong association of the phenotypes with enhanced matrix-metalloproteinase-9 (MMP-9) activity, abnormal development of parvalbumin (PV)-expressing inhibitory interneurons and reduced formation of specialized extracellular matrix structures called perineuronal nets (PNNs). Finally, we discuss how dysfunctions of inhibitory PV interneurons may contribute to cortical hyperexcitability and EEG abnormalities observed in FXS. Taken together, the studies reviewed here indicate that EEG recordings can be utilized in both pre-clinical studies and clinical trials, while at the same time, used to identify cellular and circuit mechanisms of dysfunction in FXS. New therapeutic approaches that reduce MMP-9 activity and restore functions of PV interneurons may succeed in reducing FXS sensory symptoms. Future studies should examine long-lasting benefits of developmental vs. adult interventions on sensory phenotypes.
Introduction
There is a strong association between autism spectrum disorders (ASD) and sensory processing disorders (SPD). Indeed, the latest diagnostic criteria for ASD includes atypical sensory function as a core deficit. Research findings in both humans with ASD and animal models of ASD suggest that abnormal sensory processing in early development may lead to a broader array of symptoms including abnormal anxiety, social, and hyperactive behaviors (1–5). Despite the association between ASD behaviors and SPD, little is known about underlying cellular and circuit mechanisms that links autism to sensory issues. This review focuses on recent studies of the auditory system in Fragile X Syndrome (FXS), the most common genetic cause of ASD-associated behaviors and makes the case that studying basic sensory processing has multiple advantages in terms of identifying translation-relevant neural correlates, while at the same time gaining insight into the circuit mechanisms that lead to symptoms.
Fragile X Syndrome
Fragile X syndrome is a genetic disorder that affects ~1 in 4,000 males and 1 in 8,000 females (6, 7). FXS results from the loss of Fragile X Mental Retardation protein (FMRP), an mRNA binding protein that targets key synaptic pathways. FMRP is reduced or absent in humans with FXS due to an expansion and hyper-methylation of CGG trinucleotide repeats in the promoter region of the FMR1 gene (8). Individuals with FXS experience a wide array of symptoms including intellectual impairment, language delays, seizures, repetitive behaviors, social anxiety, and hyperactivity. Consistently, abnormal sensory sensitivity (typically hypersensitivity) is seen in humans with FXS. Approximately 15–33% of individuals with FXS meet the diagnostic criteria for autism, with ~5% of autism cases attributed to FXS (9–12). Many symptoms of FXS and ASD are similar, suggesting that studies of neural mechanisms in FXS may be broadly informative.
Why Study the Auditory System in FXS?
Both humans with FXS, and a commonly used animal model of the condition, the Fmr1 knockout (KO) mouse, show auditory hypersensitivity. Neural circuits involved in auditory processing, particularly those in the early stages of processing, are likely to be more conserved across humans and rodents than circuits involved in social and cognitive symptoms. There are many similarities between humans and rodents in the basic organization of the auditory system from subcortical areas to the primary auditory cortex. There is also a rich history of studying auditory system development. Given that FXS is a neurodevelopmental disorder, existing knowledge on normal auditory circuit development provides a strong basis to study circuits that underlie hypersensitivity. The auditory system and auditory-related symptomatology offer a translation-relevant platform to identify clinically-relevant phenotypes and study circuit mechanisms of deficits in FXS. Indeed, as reviewed below, studies of auditory cortical processing in humans with FXS and mouse models have found remarkable similarities across species.
EEG Phenotypes Related to Sensory Processing in Humans With FXS
Many of the early studies of auditory hypersensitivity in humans with FXS focused on auditory event-related potential (ERP) recordings. ERP studies consistently showed enhanced amplitude of various components (e.g., N1, P2). Enhanced synchrony of population responses to individual tones is likely responsible for enlarged N1 component of ERPs observed in humans with FXS (13–20), which may be generated by specific cell types in the auditory and frontal cortex (21, 22). A study using MEG also revealed enlargement of the N100m [the MEG equivalent of the N1 in EEG (14)]. In addition, the habituation of the N1 component to repeated tones is reduced in humans with FXS (17, 23); and the P2 amplitude of the ERP is enhanced in FXS (18). The similarity in observed MEG and EEG phenotypes adds further validity to the findings. The increase in N1 and P2 amplitude may be related to neuroanatomical abnormalities in the superior temporal gyrus (STG) where the auditory cortex is located (24), and to white matter enlargement in the temporal lobe (25). The enhanced N1 amplitude is also consistent with functional imaging studies that show that the STG displays higher levels of activation in individuals with FXS (26). Behavioral auditory hypersensitivity may therefore result from altered cortical responses to sounds (cortical hyperexcitability) in humans with FXS. Both enhanced population responses to sounds and reduced habituation of cortical neurons to repeating sounds may lead to auditory hyperexcitability (27).
Human EEG Spectral Component Analysis and Relationship to Clinical Measures
More recent EEG studies in humans have examined spectral components of baseline and sound-evoked responses to identify deficits in neural oscillations that are associated with sensory and cognitive symptoms in FXS. Wang et al. (28) found that FXS patients (n = 21, mean age = 26.4, range 10–55 yrs) exhibited greater gamma frequency band power (30–80 Hz) in the resting state EEG compared to age matched controls (n = 21). There was a reduction in alpha-gamma amplitude coupling across electrodes in FXS that suggests reduced top-down cortico-cortical control in FXS (29). The gamma power abnormality was correlated with social and sensory processing difficulties as measured with Social Communication Questionnaire and Adolescent/Adult Sensory Profile scores. These data are consistent with the reduced alpha-increased gamma power trends observed across ASDs (30). Ethridge et al. (31) replicated the gamma band finding in humans with FXS (n = 17, mean age = 26.2, range = 11–55) and showed that abnormalities in gamma power were related to more severe behavioral and psychiatric features and reductions in neurocognitive functions. In addition, test-retest data shows reliability of measures in a third group of humans with FXS (n = 38, mean age = 25.5, range = 10–53) (18). Taken together, the resting EEG gamma power and ERP amplitude phenotypes have been replicated multiple times, with indications of scalability and retest reliability, which is critical for biomarker development. Importantly, these data demonstrate a close relationship between EEG measures and clinical manifestations.
Although elevated gamma power is found consistently, additional studies are needed to address its relevance. For example, Wilkinson and Nelson (32) found elevated aperiodic power in the beta-gamma range (25–50 Hz) in a younger cohort of boys with FXS age 2.5–7 (mean ~4 yrs). However, they found no association between gamma power and sensory hypersensitivity or adaptive behaviors. Rather, they found an association between elevated gamma power and improved language ability in boys with FXS, suggesting that the gamma elevation may reflect compensatory mechanisms in FXS (33). Given the links between gamma oscillations and sensory-cognitive functions, and the emerging evidence that aperiodic gamma power may reflect cortical activation and excitatory/inhibitory balance in the cortex (34), a comprehensive quantification of oscillatory and aperiodic gamma power in the resting EEG needs to be obtained and correlated with clinical scores across development to properly identify biomarkers for clinical use. Abnormal periodic and aperiodic gamma power may serve as specific biomarkers for stratification of patients and outcome measures for clinical trials.
The gamma power related to local network excitation may reduce the ability of the neural population to synchronize periodic gamma band activity. Indeed, Ethridge et al. (31) found specific deficits in the gamma synchronization by testing the ability of the neural generators of the EEG signals to phase lock to dynamic auditory stimuli called “chirp.” The chirp stimulus is a tone whose amplitude is modulated by a sinusoid of linearly increased or decreased frequency in the 1–100 Hz range. The ability of the auditory system to phase lock consistently across trials to the different frequencies (1–100 Hz range) in the chirp is quantified as the inter-trial phase coherence (ITPC). Humans with FXS including ages in 12–57 range (mean ~26 yrs) show reduced ITPC in the 30–50 Hz gamma frequencies, but enhanced non-phase locked baseline broadband gamma power as the chirp trial was ongoing. These findings were replicated in another study of control and FXS human subjects (mean ~25 yrs, range 10–53) at a different clinical site and using different EEG equipment (18) compared to the Ethridge et al. (31) paper.
The abnormal responses of auditory cortex to sound that are present from early development may affect communications and language skills (35, 36). Indeed, humans with FXS show delays and abnormalities in expressive language skills [Reynell Developmental Language Scales—Roberts et al. (37)]. Individuals with FXS experience difficulty articulating words, poor co-articulation, substitutions, and omissions of words, reduction in the number of intelligible syllables produced, difficulty sequencing sounds, and echolalia (38–42). Similar language delays seen in autism may be associated with basic auditory processing abnormalities in early sensory cortical regions (43). Schmitt et al. (44) used a “talk/listen” paradigm and EEG recordings to address possible underpinnings of the expressive language deficits in FXS. In this task, EEGs were recorded when the subject either uttered a phoneme or passively listened to the same phoneme. In a healthy individual a suppression of ERP component amplitudes is normally observed when subjects say the phoneme compared to when they listen to it (so called N1 suppression) with a negative signal in the EEGs just before the speech sound is produced (pre-speech negativity). These changes are attributed to an efference copy from the motor generators to the speech perception regions of the brain. In contrast, FXS subjects showed reduced pre-speech negativity and elevated gamma power in frontal loci that were related to speech intelligibility when frontal and temporal EEG recordings were compared between controls and humans with FXS (44). There was also reduced frontotemporal coherence in the theta-alpha frequency bands just prior to speech production, but no difference in N1 suppression was observed during the speech production. These EEG data suggest that abnormal signaling between frontal and temporal cortical regions (45) may underlie the expressive speech deficits in FXS. Elevated gamma power in the pre-speech time window indicates the gamma phenotype described above in sensory regions is also seen more broadly, can be task-related, and may relate to broader cognitive deficits in FXS.
EEG Phenotypes Related to Sensory Processing in Animal Models of FXS
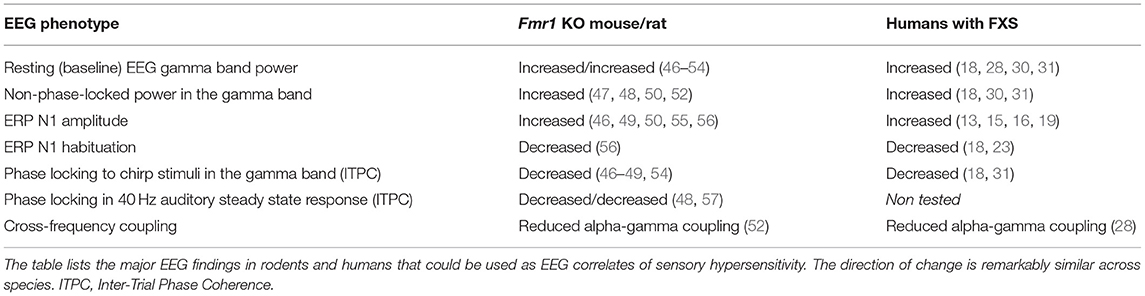
Recent implementation of new EEG technology for pre-clinical studies in awake and freely moving mice demonstrated that similar EEG phenotypes are also observed in animal models of FXS, mainly Fmr1 KO mice (Table 1) (51, 58). Lovelace et al. (55) compared EEG recordings between adult WT and Fmr1 KO mice on FVB background and showed elevated baseline gamma power, reduced phase locking at gamma band frequencies with the chirp stimuli, enhanced non-phase gamma band power during the chirp trials and enhanced N1 ERP amplitude. Enhanced gamma power, enhanced ERP amplitude and reduced gamma synchronization to chirp are also seen in adult Fmr1 KO mice on the C57BL6 background (47, 55). In addition, enhanced baseline gamma power and impaired sound-responses were observed in young P21–P28 Fmr1 KO mice from both backgrounds (52, 59), suggesting early development of the abnormal EEG phenotypes. Interestingly, the Fmr1 KO mice showed a larger increase in gamma band power during movement (46), suggesting the possibility that the motor modulation of auditory cortex may be abnormal in FXS. Although abnormal habituation to sound was not reported in awake and freely moving mice, earlier EEG studies in anesthetized adult Fmr1 KO mice on the FVB strain showed reduced habituation of N1 with repeated stimulation (56). This phenotype has not been tested in younger mice or the C57 strain. While these EEG data were obtained with epidural screw electrodes, for more immediate translation relevance, recent studies using a 30-channel skull surface multielectrode array (MEA) recording technique showed essentially the same EEG phenotypes in the Fmr1 KO mice (49). The increased number of recording sites, along with broader spatial coverage will now facilitate advanced EEG analysis, including cross-frequency and cross-region analysis in awake and freely moving mice to more closely relate to high-density human EEG studies.
Similar EEG phenotypes were also observed in the Fmr1 KO rat model of FXS, which displayed enhanced baseline gamma band power, reduced alpha power and behavioral hyperactivity (57). In addition, sound-evoked response, more specifically ITPC when tested with click trains to elicit an auditory steady state response, also showed a decrease in the gamma oscillations in the Fmr1 KO rat. The findings were consistent with reduced ITPC auditory steady state response observed in the Fmr1 KO mouse in response to a 40 Hz click train (47). Interestingly, studies in juvenile Fmr1 KO rat visual cortex showed that the typical switch from higher to lower frequency dominance in cortical response was impaired when the animal went from an active to a resting state (53). The high-frequency power remained elevated in the Fmr1 KO rat compared to the WT counterparts yet again suggesting abnormal modulation of sensory cortex responses by movement states. The species similarity (humans, mice, and rats) in the EEG phenotypes and the specific frequency bands affected is remarkable, and could prove critically useful in developing similar outcome measures between pre-clinical and clinical trials, while at the same time facilitate discovery of underlying cellular and circuit mechanisms, and new therapeutic interventions in the animal models. Future studies need to validate selected EEG phenotypes as biomarkers by performing studies on robustness, scalability, tolerance to settings and equipment and sensitivity to drug treatments.
Systems, Circuit, and Cellular Mechanisms of Auditory Hypersensitivity in FXS
Considering clinical relevance of the sensory hypersensitivity, several recent studies are focused on deciphering cellular and circuit mechanisms underlying it, utilizing both in vivo and in vitro approaches. Rotschafer and Razak (60) showed that individual neurons in the auditory cortex of Fmr1 KO mice responded with more action potentials to tones than in WT mice, using in vivo single unit recordings. Although the onset responses were similar across the genotypes, the responses were prolonged and continued well after sound offset in Fmr1 KO neurons, but not in WT neurons. This indicates an increased duration of responses in the Fmr1 KO mouse cortex, and may be related to the observed increase in baseline corrected single trial power (18, 46) and increase in resting gamma power in EEG responses (46, 50). Rotschafer and Razak (60) also showed that the frequency tuning receptive field of cortical neurons was broader in the Fmr1 KO mice. This indicates that for the same tone, more neurons will be synchronously activated in the auditory cortex of Fmr1 KO mice compared to WT mice and may underlie enhanced N1 amplitudes of ERPs, and the larger STG activation in humans with FXS (26). These increases in neural responses may arise from abnormal activation of inhibitory neurons (61, 62). In these studies, an examination of excitatory (E) and inhibitory (I) inputs to neurons in the somatosensory cortex provides important clues in terms of underlying circuit mechanisms of cortical neuron hyper-responsiveness. The strength of cortical E → E and I → E synaptic connections is shown to be relatively normal in the developing somatosensory cortex of Fmr1 KO mice. However, cortical E → I synaptic communication is reduced leading to reduced activation of inhibitory neurons, that may lead to increased excitation in the network. Local hyperconnectivity between pyramidal neurons due to deficient pruning may also lead to increased synchrony and responses in the network (63).
Development of Electrophysiological Abnormalities in Fmr1 KO Mice
To investigate developmental trajectory of the abnormal phenotypes, Wen et al. (59) compared neuronal responses to sound between Fmr1 KO and WT mice and identified the postnatal (P14–P21) window during which cortical responses began to diverge in the auditory cortex of Fmr1 KO mice. Single unit recordings showed that responses were similar in cortical neurons of WT and Fmr1 KO mice at P14. However, the responses were larger in the Fmr1 KO cortex at P21. This indicates that just after hearing onset (~P10) in mice, the abnormal development of circuits induced by auditory experience may underlie cortical hypersensitivity in the Fmr1 KO mice. The Fmr1 KO rat visual cortex, as well, shows a divergence of responses around the period of eye-opening (53). The P14–21 developmental window coincides with the age during which the excitatory and inhibitory connections mature in the mouse auditory cortex acquiring adult-like characteristics (64, 65). Perturbation of auditory experience during this window using tone exposure leads to tonotopic plasticity in the WT mouse, but such critical period plasticity is disrupted in Fmr1 KO mice (66), possibly due to impaired stability of long-term potentiation (67).
Disentangling Cortical vs. Subcortical Contributions to Auditory Hypersensitivity
Besides auditory cortex, FMRP expression is detected across the entire auditory neuraxis, with the possible exception of the cochlea (68–70). While the preponderance of studies in both humans and animal models have focused on the cortex, both subcortical site abnormalities and/or local cortical processing abnormalities may contribute to the phenotypes recorded in the cortex (70–73). Indeed, both the brainstem and midbrain auditory nuclei show abnormal synaptic markers and electrophysiological responses. The inferior colliculus shows broader frequency tuning curves, and enhanced responses to tones and amplitude modulated sounds (73). As in the cortex, these abnormalities develop between P14 and P21, a time window during which intracollicular intrinsic inhibition matures to adult-like levels (74). More neurons exhibit cFos immunoreactivity in response to sounds in the inferior colliculus, indicative of enhanced cell activation, suggesting that population synchrony may be elevated in this region. The hyperexcitability of the inferior colliculus during early development is consistent with the suggestion that this midbrain region is involved in the generation of audiogenic seizures, a commonly studied phenotype in Fmr1 KO mice (75). Supporting the role of midbrain in increased susceptibility to the audiogenic seizures, re-expression of FMRP in the glutamatergic neurons of inferior colliculus, in the Fmr1 KO mouse, prevents audiogenic seizures. Conversely, the deletion of Fmr1 in glutamatergic neurons of the inferior colliculus triggers audiogenic seizures. These data suggest that subcortical auditory sites show hyperexcitability, at least during early development.
While the brainstem and midbrain studies suggest that cortical hyperexcitability may reflect subcortical abnormalities, in vitro slice studies also indicate that local cortical processing may be abnormal. Goswami et al. (76) found that layer 2/3 circuits were hyperexcitable and showed increased gamma power in layers 2/3 and 5 in auditory cortical slices from Fmr1 KO mice following optogenetic activation of local circuits. These studies were consistent with in vivo studies of resting and sound driven activity and showed increased synchrony between layers 2/3 and 5. Considering that subcortical inputs are absent in slice electrophysiological studies, these data indicate local cortical deficits or reflect compensatory plasticity of intrinsic properties during the development of the mice from which slices were taken. To investigate the contribution of local cortical deficits in vivo, Lovelace et al. (47) examined the effects of Fmr1 deletion only from excitatory neurons in the forebrain using the Nex1 promoter. In this mouse model of FXS, FMRP expression was normal in the midbrain and thalamus, while cortical excitatory neurons showed loss of FMRP allowing for an examination of local cortical abnormalities following FMRP loss. EEG resting gamma power, and non-phase locked power in sound-evoked trials were elevated, as seen in global Fmr1 KO mice. However, the chirp-induced gamma synchronization (ITPC) was normal. These data indicate that a mixture of local cortical processing deficits and inherited deficits from subcortical sites lead to the observed cortical phenotypes, pointing to the need for a balanced investigation across the auditory neuraxis. Indeed, very little is known about subcortical auditory responses in humans with FXS. Interestingly, hyperactive locomotor behavior, but no changes in anxiety-like behaviors, was observed in mice with forebrain excitatory-specific Fmr1 deletion, pointing to combined cortical and subcortical contributions to behavioral deficits in FXS.
Cellular Mechanisms of Auditory Hypersensitivity in Fmr1 KO Mice—The MMP-9 Link
Delving more into the cellular mechanisms of abnormal cortical responses, several studies reported abnormal development and function of specific GABAergic neuron subtype parvalbumin (PV)-expressing interneurons. In particular, PV inhibitory interneurons in the cortex have been implicated in sensory hypersensitivity and abnormal sensory processing in Fmr1 KO mice in both visual and somatosensory systems (77–79). Gibson et al. (61) found a significant reduction in local excitatory drive on fast-spiking interneurons (putative PV neurons) in layer 4 of the somatosensory cortex. PV-expressing interneurons provide synchronous inhibition of multiple neighboring pyramidal cells, a process that is thought to be important in the generation of the narrowband gamma frequency rhythm (80–82). These cells may also be involved in desynchronizing higher frequency broadband gamma activity, implicating PV cells in the observed EEG phenotypes in FXS (83, 84). A characteristic structural feature of PV cells in the cortex is the preponderance of a specialized extracellular matrix structures called the perineuronal nets (PNNs) (59). PNNs are thought to increase excitability of PV cells (85) and thereby increase network inhibition. PNNs formation around PV cells also coincides with the closure of critical period plasticity windows in sensory cortices (86–89).
Auditory cortical hyperexcitability in FXS may arise from abnormal development of PV cells and PNNs during the P14–P21 window, the time window of divergence in cortical responses in Fmr1 KO mice (59). A reduced density of PV-expressing cells and the numbers of PNN-enwrapped PV cells in the Fmr1 KO mouse cortex at P21 may affect PV cell function and cortical inhibition. PNNs are dynamic structures and can be degraded by the activity of multiple proteases, including matrix metalloproteinase-9 (MMP-9). MMP-9 is a zinc-dependent endopeptidase that is found in many cell types, including neurons and glia (90). Among a large family of MMPs, MMP-9, MMP-2, and MMP-3 are widely expressed in the CNS and the expression of MMP-9 is regulated during development (90).
MMP-9 is a translational target of FMRP (91) and in the absence of FMRP, there is increased activity of MMP-9 across multiple brain regions and developmental periods in Fmr1 KO mice (59, 92, 93). Increased MMP-9 levels and activity were also observed in FXS human samples (92, 94). In addition, neural circuit deficits in Drosophila model of FXS were linked to MMPs and removal of mmp1, that encodes a secreted form of mmp in drosophila, ameliorated synaptic architecture defects at the neuromuscular junctions of dfmr1 null mutants (95). While reduction or loss of MMP-9 expression in Fmr1 KO mice reduced FXS-like symptoms (59, 92), MMP-9 overexpression in mice resulted in FXS-like symptoms (94). To test the role of MMP-9 in abnormal PV and PNN development, Wen et al. (59) utilized a genetic approach allowing to reduce MMP-9 to the normal levels in the Fmr1 KO mice. In these mice, not only PNNs were restored to normal levels, in particular around PV-expressing cells, cortical tone-driven responses were also normalized. In addition, abnormal sensory gating as tested with the pre-pulse inhibition of acoustic startle was also improved in these mice (93). Interestingly, even a complete removal of MMP-9 in the Fmr1 KO mice improved ERP habituation (56). The effectiveness of minocycline treatment in normalizing abnormal ERP habituation in FXS humans was also linked to the reduction of MMP-9 activity (17, 96), suggesting that elevated levels of MMP-9 may contribute to auditory hyperexcitability in FXS. Increased cortical MMP-9 activity and abnormal PV/PNN development were also observed in forebrain excitatory neuron-specific Fmr1 KO mice (47), suggesting a key role of cortical excitatory neurons in the dysfunction of PV interneurons, enhanced MMP-9 activity and abnormal PNN development. The loss of FMRP in excitatory neurons lead to reduced excitatory innervation of PV cells (61) and PNN loss via enhanced MMP-9 activity (47), both of which can affect PV cell functions and cortical inhibition resulting in EEG gamma band abnormalities. Consistent with the role of PV hypofunction in cortical hyperexcitability, enhancing PV cell function in the visual cortex of Fmr1 KO mice corrected orientation tuning of excitatory neurons and improved mouse performance in a visual perceptual learning task (78).
Therapeutics to Reduce Sensory Hypersensitivity
Given the strong evidence linking dysregulation of MMP-9 activity to the development of auditory cortex hyperexcitability, this pathway may serve as a potential therapeutic target to reduce sensory hypersensitivity. Minocycline is an FDA-approved antibiotic and a known inhibitor of MMP-9. Minocycline treatment in humans with FXS improved ERP habituation responses (17), and open label studies have shown significant functional improvements in FXS (97). A randomized placebo-controlled study of minocycline showed improvement in Clinical Global Impression Scale compared to placebo and greater improvement in anxiety and mood-related behaviors on the Visual Analog Scale (98).
Several studies have also shown benefits of minocycline treatment in the mouse and the drosophila models of FXS (95). For example, both minocycline treatment and genetic reduction of MMP-9 normalized the rate of ultrasonic vocalizations in Fmr1 KO mice when paired with a receptive female (99, 100). Minocycline reduced audiogenic seizures, hyperactivity and anxiety-like behaviors in both young and adult Fmr1 KO mice, but the effects lasted longer when the treatment was given at a young age (101). In contrast, adult mice had to be treated continuously for sustained benefits. These data point to an important element of treatment design –age of administration.
In addition to the improvements in mouse behaviors, a 10-day treatment of adult Fmr1 KO mice with minocycline also influenced EEG phenotypes (47). By testing resting EEG, ERPs, auditory steady state and chirp response ITPC and non-phase locked power, this study found beneficial effects of minocycline over vehicle treatment in all phenotypes, except resting gamma EEG power. Minocycline treatment increased gamma synchronization in response to auditory stimuli, and reduced sound-evoked power of auditory ERPs in Fmr1 KO mice compared to vehicle treatment. Although resting gamma power was reduced by minocycline, it was also reduced by vehicle treatment. Because minocycline has multiple targets besides MMP-9, including apoptotic pathway and microglia, it is necessary to test more specific inhibitors. Toward that goal, Pirbhoy et al. (52) tested acute treatment with SB-3CT, a MMP2/9 inhibitor, and demonstrated improved ITPC to auditory stimuli, enhanced PNN formation, and increased PV levels and TrkB phosphorylation in the auditory cortex of Fmr1 KO mice. Importantly, the reduction of MMP2/9 activity also improved mouse behavior as tested in the open field and elevated plus maze. Good sensitivity and reproducibility of EEG recordings provide a scientific justification for future use of EEG outcome measures in pre-clinical studies, including translationally relevant MEA EEG recordings. Jonak et al. (54) showed that an orally active phosphodiesterase 10A (PDE10A) inhibitor (14-day treatment) normalized the chirp ITPC in Fmr1 KO mice even at a low dose (0.5 mg/kg) without causing any sedation or effects on baseline EEG power. Taken together, these data indicate that sound-evoked EEG responses may be more sensitive measures, compared to resting EEG measures, to isolate drug effects from placebo in humans with FXS. Minocycline or other MMP-9 inhibitors show much promise in reducing sensory issues in FXS and selecting sensitive outcome measures based on the mouse EEG data may prove useful in designing statistically powerful clinical trials.
Kulinich et al. (55) also explored a non-pharmacological approach in reducing sensory hypersensitivity, in particular, therapeutic effects of reduced sound exposure during the P14–P21 developmental period, when auditory cortical hyperexcitability was first observed. Surprisingly, development of Fmr1 KO mice in a sound-attenuated environment did not reduce abnormal phenotypes, and in some cases exacerbated the symptoms (55). However, cortical correlates of auditory hypersensitivity were reduced when the mice were exposed to repeated tones at a rate of 5 Hz during this developmental window. Development of PV cells and PNNs, dendritic spines, TrkB phosphorylation and ERP amplitudes were normalized following the developmental sound exposure. These data suggest that developmental sound exposure during the critical period window, and not sound attenuation, may serve as a potential treatment option either alone, or in combination with pharmacological approaches.
Summary—Quadruple Hit Model of Auditory Hypersensitivity in FXS
Auditory hypersensitivity is a highly debilitating and commonly associated condition in humans with FXS (102). The Fmr1 KO mouse model of FXS also shows this behavioral phenotype providing a strong basis for examining mechanisms that may help to develop new therapeutic approaches in humans. At a functional level, the remarkable similarities in EEG phenotypes are evident across humans and rodents, including increased gamma band resting power, reduced phase locking to time varying and steady state auditory stimuli but increased non-phase locked power, increased ERP amplitude and reduced habituation of ERPs to repeated stimuli (Table 1). The specificity, reproducibility and sensitivity of these EEG measures provide a strong rationale for using EEG outcomes in pre-clinical trials in mice. Importantly, the scalability and clinical correlations in human EEG work supports widespread use of similar EEG outcomes in clinical studies to see real-world benefits in humans with FXS.
Based on studies of the circuit mechanisms underlying auditory hypersensitivity in FXS, we emphasize a “quadruple hit” model to explain auditory hypersensitivity: (1) individual cortical neurons are hyper-responsive to sounds (59, 60); (2) more cortical neurons respond synchronously to the same sound (60); (3) habituation of cortical neurons to repeated/continuous sounds is reduced (56); (4) background cortical activity is increased (46, 49, 50). These four phenotypes create a milieu of background noise, particularly manifesting as elevated broadband gamma noise, above which cortical neurons need to increase their responses to improve signal to noise ratio in information transfer. From a cellular and molecular perspective, recent studies from our and other groups implicated MMP-9 and PV-expressing inhibitory interneurons in abnormal circuit functions that underlie cortical hyperexcitability (47, 52, 59) as follows:
Loss of FMRP → Increased MMP-9 → Reduced PNNs around PV cells → Reduced excitability of PV cells → Reduced inhibition of cortical networks → Abnormal gamma synchrony and cortical hyperexcitability.
Future Studies
1. The functional deficits in sensory processing may emerge during specific developmental windows due to abnormal changes in circuit development providing an opportunity to target specific circuits for treatments during these windows (36). Given the vast literature on the critical role of developmental sensory experience that shapes brain structure and function over the lifespan, it is highly likely that early developmental treatments to normalize sensory circuit development will be most effective. However, it remains to be tested whether early developmental therapeutic interventions can normalize sensory processing with long-lasting benefits. It is also unclear whether early postnatal interventions to normalize sensory processing will have broader impacts and prevent abnormal behaviors in humans with FXS, such as anxiety, impaired social communication, delayed language function, and hyperactivity. Early reversal of sensory processing deficits may result in broad-acting benefits, an idea that remains untested in FXS.
2. There is a significant number of molecular targets considered as a treatment for FXS (103, 104). However, clinical trials have either failed or are inconclusive (105), contributing to mounting frustration in the FXS community. While very recent studies using phosphodiesterase inhibitors are promising (54, 106), continued efforts to understand how multiple pathways implicated in FXS interact, leading to circuit dysfunction and abnormal behaviors. One earlier theory suggests enhanced mGluR5-dependent protein synthesis in the Fmr1 KO mouse model providing a possible link between over-activated mGluR5 and enhanced protein translation in neurons lacking FMRP (107). A recent study also showed a new link between mGluR5 and MMP-9 reporting that deleting or blocking mGluR5 can decrease MMP-9 activity resulting in an elevated (almost doubling) number of PNNs in the somatosensory cortex (108). These data suggest that the increase in mGluR5 activity can lead to increased MMP-9 activity and PNN loss in FXS, suggesting a potential link between the mGluR5 and MMP-9 theories of FXS hyperexcitability. Future studies should explore these links in greater detail to determine whether MMP-9 acts downstream of mGluR5 and can be targeted therapeutically alongside or instead of mGluR5 antagonists, helping to reduce any buildup of tolerance and side-effects.
3. There is a predominant focus on the neocortex and hippocampus in studies of FXS and ASD, which is particularly true in humans. However, our investigations of the mechanisms of auditory dysfunction in FXS indicate that the cortically recorded phenotypes may reflect a mixture of local circuit deficits and subcortical deficits. A systematic investigation of deficits in subcortical processing and their developmental time course using transgenic mouse lines and promoters that allow spatial and cell-type-specific deletion or re-expression of FMRP could facilitate these studies in animal models. In humans, frequency following responses (FFR) which likely originate in the midbrain/brainstem region (109, 110) can be recorded to identify differential subcortical processing in FXS and ASD.
4. The ability of early sound exposure, but not sound attenuation, to reduce cortical hyperexcitability symptoms suggests that developmental trajectories of atypical sensory processing need to be investigated across closely spaced developmental ages. Examination of deficits at a single age or a small number of ages may miss the main cause of pathology early on, and only record manifestation of compensatory mechanisms (32, 33), which may be indirectly altered by the genetic mutation and can be beneficial (111–113).
5. The excitement around developments in the field of gene therapy indicates this approach may allow re-expression of FMRP in the near future (114, 115). However, our understanding of the function of FMRP at different ages remains underwhelming. In particular, it is unclear whether adults may benefit from FMRP re-expression, or if re-expression has to occur during embryonic or early postnatal development. There is no study comparing the developmental vs. adult effects of FMRP expression in the same model, using the same outcome measures. One published paper on this topic showed that acute expression of FMRP in adult prefrontal cortex is sufficient to elicit normal learning of adult Fmr1 KO mice in a prefrontal cortex dependent task (116). Despite the strong evidence for early developmental abnormalities in FXS, whether targeted interventions at this age provide long-lasting benefits is also unclear. In Angelman Syndrome, reactivation of Ube3A at different developmental time points has a phenotype-specific effect, but in Rett Syndrome benefits are seen for both early and late corrections of the deficits (117–119). These data from other forms of ASD indicate that a systematic study of effects of FMRP re-expression at different ages, and using a broad range of structural, functional and behavioral outcome measures is necessary. The findings reviewed here indicate that studies of sensory hypersensitivity may provide a tangible and translationally relevant niche to address these urgent issues.
Author Contributions
KR, DB, and IE contributed to the writing of the review. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Institute of Child Health and Human Development and the National Institute of Mental Health, U.S. Army Medical Research and Materiel Command and FRAXA Research Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank members of the IE, DB, and KR laboratories for helpful discussions.
Abbreviations
ASD, Autism Spectrum Disorders; Fmr1, Fragile X Mental Retardation 1 gene; FMRP, Fragile X Mental Retardation Protein; FXS, Fragile X Syndrome; EEG, Electroencephalography; ERP, Event Related Potential; ITPC, Inter-Trial Phase Coherence; KO, Knock Out; MMP-9, Matrix Metalloproteinase-9; P, Post-natal day; PNN, Perineuronal Nets; PV, Parvalbumin; SPD, Sensory Processing Disorder; STG, Superior Temporal Gyrus; STP, Single Trial Power; WT, Wild Type.
References
1. Green SA, Ben-Sasson A. Anxiety disorders and sensory over-responsivity in children with autism spectrum disorders: is there a causal relationship?. J Autism Dev Disord. (2010) 40:1495–504. doi: 10.1007/s10803-010-1007-x
2. Uljarević M, Lane A, Kelly A, Leekam S. Sensory subtypes and anxiety in older children and adolescents with autism spectrum disorder. Autism Res. (2016) 9:1073–8. doi: 10.1002/aur.1602
3. Rais M, Binder DK, Razak KA, Ethell IM. Sensory processing phenotypes in fragile X syndrome. ASN Neuro. (2018) 10:1759091418801092. doi: 10.1177/1759091418801092
4. Orefice LL, Zimmerman AL, Chirila AM, Sleboda SJ, Head JP, Ginty DD. Peripheral mechanosensory neuron dysfunction underlies tactile and behavioral deficits in mouse models of ASDs. Cell. (2016) 166:299–313. doi: 10.1016/j.cell.2016.05.033
5. Orefice LL, Mosko JR, Morency DT, Wells MF, Tasnim A, Mozeika SM, et al. Targeting peripheral somatosensory neurons to improve tactile-related phenotypes in ASD models. Cell. (2019) 178:867–86. doi: 10.1016/j.cell.2019.07.024
6. Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, et al. Advances in the treatment of fragile X syndrome. Pediatrics. (2009) 123:378–90. doi: 10.1542/peds.2008-0317
7. Hagerman RJ, Berry-Kravis E, Hazlett HC, Bailey DB, Moine H, Kooy RF, et al. Fragile X syndrome. Nat Rev Dis Primers. (2017) 3:1–19. doi: 10.1038/nrdp.2017.65
8. O'Donnell WT, Warren ST. A decade of molecular studies of fragile X syndrome. Annu Rev Neurosci. (2002) 25:315–38. doi: 10.1146/annurev.neuro.25.112701.142909
9. Bailey DB, Mesibov GB, Hatton DD, Clark RD, Roberts JE, Mayhew L. Autistic behavior in young boys with fragile X syndrome. J Autism Dev Disord. (1998) 28:499–508. doi: 10.1023/A:1026048027397
10. Cohen D, Pichard N, Tordjman S, Baumann C, Burglen L, Excoffier E, et al. Specific genetic disorders and autism: clinical contribution towards their identification. J Autism Dev Disord. (2005) 35:103–16. doi: 10.1007/s10803-004-1038-2
11. Niu M, Han Y, Dy ABC, Du J, Jin H, Qin J, et al. Autism symptoms in fragile X syndrome. J Child Neurol. (2017) 32:903–9. doi: 10.1177/0883073817712875
12. Abbeduto L, Thurman AJ, McDuffie A, Klusek J, Feigles RT, Brown WT, et al. ASD comorbidity in fragile X syndrome: symptom profile and predictors of symptom severity in adolescent and young adult males. J Autism Dev Disord. (2019) 49:960–77. doi: 10.1007/s10803-018-3796-2
13. St. Clair DMS, Blackwood DH, Oliver CJ, Dickens P. P3 abnormality in fragile X syndrome. Biol Psychiatry. (1987) 22:303–12. doi: 10.1016/0006-3223(87)90148-X
14. Rojas DC, Benkers TL, Rogers SJ, Teale PD, Reite ML, Hagerman RJ. Auditory evoked magnetic fields in adults with fragile X syndrome. Neuroreport. (2001) 12:2573–6. doi: 10.1097/00001756-200108080-00056
15. Castrén M, Pääkkönen A, Tarkka IM, Ryynänen M, Partanen J. Augmentation of auditory N1 in children with fragile X syndrome. Brain Topogr. (2003) 15:165–71. doi: 10.1023/A:1022606200636
16. Van der Molen MJW, Van der Molen MW, Ridderinkhof KR, Hamel BCJ, Curfs LMG, Ramakers GJA. Auditory change detection in fragile X syndrome males: a brain potential study. Clin Neurophysiol. (2012) 123:1309–18. doi: 10.1016/j.clinph.2011.11.039
17. Schneider A, Leigh MJ, Adams P, Nanakul R, Chechi T, Olichney J, et al. Electrocortical changes associated with minocycline treatment in fragile X syndrome. J Psychopharmacol. (2013) 27:956–63. doi: 10.1177/0269881113494105
18. Ethridge LE, De Stefano LA, Schmitt LM, Woodruff NE, Brown KL, Tran M, et al. Auditory EEG biomarkers in fragile X syndrome: clinical relevance. Front Integr Neurosci. (2019) 13:60. doi: 10.3389/fnint.2019.00060
19. Knoth IS, Lippé S. Event-related potential alterations in fragile X syndrome. Front Human Neurosci. (2012) 6:264. doi: 10.3389/fnhum.2012.00264
20. Ethridge L, Thaliath A, Kraff J, Nijhawan K, Berry-Kravis E. Development of neural response to novel sounds in fragile x syndrome: potential biomarkers. Am J Intellectual Dev Disabil. (2020) 125:449–64. doi: 10.1352/1944-7558-125.6.449
21. Hari R, Kaila K, Katila T, Tuomisto T, Varpula T. Interstimulus interval dependence of the auditory vertex response and its magnetic counterpart: implications for their neural generation. Electroencephal Clin Neurophysiol. (1982) 54:561–9. doi: 10.1016/0013-4694(82)90041-4
22. Näätänen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. (1987) 24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x
23. Ethridge LE, White SP, Mosconi MW, Wang J, Byerly MJ, Sweeney JA. Reduced habituation of auditory evoked potentials indicate cortical hyper-excitability in Fragile X Syndrome. Transl Psychiatry. (2016) 6:e787. doi: 10.1038/tp.2016.48
24. Reiss AL, Lee J, Freund L. Neuroanatomy of fragile X syndrome: the temporal lobe. Neurology. (1994) 44:1317–7. doi: 10.1212/WNL.44.7.1317
25. Hazlett HC, Poe MD, Lightbody AA, Styner M, MacFall JR, Reiss AL, et al. Trajectories of early brain volume development in fragile X syndrome and autism. J Am Acad Child Adolescent Psychiatry. (2012) 51:921–33. doi: 10.1016/j.jaac.2012.07.003
26. Hall SS, Walter E, Sherman E, Hoeft F, Reiss AL. The neural basis of auditory temporal discrimination in girls with fragile X syndrome. J Neurodev Disord. (2009) 1:91–9. doi: 10.1007/s11689-009-9007-x
27. Schmid S, Wilson DA, Rankin CH. Habituation mechanisms and their importance for cognitive function. Front Integr Neurosci. (2015) 8:97. doi: 10.3389/fnint.2014.00097
28. Wang J, Ethridge LE, Mosconi MW, White SP, Binder DK, Pedapati EV, et al. A resting EEG study of neocortical hyperexcitability and altered functional connectivity in fragile X syndrome. J Neurodev Disord. (2017) 9:1–12. doi: 10.1186/s11689-017-9191-z
29. Von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol. (2000) 38:301–13. doi: 10.1016/S0167-8760(00)00172-0
30. Wang J, Barstein J, Ethridge LE, Mosconi MW, Takarae Y, Sweeney JA. Resting state EEG abnormalities in autism spectrum disorders. J Neurodev Disord. (2013) 5:1–14. doi: 10.1186/1866-1955-5-24
31. Ethridge LE, White SP, Mosconi MW, Wang J, Pedapati EV, Erickson CA, et al. Neural synchronization deficits linked to cortical hyper-excitability and auditory hypersensitivity in fragile X syndrome. Mol Autism. (2017) 8:1–11. doi: 10.1186/s13229-017-0140-1
32. Wilkinson CL, Nelson CA. Increased aperiodic gamma power in young boys with Fragile X Syndrome is associated with better language ability. Mol Autism. (2021) 12:1–15. doi: 10.1186/s13229-021-00425-x
33. Antoine MW, Langberg T, Schnepel P, Feldman DE. Increased excitation-inhibition ratio stabilizes synapse and circuit excitability in four autism mouse models. Neuron. (2019) 101:648–61. doi: 10.1016/j.neuron.2018.12.026
34. Donoghue T, Haller M, Peterson EJ, Varma P, Sebastian P, Gao R, et al. Parameterizing neural power spectra into periodic and aperiodic components. Nat Neurosci. (2020) 23:1655–65. doi: 10.1038/s41593-020-00744-x
35. Abbeduto L, Brady N, Kover ST. Language development and fragile X syndrome: profiles, syndrome-specificity, and within-syndrome differences. Mental Retardation Dev Disabil Res Rev. (2007) 13:36–46. doi: 10.1002/mrdd.20142
36. Razak KA, Dominick KC, Erickson CA. Developmental studies in fragile X syndrome. J Neurodev Disord. (2020) 12:1–15. doi: 10.1186/s11689-020-09310-9
37. Roberts JE, Mirrett P, Burchinal M. Receptive and expressive communication development of young males with fragile X syndrome. Am J Mental Retardation. (2001) 106:216–30. doi: 10.1352/0895-8017(2001)106<0216:RAECDO>2.0.CO;2
38. Largo RH, Schinzel A. Developmental and behavioural disturbances in 13 boys with fragile X syndrome. Euro J Pediatrics. (1985) 143:269–75. doi: 10.1007/BF00442299
39. Hanson DM, Jackson AW III, Hagerman RJ, Opitz JM, Reynolds JF. Speech disturbances (cluttering) in mildly impaired males with the Martin-Bell/fragile X syndrome. Am J Med Genet. (1986) 23:195–206. doi: 10.1002/ajmg.1320230114
40. Belser RC, Sudhalter V. Conversational characteristics of children with fragile X syndrome: repetitive speech. Am J Mental Retardation. (2001) 106:28–38. doi: 10.1352/0895-8017(2001)106<0028:CCOCWF>2.0.CO;2
41. Roberts J, Price J, Barnes E, Nelson L, Burchinal M, Hennon EA, et al. Receptive vocabulary, expressive vocabulary, and speech production of boys with fragile X syndrome in comparison to boys with Down syndrome. Am J Mental Retardation. (2007) 112:177–93. doi: 10.1352/0895-8017(2007)112177:RVEVAS2.0.CO;2
42. Barnes E, Roberts J, Long SH, Martin GE, Berni MC, Mandulak KC, et al. Phonological accuracy and intelligibility in connected speech of boys with fragile X syndrome or Down syndrome. J Speech Lang Hear Res. (2009) 52:1048–61. doi: 10.1044/1092-4388(2009/08-0001)
43. Del Rincon PN. Autism: alterations in auditory perception. Rev Neurosci. (2008) 19:61–78. doi: 10.1515/REVNEURO.2008.19.1.61
44. Schmitt LM, Wang J, Pedapati EV, Thurman AJ, Abbeduto L, Erickson CA, et al. A neurophysiological model of speech production deficits in fragile X syndrome. Brain Commun. (2020) 2:fcz042. doi: 10.1093/braincomms/fcz042
45. Schneider DM, Nelson A, Mooney R. A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature. (2014) 513:189–94. doi: 10.1038/nature13724
46. Lovelace JW, Ethell IM, Binder DK, Razak KA. Translation-relevant EEG phenotypes in a mouse model of Fragile X Syndrome. Neurobiol Dis. (2018) 115:39–48. doi: 10.1016/j.nbd.2018.03.012
47. Lovelace JW, Rais M, Palacios AR, Shuai XS, Bishay S, Popa O, et al. Deletion of Fmr1 from forebrain excitatory neurons triggers abnormal cellular, EEG, and behavioral phenotypes in the auditory cortex of a mouse model of fragile X syndrome. Cereb Cortex. (2020) 30:969–88. doi: 10.1093/cercor/bhz141
48. Lovelace JW, Ethell IM, Binder DK, Razak KA. Minocycline treatment reverses sound evoked EEG abnormalities in a mouse model of Fragile X Syndrome. Front Neurosci. (2020) 14:771. doi: 10.3389/fnins.2020.00771
49. Jonak CR, Lovelace JW, Ethell IM, Razak KA, Binder DK. Multielectrode array analysis of EEG biomarkers in a mouse model of Fragile X Syndrome. Neurobiol Dis. (2020) 138:104794. doi: 10.1016/j.nbd.2020.104794
50. Wen TH, Lovelace JW, Ethell IM, Binder DK, Razak KA. Developmental changes in EEG phenotypes in a mouse model of fragile X syndrome. Neuroscience. (2019) 398:126–43. doi: 10.1016/j.neuroscience.2018.11.047
51. Sinclair D, Featherstone R, Naschek M, Nam J, Du A, Wright S, et al. GABA-B agonist baclofen normalizes auditory-evoked neural oscillations and behavioral deficits in the Fmr1 knockout mouse model of fragile X syndrome. Eneuro. (2017) 4:ENEURO.0380-16.2017. doi: 10.1523/ENEURO.0380-16.2017
52. Pirbhoy PS, Rais M, Lovelace JW, Woodard W, Razak KA, Binder DK, et al. Acute pharmacological inhibition of matrix metalloproteinase-9 activity during development restores perineuronal net formation and normalizes auditory processing in Fmr1 KO mice. J Neurochem. (2020) 155:538–58. doi: 10.1111/jnc.15037
53. Berzhanskaya J, Phillips MA, Gorin A, Lai C, Shen J, Colonnese MT. Disrupted cortical state regulation in a rat model of fragile X syndrome. Cereb Cortex. (2016) 27:bhv331. doi: 10.1093/cercor/bhv331
54. Jonak CR, Sandhu MS, Assad SA, Barbosa JA, Makhija M, Binder DK. The PDE10A inhibitor TAK-063 reverses sound-evoked EEG abnormalities in a mouse model of fragile X syndrome. Neurotherapeutics. (2021). doi: 10.1007/s13311-021-01042-5. [Epub ahead of print].
55. Kulinich AO, Reinhard SM, Rais M, Lovelace JW, Scott V, Binder DK, et al. Beneficial effects of sound exposure on auditory cortex development in a mouse model of fragile X syndrome. Neurobiol Dis. (2020) 134:104622. doi: 10.1016/j.nbd.2019.104622
56. Lovelace JW, Wen TH, Reinhard S, Hsu MS, Sidhu H, Ethell IM, et al. Matrix metalloproteinase-9 deletion rescues auditory evoked potential habituation deficit in a mouse model of Fragile X Syndrome. Neurobiol Dis. (2016) 89:126–35. doi: 10.1016/j.nbd.2016.02.002
57. Kozono N, Okamura A, Honda S, Matsumoto M, Mihara T. Gamma power abnormalities in a Fmr1-targeted transgenic rat model of fragile X syndrome. Sci Rep. (2020) 10:1–9. doi: 10.1038/s41598-020-75893-x
58. Jonak CR, Lovelace JW, Ethell IM, Razak KA, Binder DK. Reusable multielectrode array technique for electroencephalography in awake freely moving mice. Front Integr Neurosci. (2018) 12:53. doi: 10.3389/fnint.2018.00053
59. Wen TH, Afroz S, Reinhard SM, Palacios AR, Tapia K, Binder DK, et al. Genetic reduction of matrix metalloproteinase-9 promotes formation of perineuronal nets around parvalbumin-expressing interneurons and normalizes auditory cortex responses in developing Fmr1 knock-out mice. Cereb Cortex. (2018) 28:3951–64. doi: 10.1093/cercor/bhx258
60. Rotschafer S, Razak K. Altered auditory processing in a mouse model of fragile X syndrome. Brain Res. (2013) 1506:12–24. doi: 10.1016/j.brainres.2013.02.038
61. Gibson JR, Bartley AF, Hays SA, Huber KM. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J Neurophysiol. (2008) 100:2615–26. doi: 10.1152/jn.90752.2008
62. Patel AB, Hays SA, Bureau I, Huber KM, Gibson JR. A target cell-specific role for presynaptic Fmr1 in regulating glutamate release onto neocortical fast-spiking inhibitory neurons. J Neurosci. (2013) 33:2593–604. doi: 10.1523/JNEUROSCI.2447-12.2013
63. Patel AB, Loerwald KW, Huber KM, Gibson JR. Postsynaptic FMRP promotes the pruning of cell-to-cell connections among pyramidal neurons in the L5A neocortical network. J Neurosci. (2014) 34:3413–8. doi: 10.1523/JNEUROSCI.2921-13.2014
64. Oswald AMM, Reyes AD. Maturation of intrinsic and synaptic properties of layer 2/3 pyramidal neurons in mouse auditory cortex. J Neurophysiol. (2008) 99:2998–3008. doi: 10.1152/jn.01160.2007
65. Oswald AMM, Reyes AD. Development of inhibitory timescales in auditory cortex. Cereb Cortex. (2011) 21:1351–61. doi: 10.1093/cercor/bhq214
66. Kim H, Gibboni R, Kirkhart C, Bao S. Impaired critical period plasticity in primary auditory cortex of fragile X model mice. J Neurosci. (2013) 33:15686–92. doi: 10.1523/JNEUROSCI.3246-12.2013
67. Yang S, Yang S, Park JS, Kirkwood A, Bao S. Failed stabilization for long-term potentiation in the auditory cortex of FMR1 knockout mice. PLoS ONE. (2014) 9:e104691. doi: 10.1371/journal.pone.0104691
68. Wang Y, Sakano H, Beebe K, Brown MR, de Laat R, Bothwell M, et al. Intense and specialized dendritic localization of the fragile X mental retardation protein in binaural brainstem neurons: a comparative study in the alligator, chicken, gerbil, and human. J Compar Neurol. (2014) 522:2107–28. doi: 10.1002/cne.23520
69. Zorio DA, Jackson CM, Liu Y, Rubel EW, Wang Y. Cellular distribution of the fragile X mental retardation protein in the mouse brain. J Compar Neurol. (2017) 525:818–49. doi: 10.1002/cne.24100
70. McCullagh EA, Rotschafer SE, Auerbach BD, Klug A, Kaczmarek LK, Cramer KS, et al. Mechanisms underlying auditory processing deficits in Fragile X syndrome. FASEB J. (2020) 34:3501–18. doi: 10.1096/fj.201902435R
71. Rotschafer SE, Cramer KS. Developmental emergence of phenotypes in the auditory brainstem nuclei of fmr1 knockout mice. eNeuro. (2017) 4:ENEURO.0264-17.2017. doi: 10.1523/ENEURO.0264-17.2017
72. Garcia-Pino E, Gessele N, Koch U. Enhanced excitatory connectivity and disturbed sound processing in the auditory brainstem of fragile X mice. J Neurosci. (2017) 37:7403–19. doi: 10.1523/JNEUROSCI.2310-16.2017
73. Nguyen AO, Binder DK, Ethell IM, Razak KA. Abnormal development of auditory responses in the inferior colliculus of a mouse model of fragile X syndrome. J Neurophysiol. (2020) 123:2101–21. doi: 10.1152/jn.00706.2019
74. Sturm J, Nguyen T, Kandler K. Development of intrinsic connectivity in the central nucleus of the mouse inferior colliculus. J Neurosci. (2014) 34:15032–46. doi: 10.1523/JNEUROSCI.2276-14.2014
75. Gonzalez D, Tomasek M, Hays S, Sridhar V, Ammanuel S, Chang CW, et al. Audiogenic seizures in the Fmr1 knock-out mouse are induced by Fmr1 deletion in subcortical, VGlut2-expressing excitatory neurons and require deletion in the inferior colliculus. J Neurosci. (2019) 39:9852–63. doi: 10.1523/JNEUROSCI.0886-19.2019
76. Goswami S, Cavalier S, Sridhar V, Huber KM, Gibson JR. Local cortical circuit correlates of altered EEG in the mouse model of Fragile X syndrome. Neurobiol Dis. (2019) 124:563–72. doi: 10.1016/j.nbd.2019.01.002
77. Selby L, Zhang C, Sun QQ. Major defects in neocortical GABAergic inhibitory circuits in mice lacking the fragile X mental retardation protein. Neurosci Lett. (2007) 412:227–32. doi: 10.1016/j.neulet.2006.11.062
78. Goel A, Cantu DA, Guilfoyle J, Chaudhari GR, Newadkar A, Todisco B, et al. Impaired perceptual learning in a mouse model of Fragile X syndrome is mediated by parvalbumin neuron dysfunction and is reversible. Nat Neurosci. (2018) 21:1404–11. doi: 10.1038/s41593-018-0231-0
79. Nomura T, Musial TF, Marshall JJ, Zhu Y, Remmers CL, Xu J, et al. Delayed maturation of fast-spiking interneurons is rectified by activation of the TrkB receptor in the mouse model of fragile X syndrome. J Neurosci. (2017) 37:11298–310. doi: 10.1523/JNEUROSCI.2893-16.2017
80. Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circui performance. Nature. (2009) 459:698–702. doi: 10.1038/nature07991
81. Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. (2009) 459:663–7. doi: 10.1038/nature08002
82. Chen G, Zhang Y, Li X, Zhao X, Ye Q, Lin Y, et al. Distinct inhibitory circuits orchestrate cortical beta and gamma band oscillations. Neuron. (2017) 96:1403–18. doi: 10.1016/j.neuron.2017.11.033
83. Khoshkhoo S, Vogt D, Sohal VS. Dynamic, cell-type-specific roles for GABAergic interneurons in a mouse model of optogenetically inducible seizures. Neuron. (2017) 93:291–8. doi: 10.1016/j.neuron.2016.11.043
84. Guyon N, Zacharias LR, de Oliveira EF, Kim H, Leite JP, Lopes-Aguiar C, et al. Network asynchrony underlying increased broadband gamma power. J Neurosci. (2021) 41:2944–63. doi: 10.1523/JNEUROSCI.2250-20.2021
85. Balmer TS. Perineuronal nets enhance the excitability of fast-spiking neurons. Eneuro. (2016) 3:ENEURO.0112-16.2016. doi: 10.1523/ENEURO.0112-16.2016
86. Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. (2002) 298:1248–51. doi: 10.1126/science.1072699
87. Lee HHC, Bernard C, Ye Z, Acampora D, Simeone A, Prochiantz A, et al. Genetic Otx2 mis-localization delays critical period plasticity across brain regions. Mol Psychiatry. (2017) 22:680–8. doi: 10.1038/mp.2017.1
88. Lensjø KK, Lepperød ME, Dick G, Hafting T, Fyhn M. Removal of perineuronal nets unlocks juvenile plasticity through network mechanisms of decreased inhibition and increased gamma activity. J Neurosci. (2017) 37:1269–83. doi: 10.1523/JNEUROSCI.2504-16.2016
89. Murase S, Lantz CL, Quinlan EM. Light reintroduction after dark exposure reactivates plasticity in adults via perisynaptic activation of MMP-9. Elife. (2017) 6:e27345. doi: 10.7554/eLife.27345.025
90. Reinhard SM, Razak K, Ethell IM. A delicate balance: role of MMP-9 in brain development and pathophysiology of neurodevelopmental disorders. Front Cell Neurosci. (2015) 9:280. doi: 10.3389/fncel.2015.00280
91. Janusz A, Miłek J, Perycz M, Pacini L, Bagni C, Kaczmarek L, et al. The fragile X mental retardation protein regulates matrix metalloproteinase 9 mRNA at synapses. J Neurosci. (2013) 33:18234–41. doi: 10.1523/JNEUROSCI.2207-13.2013
92. Sidhu H, Dansie LE, Hickmott PW, Ethell DW, Ethell IM. Genetic removal of matrix metalloproteinase 9 rescues the symptoms of fragile X syndrome in a mouse model. J Neurosci. (2014) 34:9867–79. doi: 10.1523/JNEUROSCI.1162-14.2014
93. Kokash J, Alderson EM, Reinhard SM, Crawford CA, Binder DK, Ethell IM, et al. Genetic reduction of MMP-9 in the Fmr1 KO mouse partially rescues prepulse inhibition of acoustic startle response. Brain Res. (2019) 1719:24–9. doi: 10.1016/j.brainres.2019.05.029
94. Gkogkas CG, Khoutorsky A, Cao R, Jafarnejad SM, Prager-Khoutorsky M, Giannakas N, et al. Pharmacogenetic inhibition of eIF4E-dependent Mmp9 mRNA translation reverses fragile X syndrome-like phenotypes. Cell Rep. (2014) 9:1742–55. doi: 10.1016/j.celrep.2014.10.064
95. Siller SS, Broadie K. Neural circuit architecture defects in a Drosophila model of Fragile X syndrome are alleviated by minocycline treatment and genetic removal of matrix metalloproteinase. Dis Model Mech. (2011) 4:673–85. doi: 10.1242/dmm.008045
96. Dziembowska M, Pretto DI, Janusz A, Kaczmarek L, Leigh MJ, Gabriel N, et al. High MMP 9 activity levels in fragile X syndrome are lowered by minocycline. Am J Med Genet. (2013) 161A:1897–903. doi: 10.1002/ajmg.a.36023
97. Paribello C, Tao L, Folino A, Berry-Kravis E, Tranfaglia M, Ethell IM, et al. Open-label add-on treatment trial of minocycline in fragile X syndrome. BMC Neurol. (2010) 10:1–9. doi: 10.1186/1471-2377-10-91
98. Leigh MJS, Nguyen DV, Mu Y, Winarni TI, Schneider A, Chechi T, et al. A randomized double-blind, placebo-controlled trial of minocycline in children and adolescents with fragile x syndrome. J Dev Behav Pediatrics. (2013) 34:147. doi: 10.1097/DBP.0b013e318287cd17
99. Rotschafer SE, Trujillo MS, Dansie LE, Ethell IM, Razak KA. Minocycline treatment reverses ultrasonic vocalization production deficit in a mouse model of Fragile X Syndrome. Brain Res. (2012) 1439:7–14. doi: 10.1016/j.brainres.2011.12.041
100. Toledo MA, Wen TH, Binder DK, Ethell IM, Razak KA. Reversal of ultrasonic vocalization deficits in a mouse model of fragile X syndrome with minocycline treatment or genetic reduction of MMP-9. Behav Brain Res. (2019) 372:112068. doi: 10.1016/j.bbr.2019.112068
101. Dansie LE, Phommahaxay K, Okusanya AG, Uwadia J, Huang M, Rotschafer SE, et al. Long-lasting effects of minocycline on behavior in young but not adult Fragile X mice. Neuroscience. (2013) 246:186–98. doi: 10.1016/j.neuroscience.2013.04.058
102. Rotschafer SE, Razak KA. Auditory processing in fragile x syndrome. Front Cell Neurosci. (2014) 8:19. doi: 10.3389/fncel.2014.00019
103. Wadell MP, Hagerman J, Hessl RRD. Fragile X syndrome: psychiatric manifestations, assessment and emerging therapies. Curr Psychiatry Rev. (2013) 9:53–8. doi: 10.2174/1573400511309010008
104. Yamasue H, Aran A, Berry-Kravis E. Emerging pharmacological therapies in fragile X syndrome and autism. Curr Opin Neurol. (2019) 32:635–40. doi: 10.1097/WCO.0000000000000703
105. Berry-Kravis EM, Lindemann L, Jønch AE, Apostol G, Bear MF, Carpenter RL, et al. Drug development for neurodevelopmental disorders: lessons learned from fragile X syndrome. Nat Rev Drug Discov. (2018) 17:280–99. doi: 10.1038/nrd.2017.221
106. Berry-Kravis EM, Harnett MD, Reines SA, Reese MA, Ethridge LE, Outterson AH, et al. Inhibition of phosphodiesterase-4D in adults with fragile X syndrome: a randomized, placebo-controlled, phase 2 clinical trial. Nat Med. (2021) 27:862–70. doi: 10.1038/s41591-021-01321-w
107. Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. (2004) 27:370–7. doi: 10.1016/j.tins.2004.04.009
108. Mascio G, Bucci D, Notartomaso S, Liberatore F, Antenucci N, Scarselli P, et al. Perineuronal nets are under the control of type-5 metabotropic glutamate receptors in the developing somatosensory cortex. Transl Psychiatry. (2021) 11:1–13. doi: 10.1038/s41398-021-01210-3
109. Russo NM, Skoe E, Trommer B, Nicol T, Zecker S, Bradlow A, et al. Deficient brainstem encoding of pitch in children with autism spectrum disorders. Clin Neurophysiol. (2008) 119:1720–31. doi: 10.1016/j.clinph.2008.01.108
110. Wong PC, Skoe E, Russo NM, Dees T, Kraus N. Musical experience shapes human brainstem encoding of linguistic pitch patterns. Nat Neurosci. (2007) 10:420–2. doi: 10.1038/nn1872
111. Brager DH, Akhavan AR, Johnston D. Impaired dendritic expression and plasticity of h-channels in the fmr1–/y mouse model of fragile X syndrome. Cell Rep. (2012) 1:225–33. doi: 10.1016/j.celrep.2012.02.002
112. Zhang Z, Marro SG, Zhang Y, Arendt KL, Patzke C, Zhou B, et al. The fragile X mutation impairs homeostatic plasticity in human neurons by blocking synaptic retinoic acid signaling. Sci Transl Med. (2018) 10:eaar4338. doi: 10.1126/scitranslmed.aar4338
113. Bülow P, Murphy TJ, Bassell GJ, Wenner P. Homeostatic intrinsic plasticity is functionally altered in Fmr1 KO cortical neurons. Cell Rep. (2019) 26:1378–88. doi: 10.1016/j.celrep.2019.01.035
114. Hampson DR, Hooper AW, Niibori Y. The application of Adeno-associated viral vector gene therapy to the treatment of fragile X syndrome. Brain Sci. (2019) 9:32. doi: 10.3390/brainsci9020032
115. Shitik EM, Velmiskina AA, Dolskiy AA, Yudkin DV. Reactivation of FMR1 gene expression is a promising strategy for fragile X syndrome therapy. Gene Ther. (2020) 27:247–53. doi: 10.1038/s41434-020-0141-0
116. Siegel JJ, Chitwood RA, Ding JM, Payne C, Taylor W, Gray R, et al. Prefrontal cortex dysfunction in fragile x mice depends on the continued absence of fragile x mental retardation protein in the adult brain. J Neurosci. (2017) 37:7305–17. doi: 10.1523/JNEUROSCI.0571-17.2017
117. Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. (2007) 315:1143–7. doi: 10.1126/science.1138389
118. Silva-Santos S, Van Woerden GM, Bruinsma CF, Mientjes E, Jolfaei MA, Distel B, et al. Ube3a reinstatement identifies distinct developmental windows in a murine Angelman syndrome model. J Clin Invest. (2015) 125:2069–76. doi: 10.1172/JCI80554
Keywords: Fragile X Syndrome, autism spectrum disorders, sensory processing disorders, auditory processing, sensory hypersensitivity, matrix metalloproteinase, GABA
Citation: Razak KA, Binder DK and Ethell IM (2021) Neural Correlates of Auditory Hypersensitivity in Fragile X Syndrome. Front. Psychiatry 12:720752. doi: 10.3389/fpsyt.2021.720752
Received: 04 June 2021; Accepted: 16 August 2021;
Published: 07 October 2021.
Edited by:
Randi Jenssen Hagerman, MIND Institute, UC Davis, United StatesReviewed by:
S. M. Francis, University of Minnesota Twin Cities, United StatesBehnam Vafadari, University Medical Center Göttingen, Germany
Copyright © 2021 Razak, Binder and Ethell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khaleel A. Razak, khaleel@ucr.edu
 Khaleel A. Razak
Khaleel A. Razak Devin K. Binder2,3
Devin K. Binder2,3