- 1Department of Pharmacology, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal, India
- 2Department of Health Sciences, School of Education and Health, Cape Breton University, Sydney, NS, Canada
The Coronavirus (CoV) is a large family of viruses known to cause illnesses ranging from the common cold to acute respiratory tract infection. The severity of the infection may be visible as pneumonia, acute respiratory syndrome, and even death. Until the outbreak of SARS, this group of viruses was greatly overlooked. However, since the SARS and MERS outbreaks, these viruses have been studied in greater detail, propelling the vaccine research. On December 31, 2019, mysterious cases of pneumonia were detected in the city of Wuhan in China's Hubei Province. On January 7, 2020, the causative agent was identified as a new coronavirus (2019-nCoV), and the disease was later named as COVID-19 by the WHO. The virus spread extensively in the Wuhan region of China and has gained entry to over 210 countries and territories. Though experts suspected that the virus is transmitted from animals to humans, there are mixed reports on the origin of the virus. There are no treatment options available for the virus as such, limited to the use of anti-HIV drugs and/or other antivirals such as Remdesivir and Galidesivir. For the containment of the virus, it is recommended to quarantine the infected and to follow good hygiene practices. The virus has had a significant socio-economic impact globally. Economically, China is likely to experience a greater setback than other countries from the pandemic due to added trade war pressure, which have been discussed in this paper.
Introduction
Coronaviridae is a family of viruses with a positive-sense RNA that possess an outer viral coat. When looked at with the help of an electron microscope, there appears to be a unique corona around it. This family of viruses mainly cause respiratory diseases in humans, in the forms of common cold or pneumonia as well as respiratory infections. These viruses can infect animals as well (1, 2). Up until the year 2003, coronavirus (CoV) had attracted limited interest from researchers. However, after the SARS (severe acute respiratory syndrome) outbreak caused by the SARS-CoV, the coronavirus was looked at with renewed interest (3, 4). This also happened to be the first epidemic of the 21st century originating in the Guangdong province of China. Almost 10 years later, there was a MERS (Middle East respiratory syndrome) outbreak in 2012, which was caused by the MERS-CoV (5, 6). Both SARS and MERS have a zoonotic origin and originated from bats. A unique feature of these viruses is the ability to mutate rapidly and adapt to a new host. The zoonotic origin of these viruses allows them to jump from host to host. Coronaviruses are known to use the angiotensin-converting enzyme-2 (ACE-2) receptor or the dipeptidyl peptidase IV (DPP-4) protein to gain entry into cells for replication (7–10).
In December 2019, almost seven years after the MERS 2012 outbreak, a novel Coronavirus (2019-nCoV) surfaced in Wuhan in the Hubei region of China. The outbreak rapidly grew and spread to neighboring countries. However, rapid communication of information and the increasing scale of events led to quick quarantine and screening of travelers, thus containing the spread of the infection. The major part of the infection was restricted to China, and a second cluster was found on a cruise ship called the Diamond Princess docked in Japan (11, 12).
Origin
The new virus was identified to be a novel Coronavirus and was thus initially named 2019-nCoV; later, it was renamed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (13), and the disease it causes is now referred to as Coronavirus Disease-2019 (COVID-19) by the WHO. The virus was suspected to have begun its spread in the Huanan seafood wholesale market in the Wuhan region. It is possible that an animal that was carrying the virus was brought into or sold in the market, causing the spread of the virus in the crowded marketplace. One of the first claims made was in an article published in the Journal of Medical Virology (14), which identified snakes as the possible host. A second possibility was that pangolins could be the wild host of SARS-CoV-2 (15), though the most likely possibility is that the virus originated from bats (13, 16–19). Increasing evidence and experts are now collectively concluding the virus had a natural origin in bats, as with previous such respiratory viruses (2, 20–24).
Similarly, SARS and MERS were also suspected to originate from bats. In the case of MERS, the dromedary camel is an intermediate host (5, 10). Bats have been known to harbor coronaviruses for quite some time now. Just as in the case of avian flu, SARS, MERS, and possibly even HIV, with increasing selection and ecological pressure due to human activities, the virus made the jump from animal to man. Humans have been encroaching increasingly into forests, and this is true over much of China, as in Africa. Combined with additional ecological pressure due to climate change, such zoonotic spillovers are now more common than ever. It is likely that the next disease X will also have such an origin (25). We have learned the importance of identification of the source organism due to the Ebola virus pandemic. Viruses are unstable organisms genetically, constantly mutating by genetic shift or drift. It is not possible to predict when a cross-species jump may occur and when a seemingly harmless variant form of the virus may turn into a deadly strain. Such an incident occurred in Reston, USA, with the Reston virus (26), an alarming reminder of this possibility. The identification of the original host helps us to contain future spreads as well as to learn about the mechanism of transmission of viruses. Until the virus is isolated from a wild animal host, in this case, mostly bats, the zoonotic origin will remain hypothetical, though likely. It should further be noted that the virus has acquired several mutations, as noted by a group in China, indicating that there are more than two strains of the virus, which may have had an impact on its pathogenicity. However, this claim remains unproven, and many experts have argued otherwise; data proving this are not yet available (27). A similar finding was reported from Italy and India independently, where they found two strains (28, 29). These findings need to be further cross-verified by similar analyses globally. If true, this finding could effectively explain why some nations are more affected than others.
Transmission
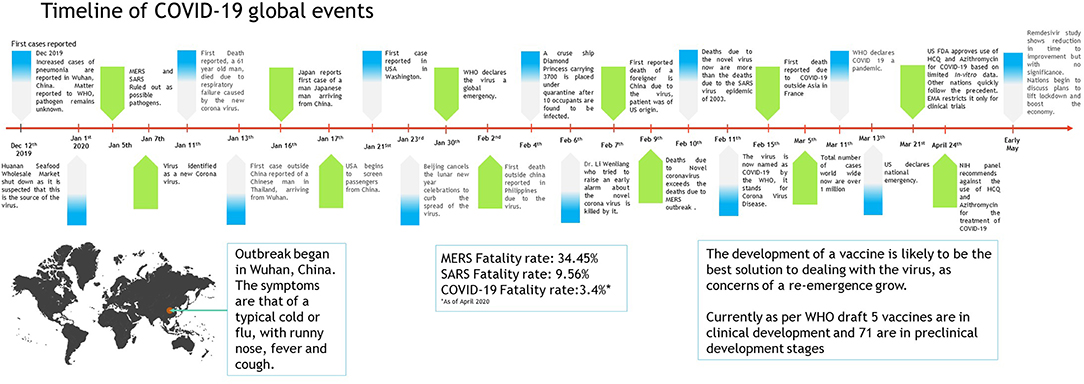
When the spread of COVID-19 began (Figure 1), the virus appeared to be contained within China and the cruise ship “Diamond Princess,” which formed the major clusters of the virus. However, as of April 2020, over 210 countries and territories are affected by the virus, with Europe, the USA, and Iran forming the new cluster of the virus. The USA (Figure 2) has the highest number of confirmed COVID-19 cases, whereas India and China, despite being among the most population-dense countries in the world, have managed to constrain the infection rate by the implementation of a complete lockdown with arrangements in place to manage the confirmed cases. Similarly, the UK has also managed to maintain a low curve of the graph by implementing similar measures, though it was not strictly enforced. Reports have indicated that the presence of different strains or strands of the virus may have had an effect on the management of the infection rate of the virus (27–29). The disease is spread by droplet transmission. As of April 2020, the total number of infected individuals stands at around 3 million, with ~200,000 deaths and more than 1 million recoveries globally (30, 34). The virus thus has a fatality rate of around 2% and an R0 of 3 based on current data. However, a more recent report from the CDC, Atlanta, USA, claims that the R0 could be as high as 5.7 (35). It has also been observed from data available from China and India that individuals likely to be infected by the virus from both these countries belong to the age groups of 20–50 years (36, 37). In both of these countries, the working class mostly belongs to this age group, making exposure more likely. Germany and Singapore are great examples of countries with a high number of cases but low fatalities as compared to their immediate neighbors. Singapore is one of the few countries that had developed a detailed plan of action after the previous SARS outbreak to deal with a similar situation in the future, and this worked in their favor during this outbreak. Both countries took swift action after the outbreak began, with Singapore banning Chinese travelers and implementing screening and quarantine measures at a time when the WHO recommended none. They ordered the elderly and the vulnerable to strictly stay at home, and they ensured that lifesaving equipment and large-scale testing facilities were available immediately (38, 39). Germany took similar measures by ramping up testing capacity quite early and by ensuring that all individuals had equal opportunity to get tested. This meant that young, old, and at-risk people all got tested, thus ensuring positive results early during disease progression and that most cases were mild like in Singapore, thus maintaining a lower death percentage (40). It allowed infected individuals to be identified and quarantined before they even had symptoms. Testing was carried out at multiple labs, reducing the load and providing massive scale, something which countries such as the USA did quite late and India restricted to select government and private labs. The German government also banned large gatherings and advocated social distancing to further reduce the spread, though unlike India and the USA, this was done quite late. South Korea is another example of how a nation has managed to contain the spread and transmission of the infection. South Korea and the USA both reported their first COVID-19 cases on the same day; however, the US administration downplayed the risks of the disease, unlike South Korean officials, who constantly informed their citizens about the developments of the disease using the media and a centralized messaging system. They also employed the Trace, Test, and Treat protocol to identify and isolate patients fast, whereas the USA restricted this to patients with severe infection and only later broadened this criterion, like many European countries as well as India. Unlike the USA, South Korea also has universal healthcare, ensuring free diagnostic testing.
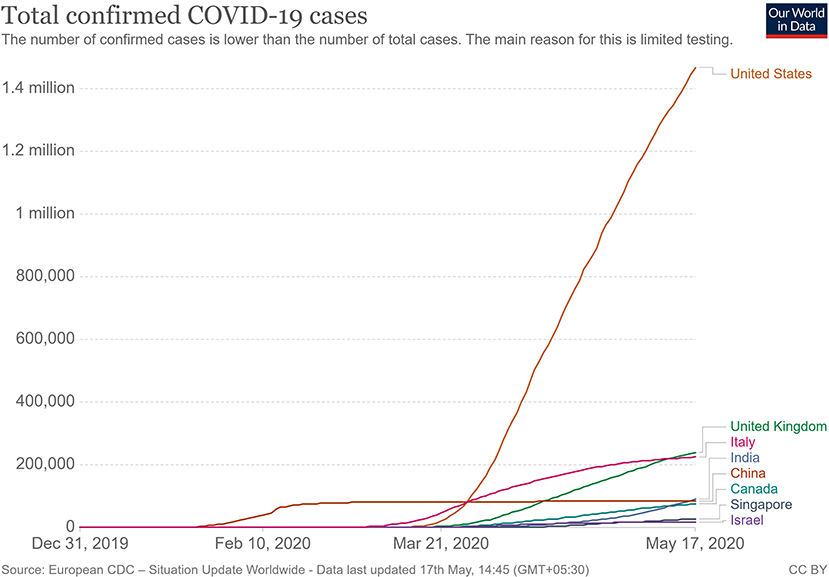
Figure 2. Total confirmed COVID 19 cases as of May 2020 (33).
The main mode of transmission of 2019-nCoV is human to human. As of now, animal-to-human transfer has not yet been confirmed. Asymptomatic carriers of the virus are at major risk of being superinfectors with this disease, as all those infected may not develop the disease (41). This is a concern that has been raised by nations globally, with the Indian government raising concerns on how to identify and contain asymptomatic carriers, who could account for 80% of those infected (42). Since current resources are directed towards understanding the hospitalized individuals showing symptoms, there is still a vast amount of information about asymptomatic individuals that has yet to be studied. For example, some questions that need to be answered include: Do asymptomatic individuals develop the disease at any point in time at all? Do they eventually develop antibodies? How long do they shed the virus for? Can any tissue of these individuals store the virus in a dormant state? Asymptomatic transmission is a gray area that encompasses major unknowns in COVID-19.
The main route of human-to-human transmission is by droplets, which are generated during coughing, talking, or sneezing and are then inhaled by a healthy individual. They can also be indirectly transmitted to a person when they land on surfaces that are touched by a healthy individual who may then touch their nose, mouth, or eyes, allowing the virus entry into the body. Fomites are also a common issue in such diseases (43).
Aerosol-based transmission of the virus has not yet been confirmed (43). Stool-based transmission via the fecal-oral route may also be possible since the SARS-CoV-2 has been found in patient feces (44, 45). Some patients with COVID-19 tend to develop diarrhea, which can become a major route of transmission if proper sanitation and personal hygiene needs are not met. There is no evidence currently available to suggest intrauterine vertical transmission of the disease in pregnant women (46).
More investigation is necessary of whether climate has played any role in the containment of the infection in countries such as India, Singapore, China, and Israel, as these are significantly warmer countries as compared with the UK, the USA, and Canada (Figure 2). Ideally, a warm climate should prevent the virus from surviving for longer periods of time on surfaces, reducing transmissibility.
Pathophysiology
On gaining entry via any of the mucus membranes, the single-stranded RNA-based virus enters the host cell using type 2 transmembrane serine protease (TMPRSS2) and ACE2 receptor protein, leading to fusion and endocytosis with the host cell (47–49). The uncoated RNA is then translated, and viral proteins are synthesized. With the help of RNA-dependant RNA polymerase, new RNA is produced for the new virions. The cell then undergoes lysis, releasing a load of new virions into the patients' body. The resultant infection causes a massive release of pro-inflammatory cytokines that causes a cytokine storm.
Clinical Presentation
The clinical presentation of the disease resembles beta coronavirus infections. The virus has an incubation time of 2–14 days, which is the reason why most patients suspected to have the illness or contact with an individual having the illness remain in quarantine for the said amount of time. Infection with SARS-CoV-2 causes severe pneumonia, intermittent fever, and cough (50, 51). Symptoms of rhinorrhoea, pharyngitis, and sneezing have been less commonly seen. Patients often develop acute respiratory distress syndrome within 2 days of hospital admission, requiring ventilatory support. It has been observed that during this phase, the mortality tends to be high. Chest CT will show indicators of pneumonia and ground-glass opacity, a feature that has helped to improve the preliminary diagnosis (51). The primary method of diagnosis for SARS-CoV-2 is with the help of PCR. For the PCR testing, the US CDC recommends testing for the N gene, whereas the Chinese CDC recommends the use of ORF lab and N gene of the viral genome for testing. Some also rely on the radiological findings for preliminary screening (52). Additionally, immunodiagnostic tests based on the presence of antibodies can also play a role in testing. While the WHO recommends the use of these tests for research use, many countries have pre-emptively deployed the use of these tests in the hope of ramping up the rate and speed of testing (52–54). Later, they noticed variations among the results, causing them to stop the use of such kits; there was also debate among the experts about the sensitivity and specificity of the tests. For immunological tests, it is beneficial to test for antibodies against the virus produced by the body rather than to test for the presence of the viral proteins, since the antibodies can be present in larger titers for a longer span of time. However, the cross-reactivity of these tests with other coronavirus antibodies is something that needs verification. Biochemical parameters such as D-dimer, C-reactive protein, and variations in neutrophil and lymphocyte counts are some other parameters that can be used to make a preliminary diagnosis; however, these parameters vary in a number of diseases and thus cannot be relied upon conclusively (51). Patients with pre-existing diseases such as asthma or similar lung disorder are at higher risk, requiring life support, as are those with other diseases such as diabetes, hypertension, or obesity. Those above the age of 60 have displayed the highest mortality rate in China, a finding that is mirrored in other nations as well (Figure 3) (55). If we cross-verify these findings with the population share that is above the age of 70, we find that Italy, the United Kingdom, Canada, and the USA have one of the highest elderly populations as compared to countries such as India and China (Figure 4), and this also reflects the case fatality rates accordingly (Figure 5) (33). This is a clear indicator that aside from comorbidities, age is also an independent risk factor for death in those infected by COVID-19. Also, in the US, it was seen that the rates of African American deaths were higher. This is probably due to the fact that the prevalence of hypertension and obesity in this community is higher than in Caucasians (56, 57). In late April 2020, there are also claims in the US media that young patients in the US with COVID-19 may be at increased risk of stroke; however, this is yet to be proven. We know that coagulopathy is a feature of COVID-19, and thus stroke is likely in this condition (58, 59). The main cause of death in COVID-19 patients was acute respiratory distress due to the inflammation in the linings of the lungs caused by the cytokine storm, which is seen in all non-survival cases and in respiratory failure. The resultant inflammation in the lungs, served as an entry point of further infection, associated with coagulopathy end-organ failure, septic shock, and secondary infections leading to death (60–63).
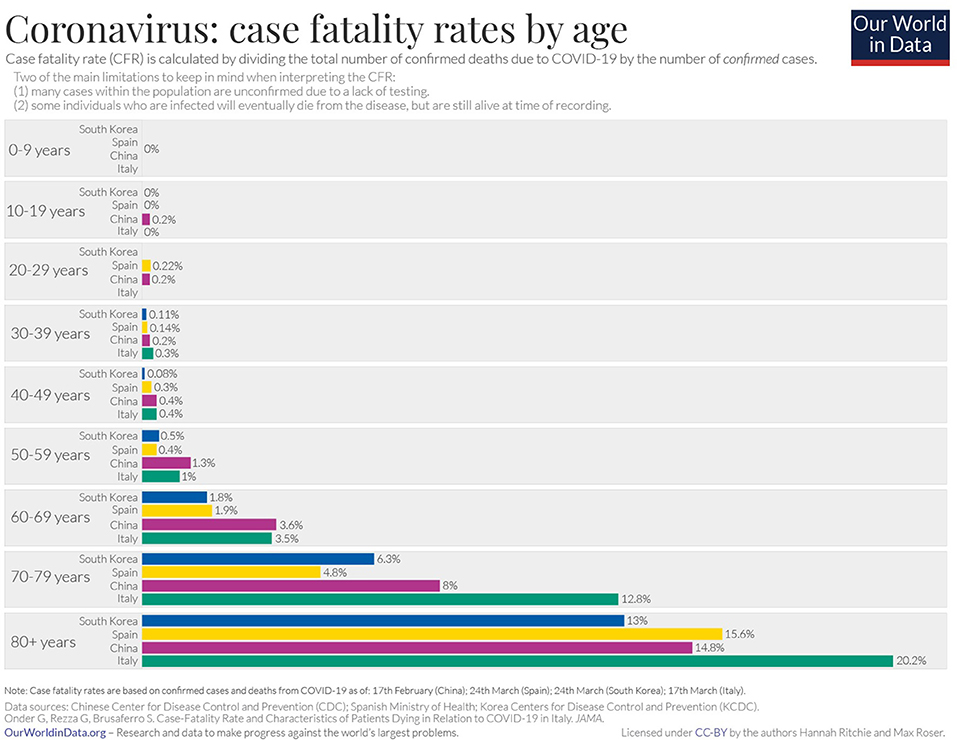
Figure 3. Case fatality rate by age in selected countries as of April 2020 (33).
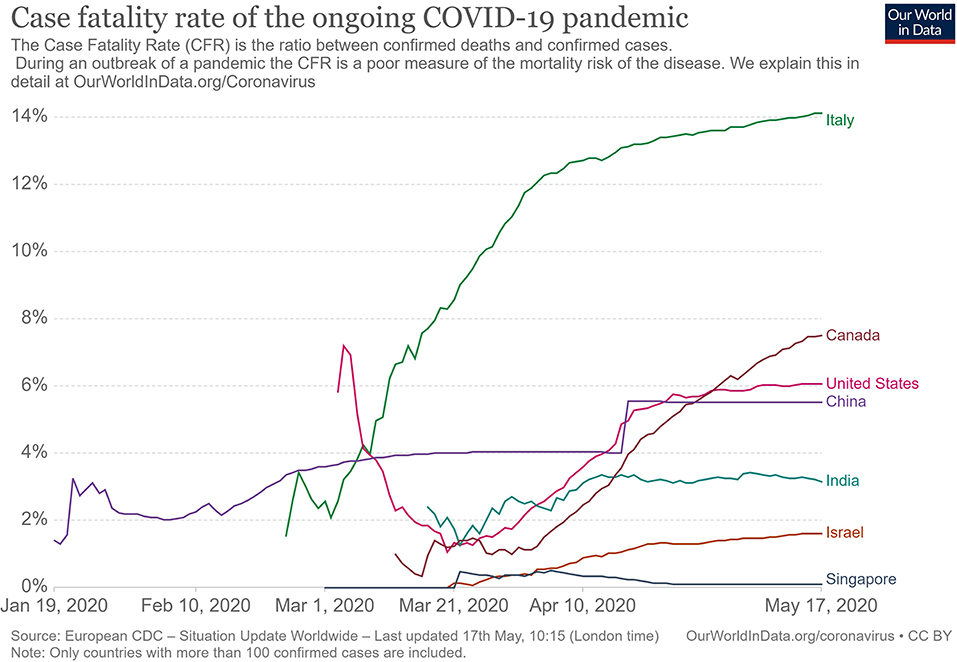
Figure 4. Case fatality rate in selected countries (33).
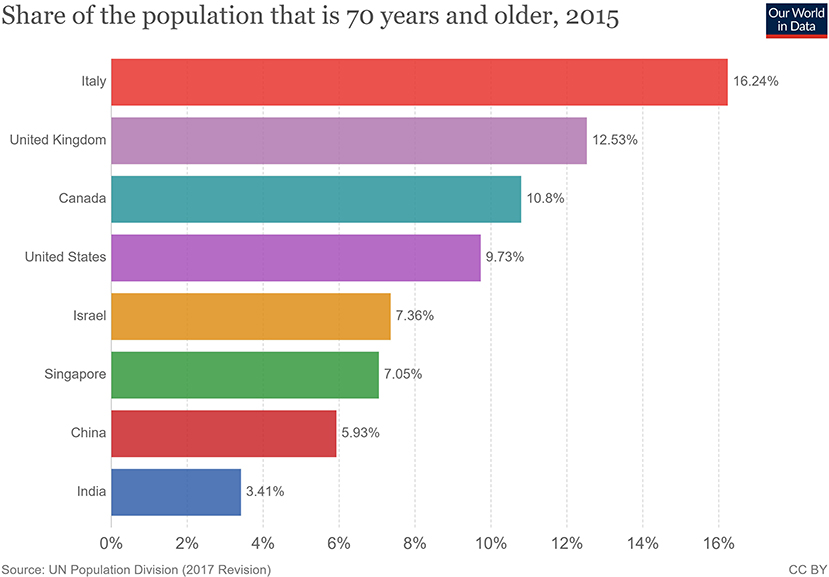
Figure 5. Population share above 70 years of age (33).
Treatment
For COVID-19, there is no specific treatment available. The WHO announced the organization of a trial dubbed the “Solidarity” clinical trial for COVID-19 treatments (64). This is an international collaborative study that investigates the use of a few prime candidate drugs for use against COVID-19, which are discussed below. The study is designed to reduce the time taken for an RCT by over 80%. There are over 1087 studies (Supplementary Data 1) for COVID-19 registered at clinicaltrials.gov, of which 657 are interventional studies (Supplementary Data 2) (65). The primary focus of the interventional studies for COVID-19 has been on antimalarial drugs and antiviral agents (Table 1), while over 200 studies deal with the use of different forms of oxygen therapy. Most trials focus on improvement of clinical status, reduction of viral load, time to improvement, and reduction of mortality rates. These studies cover both severe and mild cases.
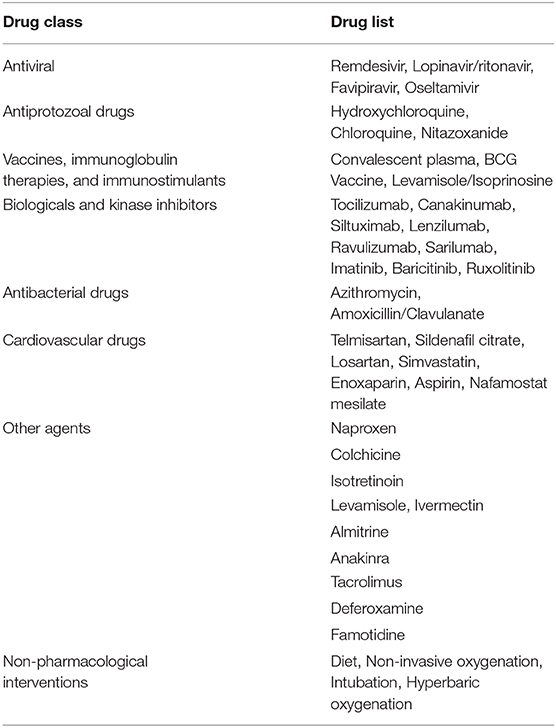
Table 1. List of therapeutic drugs under study for COVID-19 as per clinical trials registered under clinicaltrials.gov.
Use of Antimalarial Drugs Against SARS-CoV-2
The use of chloroquine for the treatment of corona virus-based infection has shown some benefit in the prevention of viral replication in the cases of SARS and MERS. However, it was not validated on a large scale in the form of a randomized control trial (50, 66–68). The drugs of choice among antimalarials are Chloroquine (CQ) and Hydroxychloroquine (HCQ). The use of CQ for COVID-19 was brought to light by the Chinese, especially by the publication of a letter to the editor of Bioscience Trends by Gao et al. (69). The letter claimed that several studies found CQ to be effective against COVID-19; however, the letter did not provide many details. Immediately, over a short span of time, interest in these two agents grew globally. Early in vitro data have revealed that chloroquine can inhibit the viral replication (70, 71).
HCQ and CQ work by raising the pH of the lysosome, the cellular organelle that is responsible for phagocytic degradation. Its function is to combine with cell contents that have been phagocytosed and break them down eventually, in some immune cells, as a downstream process to display some of the broken proteins as antigens, thus further enhancing the immune recruitment against an antigen/pathogen. The drug was to be administered alone or with azithromycin. The use of azithromycin may be advocated by the fact that it has been seen previously to have some immunomodulatory role in airway-related disease. It appears to reduce the release of pro-inflammatory cytokines in respiratory illnesses (72). However, HCQ and azithromycin are known to have a major drug interaction when co-administered, which increases the risk of QT interval prolongation (73). Quinine-based drugs are known to have adverse effects such as QT prolongation, retinal damage, hypoglycemia, and hemolysis of blood in patients with G-6-PD deficiency (66). Several preprints, including, a metanalysis now indicate that HCQ may have no benefit for severe or critically ill patients who have COVID-19 where the outcome is need for ventilation or death (74, 75). As of April 21, 2020, after having pre-emptively recommended their use for SARS-CoV-2 infection, the US now advocates against the use of these two drugs based on the new data that has become available.
Use of Antiviral Drugs Against SARS-CoV-2
The antiviral agents are mainly those used in the case of HIV/AIDS, these being Lopinavir and Ritonavir. Other agents such as nucleoside analogs like Favipiravir, Ribavirin, Remdesivir, and Galidesivir have been tested for possible activity in the prevention of viral RNA synthesis (76). Among these drugs, Lopinavir, Ritonavir, and Remdesivir are listed in the Solidarity trial by the WHO.
Remdesivir is a nucleotide analog for adenosine that gets incorporated into the viral RNA, hindering its replication and causing chain termination. This agent was originally developed for Ebola Virus Disease (77). A study was conducted with rhesus macaques infected with SARS-CoV-2 (78). In that study, after 12 h of infection, the monkeys were treated with either Remdesivir or vehicle. The drug showed good distribution in the lungs, and the animals treated with the drug showed a better clinical score than the vehicle group. The radiological findings of the study also indicated that the animals treated with Remdesivir have less lung damage. There was a reduction in viral replication but not in virus shedding. Furthermore, there were no mutations found in the RNA polymerase sequences. A randomized clinical control study that became available in late April 2020 (79), having 158 on the Remdesivir arm and 79 on the placebo arm, found that Remdesivir reduced the time to recovery in the Remdesivir-treated arm to 11 days, while the placebo-arm recovery time was 15 days. Though this was not found to be statistically significant, the agent provided a basis for further studies. The 28-days mortality was found to be similar for both groups. This has now provided us with a basis on which to develop future molecules. The study has been supported by the National Institute of Health, USA. The authors of the study advocated for more clinical trials with Remdesivir with a larger population. Such larger studies are already in progress, and their results are awaited. Remdesivir is currently one of the drugs that hold most promise against COVID-19.
An early trial in China with Lopinavir and Ritonavir showed no benefit compared with standard clinical care (80). More studies with this drug are currently underway, including one in India (81, 82).
Use of Convalescent Patient Plasma
Another possible option would be the use of serum from convalescent individuals, as this is known to contain antibodies that can neutralize the virus and aid in its elimination. This has been tried previously for other coronavirus infections (83). Early emerging case reports in this aspect look promising compared to other therapies that have been tried (84–87). A report from China indicates that five patients treated with plasma recovered and were eventually weaned off ventilators (84). They exhibited reductions in fever and viral load and improved oxygenation. The virus was not detected in the patients after 12 days of plasma transfusion. The US FDA has provided detailed recommendations for investigational COVID-19 Convalescent Plasma use (88). One of the benefits of this approach is that it can also be used for post-exposure prophylaxis. This approach is now beginning to be increasingly adopted in other countries, with over 95 trials registered on clinicaltrials.gov alone, of which at least 75 are interventional (89). The use of convalescent patient plasma, though mostly for research purposes, appears to be the best and, so far, the only successful option for treatment available.
From a future perspective, the use of monoclonal antibodies for the inhibition of the attachment of the virus to the ACE-2 receptor may be the best bet. Aside from this, ACE-2-like molecules could also be utilized to attach and inactivate the viral proteins, since inhibition of the ACE-2 receptor would not be advisable due to its negative repercussions physiologically. In the absence of drug regimens and a vaccine, the treatment is symptomatic and involves the use of non-invasive ventilation or intubation where necessary for respiratory failure patients. Patients that may go into septic shock should be managed as per existing guidelines with hemodynamic support as well as antibiotics where necessary.
Prevention
The WHO has recommended that simple personal hygiene practices can be sufficient for the prevention of spread and containment of the disease (90). Practices such as frequent washing of soiled hands or the use of sanitizer for unsoiled hands help reduce transmission. Covering of mouth while sneezing and coughing, and disinfection of surfaces that are frequently touched, such as tabletops, doorknobs, and switches with 70% isopropyl alcohol or other disinfectants are broadly recommended. It is recommended that all individuals afflicted by the disease, as well as those caring for the infected, wear a mask to avoid transmission. Healthcare works are advised to wear a complete set of personal protective equipment as per WHO-provided guidelines. Fumigation of dormitories, quarantine rooms, and washing of clothes and other fomites with detergent and warm water can help get rid of the virus. Parcels and goods are not known to transmit the virus, as per information provided by the WHO, since the virus is not able to survive sufficiently in an open, exposed environment. Quarantine of infected individuals and those who have come into contact with an infected individual is necessary to further prevent transmission of the virus (91). Quarantine is an age-old archaic practice that continues to hold relevance even today for disease containment. With the quarantine being implemented on such a large scale in some countries, taking the form of a national lockdown, the question arises of its impact on the mental health of all individuals. This topic needs to be addressed, especially in countries such as India and China, where it is still a matter of partial taboo to talk about it openly within the society.
In India, the Ministry of Ayurveda, Yoga, and Naturopathy, Unani, Siddha and Homeopathy (AYUSH), which deals with the alternative forms of medicine, issued a press release that the homeopathic, drug Arsenicum album 30, can be taken on an empty stomach for 3 days to provide protection against the infection (92). It also provided a list of herbal drugs in the same press release as per Ayurvedic and Unani systems of medicine that can boost the immune system to deal with the virus. However, there is currently no evidence to support the use of these systems of medicine against COVID-19, and they need to be tested.
The prevention of the disease with the use of a vaccine would provide a more viable solution. There are no vaccines available for any of the coronaviruses, which includes SARS and MERS. The development of a vaccine, however, is in progress at a rapid pace, though it could take about a year or two. As of April 2020, no vaccine has completed the development and testing process. A popular approach has been with the use of mRNA-based vaccine (93–96). mRNA vaccines have the advantage over conventional vaccines in terms of production, since they can be manufactured easily and do not have to be cultured, as a virus would need to be. Alternative conventional approaches to making a vaccine against SARS-CoV-2 would include the use of live attenuated virus as well as using the isolated spike proteins of the virus. Both of these approaches are in progress for vaccine development (97). Governments across the world have poured in resources and made changes in their legislation to ensure rapid development, testing, and deployment of a vaccine.
Barriers to Treatment
Lack of Transparency and Poor Media Relations
The lack of government transparency and poor reporting by the media have hampered the measures that could have been taken by healthcare systems globally to deal with the COVID-19 threat. The CDC, as well as the US administration, downplayed the threat and thus failed to stock up on essential supplies, ventilators, and test kits. An early warning system, if implemented, would have caused borders to be shut and early lockdowns. The WHO also delayed its response in sounding the alarm regarding the severity of the outbreak to allow nations globally to prepare for a pandemic. Singapore is a prime example where, despite the WHO not raising concerns and banning travel to and from China, a country banned travelers and took early measures, thus managing the outbreak quite well. South Korea is another example of how things may have played out had those measures by agencies been taken with transparency. Increased transparency would have allowed the healthcare sector to better prepare and reduced the load of patients they had to deal with, helping flatten the curve. The increased patient load and confusion among citizens arising from not following these practices has proved to be a barrier to providing effective treatments to patients with the disease elsewhere in the world.
Lack of Preparedness and Protocols
Despite the previous SARS outbreak teaching us important lessons and providing us with data on a potential outbreak, many nations did not take the important measures needed for a future outbreak. There was no allocation of sufficient funds for such an event. Many countries experienced severe lack of PPE, and the lockdown precautions hampered the logistics of supply and manufacturing of such essential equipment. Singapore and South Korea had protocols in place and were able to implement them at a moment's notice. The spurt of cases that Korea experienced was managed well, providing evidence to this effect. The lack of preparedness and lack of protocol in other nations has resulted in confusion as to how the treatment may be administered safely to the large volume of patients while dealing with diagnostics. Both of these factors have limited the accessibility to healthcare services due to sheer volume.
Socio-Economic Impact
During the SARS epidemic, China faced an economic setback, and experts were unsure if any recovery would be made. However, the global and domestic situation was then in China's favor, as it had a lower debt, allowing it to make a speedy recovery. This is not the case now. Global experts have a pessimistic outlook on the outcome of this outbreak (98). The fear of COVID-19 disease, lack of proper understanding of the dangers of the virus, and the misinformation spread on the social media (99) have caused a breakdown of the economic flow globally (100). An example of this is Indonesia, where a great amount of fear was expressed in responses to a survey when the nation was still free of COVID-19 (101). The pandemic has resulted in over 2.6 billion people being put under lockdown. This lockdown and the cancellation of the lunar year celebration has affected business at the local level. Hundreds of flights have been canceled, and tourism globally has been affected. Japan and Indonesia are estimated to lose over 2.44 billion dollars due to this (102, 103). Workers are not able to work in factories, transportation in all forms is restricted, and goods are not produced or moved. The transport of finished products and raw materials out of China is low. The Economist has published US stock market details indicating that companies in the US that have Chinese roots fell, on average, 5 points on the stock market as compared to the S&P 500 index (104). Companies such as Starbucks have had to close over 4,000 outlets due to the outbreak as a precaution. Tech and pharma companies are at higher risk since they rely on China for the supply of raw materials and active pharmaceutical ingredients. Paracetamol, for one, has reported a price increase of over 40% in India (104–106). Mass hysteria in the market has caused selling of shares of these companies, causing a tumble in the Indian stock market. Though long-term investors will not be significantly affected, short-term traders will find themselves in soup. Politically, however, this has further bolstered support for world leaders in countries such as India, Germany, and the UK, who are achieving good approval ratings, with citizens being satisfied with the government's approach. In contrast, the ratings of US President Donald Trump have dropped due to the manner in which the COVID-19 pandemic was handled. These minor impacts may be of temporary significance, and the worst and direct impact will be on China itself (107–109), as the looming trade war with the USA had a negative impact on the Chinese and Asian markets. The longer production of goods continues to remain suspended, the more adversely it will affect the Chinese economy and the global markets dependent on it (110). If this disease is not contained, more and more lockdowns by multiple nations will severely affect the economy and lead to many social complications.
Conclusion
The appearance of the 2019 Novel Coronavirus has added and will continue to add to our understanding of viruses. The pandemic has once again tested the world's preparedness for dealing with such outbreaks. It has provided an outlook on how a massive-scale biological event can cause a socio-economic disturbance through misinformation and social media. In the coming months and years, we can expect to gain further insights into SARS-CoV-2 and COVID-19.
Author Contributions
KN: conceptualization. RK, AA, JM, and KN: investigation. RK and AA: writing—original draft preparation. KN, PN, and JM: writing—review and editing. KN: supervision.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to acknowledge the contributions made by Dr. Piya Paul Mudgal, Assistant Professor, Manipal Institute of Virology, Manipal Academy of Higher Education towards inputs provided by her during the drafting of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2020.00216/full#supplementary-material
Supplementary Data 1, 2. List of all studies registered for COVID-19 on clinicaltrials.gov.
References
1. McIntosh K, Dees JH, Becker WB, Kapikian AZ, Chanock RM. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci USA. (1967) 57:933–40. doi: 10.1073/pnas.57.4.933
2. Esper F, Weibel C, Ferguson D, Landry ML, Kahn JS. Evidence of a novel human coronavirus that is associated with respiratory tract disease in infants and young children. J Infect Dis. (2005) 191:492–8. doi: 10.1086/428138
3. Stöhr K. A multicentre collaboration to investigate the cause of severe acute respiratory syndrome. Lancet. (2003) 361:1730–3. doi: 10.1016/S0140-6736(03)13376-4
4. Peiris JSM, Lai ST, Poon LLM, Guan Y, Yam LYC, Lim W, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. (2003) 361:1319–25. doi: 10.1016/S0140-6736(03)13077-2
5. Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. (2015) 386:995–1007. doi: 10.1016/S0140-6736(15)60454-8
6. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. (2012) 367:1814–20. doi: 10.1056/NEJMoa1211721
7. Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. (2009) 7:439–50. doi: 10.1038/nrmicro2147
8. Li W, Moore MJ, Vasllieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. (2003) 426:450–4. doi: 10.1038/nature02145
9. Ge X-Y, Li J-L, Yang X-L, Chmura AA, Zhu G, Epstein JH, et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. (2013) 503:535–8. doi: 10.1038/nature12711
10. Wang M, Hu Z. Bats as animal reservoirs for the SARS coronavirus: Hypothesis proved after 10 years of virus hunting. Virol Sin. (2013) 28:315–7. doi: 10.1007/s12250-013-3402-x
11. Diamond Princess Cruise Ship in Japan Confirms 99 New Coronavirus Cases | World news | The Guardian. Available online at: https://www.theguardian.com/world/2020/feb/17/coronavirus-japan-braces-for-hundreds-more-cases-as-another-china-city-locked-down (accessed February 17, 2020).
12. Diamond Princess Coronavirus & Quarantine Updates - Notices & Advisories - Princess Cruises. Available online at: https://www.princess.com/news/notices_and_advisories/notices/diamond-princess-update.html (accessed February 17, 2020).
13. Gorbalenya AE, Baker SC, Baric RS, Groot RJ, de Drosten C, Gulyaeva AA, et al. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. (2020) 5:536–44. doi: 10.1038/s41564-020-0695-z
14. Ji W, Wang W, Zhao X, Zai J, Li X. Homologous recombination within the spike glycoprotein of the newly identified coronavirus may boost cross-species transmission from snake to human. J Med Virol. (2020) 7:jmv.25682. doi: 10.1002/jmv.25682
15. Cyranoski D. Did pangolins spread the China coronavirus to people? Nature. (2020) doi: 10.1038/d41586-020-00364-2
16. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. (2020) 395:565–74. doi: 10.1016/S0140-6736(20)30251-8
17. Wassenaar TM, Zou Y. 2019_nCoV: Rapid classification of betacoronaviruses and identification of traditional Chinese medicine as potential origin of zoonotic coronaviruses. Lett Appl Microbiol. (2020) 70:324–48. doi: 10.1111/lam.13285
18. Velavan TP, Meyer CG. The Covid-19 epidemic. Trop Med Int Heal. (2020) 25:278–80. doi: 10.1111/tmi.13383
19. Guarner J. Three Emerging Coronaviruses in Two Decades. Am J Clin Pathol. (2020) 153:420–21. doi: 10.1093/ajcp/aqaa029
20. Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH, et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. (2005) 310:676–9. doi: 10.1126/science.1118391
21. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. (2020) 579:270–3. doi: 10.1038/s41586-020-2012-7
22. Menachery VD, Yount BL, Debbink K, Agnihothram S, Gralinski LE, Plante JA, et al. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat Med. (2015) 21:1508–13. doi: 10.1038/nm.3985
23. COVID-19 Coronavirus Epidemic has a Natural Origin—ScienceDaily. Available online at: https://www.sciencedaily.com/releases/2020/03/200317175442.htm (accessed April 22, 2020).
24. Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. (2020) 26:450–2. doi: 10.1038/s41591-020-0820-9
25. Prioritizing Diseases for Research and Development in Emergency Contexts. Available online at: https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts (accessed April 22, 2020).
26. Albariño CG, Guerrero LW, Jenks HM, Chakrabarti AK, Ksiazek TG, Rollin PE, et al. Insights into Reston virus spillovers and adaption from virus whole genome sequences. PLoS ONE. (2017) 12:e0178224. doi: 10.1371/journal.pone.0178224
27. Yao H, Lu X, Chen Q, Xu K, Chen Y, Cheng L, et al. Patient-derived mutations impact pathogenicity of SARS-CoV-2. medRxiv. (2020). doi: 10.2139/ssrn.3578153
28. Stefanelli P, Faggioni G, Lo Presti A, Fiore S, Marchi A, Benedetti E, et al. Whole genome and phylogenetic analysis of two SARS-CoV-2 strains isolated in Italy in January and February 2020: additional clues on multiple introductions and further circulation in Europe. Eurosurveillance. (2020) 25:2000305. doi: 10.2807/1560-7917.ES.2020.25.13.2000305
29. Yadav P, Potdar V, Choudhary M, Nyayanit D, Agrawal M, Jadhav S, et al. Full-genome sequences of the first two SARS-CoV-2 viruses from India. Indian J Med Res. (2020) 151:200–9. doi: 10.4103/ijmr.IJMR_663_20
30. WHO | Coronavirus disease 2019 (COVID-19) Situation Report – 26. Beijing (2020). Available online at: http://www.nhc.gov.cn/xcs/yqtb/list_gzbd.shtml (accessed February 16, 2020).
31. WHO | Middle East Respiratory Syndrome Coronavirus (MERS-CoV). World Health Organization (2020).
32. WHO | Summary of Probable SARS Cases With Onset of Illness From 1 November 2002 to 31 July 2003. World Health Organization (2015).
34. Update on the Outbreak of New Coronavirus Pneumonia as of 24 hours on 15 February. Beijing (2020). Available online at: http://www.nhc.gov.cn/xcs/yqtb/list_gzbd.shtml (accessed February 16, 2020).
35. Sanche S, Lin YT, Xu C, Romero-Severson E, Hengartner N, Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. (2020) 26. doi: 10.3201/eid2607.200282
36. 83% of India's Coronavirus Patients Are Below the Age of 50: Health ministry data - India News. Available online at: https://www.indiatoday.in/india/story/83-of-india-s-coronavirus-patients-are-below-the-age-of-50-health-ministry-data-1663314-2020-04-04 (accessed April 23, 2020).
37. 42% of Coronavirus Patients in 21-40 age bracket: Govt. Available online at: https://economictimes.indiatimes.com/news/politics-and-nation/42-of-coronavirus-patients-in-21-40-age-bracket-govt/articleshow/74987254.cms (accessed April 23, 2020).
38. Why COVID-19 Case Counts Are so Low in Singapore Hong Kong and Taiwan | Advisory Board Daily Briefing. Available online at: https://www.advisory.com/daily-briefing/2020/03/19/asian-countries (accessed April 29, 2020).
39. Coronavirus: Why so Few Deaths Among Singapore's 14000 Covid-19 Infections? | South China Morning Post. Available online at: https://www.scmp.com/weekasia/health-environment/article/3081772/coronavirus-why-so-few-deaths-among-singapores-14000 (accessed April 29, 2020).
40. Stafford N. Covid-19: Why Germany's case fatality rate seems so low. BMJ. (2020) 7:369. doi: 10.1136/bmj.m1395
41. Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-NCOV infection from an asymptomatic contact in Germany. N Engl J Med. (2020) 382:970–1. doi: 10.1056/NEJMc2001468
42. Coronavirus pandemic | 80% of COVID-19 Cases Either Asymptomatic or Show Mild Symptoms. Health ministry - Moneycontrol.com. Available online at: https://www.moneycontrol.com/news/india/coronavirus-pandemic-80-of-covid-19-cases-either-asymptomatic-or-show-mild-symptoms-health-ministry-5170631.html (accessed April 24, 2020).
43. WHO | Q&A on Coronaviruses. Available online at: https://www.who.int/news-room/q-a-detail/q-a-coronaviruses (accessed February 16, 2020).
44. Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. (2020) 382:929–36. doi: 10.1056/NEJMoa2001191
45. Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. (2020) 5:335–37. doi: 10.1016/S2468-1253(20)30048-0
46. Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. (2020) 395:809–15. doi: 10.1016/S0140-6736(20)30360-3
47. Fehr AR, Perlman S. Coronaviruses: An overview of their replication and pathogenesis. In: Coronaviruses: Methods and Protocols (New York: Springer), 1–23. doi: 10.1007/978-1-4939-2438-7_1
48. Chen Y, Liu Q, Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol. (2020) 92:418–23. doi: 10.1002/jmv.25681
49. Hoffmann M, Kleine-Weber H, Schroeder S, Mü MA, Drosten C, Pö S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) 181:271–80. doi: 10.1016/j.cell.2020.02.052
50. Cheng VCC, Chan JFW, To KKW, Yuen KY. Clinical management and infection control of SARS: Lessons learned. Antiviral Res. (2013) 100:407–19. doi: 10.1016/j.antiviral.2013.08.016
51. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
52. Udugama B, Kadhiresan P, Kozlowski HN, Malekjahani A, Osborne M, Li VYC, et al. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. (2020) 14:3822–35. doi: 10.1021/acsnano.0c02624
53. Testing, | CDC,. Available online at: https://www.cdc.gov/coronavirus/2019-ncov/testing/index.html (accessed April 29, 2020).
54. Advice on the Use of Point-of-Care Immunodiagnostic Tests for COVID-19. Available online at: https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-point-of-care-immunodiagnostic-tests-for-covid-19 (accessed April 29, 2020).
55. Zhang Yanping. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) — China, 2020. (2020) Available online at: http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9bfea8db1a8f51 (accessed February 18, 2020).
56. Petersen R, Pan L, Blanck HM. Racial and ethnic disparities in adult obesity in the United States: CDC's tracking to inform state and local action. Prev Chronic Dis. (2019) 16:180579. doi: 10.5888/pcd16.180579
57. Wang L, Southerland J, Wang K, Bailey BA, Alamian A, Stevens MA, et al. Ethnic differences in risk factors for obesity among adults in California, the United States. (2017) 2017:2427483. doi: 10.1155/2017/2427483
58. Covid-19 Causes Sudden Strokes in Young Adults Doctors Say - CNN. Available online at: https://edition.cnn.com/2020/04/22/health/strokes-coronavirus-young-adults/index.html (accessed April 24, 2020).
59. Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. (2020) 1–4. doi: 10.1111/jth.14828
60. Lancet T, Medicine R. Comment Understanding pathways to death in patients with. Lancet Respir Med. (2020) 2019:2019–21. doi: 10.1016/S2213-2600(20)30165-X
61. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. (2020) 323:1775–6. doi: 10.1001/jama.2020.4683
62. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
63. Doctors Warn COVID-19 Might Cause Strokes in Young Adults. Available online at: https://nypost.com/2020/04/23/doctors-warn-covid-19-might-cause-strokes-in-young-adults/ (accessed April 24, 2020).
64. “Solidarity” Clinical Trial for COVID-19 Treatments. Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments (accessed April 23, 2020).
65. Search, of: COVID-19 - List Results - ClinicalTrials.gov. Available online at: https://www.clinicaltrials.gov/ct2/results?cond=COVID-19&term=&cntry=&state=&city=&dist= (accessed May 1, 2020).
66. De Wilde AH, Jochmans D, Posthuma CC, Zevenhoven-Dobbe JC, Van Nieuwkoop S, Bestebroer TM, et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. (2014) 58:4875–84. doi: 10.1128/AAC.03011-14
67. Tai DYH. Pharmacologic treatment of SARS: current knowledge and recommendations. Ann Acad Med Singapore. (2007) 36:438–43.
68. Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. (2020) 11:222. doi: 10.1038/s41467-019-13940-6
69. Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. (2020) 14:72–73. doi: 10.5582/bst.2020.01047
70. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. (2020) 30:269–71. doi: 10.1038/s41422-020-0282-0
71. Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. (2005) 2:1–0. doi: 10.1186/1743-422X-2-69
72. Cramer CL, Patterson A, Alchakaki A, Soubani AO. Immunomodulatory indications of azithromycin in respiratory disease: a concise review for the clinician. Postgrad Med. (2017) 129:493–9. doi: 10.1080/00325481.2017.1285677
73. Plaquenil™ Hydroxychloroquine Sulfate Tablets USP Description. Available online at: http://www.cdc.gov/malaria (accessed April 21, 2020).
74. Magagnoli J, Narendran S, Pereira F, Cummings T, Hardin JW, Sutton SS, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv. (2020) doi: 10.1101/2020.04.16.20065920
75. Shamshirian A, Hessami A, Heydari K, Alizadeh-Navaei R, Ebrahimzadeh MA, Ghasemian R, et al. Hydroxychloroquine Versus COVID-19: A Rapid Systematic Review and Meta-Analysis. medRxiv. (2020) doi: 10.1101/2020.04.14.20065276
76. Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov. (2020) 19:149–50. doi: 10.1038/d41573-020-00016-0
77. Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. (2016) 531:381–5. doi: 10.1038/nature17180
78. Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. bioRxiv. (2020) doi: 10.1101/2020.04.15.043166
79. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. (2020) 395:1569–78. doi: 10.1016/S0140-6736(20)31022-9
80. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. N Engl J Med. (2020) 382:1787–99. doi: 10.1056/NEJMoa2001282
81. Search, of: Lopinavir, Ritonavir, | COVID - List Results - ClinicalTrials.gov. Available online at: https://clinicaltrials.gov/ct2/results?term=Lopinavir%2CRitonavir&cond=COVID&draw=4&rank=22#rowId21 (accessed April 23, 2020).
82. Bhatnagar T, Murhekar M, Soneja M, Gupta N, Giri S, Wig N, et al. Lopinavir/ritonavir combination therapy amongst symptomatic coronavirus disease 2019 patients in India: protocol for restricted public health emergency use. Indian J Med Res. (2020) 151:184–9. doi: 10.4103/ijmr.IJMR_502_20
83. Yeh K-M, Chiueh T-S, Siu LK, Lin J-C, Chan PKS, Peng M-Y, et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. (2005) 56:919–22. doi: 10.1093/jac/dki346
84. Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA. (2020) 323:1582–9. doi: 10.1001/jama.2020.4783
85. Cao W, Liu X, Bai T, Fan H, Hong K, Song H, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. (2020) 7:1–6. doi: 10.1093/ofid/ofaa102
86. Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. (2020) 138745:1–22. doi: 10.1172/JCI138745
87. Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. (2020) doi: 10.1002/jmv.25882
88. Recommendations for Investigational COVID-19 Convalescent Plasma | FDA. Available online at: https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma (accessed April 23, 2020).
89. Search, of: plasma | Interventional Studies | COVID - List Results - ClinicalTrials.gov. Available online at: https://clinicaltrials.gov/ct2/results?term=plasma&cond=COVID&Search=Apply&age_v=&gndr=&type=Intr&rslt= (accessed April 23, 2020).
90. Infection Prevention and Control. Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/infection-prevention-and-control (accessed February 17, 2020).
91. Wilder-Smith A, Freedman DO. Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J Travel Med. (2020) 27:taaa020. doi: 10.1093/jtm/taaa020
92. Press Information Bureau AYUSH Advisory for Corona virus. Press Inf Bereau. Available online at: https://pib.gov.in/PressReleasePage.aspx?PRID=1600895 (accessed February 17, 2020).
93. CanSino, Biologics : China Announces First Human Trials of Covid-19 Vaccine | MarketScreener,. Available online at: https://www.marketscreener.com/CANSINO-BIOLOGICS-INC-59318312/news/CanSino-Biologics-China-announces-first-human-trials-of-Covid-19-vaccine-30183232/ (accessed April 7, 2020).
94. Safety, and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis SARS CoV-2 Infection - Full Text View - ClinicalTrials.gov. Available online at: https://clinicaltrials.gov/ct2/show/NCT04283461 (accessed April 7, 2020).
95. NIH, Clinical Trial of Investigational Vaccine for COVID-19 Begins | National Institutes of Health (NIH),. Available at: https://www.nih.gov/news-events/news-releases/nih-clinical-trial-investigational-vaccine-covid-19-begins (accessed April 7, 2020).
96. Novel, Coronavirus vaccine manufacturing contract signed — The Jenner Institute,. Available online at: https://www.jenner.ac.uk/about/news/novel-coronavirus-vaccine-manufacturing-contract-signed (accessed April 7, 2020).
97. Xie L, Sun C, Luo C, Zhang Y, Zhang J, Yang J, et al. SARS-CoV-2 and SARS-CoV Spike-RBD structure and receptor binding comparison and potential implications on neutralizing antibody and vaccine development. bioRxiv [Preprint]. (2020). doi: 10.1101/2020.02.16.951723
98. The Global Economic Impact of the Coronavirus Outbreak – Harvard Gazette. Available online at: https://news.harvard.edu/gazette/story/2020/02/the-global-economic-impact-of-the-coronavirus-outbreak/ (accessed February 17, 2020).
99. Shimizu K. 2019-nCoV, fake news, and racism. Lancet. (2020) 395:685–6. doi: 10.1016/S0140-6736(20)30357-3
100. ROHDE RODNEY. 2019 Novel Coronavirus (2019-nCoV) Update: Uncoating the Virus. Am Soc Microbiol. (2020). Available online at: https://asm.org/Articles/2020/January/2019-Novel-Coronavirus-2019-nCoV-Update-Uncoating
101. Virus-free Indonesia more threatened by COVID-19 than Singapore Malaysia: Survey - World - The Jakarta Post. Available online at: https://www.thejakartapost.com/news/2020/02/18/virus-free-indonesia-more-threatened-by-covid-19-than-singapore-malaysia-survey.html (accessed February 18, 2020).
102. Japan, May Lose $1,.29 Billion in Tourism Revenue Due to COVID-19 Outbreak | The Japan Times. Available online at: https://www.japantimes.co.jp/news/2020/02/16/business/economy-business/japan-lose-billion-tourism-revenue-covid19-outbreak/#.XkvxX0fitPY (accessed February 18, 2020).
103. Coronavirus's Effect on Tourism Will Carry Into 2021 Experts Say - Bloomberg. Available online at: https://www.bloomberg.com/news/articles/2020-02-13/coronavirus-s-effect-on-tourism-will-carry-into-2021-experts-say (accessed February 18, 2020).
104. The, week in charts - The cost of covid-19 | Graphic detail | The Economist,. Available online at: https://www.economist.com/graphic-detail/2020/02/14/the-cost-of-covid-19 (accessed February 17, 2020).
105. coronavirus: Covid-19 Impact: Pharma Companies Feel the Pain as Prices of Key Inputs Shoot Up - The Economic Times. Available online at: https://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/covid-19-impact-pharma-companies-feel-the-pain-as-prices-of-key-inputs-shoot-up/articleshow/74144044.cms?from=mdr (accessed February 17, 2020).
106. Coronavirus Outbreak: Paracetamol Prices Jump 40% In India As Coronavirus Shuts Down China. Available online at: https://www.ndtv.com/india-news/coronavirus-outbreak-paracetamol-prices-jump-40-in-india-as-coronavirus-shuts-down-china-2181480 (accessed February 18, 2020).
107. The coronavirus could cripple China's economy for longer than Wall Street wants to believe | Business Insider India. Available online at: https://www.businessinsider.in/international/news/the-coronavirus-could-cripple-chinas-economy-for-longer-than-wall-street-wants-to-believe/articleshow/74162183.cms (accessed February 17, 2020).
108. Viral Slowdown - How China's Coronavirus Epidemic Could Hurt the World Economy | Leaders | The Economist. Available online at: https://www.economist.com/leaders/2020/02/13/how-chinas-coronavirus-epidemic-could-hurt-the-world-economy (accessed February 17, 2020).
109. China's Economic Battle With COVID-19 | The ASEAN Post. Available online at: https://theaseanpost.com/article/chinas-economic-battle-covid-19 (accessed February 17, 2020).
110. The Coronavirus Could Cost China's Economy $60 Billion this Quarter. - CNN. Available online at: https://edition.cnn.com/2020/01/31/economy/china-economy-coronavirus/index.html (accessed February 18, 2020).
Keywords: 2019-nCoV, COVID-19, SARS-CoV-2, coronavirus, pandemic, SARS
Citation: Keni R, Alexander A, Nayak PG, Mudgal J and Nandakumar K (2020) COVID-19: Emergence, Spread, Possible Treatments, and Global Burden. Front. Public Health 8:216. doi: 10.3389/fpubh.2020.00216
Received: 21 February 2020; Accepted: 11 May 2020;
Published: 28 May 2020.
Edited by:
Murat Akova, Hacettepe University, TurkeyReviewed by:
Tarek Adnan Ahmad, Bibliotheca Alexandrina, EgyptSriSowmya Sanisetty, Independent Researcher, Cambridge, MA, United States
Copyright © 2020 Keni, Alexander, Nayak, Mudgal and Nandakumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Krishnadas Nandakumar, mailnandakumar77@gmail.com
 Raghuvir Keni
Raghuvir Keni Anila Alexander
Anila Alexander Pawan Ganesh Nayak
Pawan Ganesh Nayak Jayesh Mudgal
Jayesh Mudgal Krishnadas Nandakumar
Krishnadas Nandakumar