- 1Department of Clinical and Experimental Medicine, University of Foggia, Foggia, Italy
- 2Department of Advanced Medical and Surgical Sciences, University of Campania “Luigi Vanvitelli,” Naples, Italy
- 3Section of Human Physiology and Unit of Dietetics and Sports Medicine, Department of Experimental Medicine, Università degli Studi della Campania “Luigi Vanvitelli,” Naples, Italy
- 4Department of Education Sciences, Psychology, and Communication, University of Bari, Bari, Italy
- 5Department of Medical, Surgical and Advanced Technologies “G.F. Ingrassia,” University of Catania, Catania, Italy
- 6Clinic of Child and Adolescent Neuropsychiatry, Department of Mental Health, Physical and Preventive Medicine, Università degli Studi della Campania “Luigi Vanvitelli,” Naples, Italy
- 7Pediatrics, Department of Medical and Surgical Sciences, University of Foggia, Foggia, Italy
Bisphenol A (BPA) is an endocrine disruptor (ED) frequently used in food packaging. BPA is used as a monomer in the manufacture of some food packaging. This study aimed to evaluate the urinary BPA concentration in an Italian pediatric cohort, testing the levels of this ED over a period of 6 months, evaluating the effects of a diet regimen with a reduction of Plastic Food Packaging (PFP). One hundred thirty Italian children were enrolled and divided into two groups “School Canteen” and “No School Canteen.” The first group consumed one meal at school using a plastic-free service for 5 days/weeks, while the other group did not modify their normal meal-time habits. The BPA levels were tested in urine samples at three time points: T0, is the time before the application of the plastic-free regimen diet; T3, 3 months later; and T6, 6 months later. A reduction of urine BPA levels was detected in the “School Canteen” group. In particular, the reduction was significant analyzing both the intra (among the three testing times) group and inter (between “School Canteen” and “No School Canteen”) group variability. Our results show the effects of a diet regimen with a reduction of PFP, demonstrating a connection between urinary BPA levels and food packaging.
Introduction
Over the last few years, a new health concept has led us to consider a person's well-being in a broader way than in the past (1). Human wellness is conditioned by different essential factors, altering its homeostasis. Indeed, safety and human survival are strictly linked to environmental conditions. Many toxic substances act on human health, generating adverse effects. In this regard, the protracted exposure to minimal chemical substances can progressively alter the functioning of cells, tissues, and organs, as well as interfering with DNA expression (2).
Plastic Food Packaging (PFP) can interact with the food, generating the subsequent diffusion process of chemical compounds that could be transferred from packaging to food (3). This inconvenience is strictly related to different conditions such as the chemical properties of the PFP, storage temperature, UV exposure and the shelf life of the product. All these characteristics can determine the amount of the chemical substances that could be transferred: this process is named “migration” (4–6).
There is a growing interest in the possible health threat posed by chemical compounds of PFP known as endocrine disruptors (EDs). EDs are a heterogeneous group of substances that interfere with the endocrine system function (acting on the enzymes involved in steroidogenesis or interacting with the binding sites of sex hormones). They frequently act on steroid and thyroid homeostasis, generating several adverse outcomes such as behavioral disorders, obesity, some types of cancer (testicle, breast) and different problems of the reproductive systems (infertility, abortion, endometriosis). For these reasons, fetus development and childhood are considered the most susceptible periods (7).
Bisphenol A (BPA) is used as a monomer in the manufacture of some food packaging (such as polycarbonates) because it is regulated and has been approved to be used in the European union (EU) (8). Considering its characteristics, such as transparency, thermal and mechanical properties, it is also contained in epoxy resins (9, 10). Human exposure occurs through multiple sources, for example, drinking the water contained in plastic bottles, eating food contained in plastic packaging or food cans, and using dental devices (11).
To date, even if the safety of BPA is controversial, it can be used in the manufacture of PFP following the EU regulation on plastic material (8). Indeed, it is still not clear that BPA coming from food contact articles (very low levels) may pose a food safety issue. Several papers described that BPA may have estrogenic effects and may alter the thyroid function and the reproductive, nervous, and immune systems (12–14). Concerning the adverse effects on thyroid function, Andrianou et al. reported a positive association of BPA with thyroid-stimulating hormone (TSH), even if further studies are needed to confirm these interesting findings (15). Moreover, a recent post-mortem study performed on obese subjects showed the presence of BPA in two distinct regions of the human brain, suggesting that it may be able to cross the blood-brain barrier (16). Its adverse effects could be very important during puberty, particularly in females (17, 18). Furthermore, BPA levels could be linked to the development of different kinds of cancers (19–22). Moreover, several papers have described a direct link between BPA levels and increased risks of cardiovascular diseases and diabetes (23, 24): a recent study described an involvement of BPA in the development of both obesity and insulin resistance (25–27). Finally, several papers reported a pivotal role for BPA in obesogenic activity: it is able to act on adipocytes, reducing both the production and the secretion of adiponectin (28–32). Moreover, it increases the expression of the genes involved both in pro-inflammatory cytokine production and in lipid accumulation (30, 33). A recent review of Andra et al. suggested performing research on BPA and its analogs in order to better clarify the effects on human health (34).
This study aimed to evaluate the urinary BPA concentration in an Italian pediatric cohort, testing the levels of this ED over a 6-months period, evaluating the effects of a diet regimen with a reduction of food in plastic packaging. Moreover, the relationships among BPA, BMI, and plastic packaging use were investigated.
Materials and Methods
Participants
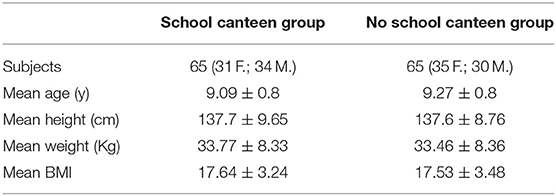
One hundred and thirty Italian children were enrolled for this research study. Researchers provided the children's parents/guardians with information explaining the proposed research project and gave them the opportunity to opt their children out by filling in and returning a form. Written informed consent for publication was obtained from the parents/guardians of all the children enrolled. This research project was approved by the Ethics Committee of “Riuniti” Hospital (code: 28_09_2018_RH) and all procedures were performed in accordance with the Declaration of Helsinki. Children were divided into the “School Canteen” Group (65 children) and the “No School Canteen” Group (65 Children), and enrolled in September 2018. The main characteristics and the experimental model are summarized in Figure 1.
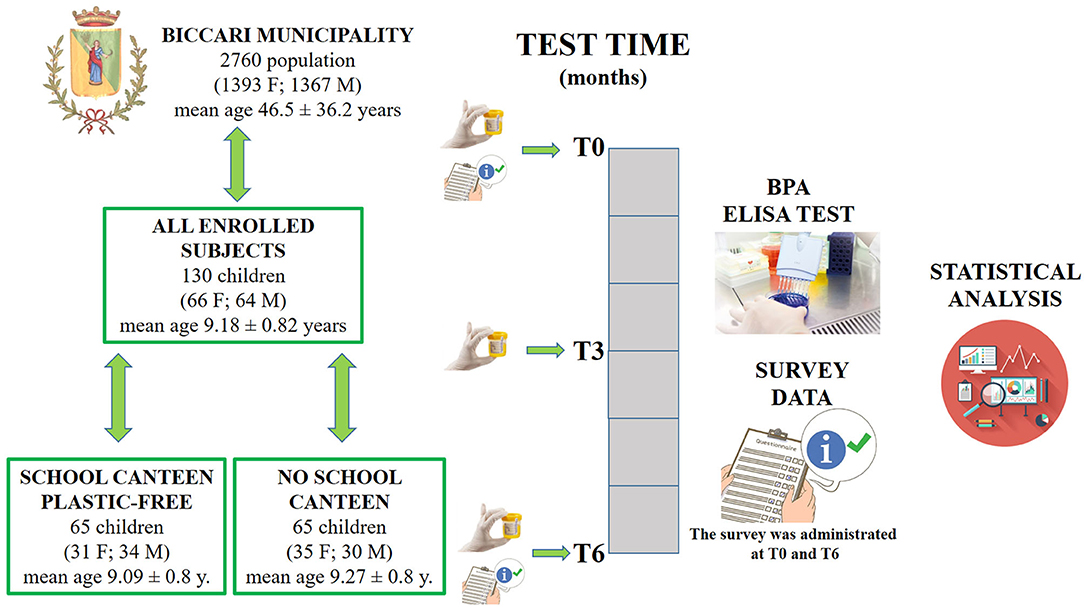
Figure 1. Three urine samples were collected at T0, T3, and T6. At T0 and T6 a questionnaire was filled in by the parents/guardians of each participant. At the end of the study, 390 urine samples had been analyzed.
Study Protocol
The study was performed in a small town in southern Italy (Biccari, Foggia, Apulia region, Italy). Before the school year, based on their own necessity, the parents/guardians of the children were free to choose a different diet regimen for their children. For this reason, the enrolled children were divided into two groups “School Canteen” and “No School Canteen”; the anthropometric data of the study population is summarized in Table 1; it is important to note that BMI was calculated using the child's weight and height and was then used to find the corresponding BMI-for-age percentile for the child's age and sex based on CDC growth charts for children and teens ages 2 through 19 years old (available at https://www.cdc.gov/healthyweight/bmi/calculator.html).
The “School Canteen” group was made up of 65 children who ate 1 meal at school using a plastic-free service for 5 days/weeks: particularly, during school lunch they ate in the canteen, they used certified compostable materials, while in the previous school years, they had used all plastic material. The other group (65 children from the same school) did not modify their normal eating habits (e.g., using plastic bottles for water or another liquid, plastic plates, etc.). All parents/guardians of both groups declared that they avoided the usage of a microwave oven with plastic material for all the study period. No differences were detected between groups evaluating numerous measures of self-reported socioeconomic position, assessed through interviews conducted in-person by trained interviewers (35). Based on a certified release form from the family medical doctor, all participants were healthy. Three sampling points were identified to collect urine samples: - T0, is the time before the application of the plastic-free regimen diet; T3, 3 months later; and T6 6 months later. The survey cocerning the food habits related to the use of food packaging was administrated at T0 and T6.
The experimental model is illustrated in Figure 1.
Sample Collection
Urine samples were taken from each participant after an overnight fast. Urine samples were collected in a glass tube. After the urine volume was determined, 5 mL were stored at −80°C in glass vials and analyzed within 1 month.
BPA Determination in Urine
Total urinary BPA concentrations were determined by enzyme-linked immunoassay (ELISA), using a commercial kit, according to the manufacturer's instructions (Human Bisphenol A (BPA) ELISA kit MBS269957). Briefly, we added 100 μl of the sample and different concentrations of human BPA standard samples to corresponding wells and incubated them at 37°C for 90 min. After washing, we added 100 ml of the biotinylated human BPA antibody liquid to each well and incubated at 37°C for 60 min. We then added 100 μl of enzyme-conjugate liquid to the incubator at 37°C for 30 min. Finally, we added 100 μl of the color reagent to each well for the chromogenic reaction, and we read the plate at 450 nm. The urinary BPA levels are expressed in ng/ml, as indicated by the manufacturer.
Moreover, urinary creatinine concentrations were measured using a Creatinine Urinary Detection Kit following the user manual (Life Technologies Corporation, Carlsbad, CA, USA). Finally, the creatinine-standardized values (individual BPA concentrations divided by creatinine) were evaluated. The concentrations of the standard curve were 200, 100, 50, 25, 12.5, 6.25, ad 3.12 ng/ml. The blank had a reading equal to a BPA concentration lower than 0.05 ng/.
Questionnaire
A questionnaire was administered to the parents/guardians of the enrolled children with the aim of investigating the food habits related to the use of food packaging. This survey had been adopted for a previous Italian study (36).
Six questions were investigated: Use of products with plasticized packaging for breakfast; Use of products with plasticized packaging for lunch/dinner; Use of daily snacks with plasticized packaging; Use of water in plastic bottles; Use of carbonated drinks in plastic bottles; Use of juices in plastic packs. For each answer a score was attributed: 0 (never), 1 (sometimes), 2 (Always). Therefore, the maximum score was 12 (the subject always used plastic packaging) and the minimum score 0 (the subject never used plastic packaging).
The questionnaire was administrated at T0 and T6.
Statistical Analysis
As suggested by Stacy et al. (37), Log10-transformed urinary BPA concentrations were the outcome in all statistical analyses. Data were analyzed with the software package SPSS 22.0 for Windows. The two-way variance analysis (ANOVA) was used to determine any statistically significant differences among the groups. If needed, Tukey post-hoc test was used to calculate multiple comparisons.
Results
As summarized in Supplementary Tables 1–3 and in the relative box plot (Figure 2), a reduction of urinary BPA levels was detected after 6 months in the “School Canteen” group: the ANOVA test showed significant differences both for unstandardized/unadjusted BPA values [F = 3.066, p < 0.05, 0.00032, Figure 2A] and for creatinine-standardized BPA values [F = 3.066, p < 0.05, 5.93 × 10−17, Figure 2B]. No statistical differences were reported in the BPA values in the “No School Canteen” group (Figures 2C,D; Supplementary Table 1). The BPA values are summarized in two different forms: unstandardized/unadjusted BPA values (Figures 2A,C) and for creatinine-standardized BPA values (Figures 2B,D). It is interesting to note that analyzing the intra-group variability in the “School Canteen” group, the post hoc analysis highlighted significant differences analyzing the unstandardized/unadjusted BPA values between T0 and T6, while no significant differences were found at T0 vs. T3, and between T3 and T6 values (Supplementary Table 1). Moreover, as reported in Supplementary Tables 2, 3, the post hoc analysis ascertained that there were no significant differences between the two tested groups at T0 (p > 0.05), this became significant at T3 (p < 0.05) and T6 (p < 0.05) both for unstandardized/unadjusted BPA values and for creatinine-standardized BPA values.
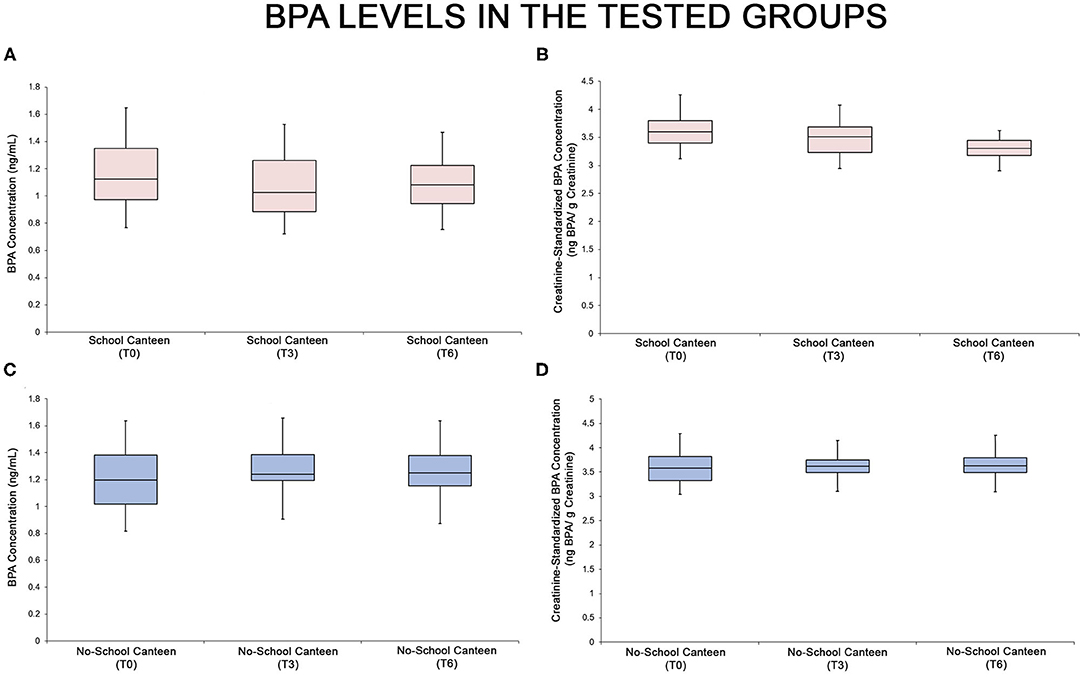
Figure 2. BPA levels in the two tested groups, “School Canteen” (A,B, pink box plots) and “No School Canteen” (C,D, blue box plots), at the different tested times. For each group, the BPA values are summarized in two different forms: unstandardized/unadjusted BPA values (A,C) and for creatinine-standardized BPA values (B,D).
The “School Canteen” group was subdivided under the BMI-for-age percentile criteria (using CDC growth charts for children and teens aged two through 19 years old into four groups: lower (13/65, less than the 5th percentile), normal (30/65, 5th percentile up to the 85th percentile), overweight (14/65, 85th to less than the 95th percentile) and obese (8/65, equal to or greater than the 95th percentile) (Table 2).

Table 2. The “School Canteen” group was subdivided under BMI criteria: the average BMI ± SD is summarized in the table.
The urine BPA levels of each sub-group are summarized through the box plot analysis (Figure 3): even if the box plot analysis shows a reduction of creatinine-standardized BPA values in all sub-groups, significant differences were found in two of the four categories analyzed (normal weight and overweight).
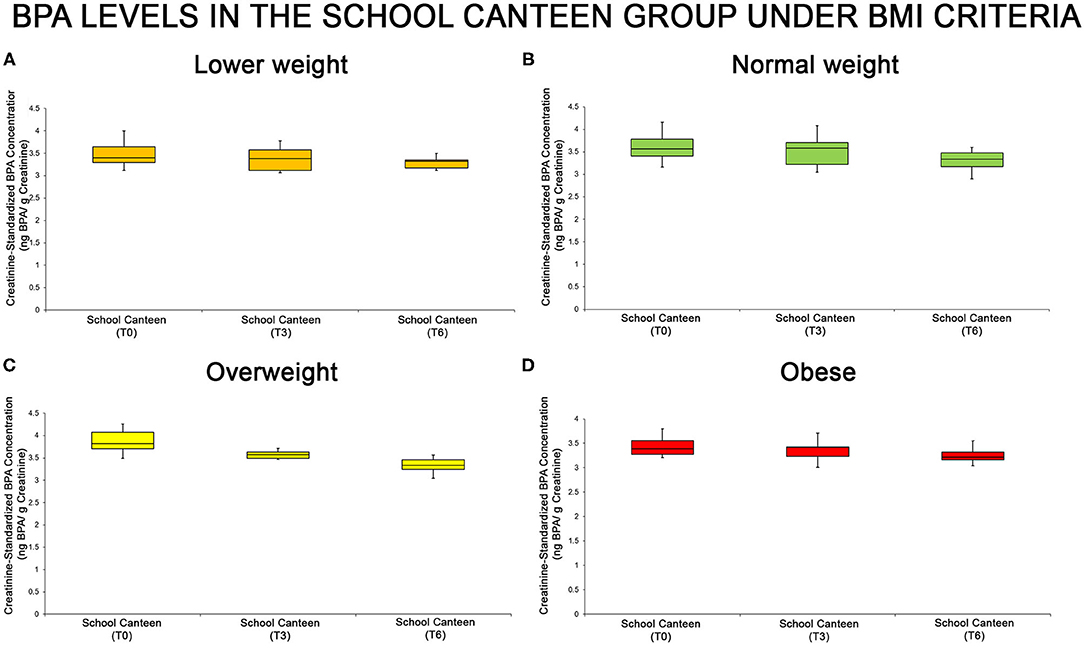
Figure 3. BPA levels at the different tested times in the “School Canteen” group, summarized under the BMI-for-age percentile criteria [lower weight, (A), normal weight, (B), overweight, (C), obese, (D)]: even if the box plot analysis shows a reduction of creatinine-standardized BPA values in all sub-groups, significant differences were found in two of the four categories analyzed (normal weight and overweight).
Moreover, the statistical analysis was performed analyzing the intra group variation. In the “Normal weight” and “Overweight” groups there were significant differences in the creatinine-standardized BPA levels as reported in Supplementary Table 4. As summarized in Supplementary Table 5, in the “Normal weight” group there are significant differences at T0 compared with T6, and at T3 compared with T6 (P < 0.05). No statistical differences were reported comparing T0 with T3 values. In the “Overweight” group, there were significant differences among all sampling points T0 vs. T3, T3 vs. T6, T0 vs. T6 (P > 0.05). In the “Lower weight” and “Obese” groups no significant differences were reported (T0 vs. T3, p > 0.05; T3 vs. T6, p > 0.05; T0 vs. T6, p > 0.05).
The same subdivision (BMI-for-age percentile criteria) was performed in the “No-School Canteen” group, generating four groups: lower (15/65), normal (26/65), overweight (14/65) and obese (10/65) (Table 3).

Table 3. The “No-School Canteen” group was subdivided under BMI criteria: the average BMI ± SD is summarized in the table.
Moreover, in the same group the statistical analysis was performed evaluating the BPA levels; nevertheless, no significant differences were observed (Supplementary Table 7).
The “School Canteen” group was subdivided under packaging use criteria, analyzing the data of the survey in four groups: Score 0–3 (low plastic packaging use, 0/65); score 4–6 (medium plastic packaging use, 8/65); score 7–9 (medium-high plastic packaging use, 39/65); score 10–12 (high plastic packaging use, 18/65). As reported, only 12.3% of this group could be classified as having a virtuous behavior regarding the use of plastic packaging. The urine BPA levels of each sub-group were summarized through the box plot analysis (Figure 4): even if the box plot analysis showed a reduction of creatinine-standardized BPA values in all sub-groups, significant differences were found in two of the three categories analyzed (medium-high packaging use and high packaging use).
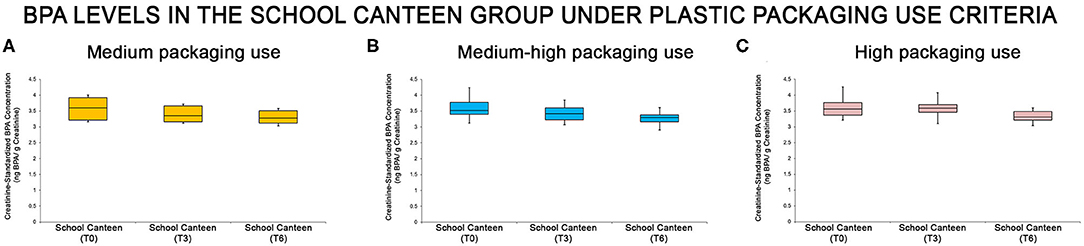
Figure 4. BPA levels at the different tested times in the “School Canteen” group, summarized under the plastic packaging use criteria [medium packaging use, (A), medium-high packaging use, (B), high packaging use, (C)]: even if the box plot analysis showed a reduction of creatinine-standardized BPA values in all sub-groups, significant differences were found in two of the three categories analyzed (medium-high packaging use and high packaging use).
Statistical analysis was performed analyzing the intra group variation. In the “Medium-high packaging use” and “high packaging use” groups there were significant differences in the creatinine-standardized BPA levels, as reported in Supplementary Table 6. As summarized in Supplementary Table 7, in the “Medium-high packaging use” group there are significant differences at T0 compared with T6, and at T3 compared with T6 (P < 0.05). No statistical differences were reported comparing T0 with T3 values. In the “high packaging use” group, there are significant differences among all sampling points T0 vs. T3, T3 vs. T6, T0 vs. T6 (P > 0.05). In the “Medium packaging use” group no significant differences were reported (T0 vs. T3, p > 0.05; T3 vs. T6, p > 0.05; T0 vs. T6, p > 0.05). It is important to note that the number of subjects in this subgroup (8/65) is limited.
Discussion
The international data concerning the transfer between packaging and the food it contains are constantly growing. The so-called “migration” process is related to different parameters such as the nature of the food, the concentration of substances in the packaging composition, storage temperature, and contact duration (38–40). The adverse effects of these substances affect the reproductive systems, exerting (41) their action particularly during puberty (42, 43). In several countries, such as Italy, even if the public water supply is potable, a lot of people drink from plastic bottles rather than drinking tap water (44). Plastic bottles usually contain a higher concentration of several chemical compounds that could be transferred to the contained liquid such as water, alcoholic and nonalcoholic beverages (45). These chemical compounds are released under different conditions such as high temperatures or UV exposure; the owners of storage facilities for bottled water often disregard warnings, storing the bottles in open spaces under these adverse environmental conditions (46–48). This concept is shown by Makris et al. in their experimental model where they showed that high urinary BPA levels were partially ascribed to the high polycarbonate water consumption in the summer and weather characteristics (high temperatures, >40°C; very high UV index values, >8), which could be causing BPA leaching from polycarbonate (41). The presence of BPA in drinking water is controversial, even if it is certainly released by polycarbonate food packaging (49).
In light of this evidence, in this study, we evaluated, for the first time, the urinary BPA concentration in an Italian pediatric cohort over 6 months, assessing the effects of a diet regimen with a reduction of PFP. Our results show a reduction of urine BPA levels after 6 months in the “School Canteen” group: the reduction is significant analyzing both the intra (among the tested periods, T0, T3, and T6) group and inter (between “School Canteen” and “No School Canteen”) group variability. We found that there were significant differences in urinary BPA concentrations in the “School Canteen” group after 6 months compared to T0 and the BPA reduction correlated with the score of the group, which could be classified as having a virtuous behavior compared to the use of plastic packaging. These findings are very interesting, highlighting how lifestyle habits can change and improve our health status.
The “School Canteen” group was subdivided under the BMI criteria, generating lower, normal, overweight, and obese groups. The results show that the reduction of urinary BPA values was detected in children in two categories (normal and overweight), while no statistically significant differences were detected in the lower and obese groups. In the lower weight group this result was related to the degree of BMI: indeed, in consideration of their diet habit, it could be supposed that a single correction in diet regimen should not influence the BPA levels. Moreover, in the obese subjects BPA remained about the same: for this group, a correction in the diet regimen did not significantly influence the BPA urinary levels. It is important to remark that the data about the “obese” group could be influenced by the small number of subjects in this group. Fernandez et al. also reported a non-significant negative association between BMI and adipose BPA concentration, which suggests that even if BPA is stored in adipose tissue, a higher BMI may not predict elevated BPA biomarker concentrations (50). Previous studies reported that perinatal exposure to BPA can predispose both to obesity and insulin resistance, particularly in older age (51–53). Furthermore, in pediatric populations higher levels of BPA are strictly associated with obesity and insulin resistance (54). Several recent epidemiological studies have demonstrated a correlation between urinary BPA levels and obesity (55, 56). Moreover, as previously described, the exposure to BPA can induce adverse effects on neurological control, suggesting an involvement in behavior management (57–59). A very recent study performed on obese subjects demonstrated a reduction in most phthalate levels after a dietary intervention (60). The results of the present study are in agreement with these studies, even if this indication should be confirmed by further studies. Moreover, analyzing the survey results, the urinary BPA levels of the “School Canteen” group was further subdivided under packaging use criteria. In particular, for each test time, 4 subgroups were obtained: low plastic packaging use group; medium plastic packaging use group; medium-high plastic packaging use group; and high plastic packaging use group. The urinary BPA levels were significantly reduced at the three sampling points in the groups with medium-high and high plastic packaging use. No significant differences at the three sampling points were found in the other groups, even if it is important to remark that the results of “Medium packaging use” group could be influenced by the small number of subjects. These results suggest that a few changes in the use of plastic packaging can influence the urinary BPA levels in the children that had intensively used plastic packaging, while no significant changes were found in the “Medium packaging use” group. In the same way, children who rarely consumed food in plastic packaging showed no significant reduction of their BPA levels. These data suggest that BPA urinary levels are strictly related to PFP use. As reported by Heras-Gonzales et al., there is a strong correlation between obesity, lifestyle and diet and exposure to ED chemicals. Several studies reported that the migration of BPA from drink and food packaging, plastic baby bottles, and the coating of cans is associated with obesity, classifying them as obesogens (61). Diet also strongly influences urinary BPA levels and changes in diet are attributable to alterations in urinary BPA levels: the results of the present study suggest that a plastic-free lifestyle may be related to a reduction in urinary BPA levels. In fact, as reported by Carnile and Michels, the vast majority of BPA exposure occurs through the ingestion of contaminated food and drink and metabolization is rapid and complete (55). The dosage of BPA urinary levels represents the prevalent way to determine BPA levels in the human body, not only because it potentially reaches more people without age differentiation (children and adults), but also because it inadvertently occurs over long time periods, almost certainly over one's entire life (62). The data obtained in the present study are in agreement with previous published papers. Nevertheless, it is important to note that these considerations are based on the questionnaire results: this represents a limit for the study, because the answers provided by parents/guardians may not completely reflect the child's BPA exposure. In light of these results, it may be desirable that several countermeasures should be taken in order to develop effective interventions that are feasible in the general population. In this way, each action should be carefully evaluated because it could be ineffective (63). At this regard, Rudel et al. reported that a dietary intervention of 3 days of eating food with limited food packaging was associated with substantial reductions in BPA exposure (64). The statistical reduction of BPA concentration that we found in our pediatric population confirmed these data, showing the effects of a diet regimen with a decrease of food in plastic packaging, demonstrating that there is a connection between urinary BPA levels and PFP. Many literature data also reported that urinary BPA concentrations are associated with behavior problems, suggesting that BPA may predominantly affect the behavior of children rather than their cognitive function (65, 66). In addition, several studies reported the obsogenic effects of BPA, suggesting that BPA may cause lipid accumulation and promote the differentiation of pre-adipose cells via the peroxisome proliferator-activated receptor-γ (PPARG) pathway (67). It has also been reported that BPA increased adipogenic markers in Murine 3T3-L1 preadipocytes. Moreover, in children, BPA levels were significantly correlated with insulin resistance, albuminuria, and irregular vascular function (68).
This study has several strengths: the urine samples were obtained in homogenous groups following both age and sociodemographic criteria. Indeed, several previous studies reported that children or adolescents also have decreased urinary BPA concentrations with increasing age (69–71). Other studies reported that sociodemographic factors such as race, education, and household income may influence childhood exposure to BPA (37, 72). Moreover, the BPA urinary levels were evaluated using the creatinine-standardized BPA concentrations (73).
It is important to note that this study has several limitations. The urinary BPA concentrations were tested through ELISA-test. Even if a series of highly sensitive ELISA tests in direct and indirect assay formats with high specificity has been developed (74), the high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) method remains the best method (75); however, the ELISA-test represents a reliable, cheaper, and faster quantitative test; moreover, it is available in all laboratories and does not require specialized personnel. Moreover, the data about the use of plastic packaging were obtained through a questionnaire: this can be considered an inherent limitation because the answers provided by parents/guardians may not reflect the child's real exposure; furthermore, even if the same questionnaire had been adopted for a previous national study, the BPA exposure values might be influenced by the scoring system. Moreover, analyzing the data about the packaging use criteria the results of the “Medium packaging use” group could be influenced by the small number of the subjects I this group (8/65). Finally, the data about the BMI criteria discussed in the present study could be influenced by the small number of children in the “obese” group (8/65).
Conclusions
The most recent studies, both in vitro and in vivo, demonstrate that human exposure to BPA is related to several adverse effects, particularly in the first period of life. It is commonly ingested involuntarily through diet, both drinking and eating substances packaged in plastic containers. Even if further research is needed, the results of the present study suggest that a substantial reduction in the use of PFP directly correlates with a decrease of urinary BPA concentration and an active lifestyle. The idea that increasing awareness of how health and human safety could be linked to environmental conditions and chemical compound exposure represents undoubtful an important concept to develop future studies and applications. Particularly, in the new concept to life transitioning toward a plastic-free world.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Riuniti Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
FS and GM: conceptualization and project administration. FS, RP, VM, MM, and GM: methodology and resources. FS, RP, AS, MS, MC, GC, AV, MPM, and GM: software, formal analysis, and investigation. FS, RP, VM, AC, MM, and GM: validation. FS and RP: data curation. FS, RP, and GM: writing—original draft preparation and writing—review and editing. MM and GM: supervision. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research has been inspired by the Plastic Free Challenge, conceived by the Italian Ministry for Environment, Dr. Sergio Costa. It has been realized after the protocol signed in 2018 by the Rector of the University of Foggia, Prof. Maurizio Ricci and the Mayor of Biccari Dr. Gianfilippo Mignogna. We wish to thank the Municipality of Biccari, the headmaster of the school, Rosa Manella, and all those involved who contributed to this research project. Moreover, we wish to thank the Scientific Bureau of the University of Catania for language support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2021.626070/full#supplementary-material
References
1. Goodman RA, Bunnell R, Posner SF. What is “community health”? Examining the meaning of an evolving field in public health. Prev Med (Baltim). (2014) 67 (Suppl 1):S58–61. doi: 10.1016/j.ypmed.2014.07.028
2. Singla VI, Sutton PM, Woodruff TJ. The environmental protection agency toxic substances control act systematic review method may curtail science used to inform policies, with profound implications for public health. Am J Public Health. (2019) 109:982–4. doi: 10.2105/AJPH.2019.305068
3. Maia J, Rodríguez-Bernaldo de Quirós A, Sendón R, Cruz JM, Seiler A, Franz R, et al. Determination of key diffusion and partition parameters and their use in migration modelling of benzophenone from low-density polyethylene (LDPE) into different foodstuffs. Food Addit Contam - Part A Chem Anal Control Expo Risk Assess. (2016) 33:715–24. doi: 10.1080/19440049.2016.1156165
4. Groh KJ, Backhaus T, Carney-Almroth B, Geueke B, Inostroza PA, Lennquist A, et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci Total Environ. (2019) 651:3253–68. doi: 10.1016/j.scitotenv.2018.10.015
5. Hong SH, Shim WJ, Jang M. Chemicals associated with marine plastic debris and microplastics: analyses and contaminant levels. In: Microplastic Contamination in Aquatic Environments. (2018) Chapter 9; 271–315. doi: 10.1016/b978-0-12-813747-5.00009-6. Available online at: https://www.sciencedirect.com/book/9780128137475/microplastic-contamination-in-aquatic-environments?via=ihub=#book-description
6. Deshwal GK, Panjagari NR, Alam T. An overview of paper and paper based food packaging materials: health safety and environmental concerns. J Food Sci Technol. (2019) 56:4391–403. doi: 10.1007/s13197-019-03950-z
7. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocr Rev. (2009) 30:293–342. doi: 10.1210/er.2009-0002
8. European Commission. Commission Regulation (EU) N° 10/2011. 14 January 2011 Plast Mater Artic Intend to Come Into Contact With Food Off J Eur Union. Bruxelles (2011) 1–89.
9. Chin KY, Pang KL, Mark-Lee WF. A review on the effects of bisphenol a and its derivatives on skeletal health. Int J Med Sci. (2018) 15:1043–50. doi: 10.7150/ijms.25634
10. Parisi L, Faraldo M, Ruberto M, Salerno M, Maltese A, Folco A Di, et al. Life events and primary monosymptomatic nocturnal enuresis: a pediatric pilot study. Acta Medica Mediterr. (2017) 33:23–27. doi: 10.19193/0393-6384_2017_1_003
11. Andújar N, Gálvez-Ontiveros Y, Zafra-Gómez A, Rodrigo L, Álvarez-Cubero MJ, Aguilera M, Monteagudo C, et al. Bisphenol A analogues in food and their hormonal and obesogenic effects: a review. Nutrients. (2019) 11:2136. doi: 10.3390/nu11092136
12. Maffini MV, Rubin BS, Sonnenschein C, Soto AM. Endocrine disruptors and reproductive health: The case of bisphenol-A. Mol Cell Endocrinol. (2006) 254–55:179–86. doi: 10.1016/j.mce.2006.04.033
13. Pomara C, Neri M, Bello S, Fiore C, Riezzo I, Turillazzi E. Neurotoxicity by synthetic androgen steroids: oxidative stress, apoptosis, and neuropathology: a review. Curr Neuropharmacol. (2014) 13:132–45. doi: 10.2174/1570159x13666141210221434
14. Bertozzi G, Sessa F, Albano GD, Sani G, Maglietta F, Roshan MHK, et al. The role of anabolic androgenic steroids in disruption of the physiological function in discrete areas of the central nervous system. Mol Neurobiol. (2017) 55:5548–56. doi: 10.1007/s12035-017-0774-1
15. Andrianou XD, Gangler S, Piciu A, Charisiadis P, Zira C, Aristidou K, et al. Human exposures to Bisphenol A, Bisphenol F and Chlorinated Bisphenol a derivatives and thyroid function. PLoS ONE. (2016) 11:e0155237. doi: 10.1371/journal.pone.0155237
16. Charisiadis P, Andrianou XD, Van Der Meer TP, Den Dunnen WFA, Swaab DF, Wolffenbuttel BHR, et al. Possible obesogenic effects of bisphenols accumulation in the human brain. Sci Rep. (2018) 8:8186.doi: 10.1038/s41598-018-26498-y
17. Rasier G, Toppari J, Parent AS, Bourguignon JP. Female sexual maturation and reproduction after prepubertal exposure to estrogens and endocrine disrupting chemicals: a review of rodent and human data. Mol Cell Endocrinol. (2006) 254-255:187–201. doi: 10.1016/j.mce.2006.04.002
18. Fineschi V, Neri M, Di Donato S, Pomara C, Riezzo I, Turillazzi E. An immunohistochemical study in a fatality due to ovarian hyperstimulation syndrome. Int J Legal Med. (2006) 120:293–9. doi: 10.1007/s00414-006-0104-z
19. Keri RA, Ho SM, Hunt PA, Knudsen KE, Soto AM, Prins GS. An evaluation of evidence for the carcinogenic activity of bisphenol A. Reprod Toxicol. (2007) 24:240–52. doi: 10.1016/j.reprotox.2007.06.008
20. Ledda C, Loreto C, Zammit C, Marconi A, Fago L, Matera S, et al. Non-infective occupational risk factors for hepatocellular carcinoma: a review (Review). Mol Med Rep. (2017) 15:511–33. doi: 10.3892/mmr.2016.6046
21. Monda V, Salerno M, Sessa F, Bernardini R, Valenzano A, Marsala G, et al. Functional changes of orexinergic reaction to psychoactive substances. Mol Neurobiol. (2018) 55:6362–8. doi: 10.1007/s12035-017-0865-z
22. Monda V, Salerno M, Fiorenzo M, Villano I, Viggiano A, Sessa F, et al. Role of sex hormones in the control of vegetative and metabolic functions of middle-aged women. Front Physiol. (2017) 8:773. doi: 10.3389/fphys.2017.00773
23. Rezg R, El-Fazaa S, Gharbi N, Mornagui B. Bisphenol A and human chronic diseases: current evidences, possible mechanisms, and future perspectives. Environ Int. (2014) 64:83–90. doi: 10.1016/j.envint.2013.12.007
24. Sessa F, Anna V, Messina G, Cibelli G, Monda V, Marsala G, et al. Heart rate variability as predictive factor for sudden cardiac death. Aging (Albany NY). (2018) 10:166–77. doi: 10.18632/aging.101386
25. Baillie-Hamilton PF. Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med. (2002) 8:185–92. doi: 10.1089/107555302317371479
26. Mazzeo F, Motti ML, Messina G, Monda V, Ascione A, Tafuri D, et al. Use of nutritional supplements among south Italian students of physical training and sport university. Curr Top Toxicol. (2013) 9:21–26.
27. Cavaliere G, Viggiano E, Trinchese G, De Filippo C, Messina A, Monda V, et al. Long feeding high-fat diet induces hypothalamic oxidative stress and inflammation, and prolonged hypothalamic AMPK activation in rat animal model. Front Physiol. (2018) 9:818. doi: 10.3389/fphys.2018.00818
28. Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect. (2008) 116:1642–7. doi: 10.1289/ehp.11537
29. Ben-Jonathan N, Hugo ER, Brandebourg TD. Effects of bisphenol A on adipokine release from human adipose tissue: implications for the metabolic syndrome. Mol Cell Endocrinol. (2009) 304:49–54. doi: 10.1016/j.mce.2009.02.022
30. Sperandeo R, Maldonato MN, Messina A, Cozzolino P, Monda M, Cerroni F, et al. Orexin system: network multi-tasking. Acta Medica Mediterr. (2018) 34:349–56. doi: 10.19193/0393-6384_2018_2_55
31. Bukvic N, Delli Carri V, Di Cosola ML, Pustorino G, Cesarano C, Chetta M, et al. Familial X;Y translocation with distinct phenotypic consequences: characterization using FISH and array CGH. Am J Med Genet Part A. (2010) 152:1730–1734. doi: 10.1002/ajmg.a.33437
32. Santacroce R, Santoro R, Sessa F, Iannaccaro P, Sarno M, Longo V, et al. Screening of mutations of hemophilia A in 40 Italian patients: a novel G-to-A mutation in intron 10 of the F8 gene as a putative cause of mild hemophilia a in southern Italy. Blood Coagul Fibrinolysis. (2008) 19:197–202. doi: 10.1097/MBC.0b013e3282f234ab
33. Menale C, Piccolo MT, Cirillo G, Calogero RA, Papparella A, Mita L, et al. Bisphenol A effects on gene expression in adipocytes from children: association with metabolic disorders. J Mol Endocrinol. (2015) 54:289–303. doi: 10.1530/JME-14-0282
34. Andra SS, Charisiadis P, Arora M, van Vliet-Ostaptchouk JV, Makris KC. Biomonitoring of human exposures to chlorinated derivatives and structural analogs of bisphenol A. Environ Int. (2015) 85:352–79. doi: 10.1016/j.envint.2015.09.011
35. Centers for Disease Control and Prevention. MEC In-Person Dietary Interviewers Procedures Manual. (2017) Available online at: https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_dietarymec.pdf.
36. ANCOS. National Study “Manteniamo il Passso”—Survey. Torino (2017). Available online at: https://www.ancos.it/progetti/manteniamo-il-passo.
37. Stacy SL, Eliot M, Calafat AM, Chen A, Lanphear BP, Hauser R, et al. Patterns, variability, and predictors of urinary bisphenol a concentrations during childhood. Environ Sci Technol. (2016) 50:5981–90. doi: 10.1021/acs.est.6b00794
38. Arvanitoyannis IS, Bosnea L. Migration of substances from food packaging materials to foods. Crit Rev Food Sci Nutr. (2004) 44:63–76. doi: 10.1080/10408690490424621
39. Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P. An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater. (2018) 344:179–99. doi: 10.1016/j.jhazmat.2017.10.014
40. Bhunia K, Sablani SS, Tang J, Rasco B. Migration of chemical compounds from packaging polymers during microwave, conventional heat treatment, and storage. Compr Rev Food Sci Food Saf. (2013) 12:523–45. doi: 10.1111/1541-4337.12028
41. Makris KC, Andra SS, Jia A, Herrick L, Christophi CA, Snyder SA, et al. Association between water consumption from polycarbonate containers and bisphenol a intake during harsh environmental conditions in summer. Environ Sci Technol. (2013) 47:3333–43. doi: 10.1021/es304038k
42. Zawatski W, Lee MM. Male pubertal development: are endocrine-disrupting compounds shifting the norms? J Endocrinol. (2013) 218:R1–12. doi: 10.1530/JOE-12-0449
43. Giulivo M, Lopez de Alda M, Capri E, Barceló D. Human exposure to endocrine disrupting compounds: their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ Res. (2016) 151:251–64. doi: 10.1016/j.envres.2016.07.011
44. De Giglio O, Quaranta A, Lovero G, Caggiano G, Montagna MT. Mineral water or tap water? An endless debate. Ann Ig. (2015) 27:58–65. doi: 10.7416/ai.2015.2023
45. Maggioni S, Balaguer P, Chiozzotto C, Benfenati E. Screening of endocrine-disrupting phenols, herbicides, steroid estrogens, and estrogenicity in drinking water from the waterworks of 35 Italian cities and from PET-bottled mineral water. Environ Sci Pollut Res. (2013) 20:1649–60. doi: 10.1007/s11356-012-1075-x
46. Bach C, Dauchy X, Chagnon MC, Etienne S. Chemical compounds and toxicological assessments of drinking water stored in polyethylene terephthalate (PET) bottles: a source of controversy reviewed. Water Res. (2012) 46:571–83. doi: 10.1016/j.watres.2011.11.062
47. Bach C, Dauchy X, Severin I, Munoz JF, Etienne S, Chagnon MC. Effect of temperature on the release of intentionally and non-intentionally added substances from polyethylene terephthalate (PET) bottles into water: chemical analysis and potential toxicity. Food Chem. (2013) 139:672–80. doi: 10.1016/j.foodchem.2013.01.046
48. Bach C, Dauchy X, Severin I, Munoz JF, Etienne S, Chagnon MC. Effect of sunlight exposure on the release of intentionally and/or non-intentionally added substances from polyethylene terephthalate (PET) bottles into water: chemical analysis and in vitro toxicity. Food Chem. (2014) 162:63–71. doi: 10.1016/j.foodchem.2014.04.020
49. Le HH, Carlson EM, Chua JP, Belcher SM. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol Lett. (2008) 176:149–56. doi: 10.1016/j.toxlet.2007.11.001
50. Fernandez MF, Arrebola JP, Taoufiki J, Navalón A, Ballesteros O, Pulgar R, et al. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod Toxicol. (2007) 24:259–64. doi: 10.1016/j.reprotox.2007.06.007
51. Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S, et al. Perinatal exposure to bisphenol A alters early adipogenesis in the rat. Environ Health Perspect. (2009) 117:1549–55. doi: 10.1289/ehp.11342
52. Cao J, Rebuli ME, Rogers J, Todd KL, Leyrer SM, Ferguson SA, et al. Prenatal bisphenol a exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol Sci. (2013) 133:157–73. doi: 10.1093/toxsci/kft035
53. McCaffrey KA, Jones B, Mabrey N, Weiss B, Swan SH, Patisaul HB. Sex specific impact of perinatal bisphenol A (BPA) exposure over a range of orally administered doses on rat hypothalamic sexual differentiation. Neurotoxicology. (2013) 36:55–62. doi: 10.1016/j.neuro.2013.03.001
54. Lee HA, Kim YJ, Lee H, Gwak HS, Park EA, Cho SJ, et al. Effect of urinary bisphenol a on androgenic hormones and insulin resistance in preadolescent girls:. doi: 10.3390/ijerph10115737
55. Carwile JL, Michels KB. Urinary bisphenol A and obesity: NHANES 2003-2006. Environ Res. (2011) 111:825–30. doi: 10.1016/j.envres.2011.05.014
56. Wang T, Li M, Chen B, Xu M, Xu Y, Huang Y, et al. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J Clin Endocrinol Metab. (2012) 86:506–12. doi: 10.1210/jc.2011-1989
57. Fan Y, Tian C, Liu Q, Zhen X, Zhang H, Zhou L, et al. Preconception paternal bisphenol A exposure induces sex-specific anxiety and depression behaviors in adult rats. PLoS ONE. (2018) 13:e0192434. doi: 10.1371/journal.pone.0192434
58. Petito A, Altamura M, Iuso S, Padalino FA, Sessa F, D'Andrea G, et al. The relationship between personality traits, the 5HTT polymorphisms, and the occurrence of anxiety and depressive symptoms in elite athletes. PLoS ONE. (2016) 11:e0156601. doi: 10.1371/journal.pone.0156601
59. Altamura M, Iuso S, D'andrea G, D'urso F, Piccininni C, Angelini E, et al. Maladaptive coping strategies and neuroticism mediate the relationship between 5HTT-LPR polymorphisms and symptoms of anxiety in elite athletes. Clin Neuropsychiatry. (2019) 16:62–71 doi: 10.1101/493320
60. van der Meer TP, Thio CHL, van Faassen M, van Beek AP, Snieder H, van Berkum FNR, et al. Endocrine disrupting chemicals during diet-induced weight loss – a post-hoc analysis of the LOWER study. Environ Res. (2020) 192:110262. doi: 10.1016/j.envres.2020.110262
61. Heras-González L, Latorre JA, Martinez-Bebia M, Espino D, Olea-Serrano F, Mariscal-Arcas M. The relationship of obesity with lifestyle and dietary exposure to endocrine-disrupting chemicals. Food Chem Toxicol. (2020) 136:110983. doi: 10.1016/j.fct.2019.110983
62. Almeida S, Raposo A, Almeida-González M, Carrascosa C. Bisphenol a: food exposure and impact on human health. Compr Rev Food Sci Food Saf. (2018) 17:1503–17. doi: 10.1111/1541-4337.12388
63. Sathyanarayana S, Alcedo G, Saelens BE, Zhou C, Dills RL, Yu J, et al. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures. J Expo Sci Environ Epidemiol. (2013) 23:378–84. doi: 10.1038/jes.2013.9
64. Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, et al. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect. (2011) 119:914–20. doi: 10.1289/ehp.1003170
65. Ejaredar M, Lee Y, Roberts DJ, Sauve R, Dewey D. Bisphenol A exposure and children's behavior: a systematic review. J Expo Sci Environ Epidemiol. (2017) 27:175–83. doi: 10.1038/jes.2016.8
66. Grohs MN, Reynolds JE, Liu J, Martin JW, Pollock T, Lebel C, et al. Prenatal maternal and childhood bisphenol a exposure and brain structure and behavior of young children. Environ Heal A Glob Access Sci Source. (2019) 18:85. doi: 10.1186/s12940-019-0528-9
67. Thoene M, Dzika E, Gonkowski S, Wojtkiewicz J. Bisphenol S in food causes hormonal and obesogenic effects comparable to or worse than bisphenol a: A literature review. Nutrients. (2020) 12:532. doi: 10.3390/nu12020532
68. Kataria A, Levine D, Wertenteil S, Vento S, Xue J, Rajendiran K, et al. Exposure to bisphenols and phthalates and association with oxidant stress, insulin resistance, and endothelial dysfunction in children. Pediatr Res. (2017) 81:857–64. doi: 10.1038/pr.2017.16
69. Frederiksen H, Aksglaede L, Sorensen K, Nielsen O, Main KM, Skakkebaek NE, et al. Bisphenol A and other phenols in urine from Danish children and adolescents analyzed by isotope diluted TURBOFLOW-LC-MS/MS. Int J Hyg Environ Health. (2013) 216:710–20. doi: 10.1016/j.ijheh.2013.01.007
70. Hoepner LA, Whyatt RM, Just AC, Calafat AM, Perera FP, Rundle AG. Urinary concentrations of bisphenol A in an urban minority birth cohort in New York City, prenatal through age 7 years. Environ Res. (2013) 122:38–44. doi: 10.1016/j.envres.2012.12.003
71. Larsson K, Ljung Björklund K, Palm B, Wennberg M, Kaj L, Lindh CH, et al. Exposure determinants of phthalates, parabens, bisphenol A and triclosan in Swedish mothers and their children. Environ Int. (2014) 73:323–33. doi: 10.1016/j.envint.2014.08.014
72. Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to Bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. (2008) 116:39–44. doi: 10.1289/ehp.10753
73. Watkins DJ, Eliot M, Sathyanarayana S, Calafat AM, Yolton K, Lanphear BP, et al. Variability and predictors of urinary concentrations of phthalate metabolites during early childhood. Environ Sci Technol. (2014) 48:8881–90. doi: 10.1021/es501744v
74. Lu Y, Peterson JR, Gooding JJ, Lee NA. Development of sensitive direct and indirect enzyme-linked immunosorbent assays (ELISAs) for monitoring bisphenol-A in canned foods and beverages. Anal Bioanal Chem. (2012) 403:1607–18. doi: 10.1007/s00216-012-5969-8
Keywords: bisphenol A (BPA), urinary BPA concentration, pediatric population, eating behavior, endocrine disruptors (EDs), plastic food packaging (PFP)
Citation: Sessa F, Polito R, Monda V, Scarinci A, Salerno M, Carotenuto M, Cibelli G, Valenzano A, Campanozzi A, Mollica MP, Monda M and Messina G (2021) Effects of a Plastic-Free Lifestyle on Urinary Bisphenol A Levels in School-Aged Children of Southern Italy: A Pilot Study. Front. Public Health 9:626070. doi: 10.3389/fpubh.2021.626070
Received: 05 November 2020; Accepted: 05 January 2021;
Published: 01 February 2021.
Edited by:
Oliver Robinson, Imperial College London, United KingdomReviewed by:
Konstantinos C. Makris, Cyprus University of Technology, CyprusCinzia La Rocca, National Institute of Health (ISS), Italy
Copyright © 2021 Sessa, Polito, Monda, Scarinci, Salerno, Carotenuto, Cibelli, Valenzano, Campanozzi, Mollica, Monda and Messina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanni Messina, giovanni.messina@unifg.it; Francesco Sessa, francesco.sessa@unifg.it
 Francesco Sessa
Francesco Sessa Rita Polito1,2
Rita Polito1,2 Vincenzo Monda
Vincenzo Monda Marco Carotenuto
Marco Carotenuto Giuseppe Cibelli
Giuseppe Cibelli Anna Valenzano
Anna Valenzano Angelo Campanozzi
Angelo Campanozzi Marcellino Monda
Marcellino Monda Giovanni Messina
Giovanni Messina