- 1Department of Dermatology, First Affiliated Hospital of Kunming Medical University, Kunming, China
- 2The Centre for Modern Chinese City Studies, East China Normal University, Shanghai, China
- 3Faculty of Land Resources Engineering, Kunming University of Science and Technology, Kunming, China
- 4Faculty of Environmental Science and Engineering, Kunming University of Science and Technology, Kunming, China
- 5Yunnan Institute of Skin Health, Kunming, China
Background: Sensitive skin (SS) is a condition characterized by hyperreactivity. Impacting around 37 percent of the worldwide population and exerting an influence on the quality of life for affected individuals. Its prevalence rate has increased due to factors such as elevating stress levels and deteriorating environmental conditions. The exposome factors influencing SS have extended from demographic, biological attributes, and lifestyle to external environments. Built environments (BEs) have demonstrated as root drivers for changes in behaviors and environmental exposure which have the potential to trigger SS, but the review of the associations between BEs and SS is currently lacking.
Objective: This review aims to achieve two primary objectives: (1) Examine exposome factors that exert influence on SS at the individual and environmental levels. (2) Develop a theoretical framework that establishes a connection between BEs and SS, thereby offering valuable insights into the impact of the built environment on this condition.
Methods: An extensive literature search was carried out across multiple fields, including sociology, epidemiology, basic medicine, clinical medicine, and environmental research, with a focus on SS. To identify pertinent references, renowned databases such as PubMed, Web of Science, and CNKI were utilized.
Results: SS is the outcome of interactions between individual attributes and environmental factors. These influencing factors can be categorized into five distinct classes: (1) demographic and socioeconomic characteristics including age, gender, and race; (2) physiological and biological attributes such as emotional changes, skin types, sleep disorders, and menstrual cycles in women; (3) behavioral factors, such as spicy diet, cosmetic use, alcohol consumption, and physical exercise; (4) natural environmental features, including climate conditions and air pollution; (5) built environmental features such as population density, green space availability, road network density, and access to public transportation, also have the potential to affect the condition.
Conclusion: The importance of interdisciplinary integration lies in its ability to ascertain whether and how BEs are impacting SS. By elucidating the role of BEs in conjunction with other factors in the onset of SS, we can provide guidance for future research endeavors and the formulation of interventions aimed at mitigating the prevalence of SS.
1 Introduction
According to the World Health Organization’s projections, chronic non-communicable diseases (NCDs) are anticipated to account for a staggering 80% of annually global deaths by 2030 (1). This shift signifies a remarkable transition, with NCDs superseding infectious diseases as the primary drivers of the overall disease burden (2). Conditions such as obesity, cardiovascular diseases, and respiratory ailments are at the forefront of this transformative landscape.
Skin disease stands as one of the most prevalent human ailments (3), and many of them are also NCDs. Notably, an analysis conducted for the Global Burden of Disease Study in 2017 revealed that the rising population growth and aging demographics in China have led to a substantial disease burden associated with skin conditions (4). Among these conditions, sensitive skin (SS) represents a commonly encountered clinical symptom and sign, primarily manifesting on the facial region. When exposed to physical, chemical, and psychological stimuli, individuals often experience subjective sensations such as burning, tingling, itching, and tightness. Objective signs such as erythema, scales, and telangiectasia may or may not accompany these symptoms (5). In 2016, the International Pruritus Research Forum defined SS as the unpleasant sensation triggered by external stimuli that typically do not elicit skin symptoms (6). The etiology of SS remains elusive, potentially involving sensory nerve dysfunction (7), heightened vascular reactivity (8), barrier impairments (9), and immune-inflammatory mechanisms of the skin (10). Additionally, the surge in SS cases is associated with environmental pollution and unhealthy lifestyles. Globally, the prevalence of SS is estimated at 36.9% (11), with some regions reporting prevalence rate as high as 50–70%. The presence of SS may negatively impact the quality of life for affected individuals (12). Lower self-confidence, mental disorders (13) and the economic burdens resulting from its high prevalence and recurrent nature impose substantial hardships on patients.
The interconnection between health and the environment has garnered increasing attention, particularly in the realm of public health research concerning the built environments (BEs). The BEs encompass the man-made spaces in which individuals engage in daily activities, work and leisure (14). Empirical studies have extensively linked BEs to a range of health-related behaviors (15, 16), mental conditions (17, 18), NCDs such as obesity (19), cardiovascular disease (20), and respiratory disease (21). Despite the growing body of research, the relationship between the BE and SS remains understudied. Previous epidemiological investigations on SS predominantly focus on individual sociology and lifestyles of the population, including age, gender, dietary habits and cosmetic Usage as well as natural environmental stressors such as sun exposure, air pollution and temperature (22, 23). This review aims to address the aforementioned research gap by providing a comprehensive profile of the influencing factors at individual and environmental levels. Furthermore, this study also puts forward a theoretical framework to elucidate the potential pathways linking the BEs to SS based on the established mechanisms that underpin the associations between BEs and health (24, 25).
2 Search strategies and selection of studies
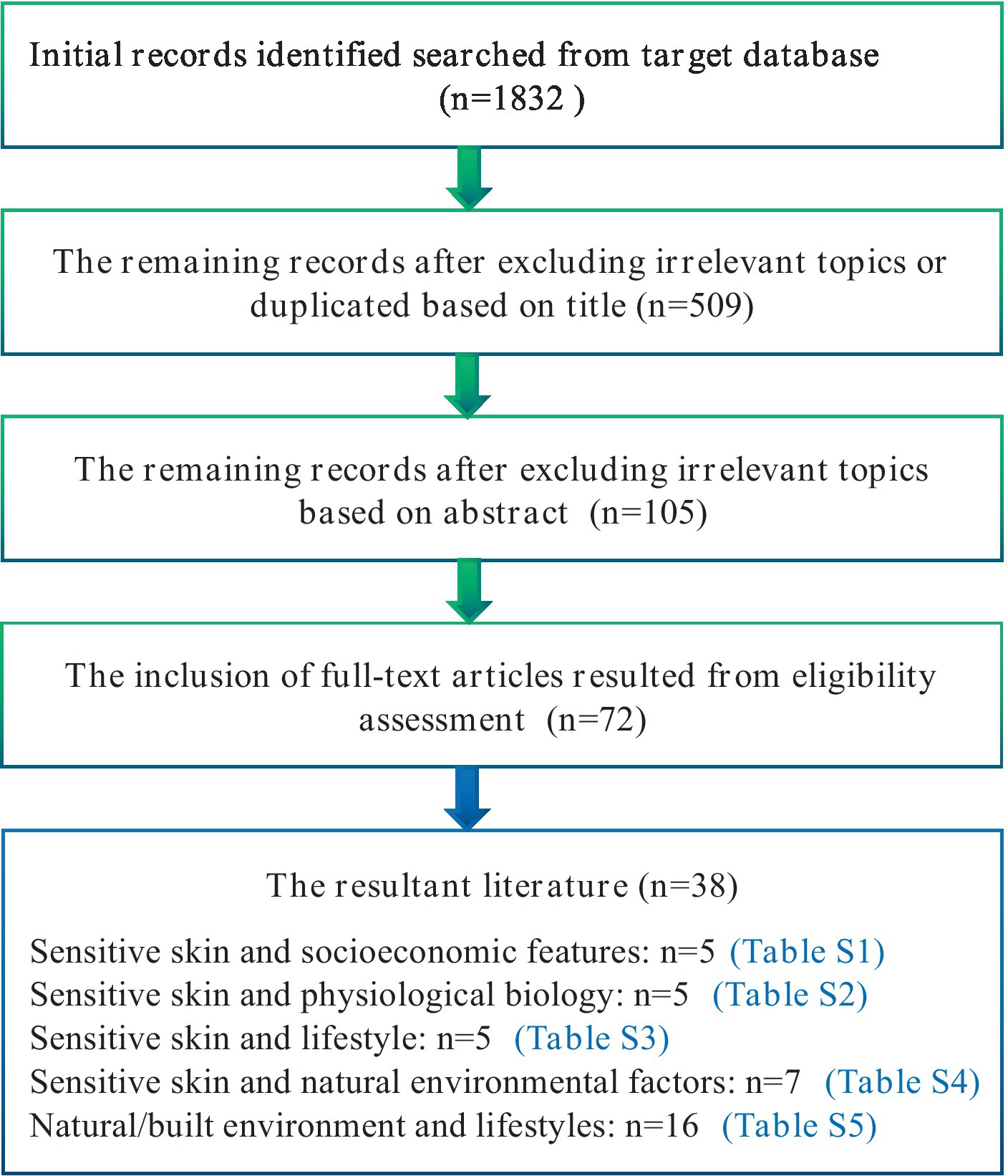
We conducted systematic searches in reputable databases, including PubMed, Web of Science, and CNKI (China National Knowledge Infrastructure), to identify relevant references published since 1997. The search strategy employed multiple combinations of the terms “sensitive skin” within the title/abstract, encompassing epidemiology, prevalence, socioeconomics, gender, ethnicity, skin type, emotional change, menstrual cycle, sleep, diet, spicy food, cosmetics, smoking, drinking, exposure, sun exposure, temperature, humidity, particles, pollution and the built environment. Our literature review did not uncover any studies directly addressing the built environment and SS. However, we did find evidence suggesting that the occurrence of SS is associated with exposure to natural environment, mental conditions and patterns of sleep and diet, all of which have been linked with BEs. Additionally, we checked the references cited in the identified papers to ensure the inclusion of all pertinent literature. The evaluation process involved assessing the title and abstract against our inclusion criteria. Subsequently, a full-text review was conducted to determine if the article met our standards. Inclusion criteria comprised studies written in English that focused on the epidemiology of SS. Studies that did not specifically focus on SS were excluded. The flowchart (Figure 1) depicted the process for the inclusion and exclusion of literature.
As illustrated in Figure 1, our literature review did not uncover any studies directly addressing the built environment and SS. However, we did find evidence suggesting that the occurrence of SS is associated with exposure to natural environment, mental conditions and patterns of sleep and diet, all of which have been linked with BEs. As a result, the scope of our search was broadened to incorporate the term “built environment” in conjunction with factors such as air pollution, sun exposure, mental conditions such as anxiety and depression, and lifestyle elements like sleep and diet (Figure 1). The influencing factors can be classified into five categories: (I) demographic and socioeconomic attributes; (II) physiological and biological characteristics; (III) lifestyles; (IV) natural environmental features; (V) built environmental features.
3 Demographic and socioeconomic attributes
3.1 Age
Research indicates a higher prevalence of SS in the young group than their elder counterparts. Notably, a French epidemiological survey comprising 5,000 individuals revealed that individuals under the age of 35 exhibit a higher prevalence of SS, surpassing 60% of the population within this age range (26). Similarly, a survey conducted in South Korea found that the younger group ranging from 15 to 44 years old displays a greater likelihood of developing SS compared to individuals over the age of 45 (27). Correspondingly, another study involving 22,085 women in China unveiled that the younger group, specifically those aged 20 to 30, is more susceptible to sensitive or highly SS in comparison to the elder group, aged 30 and above (28). These findings suggest a plausible mechanism wherein the nerve innervation function in the human body, related to touch and pain, diminishes as individual age (29).
3.2 Gender
Extensive empirical studies have consistently shown that women are more prone to SS than men. Notably, a study based on 3,012 participants in India found that the proportion of women experiencing “sensitive” or “very sensitive” skin is significantly higher (36.7%) compared to the men (27.9%) (30). These findings are consistent with multiple investigations conducted domestically and internationally, which consistently demonstrated that women are more likely to develop SS than men (23, 31, 32). One plausible explanation for this disparity could be the significant differences in epidermis and dermis thickness between males and females (33), with males exhibiting a greater thickness. Consequently, the skin of males is less permeable than that of females, making it less susceptible to the effects of irritants or allergens. Additionally, the periodic hormonal fluctuations experienced by women contribute to increased SS (34).
3.3 Race
While no significant racial differences were observed in the prevalence of SS, variances in sensory perception among different races have been documented. For instance, European Americans tend to exhibit heightened sensitivity to wind, Asians are more sensitive to spicy food, and Hispanics display relatively lower responsiveness to alcohol (35). There are also reports suggesting that African Americans have a higher incidence of SS compared to European Americans (36). As human skin exhibits individual differences, further parallel studies are required to ascertain the true racial disparities in SS (37).
3.4 Occupation
Medical personnel wear masks for extended durations while on duty, and studies have documented skin-related symptoms, such as redness and itching, associated with mask-wearing among medical staff (38). However, a survey revealed a prevalence of SS found that there was no significant difference between the socio-professional categories in SS (39). Hence, further research is required to shed light on the influence of occupation on SS.
4 Physiological and biological characteristics
4.1 Emotional change
Emotional change has been identified as a contributing factor to the development of SS (32, 34). Individuals with SS are more likely to experience discomfort, such as tingling and itching, during the periods of emotional fluctuation compared to individuals without SS (27). Moreover, there is a significant correlation between the occurrence of SS and anxiety, suggesting that anxiety may play a role in the manifestation of SS symptoms (13). Additionally, depression has been associated with SS, where facial flushing is recognized as the primary clinical manifestation (31).
4.2 Skin type
Compared to neutral skin, dry, oily, and mixed skin exhibit varying degrees of damage to the skin barrier function, rendering them more susceptible to SS and inflammatory skin diseases (40, 41). Epidemiological studies have further confirmed that these skin types are risk factors for SS diseases (28, 42).
4.3 Sleep disorder
Individuals with poor sleep quality exhibit an increase in transepidermal water loss (TEWL) compared to those with good sleep quality, consequently leading to the damage of skin barrier (43). Sleep disorders contribute to an elevation in oxidative stress, resulting in alterations in skin homeostasis and the disruption of inflammatory pathways, ultimately leading to the dysfunction of the skin barrier (44, 45). Impaired skin barrier function and reduced water content in the stratum corneum are significant pathophysiological characteristics observed in individuals with SS (46). An epidemiological survey conducted across multiple countries, including Brazil, China, France, Russia and the United States, found a higher prevalence of SS among subjects with sleep disorders (47).
4.4 Menstrual cycle for female
The presence of estrogen receptors in the epidermis impacts skin hydration and the functionality of skin barrier, as estrogen levels directly influence these factors (48). Muizzuddin’s research demonstrated that the strength and dryness of skin barrier, as well as the sensitivity to lactic acid stinging, vary in response to fluctuations in estrogen levels during the menstrual cycle (49). A questionnaire survey involving Dutch women revealed that approximately 42% of premenopausal women experienced an increased sense of SS prior to and during the menstrual cycle (34).
5 Lifestyles
5.1 Spicy food
Capsaicin, a biologically active component found abundantly in peppers, interacts with the Capsaicin receptor, a transmembrane channel protein widely expressed in skin tissue (50). The activation of the Capsaicin receptor, known as TRPV1, stimulates the release of neuropeptides and excitatory amino acids from free nerve endings, ultimately leading to the sensation of pain in the cerebral cortex (51). Studies have revealed that individuals of Asian descent exhibit increased susceptibility to SS when exposed to the stimulation of a spicy diet (35). A survey conducted among college students in China further confirmed that a spicy diet serves as a risk factor for SS (32). Moreover, experimental evidence has demonstrated that inhibiting the expression of TRPV1 can be effective in treating SS (52).
5.2 Cosmetic usage
Research indicates a positive correlation between the frequency of cosmetic usage and the prevalence of SS (53). Specifically, when women utilize multiple types of cosmetics and makeup removers on a daily basis, it can easily trigger SS (28). Brenaut carried out a meta-analysis of 13 surveys, which collectively identified cosmetics as a common trigger for SS, and excessive or improper usage exacerbating this reaction (54). One potential explanation for this phenomenon is that frequent and simultaneous use of cosmetics may thin the stratum corneum on free ending is compromised, leading to a significant increase in nerve sensation input and signal release, resulting in uncomfortable sensations (55). Additionally, a thinner stratum corneum can enhance the penetration of capsaicin, thereby increasing SS (56).
5.3 Smoking
Pavithra carried out a proteomic analysis on primary human keratinocytes exposed to long-term cigarette smoke condensate, revealing that the differentially expressed proteins were predominantly associated with the integrity of the epithelial barrier and the anti-inflammatory response (57). Moreover, excessive smoking over 20 cigarettes per day for at least 2 years can disrupt the homeostasis of the epidermal permeability barrier (58). Studies simulating cigarette smoke exposure have further shown that prolonged and/or repeated exposure to high levels of toxic smoke pollutants can impair the function of the epidermal barrier (59). Multiple epidemiological investigations have also confirmed a positive correlation between smoking and the prevalence of SS (60, 61).
5.4 Alcohol consumption
Epidemiological studies have established a link between alcohol consumption and an increase in the prevalence of SS (35). It is believed that the decomposition of ethanol into acetaldehyde induces vasodilation, leading to facial burning and flushing (62, 63).
5.5 Physical exercise
Prior research has demonstrated that various stimuli, including pain sensation, may undergo changes during and after exercise, potentially attributed to the activation of exercise-induced opioid substances (64). Limbs exhibit a decrease in skin thermal sensitivity only after high-intensity exercise (65). The tingling, burning and itching symptoms associated with SS are also connected to sensory nerve dysfunction in the skin (66). In addition, research has found that spending more time exercising is associated with a lower risk of developing depression (67), which potentially reducing susceptibility to SS (31).
6 Environment features
Environmental features encompass natural elements, which are those aspects of the environment existing without significant human modification or intervention. This category comprises variables such as sunlight exposure, temperature and humidity, air pollutions (68).
6.1 Sunshine
A large body of literature has examined the effects of ultraviolet (UV) rays from sunlight on the skin. UV rays are categorized into UVA, UVB, and UVC based on their wavelengths, with UVC being absorbed by the ozone layer (69). UVA primarily impacts the dermis and promotes oxidative damage to DNA, while UVB mainly affects the epidermis (70). Studies have indicated that exposure of human keratinocyte to UVB radiation can disrupt the hydration of stratum corneum (71). Numerous experiments utilizing UVB irradiation on epidermal mice have revealed epidermal barrier dysfunction, including increased epidermal thickness, elevated TEWL, and reduced water content in the stratum corneum (72, 73). Furthermore, UVB radiation induces oxidative damage and triggers inflammatory reactions to keratinocyte on the skin surface (74–76), herein contributing to the development of SS. A survey performed on the Chinese population highlighted the gender and radiation dose-related changes in stratum corneum function caused by ultraviolet radiation (77). Building upon this, Francesca and his collaborators discovered a significant cumulative effect of UV and O3 in reducing skin barrier-related proteins, such as silk fibroin and skin protein (78). Epidemiological investigations have confirmed that sunlight represents a significant factor influencing the prevalence of SS (32, 79, 80).
6.2 Temperature and humidity
Temperatures exceeding 43°C have been shown to directly activate TRPV1 (81). TRPV1 is a specific member of the transient receptor potential (TRP) superfamily of ion channels. Which are found in various tissues throughout the body. TRPV1 leading to the sensation of heat and pain (50). Research has found that the expression of TRPV1 in SS is enhanced, leading to overload of harmful stimuli (82). Therefore, patients are prone to experiencing abnormal heat and pain sensations. In the case of SS, inflammatory mediators can significantly lower the activation threshold of TRPV1 (83). Epidemiological studies have identified changes in temperature and humidity as triggering factors for SS (54, 80). Individuals with SS are particularly sensitive to TEWL and ambient temperature (84). Research has demonstrated that low humidity is more likely to induce SS (23, 54).
6.3 Air pollution
The prevalence of SS has been associated with air pollution (22, 85). Among air pollutants, fine particulate matter (PM2.5) has been extensively studied. PM2.5 exposure in human keratinocytes has been shown to increase Cyclooxygenase2 (COX-2)/Prostaglandin E2 (PGE2) levels, leading to the downregulation of expression of silk fibroin (86, 87). Treatment of human and mouse skin equivalents with PM2.5 has also been found to inhibit the expression of fibronectin and increase TEWL, thus compromising the integrity of the skin barrier (88). Additionally, PM2.5 can disrupt the morphology and structure of keratinocyte, further compromising the skin barrier (89). Previously, it has been found that exposure to nitrogen dioxide (NO2) increases the TEWL value of skin, leading to damage to the skin barrier (90). Low concentration of carbon monoxide (CO) can inhibit the release of tumor necrosis factor-α,interleukin-1β and interleukin-10 reducing inflammatory response, therefore CO may reduce damage to the skin barrier (91), It has been shown that ozone (O3) exposure will lead to various skin inflammation through redox pathway (92), At the same time, O3 therapy can be applied to the clinical treatment of various inflammatory skin (93, 94), but its role in SS needs further research. Air pollution can cause anxiety in people (17), and the prevalence of SS is significantly correlated with anxiety (13).
7 Discussion
Environment encompasses natural environment and built environment, the built environment is defined as encompassing all buildings, spaces, and products that are created or modified by people (14). Factors within this domain include population density, the availability of green spaces, access to food stores, and the connectivity of streets (95).
7.1 Empirical associations between BEs and NCDs
The growing body of evidence supports the notion that the BEs plays a significant role in development of NCDs through changes in environmental exposure and health-related lifestyles. For instance, individuals residing in densely populated communities often face a higher risk of obesity (96). As reported, a densely populated community are likely to emit more air pollutants (97) that contribute to an increase in risk of obesity (98, 99). Moreover, population density has a positive correlation with PM10 concentration (100), and elevated PM10 levels have been shown to compromise lung function (101, 102), consequently impacting cardiovascular health (103). Interestingly, research have indicated that an increase in coverage ratio of greenspaces benefits to lower the prevalence of obesity (104), hypertension and cardiovascular disease (105, 106), as well as reduce the mortality of cardiovascular disease (107, 108). This relationship can be attributable for that urban greenness promotes physical activity (109) and mitigates exposure to PM2.5 (110). A survey conducted in Apulia found the impact of green space use on depressive symptoms, and the mediating role of perceived social support in the association (111). Nighttime artificial light (ALAN) has emerged as a risk factor for physiological functions, with a significant correlation observed between higher levels of nighttime outdoor light and poor sleep patterns (112). Notably, ALAN is likely to reduce sleep duration (113), which is more prevalent in communities with higher poverty levels (114). Moreover, sleep disturbance may play a role in anxiety and depressive disorders (111). Besides, research studies have demonstrated that the exposure to Artificial Light at Night (ALAN) during adolescence could potentially elevate the risk of developing atopic diseases during youth (115). Further research is warranted to substantiate causal relationships, particularly those related to circadian rhythm disorders and endocrine pathways. Additionally, a high density of road intersections increases the likelihood of walking and cycling (116). Utilizing public transportation necessitates walking or cycling to bus transits, resulting in elevated levels of moderate physical activity (117, 118). As observed, physical inactivity in both adults and children elevates the risk of overweight/obesity (119–121). Conversely, engaging in physical activity helps to decrease the prevalence and mortality of cardiovascular disease (122, 123). Furthermore, a higher density of fast food restaurants has been associated with a greater prevalence of obesity (124) due to the residential surrounding food BEs have potentials to alter residents’ dietary behavior and quality (125, 126), thereby affecting their health.
Overall, the BEs exerts an influence on individual health through changes in exposure to environmental hazardous stressors and health-related lifestyles (25). SS, identified as a common clinical symptom, results from various internal and external factors. Given the close relationships between BEs and individuals’ daily routines, it is plausible that BEs may also have potential impacts on SS.
7.2 Theoretical pathways from BEs to SS
The relationship between the BEs and the development of SS has not yet been fully explored. Based on identified pathways from BEs to other NCDs, our study aims to establish a theoretical framework connecting BEs and SS, providing a scientific foundation for future epidemiological investigations, basic research, and clinical studies. As previously mentioned, SS development may be influenced by factors such as population density, the availability of green space, street design, and the food environment, Additionally, natural environmental exposure and individual behaviors serve as mediating pathways that can contribute to human skin inflammation and the emergence of adverse emotions, ultimately culminating in the manifestation of SS. When skin inflammation occurs, a substantial quantity of inflammatory cytokines and chemokines is released, leading to heightened sensitivity of nerve fibers within the epidermis and an increased responsiveness to external stimuli (127, 128). The association between negative emotions, such as anxiety and depression, and SS has primarily been explored through epidemiological surveys, which have demonstrated a positive correlation. However, there remains a dearth of experimental research evidence on this subject (Figure 2).
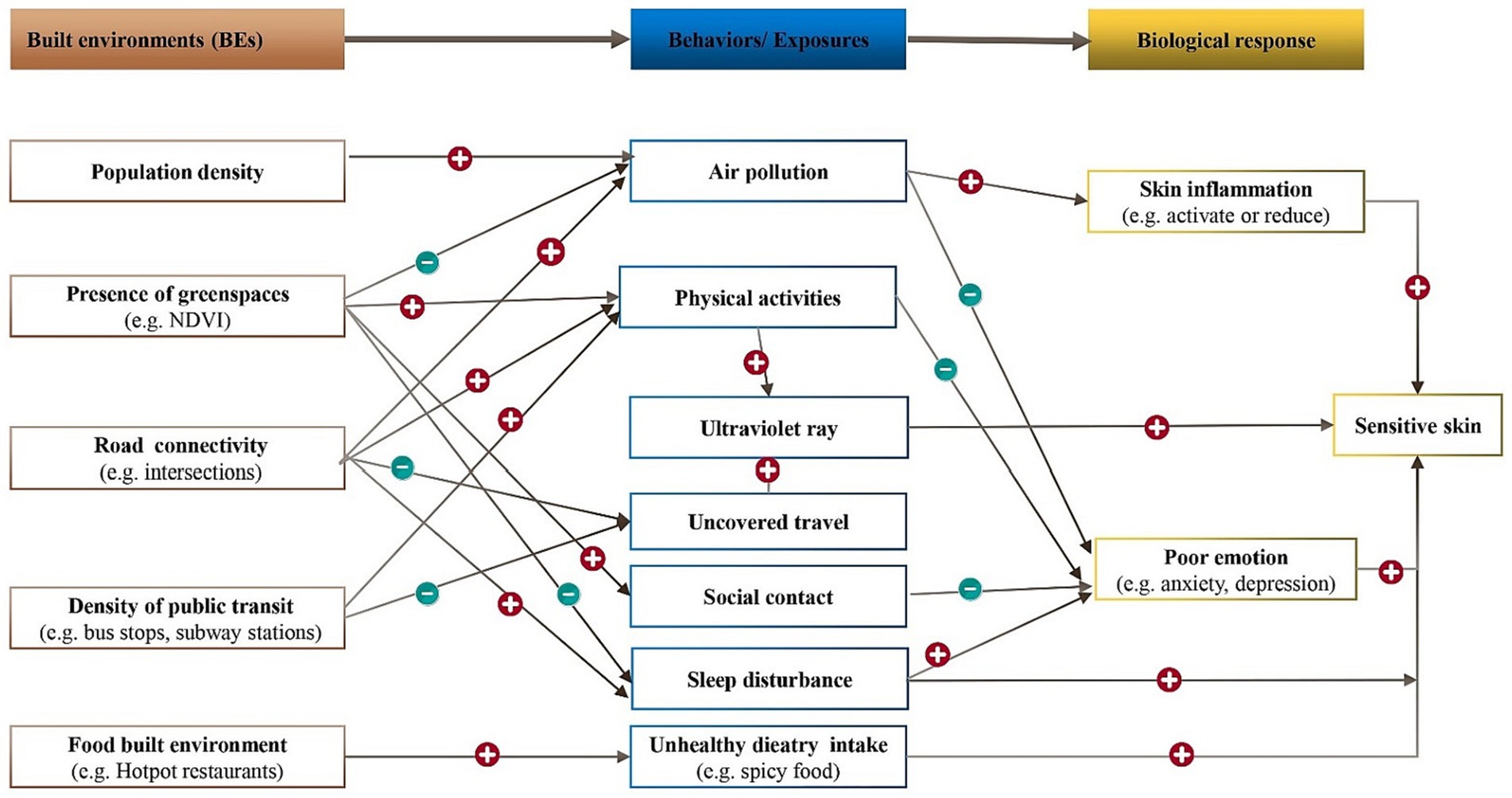
Figure 2. Potential pathways linking BEs to SS. The plus and minus signs in the chart represents positive and negative associations between the two linked variables. NDVI corresponds to the normalized difference vegetation index.
7.2.1 Population density
Exposure to PM2.5 and emotional change are likely to exhibit mediating role in the association between Population density and SS. High population density has been found to be correlated with an elevated risk of anxiety (129, 130). Notably, anxiety itself has been linked to a higher likelihood of developing SS (13). Furthermore, studies have indicated that as the population density of cities increases, so does the concentration of PM2.5 (131). Through experimental verification, researchers have demonstrated that PM2.5 can activate the skin’s inflammatory process, resulting in the release of inflammatory factors such as TNF-a and IL-1. This activation can trigger skin nerve fiber sensitivity and impede the repair of the damaged skin barrier (132, 133). Hence, it can be inferred that high population density may contribute to an increased prevalence of SS by elevating anxiety levels and raising PM2.5 concentrations.
7.2.2 Green spaces
Urban intervention of greenspace was anticipated to promoting general, mental and physical health. The influence of green spaces on mitigation of the particulate matter concentrations has been extensively researched, revealing various mechanism that are contingent upon environmental and vegetation characteristics (134). A study conducted in Spain involving 39 schools found that higher levels of greenery within and surrounding schools are correlated with reduced levels of traffic-related pollutants (135). Increased levels of ambient particulate matter have been linked to a higher prevalence of SS (136). Moreover, poor emotions such as depression, anxiety, and stress (137) have been recognized as risk factors influencing SS (138). Empirically, urban green spaces have been reported to encourage residents to engage in physical activities (139, 140), which helps to alleviate anxiety (141), thereby protecting against the occurrence of SS. Given the aforementioned association, it is speculated that green spaces could reduce ambient air pollution and increase outdoor activity time, thereby lowering the prevalence of SS through the reduction of exposure to air pollution and alleviation of anxiety levels. In addition, higher tree canopy cover was associated with more favorable sleep (16), so it is speculated that green spaces can reduce SS by improving sleep.
Additionally, a significant statistical correlation has been observed between the proximity of green spaces to residences and the indoor microbial diversity index (142). Exposure to urban green spaces has the potential to increase the diversity of skin microorganisms and alter the composition of human microbiota (143). However, the results from different studies examining the differences in microorganisms between SS patients and normal individuals have been inconsistent (144, 145). Consequently, further research is warranted to ascertain the alterations in microorganisms associated with SS.
7.2.3 Street designs
The density and connectivity of the road network and the availability of public transits play a significant role in influencing residents’ travel mode (146, 147). Although encouraging physical activities, active modes of travel, such as walking and cycling, can increase exposure to ultraviolet radiation, which has been shown to potentially trigger SS conditions. Conversely, motorized modes of travel like busses, self-driving, and subways reduce physical activities but can minimize exposure to ultraviolet radiation. People have experienced higher exposure to air pollutants at intersections (148), and dense intersections have adverse impacts on sleep (149). Therefore, it is speculated that intersections could increase ambient air pollution and sleep disturbance, thereby potentially increasing the prevalence of SS.
7.2.4 Food environment
A spicy diet is a potential risk factor for SS. Health status is associated with the local food environment (150, 151). The proliferation of hotpot and barbecue establishments in residential and workplace areas may contribute to increased consumption of spicy foods, thereby potentially influencing the occurrence of SS. The number of hotpot and barbecue shops serves as an indicator of the built environment, but empirical research is necessary to determine their impact on SS.
8 Conclusion
SS, characterized by heightened skin reactivity, is influenced by various internal and external factors. Our study indicates a higher prevalence of SS among women compared to men, with its occurrence diminishing with age. Emotional fluctuations, skin type (dry, oily, or mixed), sleep disturbances, menstrual cycles, consumption of spicy foods, improper cosmetic use, smoking, alcohol consumption, and physical activity also impact SS. Furthermore, environmental factors such as sun exposure, airborne particulate matter, temperature, and humidity contribute significantly. Notably, the connection between the built environment and SS has not been previously explored. Hence, an analysis has been undertaken to summarize the influence of the built environment on individual behavior and the natural environment, with the objective of uncovering a potential indirect connection between the built environment and SS (refer to Figure 2 for comprehensive details).
Health outcomes result from intricate interactions between human biology and the environment. Existing research demonstrates that gene–environment interactions can influence various chronic conditions like obesity (152), cardiovascular diseases (153), and respiratory disorders (154). Recent genome-wide association studies have identified genetic variations in the 2p21 region associated with self-reported SS in the Han population (155). Moreover, Yang et al. reported reduced expression of CLDN5 in the facial skin lesions of SS patients from the Han population compared to unaffected individuals (156). Nonetheless, further scientific validation is required to ascertain the extent to which gene–environment interactions contribute to the development of SS.
The comprehension of how the built environment influences health constitutes an essential component in the development of effective strategies geared toward the promotion of physical and mental well-being. Lifestyle factors, encompassing engagement in sports activities, dietary choices, and stress levels, are all subject to influence by the building environment, and they hold a pivotal role in the management of chronic diseases. The creation of an environment that fosters healthy behavior has the potential to contribute to the management of diseases such as obesity, cardiovascular disease, and skin diseases. The skin, functioning as the interface between the human body and the environment, is notably susceptible to the influences of the built environment. Understanding how building environments impact skin diseases is crucial for comprehending the role of environmental factors in skin health. This knowledge, once acquired, can provide valuable insights for urban planning decisions, inform public health interventions, and guide individual behaviors to cultivate a skin-friendly environment, thereby contributing to the reduction of the prevalence and severity of skin diseases.
This article explores the potential indirect relationship between the built environment and SS by investigating traditional factors influencing SS and the impact of the built environment on individual behavior and the natural surroundings. To establish such associations, a comprehensive approach encompassing geography, public health, and clinical medicine is necessary. We recommend conducting a large-scale epidemiological survey on SS, analyzing epidemiological data, and delving into the pathways and mechanisms through which the built environment influences SS. Ultimately, enhancing the built environment can serve as an effective strategy for the prevention and management of SS.
Author contributions
XCh: Writing – original draft, Data curation, Formal analysis, Methodology, Supervision, Writing – review & editing. JW: Data curation, Methodology, Writing – review & editing, Formal analysis. WW: Data curation, Formal analysis, Methodology, Writing – review & editing. QP: Methodology, Writing – review & editing. XCu: Methodology, Resources, Supervision, Writing – review & editing. LH: Funding acquisition, Methodology, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Yunnan Province Clinical Center for Skin Immune Diseases (ZX2019-03-02) and Yunnan Province Clinical Research Center for Skin Immune Diseases (2019ZF012).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1269314/full#supplementary-material
References
1. Hughes, BB, Kuhn, R, Peterson, CM, Rothman, DS, Solórzano, JR, Mathers, CD, et al. Projections of Global Health outcomes from 2005 to 2060 using the international futures integrated forecasting model. Bull World Health Organ. (2011) 89:478–86. doi: 10.2471/BLT.10.083766
2. Yang, J, Siri, JG, Remais, JV, Cheng, Q, Zhang, H, Chan, KKY, et al. The Tsinghua-lancet commission on healthy cities in China: unlocking the power of cities for a healthy China. Lancet. (2018) 391:2140–84. doi: 10.1016/S0140-6736(18)30486-0
3. Karimkhani, C, Dellavalle, RP, Karimi, SM, Rahimi-Movaghar, V, Pourmalek, F, Kiadaliri, AA, et al. Burden of skin and subcutaneous diseases in Iran and neighboring countries: results from the global burden of disease study 2015. Arch Iran Med. (2017) 20:429–40.
4. Dong, W, An, J, Geng, P, Zeng, X, Chen, Y, Zhao, Z, et al. Years lost due to disability from skin diseases in China 1990-2017: findings from the global burden of disease study 2017. Br J Dermatol. (2020) 182:248–50. doi: 10.1111/bjd.18329
5. Do, LHD, Azizi, N, and Maibach, H. Sensitive skin syndrome: an update. Am J Clin Dermatol. (2020) 21:401–9. doi: 10.1007/s40257-019-00499-7
6. Misery, L, Ständer, S, Szepietowski, JC, Reich, A, Wallengren, J, Evers, AW, et al. Definition of sensitive skin: an expert position paper from the special interest group on sensitive skin of the international forum for the study of itch. Acta Derm Venereol. (2017) 97:4–6. doi: 10.2340/00015555-2397
7. Talagas, M, and Misery, L. Role of keratinocytes in sensitive skin. Front Med. (2019) 6:108. doi: 10.3389/fmed.2019.00108
8. Richters, R, Falcone, D, Uzunbajakava, N, Verkruysse, W, van Erp, P, and van de Kerkhof, P. What is sensitive skin? A systematic literature review of objective measurements. Skin Pharmacol Physiol. (2015) 28:75–83. doi: 10.1159/000363149
9. Nojiri, H, Ishida, K, Yao, X, Liu, W, and Imokawa, G. Amelioration of lactic acid sensations in sensitive skin by stimulating the barrier function and improving the ceramide profile. Arch Dermatol Res. (2018) 310:495–504. doi: 10.1007/s00403-018-1833-9
10. Li, DG, Du, HY, Gerhard, S, Imke, M, and Liu, W. Inhibition of TRPV1 prevented skin irritancy induced by Phenoxyethanol. A preliminary in vitro and in vivo study. Int J Cosmet Sci. (2017) 39:11–6. doi: 10.1111/ics.12340
11. Honari, G, Andersen, R, and Maibach, HL. Sensitive skin syndrome. 2nd ed Taylor & Francis; CRC Publisher (2017).
12. Farage, MA. The prevalence of sensitive skin. Front Med. (2019) 6:98. doi: 10.3389/fmed.2019.00098
13. Manav, V, Karaali, MG, Erdem, O, and Koku Aksu, AE. Association between biophysical properties and anxiety in patients with sensitive skin. Skin Res Technol. (2022) 28:556–63. doi: 10.1111/srt.13156
14. Rao, M, Prasad, S, Adshead, F, and Tissera, H. The built environment and health. Lancet. (2007) 370:1111–3. doi: 10.1016/S0140-6736(07)61260-4
15. Zhou, P, Li, R, and Liu, K. The neighborhood food environment and the onset of childhood obesity: a retrospective time-trend study in a mid-Sized City in China. Front Public Health. (2021) 9:688767. doi: 10.3389/fpubh.2021.688767
16. Mayne, SL, Morales, KH, Williamson, AA, Grant, SFA, Fiks, AG, Basner, M, et al. Associations of the residential built environment with adolescent sleep outcomes. Sleep. (2021) 44:zsaa276. doi: 10.1093/sleep/zsaa276
17. Xu, J, Liu, N, Polemiti, E, Garcia-Mondragon, L, Tang, J, Liu, X, et al. Effects of urban living environments on mental health in adults. Nat Med. (2023) 29:1456–67. doi: 10.1038/s41591-023-02365-w
18. Franklin, M, Yin, X, McConnell, R, and Fruin, S. Association of the built environment with childhood psychosocial stress. JAMA Netw Open. (2020) 3:e2017634. doi: 10.1001/jamanetworkopen.2020.17634
19. Mason, KE, Pearce, N, and Cummins, S. Associations between fast food and physical activity environments and adiposity in mid-life: cross-sectional, observational evidence from UK biobank. Lancet Public Health. (2018) 3:e24–33. doi: 10.1016/S2468-2667(17)30212-8
20. Poelman, M, Strak, M, Schmitz, O, Hoek, G, Karssenberg, D, Helbich, M, et al. Relations between the residential fast-food environment and the individual risk of cardiovascular diseases in the Netherlands: a nationwide follow-up study. Eur J Prev Cardiol. (2018) 25:1397–405. doi: 10.1177/2047487318769458
21. Wang, L, Sun, W, Zhou, K, Zhang, M, and Bao, P. Spatial analysis of built environment risk for respiratory health and its implication for urban planning: a case study of Shanghai. Int J Environ Res Public Health. (2019) 16:1455. doi: 10.3390/ijerph16081455
22. Kamide, R, Misery, L, Perez-Cullell, N, Sibaud, V, and Taïeb, C. Sensitive skin evaluation in the Japanese population. J Dermatol. (2013) 40:177–81. doi: 10.1111/1346-8138.12027
23. Wang, X, Su, Y, Zheng, B, Wen, S, Liu, D, Ye, L, et al. Gender-related characterization of sensitive skin in normal young Chinese. J Cosmet Dermatol. (2020) 19:1137–42. doi: 10.1111/jocd.13123
24. Markevych, I, Schoierer, J, Hartig, T, Chudnovsky, A, Hystad, P, Dzhambov, AM, et al. Exploring pathways linking greenspace to health: theoretical and methodological guidance. Environ Res. (2017) 158:301–17. doi: 10.1016/j.envres.2017.06.028
25. Frank, LD, Iroz-Elardo, N, Mac Leod, KE, and Hong, A. Pathways from built environment to health: a conceptual framework linking behavior and exposure-based impacts. J Transp Health. (2019) 12:319–35. doi: 10.1016/j.jth.2018.11.008
26. Misery, L, Jourdan, E, Huet, F, Brenaut, E, Cadars, B, Virassamynaïk, S, et al. Sensitive skin in France: a study on prevalence, relationship with age and skin type and impact on quality of life. J Eur Acad Dermatol Venereol. (2018) 32:791–5. doi: 10.1111/jdv.14837
27. Kim, YR, Cheon, HI, Misery, L, Taieb, C, and Lee, YW. Sensitive skin in Korean population: an epidemiological approach. Skin Res Technol. (2018) 24:229–34. doi: 10.1111/srt.12418
28. Xiao, X, Qiao, L, Ye, R, and Zuo, F. Nationwide survey and identification of potential stress factor in sensitive skin of Chinese women. Clin Cosmet Investig Dermatol. (2020) 13:867–74. doi: 10.2147/CCID.S284359
29. de Bengy, AF, Lamartine, J, Sigaudo-Roussel, D, and Fromy, B. Newborn and elderly skin: two fragile skins at higher risk of pressure injury. Biol Rev. (2022) 97:874–95. doi: 10.1111/brv.12827
30. Brenaut, E, Misery, L, and Taieb, C. Sensitive skin in the Indian population: an epidemiological approach. Front Med. (2019) 6:29. doi: 10.3389/fmed.2019.00029
31. Misery, L, Myon, E, Martin, N, Consoli, S, Boussetta, S, Nocera, T, et al. Sensitive skin: psychological effects and seasonal changes. J Eur Acad Dermatol Venereol. (2007) 21:620–8. doi: 10.1111/j.1468-3083.2006.02027.x
32. Feng, Y, Shi, Q, Ding, Y, Xiang, F, Liu, J, and Yu, J. The prevalence and characterization of self-perceived sensitive facial skin among freshmen in Urumqi, China. J Cosmet Dermatol. (2022) 21:2516–22. doi: 10.1111/jocd.14474
33. Meng, Y, Feng, L, Shan, J, Yuan, Z, and Jin, L. Application of high-frequency ultrasound to assess facial skin thickness in association with gender, age, and BMI in healthy adults. BMC Med Imaging. (2022) 22:113. doi: 10.1186/s12880-022-00839-w
34. Falcone, D, Richters, RJ, Uzunbajakava, NE, Van Erp, PE, and Van De Kerkhof, PC. Sensitive skin and the influence of female hormone fluctuations: results from a cross-sectional digital survey in the Dutch population. Eur J Dermatol. (2017) 27:42–8. doi: 10.1684/ejd.2016.2913
35. Jourdain, R, de Lacharrière, O, Bastien, P, and Maibach, HI. Ethnic variations in self-perceived sensitive skin: epidemiological survey. Contact Dermatitis. (2002) 46:162–9. doi: 10.1034/j.1600-0536.2002.460307.x
36. Farage, MA. Does sensitive skin differ between men and women? Cutan Ocul Toxicol. (2010) 29:153–63. doi: 10.3109/15569521003774990
37. Robinson, MK. Racial differences in acute and cumulative skin irritation responses between Caucasian and Asian populations. Contact Dermatitis. (2000) 42:134–43. doi: 10.1034/j.1600-0536.2000.042003134.x
38. Zuo, Y, Hua, W, Luo, Y, and Li, L. Skin reactions of N95 masks and medial masks among health-care personnel: a self-report questionnaire survey in China. Contact Dermatitis. (2020) 83:145–7. doi: 10.1111/cod.13555
39. Misery, L, Myon, E, Martin, N, Verrière, F, Nocera, T, and Taieb, C. Sensitive skin in France: an epidemiological approach. Ann Dermatol Venereol. (2005) 132:425–9. doi: 10.1016/S0151-9638(05)79303-0
40. Bize, C, Le Gélébart, E, Moga, A, Payré, B, and Garcia, C. Barrier disruption, dehydration and inflammation: investigation of the vicious circle underlying dry skin. Int J Cosmet Sci. (2021) 43:729–37. doi: 10.1111/ics.12748
41. Yosipovitch, G, Misery, L, Proksch, E, Metz, M, Ständer, S, and Schmelz, M. Skin barrier damage and itch: review of mechanisms, topical management and future directions. Acta Derm Venereol. (2019) 99:1201–9. doi: 10.2340/00015555-3296
42. Legeas, C, Misery, L, Fluhr, JW, Roudot, AC, Ficheux, AS, and Brenaut, E. Proposal for cut-off scores for sensitive skin on sensitive Scale-10 in a group of adult women. Acta Derm Venereol. (2021) 101:adv00373. doi: 10.2340/00015555-3741
43. Oyetakin-White, P, Suggs, A, Koo, B, Matsui, MS, Yarosh, D, Cooper, KD, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. (2015) 40:17–22. doi: 10.1111/ced.12455
44. Xerfan, EMS, Tomimori, J, Andersen, ML, Tufik, S, and Facina, AS. Sleep disturbance and atopic dermatitis: a bidirectional relationship? Med Hypotheses. (2020) 140:109637. doi: 10.1016/j.mehy.2020.109637
45. Khmaladze, I, Leonardi, M, Fabre, S, Messaraa, C, and Mavon, A. The skin Interactome: a holistic "genome-microbiome-exposome" approach to understand and modulate skin health and aging. Clin Cosmet Investig Dermatol. (2020) 13:1021–40. doi: 10.2147/CCID.S239367
46. Proksch, E, and Weidinger, S. New insights into the pathogenesis of sensitive skin. Hautarzt. (2011) 62:900–5. doi: 10.1007/s00105-011-2209-7
47. Misery, L, Morisset, S, Séité, S, Brenaut, E, Ficheux, AS, Fluhr, JW, et al. Relationship between sensitive skin and sleep disorders, fatigue, dust, sweating, food, tobacco consumption or female hormonal changes: results from a worldwide survey of 10 743 individuals. J Eur Acad Dermatol Venereol. (2021) 35:1371–6. doi: 10.1111/jdv.17162
48. Farage, MA, Neill, S, and Mac Lean, AB. Physiological changes associated with the menstrual cycle: a review. Obstet Gynecol Surv. (2009) 64:58–72. doi: 10.1097/OGX.0b013e3181932a37
49. Muizzuddin, N, Marenus, KD, Schnittger, SF, Sullivan, M, and Maes, DH. Effect of systemic hormonal cyclicity on skin. J Cosmet Sci. (2005) 56:311–21.
50. Xiao, T, Sun, M, Zhao, C, and Kang, J. TRPV1: a promising therapeutic target for skin aging and inflammatory skin diseases. Front Pharmacol. (2023) 14:1037925. doi: 10.3389/fphar.2023.1037925
51. Chuang, HH, Prescott, ED, Kong, H, Shields, S, Jordt, SE, Basbaum, AI, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns (4, 5) P 2-mediated inhibition. Nature. (2001) 411:957–62. doi: 10.1038/35082088
52. Kueper, T, Krohn, M, Haustedt, LO, Hatt, H, Schmaus, G, and Vielhaber, G. Inhibition of TRPV 1 for the treatment of sensitive skin. Exp Dermatol. (2010) 19:980–6. doi: 10.1111/j.1600-0625.2010.01122.x
53. Yasak Guner, R, Tosun, M, Akyol, M, and Hayta, SB. Demodex infestation as a cause of sensitive skin in a dermatology outpatient clinic. J Cosmet Dermatol. (2022) 21:1610–5. doi: 10.1111/jocd.14246
54. Brenaut, E, Barnetche, T, Le Gall-Ianotto, C, Roudot, AC, Misery, L, and Ficheux, AS. Triggering factors in sensitive skin from the worldwide patients' point of view: a systematic literature review and meta-analysis. J Eur Acad Dermatol Venereol. (2020) 34:230–8. doi: 10.1111/jdv.15985
55. Pinto, P, Rosado, C, Parreirão, C, and Rodrigues, LM. Is there any barrier impairment in sensitive skin? A quantitative analysis of sensitive skin by mathematical modeling of transepidermal water loss desorption curves. Skin Res Technol. (2011) 17:181–5. doi: 10.1111/j.1600-0846.2010.00478.x
56. Raj, N, Voegeli, R, Rawlings, AV, Doppler, S, Imfeld, D, Munday, MR, et al. A fundamental investigation into aspects of the physiology and biochemistry of the stratum Corneum in subjects with sensitive skin. Int J Cosmet Sci. (2017) 39:2–10. doi: 10.1111/ics.12334
57. Rajagopalan, P, Nanjappa, V, Raja, R, Jain, AP, Mangalaparthi, KK, Sathe, GJ, et al. How does chronic cigarette smoke exposure affect human skin? A global proteomics study in primary human keratinocytes. OMICS. (2016) 20:615–26. doi: 10.1089/omi.2016.0123
58. Xin, S, Ye, L, Man, G, Lv, C, Elias, PM, and Man, MQ. Heavy cigarette smokers in a Chinese population display a compromised permeability barrier. Biomed Res Int. (2016) 2016:1–7. doi: 10.1155/2016/9704598
59. Prieux, R, Eeman, M, Rothen-Rutishauser, B, and Valacchi, G. Mimicking cigarette smoke exposure to assess cutaneous toxicity. Toxicol In Vitro. (2020) 62:104664. doi: 10.1016/j.tiv.2019.104664
60. Falcone, D, Richters, RJH, Uzunbajakava, NE, van Erp, PEJ, and van de Kerkhof, PCM. Risk factors associated with sensitive skin and potential role of lifestyle habits: a cross-sectional study. Clin Exp Dermatol. (2017) 42:656–8. doi: 10.1111/ced.13133
61. Fawkes, N, Tselenti, E, Shah, N, Lappin, V, Smith, N, Narasimhan, A, et al. A survey to identify determinants that influence self-perceived sensitive skin in a British population: clues to developing a reliable screening tool for sensitive skin. Clin Cosmet Investig Dermatol. (2021) 14:1201–10. doi: 10.2147/CCID.S317970
62. Eriksson, CJ. The role of acetaldehyde in the actions of alcohol (update 2000). Alcohol Clin Exp Res. (2001) 25:15s–32s. doi: 10.1111/j.1530-0277.2001.tb02369.x
63. Aoki, Y, Wehage, SL, and Talalay, P. Quantification of skin erythema response to topical alcohol in alcohol-intolerant east Asians. Skin Res Technol. (2017) 23:593–6. doi: 10.1111/srt.12376
64. Nijs, J, Kosek, E, Van Oosterwijck, J, and Meeus, M. Dysfunctional endogenous analgesia during exercise in patients with chronic pain: to exercise or not to exercise? Pain Physician. (2012) 15:Es205–13. doi: 10.36076/ppj.2012/15/ES205
65. Thomas, SD, Carter, HH, Jones, H, Thijssen, DHJ, and Low, DA. Effects of acute exercise on cutaneous thermal sensation. Int J Environ Res Public Health. (2020) 17:2491. doi: 10.3390/ijerph17072491
66. Schmelz, M. Neuronal sensitivity of the skin. Eur J Dermatol. (2011) 21:43–7. doi: 10.1684/ejd.2011.1265
67. von Zimmermann, C, Winkelmann, M, Richter-Schmidinger, T, Mühle, C, Kornhuber, J, and Lenz, B. Physical activity and body composition are associated with severity and risk of depression, and serum lipids. Front Psych. (2020) 11:494. doi: 10.3389/fpsyt.2020.00494
68. Jia, P, Dai, S, Rohli, KE, Rohli, RV, Ma, Y, Yu, C, et al. Natural environment and childhood obesity: a systematic review. Obes Rev. (2021) 22:e13097. doi: 10.1111/obr.13097
69. Mohania, D, Chandel, S, Kumar, P, Verma, V, Digvijay, K, Tripathi, D, et al. Ultraviolet radiations: skin defense-damage mechanism. Adv Exp Med Biol. (2017) 996:71–87. doi: 10.1007/978-3-319-56017-5_7
70. Wang, PW, Hung, YC, Lin, TY, Fang, JY, Yang, PM, Chen, MH, et al. Comparison of the biological impact of UVA and UVB upon the skin with functional proteomics and immunohistochemistry. Antioxidants. (2019) 8:569. doi: 10.3390/antiox8120569
71. Fernando, IPS, Dias, M, Madusanka, DMD, Han, EJ, Kim, MJ, Jeon, YJ, et al. Fucoidan refined by Sargassum confusum indicate protective effects suppressing photo-oxidative stress and skin barrier perturbation in UVB-induced human keratinocytes. Int J Biol Macromol. (2020) 164:149–61. doi: 10.1016/j.ijbiomac.2020.07.136
72. Li, Z, Jiang, R, Wang, M, Zhai, L, Liu, J, Xu, X, et al. Ginsenosides repair UVB-induced skin barrier damage in BALB/c hairless mice and HaCaT keratinocytes. J Ginseng Res. (2022) 46:115–25. doi: 10.1016/j.jgr.2021.05.001
73. Kim, H, Park, SY, and Chung, DK. Effect of the oral administration of common evening primrose sprout (Oenothera biennis L.) extract on skin function improvement in UVB-irradiated hairless mice. Pharmaceuticals. (2021) 14:222. doi: 10.3390/ph14030222
74. Lee, TA, Huang, YT, Hsiao, PF, Chiu, LY, Chern, SR, and Wu, NL. Critical roles of irradiance in the regulation of UVB-induced Inflammasome activation and skin inflammation in human skin keratinocytes. J Photochem Photobiol B. (2022) 226:112373. doi: 10.1016/j.jphotobiol.2021.112373
75. Park, C, Park, J, Kim, WJ, Kim, W, Cheong, H, and Kim, SJ. Malonic acid isolated from Pinus densiflora inhibits UVB-induced oxidative stress and inflammation in HaCaT keratinocytes. Polymers. (2021) 13:816. doi: 10.3390/polym13050816
76. Lu, PH, Wang, JY, Chiu, LY, Huang, YT, Hung, CF, and Wu, NL. Spleen tyrosine kinase regulates keratinocyte inflammasome activation and skin inflammation induced by UVB irradiation. Free Radic Biol Med. (2022) 180:121–33. doi: 10.1016/j.freeradbiomed.2022.01.004
77. Liu, Z, Song, S, Luo, W, Elias, PM, and Man, MQ. Sun-induced changes of stratum Corneum hydration vary with age and gender in a normal Chinese population. Skin Res Technol. (2012) 18:22–8. doi: 10.1111/j.1600-0846.2011.00536.x
78. Ferrara, F, Woodby, B, Pecorelli, A, Schiavone, ML, Pambianchi, E, Messano, N, et al. Additive effect of combined pollutants to UV induced skin oxinflammation damage. Evaluating the protective topical application of a cosmeceutical mixture formulation. Redox Biol. (2020) 34:101481. doi: 10.1016/j.redox.2020.101481
79. Ma, L, Guichard, A, Humbert, P, Zheng, S, Tan, Y, Yu, L, et al. Evaluation of the severity and triggering factors of sensitive scalp in Chinese females. J Cosmet Dermatol. (2016) 15:219–25. doi: 10.1111/jocd.12203
80. Vanoosthuyze, K, Zupkosky, PJ, and Buckley, K. Survey of practicing dermatologists on the prevalence of sensitive skin in men. Int J Cosmet Sci. (2013) 35:388–93. doi: 10.1111/ics.12056
81. Caterina, MJ, Schumacher, MA, Tominaga, M, Rosen, TA, Levine, JD, and Julius, D. The capsaicin receptor: a heat-activated Ion Channel in the pain pathway. Nature. (1997) 389:816–24. doi: 10.1038/39807
82. Facer, P, Casula, MA, Smith, GD, Benham, CD, Chessell, IP, Bountra, C, et al. Differential expression of the capsaicin receptor TRPV 1 and related novel receptors TRPV 3, TRPV 4 and TRPM 8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. (2007) 7:11. doi: 10.1186/1471-2377-7-11
83. Szallasi, A, Cortright, DN, Blum, CA, and Eid, SR. The Vanilloid receptor TRPV 1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. (2007) 6:357–72. doi: 10.1038/nrd2280
84. Singh, B, and Maibach, H. Climate and skin function: an overview. Skin Res Technol. (2013) 19:207–12. doi: 10.1111/srt.12043
85. Misery, L, Sibaud, V, Merial-Kieny, C, and Taieb, C. Sensitive skin in the American population: prevalence, clinical data, and role of the dermatologist. Int J Dermatol. (2011) 50:961–7. doi: 10.1111/j.1365-4632.2011.04884.x
86. Lee, CW, Lin, ZC, Hu, SC, Chiang, YC, Hsu, LF, Lin, YC, et al. Urban particulate matter down-regulates filaggrin via COX 2 expression/PGE 2 production leading to skin barrier dysfunction. Sci Rep. (2016) 6:27995. doi: 10.1038/srep27995
87. Bae, JE, Choi, H, Shin, DW, Na, HW, Park, NY, Kim, JB, et al. Fine particulate matter (PM2.5) inhibits ciliogenesis by increasing SPRR 3 expression via c-Jun activation in RPE cells and skin keratinocytes. Sci Rep. (2019) 9:3994. doi: 10.1038/s41598-019-40670-y
88. Kim, BE, Kim, J, Goleva, E, Berdyshev, E, Lee, J, Vang, KA, et al. Particulate matter causes skin barrier dysfunction. JCI Insight. (2021) 6:e145185. doi: 10.1172/jci.insight.145185
89. Liao, Z, Nie, J, and Sun, P. The impact of particulate matter (PM2.5) on skin barrier revealed by transcriptome analysis: focusing on cholesterol metabolism. Toxicol Rep. (2020) 7:1–9. Epub 2019/12/24. doi: 10.1016/j.toxrep.2019.11.014
90. Shamsipour, M, Nasrollahi, SA, Hassanvand, MS, Yazdanparast, T, Samadi, A, Yunesian, M, et al. Short-term effects of exposure to air pollution on biophysical parameters of skin in a panel of healthy adults. Dermatol Ther. (2020) 33:e14536. doi: 10.1111/dth.14536
91. Otterbein, LE, Bach, FH, Alam, J, Soares, M, Tao, LH, Wysk, M, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. (2000) 6:422–8. doi: 10.1038/74680
92. Woodby, B, Pambianchi, E, Ferrara, F, Therrien, JP, Pecorelli, A, Messano, N, et al. Cutaneous antimicrobial peptides: new "actors" in pollution related inflammatory conditions. Redox Biol. (2021) 41:101952. doi: 10.1016/j.redox.2021.101952
93. Machado, AU, and Contri, RV. Effectiveness and safety of ozone therapy for dermatological disorders: a literature review of clinical trials. Indian J Dermatol. (2022) 67:479.
94. Zeng, J, Lei, L, Zeng, Q, Yao, Y, Wu, Y, Li, Q, et al. Ozone therapy attenuates NF-ΚB-mediated local inflammatory response and activation of Th17 cells in treatment for psoriasis. Int J Biol Sci. (2020) 16:1833–45. doi: 10.7150/ijbs.41940
95. Figueiredo, M, Eloy, S, Marques, S, and Dias, L. Older people perceptions on the built environment: a scoping review. Appl Ergon. (2023) 108:103951. doi: 10.1016/j.apergo.2022.103951
96. Sun, B, and Yin, C. Relationship between multi-scale urban built environments and body mass index: a study of China. Appl Geogr. (2018) 94:230–40. doi: 10.1016/j.apgeog.2018.03.012
97. Chen, H, Jia, B, and Lau, SSY. Sustainable urban form for Chinese compact cities: challenges of a rapid urbanized economy. Habitat Int. (2008) 32:28–40. doi: 10.1016/j.habitatint.2007.06.005
98. Yang, Z, Song, Q, Li, J, and Zhang, Y. Air pollution as a cause of obesity: micro-level evidence from Chinese cities. Int J Environ Res Public Health. (2019) 16:4296. doi: 10.3390/ijerph16214296
99. Furlong, MA, and Klimentidis, YC. Associations of air pollution with obesity and body fat percentage, and modification by polygenic risk score for BMI in the UK biobank. Environ Res. (2020) 185:109364. doi: 10.1016/j.envres.2020.109364
100. Choi, H, and Myong, JP. Association between air pollution in the 2015 winter in South Korea and population size, car emissions, industrial activity, and fossil-fuel power plants: an ecological study. Ann Occup Environ Med. (2018) 30:60. doi: 10.1186/s40557-018-0273-5
101. Liu, Q, Pan, L, Yang, T, Ou, Q, Sun, Z, He, H, et al. Association between long-term exposure to ambient particulate matter and pulmonary function among men and women in typical areas of south and North China. Front Public Health. (2023) 11:1170584. doi: 10.3389/fpubh.2023.1170584
102. Badyda, A, Gayer, A, Czechowski, PO, Majewski, G, and Dąbrowiecki, P. Pulmonary function and incidence of selected respiratory diseases depending on the exposure to ambient PM10. Int J Mol Sci. (2016) 17:1954. doi: 10.3390/ijms17111954
103. Li, H, Wu, S, Pan, L, Xu, J, Shan, J, Yang, X, et al. Short-term effects of various ozone metrics on cardiopulmonary function in chronic obstructive pulmonary disease patients: results from a panel study in Beijing, China. Environ Pollut. (2018) 232:358–66. doi: 10.1016/j.envpol.2017.09.030
104. Li, G, Liu, J, Lu, H, Hu, W, Hu, M, He, J, et al. Multiple environmental exposures and obesity in eastern China: an individual exposure evaluation model. Chemosphere. (2022) 298:134316. doi: 10.1016/j.chemosphere.2022.134316
105. Astell-Burt, T, and Feng, X. Urban green space, tree canopy and prevention of cardiometabolic diseases: a multilevel longitudinal study of 46 786 Australians. Int J Epidemiol. (2020) 49:926–33. doi: 10.1093/ije/dyz239
106. Seo, S, Choi, S, Kim, K, Kim, SM, and Park, SM. Association between urban green space and the risk of cardiovascular disease: a longitudinal study in seven Korean metropolitan areas. Environ Int. (2019) 125:51–7. doi: 10.1016/j.envint.2019.01.038
107. Gascon, M, Triguero-Mas, M, Martínez, D, Dadvand, P, Rojas-Rueda, D, Plasència, A, et al. Residential green spaces and mortality: a systematic review. Environ Int. (2016) 86:60–7. doi: 10.1016/j.envint.2015.10.013
108. Liu, XX, Ma, XL, Huang, WZ, Luo, YN, He, CJ, Zhong, XM, et al. Green space and cardiovascular disease: a systematic review with meta-analysis. Environ Pollut. (2022) 301:118990. doi: 10.1016/j.envpol.2022.118990
109. Kim, J, Lee, S, and Ramos, W. Investigating the relationship between accessibility of green space and adult obesity rates: a secondary data analysis in the United States. J Prevent Med Public Health. (2021) 54:208–17. doi: 10.3961/jpmph.20.625
110. Huang, YJ, Lee, PH, Chen, LC, Lin, BC, Lin, C, and Chan, TC. Relationships among green space, ambient fine particulate matter, and cancer incidence in Taiwan: a 16-year retrospective cohort study. Environ Res. (2022) 212:113416. doi: 10.1016/j.envres.2022.113416
111. Klumpp, H, Roberts, J, Kapella, MC, Kennedy, AE, Kumar, A, and Phan, KL. Subjective and objective sleep quality modulate emotion regulatory brain function in anxiety and depression. Depress Anxiety. (2017) 34:651–60. doi: 10.1002/da.22622
112. Paksarian, D, Rudolph, KE, Stapp, EK, Dunster, GP, He, J, Mennitt, D, et al. Association of outdoor artificial light at night with mental disorders and sleep patterns among US adolescents. JAMA Psychiatry. (2020) 77:1266–75. doi: 10.1001/jamapsychiatry.2020.1935
113. Ohayon, MM, and Milesi, C. Artificial outdoor nighttime lights associate with altered sleep behavior in the American general population. Sleep. (2016) 39:1311–20. doi: 10.5665/sleep.5860
114. Xiao, Q, Gee, G, Jones, RR, Jia, P, James, P, and Hale, L. Cross-sectional association between outdoor artificial light at night and sleep duration in middle-to-older aged adults: the NIH-AARP diet and health study. Environ Res. (2020) 180:108823. doi: 10.1016/j.envres.2019.108823
115. Tang, Z, Li, S, Shen, M, Xiao, Y, Su, J, Tao, J, et al. Association of Exposure to artificial light at night with atopic diseases: a cross-sectional study in college students. Int J Hyg Environ Health. (2022) 241:113932. doi: 10.1016/j.ijheh.2022.113932
116. Winters, M, Brauer, M, Setton, EM, and Teschke, K. Built environment influences on healthy transportation choices: bicycling versus driving. J Urban Health. (2010) 87:969–93. doi: 10.1007/s11524-010-9509-6
117. Djurhuus, S, Hansen, HS, Aadahl, M, and Glümer, C. The association between access to public transportation and self-reported active commuting. Int J Environ Res Public Health. (2014) 11:12632–51. doi: 10.3390/ijerph111212632
118. Villanueva, K, Giles-Corti, B, and McCormack, G. Achieving 10, 000 steps: a comparison of public transport users and drivers in a university setting. Prev Med. (2008) 47:338–41. doi: 10.1016/j.ypmed.2008.03.005
119. Babak, A, Rouzbahani, R, Khalili Nejad, R, and Rafiee, ZA. Comparison of nutritional behaviors and physical activities between overweight/obese and normal-weight adults. Adv Biomed Res. (2019) 8:62. doi: 10.4103/abr.abr_134_19
120. Paduano, S, Greco, A, Borsari, L, Salvia, C, Tancredi, S, Pinca, J, et al. Physical and sedentary activities and childhood overweight/obesity: a cross-sectional study among first-year children of primary schools in Modena, Italy. Int J Environ Res Public Health. (2021) 18:3221. doi: 10.3390/ijerph18063221
121. Thomas-Eapen, N. Childhood obesity. Prim Care. (2021) 48:505–15. doi: 10.1016/j.pop.2021.04.002
122. Schneider, S, Diehl, K, Bock, C, Herr, RM, Mayer, M, and Görig, T. Modifying health behavior to prevent cardiovascular diseases: a Nationwide survey among German primary care physicians. Int J Environ Res Public Health. (2014) 11:4218–32. doi: 10.3390/ijerph110404218
123. Gero, K, Iso, H, Kitamura, A, Yamagishi, K, Yatsuya, H, and Tamakoshi, A. Cardiovascular disease mortality in relation to physical activity during adolescence and adulthood in Japan: does school-based sport Club participation matter? Prev Med. (2018) 113:102–8. doi: 10.1016/j.ypmed.2018.05.012
124. Dwicaksono, A, Brissette, I, Birkhead, GS, Bozlak, CT, and Martin, EG. Evaluating the contribution of the built environment on obesity among New York state students. Health Educ Behav. (2018) 45:480–91. doi: 10.1177/1090198117742440
125. McInerney, M, Csizmadi, I, Friedenreich, CM, Uribe, FA, Nettel-Aguirre, A, McLaren, L, et al. Associations between the neighbourhood food environment, neighbourhood socioeconomic status, and diet quality: an observational study. BMC Public Health. (2016) 16:984. doi: 10.1186/s12889-016-3631-7
126. Rahmanian, E, Gasevic, D, Vukmirovich, I, and Lear, SA. The association between the built environment and dietary intake - a systematic review. Asia Pac J Clin Nutr. (2014) 23:183–96.
127. Huet, F, and Misery, L. Sensitive skin is a neuropathic disorder. Exp Dermatol. (2019) 28:1470–3. doi: 10.1111/exd.13991
128. Gouin, O, L'Herondelle, K, Lebonvallet, N, Le Gall-Ianotto, C, Sakka, M, Buhé, V, et al. TRPV 1 and TRPA 1 in cutaneous neurogenic and chronic inflammation: pro-inflammatory response induced by their activation and their sensitization. Protein Cell. (2017) 8:644–61. doi: 10.1007/s13238-017-0395-5
129. Tao, Z, Wu, G, and Wang, Z. The relationship between high residential density in student dormitories and anxiety, binge eating and internet addiction: a study of Chinese college students. Springer Plus. (2016) 5:1579. doi: 10.1186/s40064-016-3246-6
130. Chan, SM, Wong, H, Chung, RY, and Au-Yeung, TC. Association of living density with anxiety and stress: a cross-sectional population study in Hong Kong. Health Soc Care Commun. (2021) 29:1019–29. doi: 10.1111/hsc.13136
131. Kim, MJ, Chang, YS, and Kim, SM. Impact of income, density, and population size on PM2.5 pollutions: a scaling analysis of 254 large cities in six developed countries. Int J Environ Res Public Health. (2021) 18:9019. doi: 10.3390/ijerph18179019
132. Li, Q, Kang, Z, Jiang, S, Zhao, J, Yan, S, Xu, F, et al. Effects of ambient fine particles PM2.5 on human HaCaT cells. Int J Environ Res Public Health. (2017) 14:72. doi: 10.3390/ijerph14010072
133. Dong, L, Hu, R, Yang, D, Zhao, J, Kan, H, Tan, J, et al. Fine particulate matter (PM2.5) upregulates expression of Inflammasome NLRP 1 via Ros/NF-ΚB signaling in HaCaT cells. Int J Med Sci. (2020) 17:2200–6. doi: 10.7150/ijms.46962
134. Diener, A, and Mudu, P. How can vegetation protect us from air pollution? A critical review on green Spaces' mitigation abilities for air-borne particles from a public health perspective - with implications for urban planning. Sci Total Environ. (2021) 796:148605. doi: 10.1016/j.scitotenv.2021.148605
135. Dadvand, P, Rivas, I, Basagaña, X, Alvarez-Pedrerol, M, Su, J, De Castro, PM, et al. The association between greenness and traffic-related air pollution at schools. Sci Total Environ. (2015) 523:59–63. doi: 10.1016/j.scitotenv.2015.03.103
136. Zhang, X, Li, F, Zhang, L, Zhao, Z, and Norback, D. A longitudinal study of sick building syndrome (SBS) among pupils in relation to SO2, NO2, O3 and PM10 in schools in China. PLoS One. (2014) 9:e112933. doi: 10.1371/journal.pone.0112933
137. Beyer, KM, Kaltenbach, A, Szabo, A, Bogar, S, Nieto, FJ, and Malecki, KM. Exposure to neighborhood green space and mental health: evidence from the survey of the health of Wisconsin. Int J Environ Res Public Health. (2014) 11:3453–72. doi: 10.3390/ijerph110303453
138. Zafiriou, E, Angelopoulos, NV, Zintzaras, E, Rallis, E, and Roussaki-Schulze, AV. Psychiatric factors in patients with sensitive skin. Drugs Exp Clin Res. (2005) 31:25–30.
139. Wang, H, Dai, X, Wu, J, Wu, X, and Nie, X. Influence of urban green open space on Residents' physical activity in China. BMC Public Health. (2019) 19:1093. doi: 10.1186/s12889-019-7416-7
140. Yuen, JWM, Chang, KKP, Wong, FKY, Wong, FY, Siu, JYM, Ho, HC, et al. Influence of urban green space and facility accessibility on exercise and healthy diet in Hong Kong. Int J Environ Res Public Health. (2019) 16:1514. doi: 10.3390/ijerph16091514
141. Gerdes, ME, Aistis, LA, Sachs, NA, Williams, M, Roberts, JD, and Rosenberg Goldstein, RE. Reducing anxiety with nature and gardening (rang): evaluating the impacts of gardening and outdoor activities on anxiety among U.S. adults during the Covid-19 pandemic. Int J Environ Res Public Health. (2022) 19:5121. doi: 10.3390/ijerph19095121
142. Dockx, Y, Täubel, M, Bijnens, EM, Witters, K, Valkonen, M, Jayaprakash, B, et al. Residential green space can shape the indoor microbial environment. Environ Res. (2021) 201:111543. doi: 10.1016/j.envres.2021.111543
143. Selway, CA, Mills, JG, Weinstein, P, Skelly, C, Yadav, S, Lowe, A, et al. Transfer of environmental microbes to the skin and respiratory tract of humans after urban green space exposure. Environ Int. (2020) 145:106084. doi: 10.1016/j.envint.2020.106084
144. Bai, Y, Wang, Y, Zheng, H, Tan, F, and Yuan, C. Correlation between facial skin microbiota and skin barriers in a Chinese female population with sensitive skin. Infect Drug Resist. (2021) 14:219–26. doi: 10.2147/IDR.S287844
145. Seite, S, and Misery, L. Skin sensitivity and skin microbiota: is there a link? Exp Dermatol. (2018) 27:1061–4. doi: 10.1111/exd.13686
146. Li, H, Han, L, Ao, Y, Wang, Y, and Wang, T. Influences of the built environment on rural school Children's travel mode choice: the case of Chengdu. Int J Environ Res Public Health. (2022) 19:9008. doi: 10.3390/ijerph19159008
147. Wang, W, Zhang, Y, Zhao, C, Liu, X, Chen, X, Li, C, et al. Nonlinear associations of the built environment with cycling frequency among older adults in Zhongshan, China. Int J Environ Res Public Health. (2021) 18:10723. doi: 10.3390/ijerph182010723
148. Dirks, KN, Wang, JY, Khan, A, and Rushton, C. Air pollution exposure in relation to the commute to school: a Bradford UK case study. Int J Environ Res Public Health. (2016) 13:1064. doi: 10.3390/ijerph13111064
149. Johnson, DA, Hirsch, JA, Moore, KA, Redline, S, and Diez Roux, AV. Associations between the built environment and objective measures of sleep: the multi-ethnic study of atherosclerosis. Am J Epidemiol. (2018) 187:941–50. doi: 10.1093/aje/kwx302
150. Kanchi, R, Lopez, P, Rummo, PE, Lee, DC, Adhikari, S, Schwartz, MD, et al. Longitudinal analysis of neighborhood food environment and diabetes risk in the veterans administration diabetes risk cohort. JAMA Netw Open. (2021) 4:e2130789. doi: 10.1001/jamanetworkopen.2021.30789
151. Jia, P, Xue, H, Cheng, X, and Wang, Y. Effects of school neighborhood food environments on childhood obesity at multiple scales: a longitudinal kindergarten cohort study in the USA. BMC Med. (2019) 17:99. doi: 10.1186/s12916-019-1329-2
152. Reddon, H, Guéant, JL, and Meyre, D. The importance of gene-environment interactions in human obesity. Clin Sci. (2016) 130:1571–97. doi: 10.1042/CS20160221
153. Hartiala, JA, Hilser, JR, Biswas, S, Lusis, AJ, and Allayee, H. Gene-environment interactions for cardiovascular disease. Curr Atheroscler Rep. (2021) 23:75. doi: 10.1007/s11883-021-00974-9
154. Kleeberger, SR, and Peden, D. Gene-environment interactions in asthma and other respiratory diseases. Annu Rev Med. (2005) 56:383–400. doi: 10.1146/annurev.med.56.062904.144908
155. Li, B, Cai, X, Wang, L, Li, J, Zou, Y, Chen, G, et al. A Gwas finds variants at 2p21 associated with self-reported sensitive skin in the Han Chinese population. J Invest Dermatol. (2022) 142:243–7.e9. doi: 10.1016/j.jid.2021.04.021
Keywords: sensitive skin, influencing factors, socioeconomic attributes, behavioral characteristics, natural environment, built environment
Citation: Chen X, Wen J, Wu W, Peng Q, Cui X and He L (2023) A review of factors influencing sensitive skin: an emphasis on built environment characteristics. Front. Public Health. 11:1269314. doi: 10.3389/fpubh.2023.1269314
Edited by:
Chun Yin, Wuhan University, ChinaReviewed by:
Linjing Deng, Jiangsu University, ChinaJing Cai, Fudan University, China
Jayanta Gupta, Florida Gulf Coast University, United States
Copyright © 2023 Chen, Wen, Wu, Peng, Cui and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li He, drheli2662@126.com; Xiangfen Cui, cui1987rainny@163.com
 Xiangfeng Chen
Xiangfeng Chen Jing Wen
Jing Wen Wenjuan Wu
Wenjuan Wu Qiuzhi Peng3
Qiuzhi Peng3 Xiangfen Cui
Xiangfen Cui Li He
Li He