First Molecular Identification of Canine Parvovirus Type 2 (CPV2) in Chile Reveals High Occurrence of CPV2c Antigenic Variant
- 1Departamento de Patología y Medicina Preventiva, Facultad de Ciencias Veterinarias, Universidad de Concepción, Chillán, Chile
- 2Departamento de Medicina Preventiva Animal, Facultad de Ciencias Veterinarias y Pecuarias, Universidad de Chile, Santiago, Chile
- 3Sección Genética Evolutiva, Departamento de Biología Animal, Instituto de Biología, Facultad de Ciencias, Universidad de la República, Montevideo, Uruguay
- 4Departamento de Ciencias Clínicas, Facultad de Ciencias Veterinarias Universidad de Concepción, Chillán, Chile
Canine parvovirus type 2 (CPV2) is one of the most important intestinal pathogens in dogs and puppies. CPV2 has been evolved into three genetic and antigenic variants (2a, 2b, and 2c), which are distributed worldwide. We reported the first study of genetic diversity of CPV2 in Chile. Sixty-five samples were collected from puppies presenting with severe gastroenteritis and different vaccination statuses. PCR, restriction fragment length polymorphism (RFLP), and partial sequencing of the coding region of the structural viral protein VP2 was performed. Thirty of a total of 65 samples tested positive by PCR out of which 19 were further classified as CPV2c and one as CPV2a using RFLP and Sanger sequencing. The phylogeny was in concordance with the RFLP analysis. This is the first report of the genetic characterization of CPV2 in Chile and reveals a high occurrence of CPV2c.
Introduction
Canine parvovirus (CPV) is a non-enveloped, linear, single-stranded DNA virus that belongs to the family Parvoviridae, genus Protoparvovirus. The CPV2 genome is 5.2 kb long containing two open reading frames (ORFs) (1). The non-structural ORF encodes two non-structural proteins (NS1 and NS2), the structural ORF encodes the capsid proteins VP1 and VP2 (2). The original CPV strain designated as type-2 (CPV2) was reported in the 1970s and soon after that in the 1980s, two antigenic variants termed CPV types 2a (CPV2a) and 2b (CPV2b) were reported (3). Today, the antigenic variants of CPV2 are classified based on the amino acid present at position 426 of the VP2 capsid protein, asparagine (Asn) for CVP2a, aspartic acid (Asp) for CPV2b, and glutamic acid (Glu) for CPV2c (3). The CVP2a was the first antigenic variant identified and is still ubiquitous. The CVP2b was first reported in 1984 in the United States and is now detected worldwide (4). The CVP2c was first identified in the 2000s in Italy (3) and later reported in Europe, Asia, and South America (5). Most recently, new antigenic variants of CPV2a/b emerged, generated by a specific mutation at position 297 (Ser-Ala), which has been denominated as new CPV2a/b variants (6–9).
In Chile, serologic evidence of CPV2 has been reported in both domestic dogs and wild canids (10, 11). CPV2 has previously been described in Chile; however, information about the genetic and antigenic diversity is not known. The aim of this study was to determine the genetic diversity of CPV2 in Chile.
Materials and Methods
Small-animal veterinarians were recruited to participate in sample collection during 2016–2017. Fecal samples from diarrhea cases suspected for CPV were collected with detailed clinical history and stored properly at −20°C until used. Data about age, gender, and vaccination status were recorded for each sample. DNA was extracted by fast boiling preparation protocol (12). Briefly, 100 μl of diarrheic fecal material was homogenized in 1 ml of phosphate buffered saline and boiled for 10 min. The preparation was centrifuged at 1,600G for 5 min, and the supernatant containing the genetic material was collected and stored at −20°C for further use. For CPV2 diagnosis, a fragment of VP2 (583 bp) was amplified following the protocol described by Buonavoglia et al. (13). The CPV-positive samples were further processed for another PCR, restriction fragment length polymorphism (RFLP) analysis, and Sanger sequencing. Briefly, a fragment of VP2 (1,315 bp) was amplified by PCR using primers forward 5′-GGA AAC CAA CCA TAC CAA CTC C-3′ and reverse 5′-GGA TTC CAA GTA TGA GAG GC-3′. The RFLP was performed with the restriction enzyme MboII for 2 h at 37°C. Digested products (10 μl) were run on a 0.8% agarose gel to see the RFLP pattern. All positive samples were submitted for Sanger sequencing. The sequences were aligned and trimmed in Clustal W using the MEGA 7.0 analysis software (14). The phylogenetic tree was constructed using CPV2 reference sequences including those reported from neighboring countries. The phylogenetic tree was generated by the Bayesian method using MrBayes 3.2 software (15, 16) running 2 million iterations, sampling every 50 iterations, with the first 25% of samples discarded as burn-in. The phylogenetic tree was visualized and edited with FigTree (17).
Results and Discussion
Sixty-five clinical cases compatible with CPV2 infection were collected from three different locations in Chile, separated by distances ranging from 70 to 470 km. All samples were obtained from puppies younger than 7 months of age, which presented with severe diarrhea and other symptoms such as fever, vomiting, anorexia, and dehydration. Data about vaccination status, gender, and age are described in Supplementary Table 1.
The 46% (30 out of 65) of the samples that tested positive by PCR indicate the important role of CPV2 in causing gastroenteritis in puppies. In general, positive animals were unvaccinated or had an incomplete vaccination schedule, reinforcing the need to increase vaccination efforts to reduce CPV2 prevalence. However, the results suggested that there may be other causative agents associated with severe diarrhea that require further investigation. RFLP was performed successfully on 20 samples only, and the remaining 10 samples which were weak positive by PCR could not be characterized. The RFLP analysis classified 19 samples as CPV2c and one as CPV2a (Supplementary Figure 1). CPV2b variant was not identified.
Sanger sequencing was attempted for 30 PCR positive samples; however, only 13 were successfully sequenced and deposited in GenBank (accession numbers MN389736–MN389748). The alignment analysis confirmed the identify of CPV2 variants in concordance with the RFLP analysis (Supplementary Table 2 and Supplementary Figure 2). Chilean CPV2c sequences showed high identity between them (99.9%), and NCBI BLAST had 99.7 to 100% pairwise nucleotide identity values when compared with sequences reported in Uruguay, Argentina, and Mexico. Three samples had an Ala440Thr amino acid substitution, which is antigenically relevant because of its external position in the viral capsid (3, 18, 19). The same amino acid substitution has been observed in Argentina (19) and may suggest that Chilean and Argentine CPV2c populations may have the same origin. Finally, the phylogeny was in concordance of RFLP and alignment analysis. All the Chilean CPV2c were grouped with sequences from Uruguay, Argentina, Paraguay, Ecuador, and other countries all antigenically classified as CVP2c (Figure 1). On the other hand, the closest sequence to the CPV2a strain using nucleotide BLAST was EC/01/2017 (MG264075.1) with 100% identity. The CVP2a strain was identified as a singleton which is genetically related with both CPV2a and CPV2b sequences.
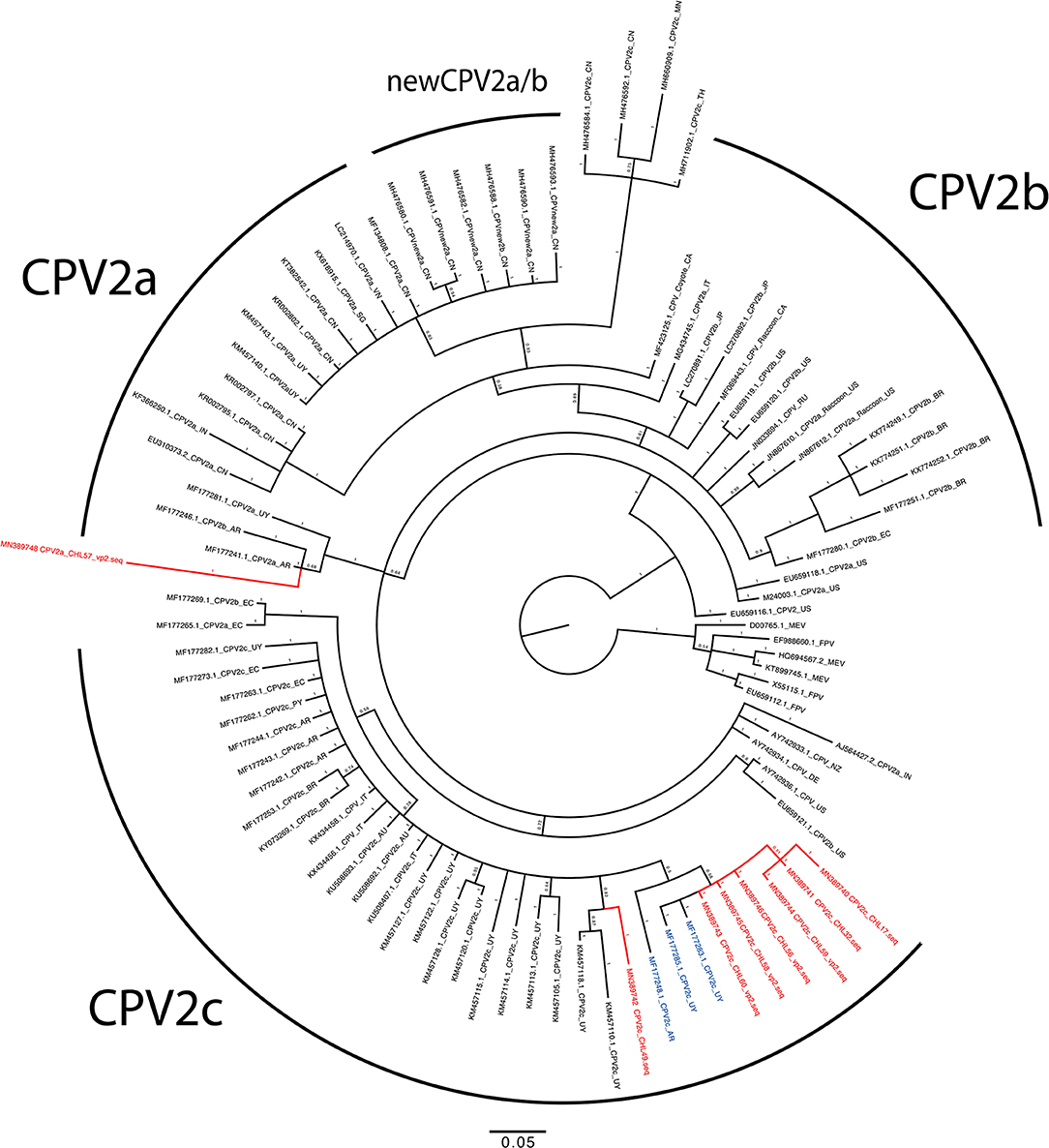
Figure 1. Phylogenetic tree of partial genome of VP2 gene of canine parvovirus (CPV) from Chile, South American strains, and references strains. The phylogenetic tree was constructed by using the Bayesian method using Mr. Bayes running 2 million iterations, sampling every 50 iterations, with the first 25% samples discarded as burn-in. The posterior probability values are indicated in each node. Chilean sequences are highlighted in red, and relevant sequences from Argentina and Uruguay are depicted in blue.
This is the first study describing the genetic diversity of CPV2 in Chile. Previous studies in Chile have reported the serologic identification of CPV2 in both domestic and wild canids, demonstrating that the virus is ubiquitous in Chile. However, genetic variants of CPV2 have been reported from different countries in South America, indicating diversity of this virus (5, 20–22). This study highlighted the high occurrence of CPV2c antigenic variant in puppies affected with severe diarrhea from Chillan city and its widespread presence in three distant locations (470 km) (Supplementary Figure 3). CPV2c variant is considered an emerging pathogen causing epizootics in several countries around the world (3, 23, 24). The occurrence of CPV2c variant has been described in other South American countries including Uruguay, Argentina (19, 25), and Brazil (7, 26, 27). Recently, it has been reported that the CPV2c variants from Argentina originated from Europe and later spread to Brazil and Uruguay (5) and, based on our results, also probably to Chile. On the other hand, CPV2a is still circulating in domestic dogs in South America and predominant in Peru (21), Colombia (22), and recently an increase in the number of cases of this strain has been reported in Uruguay (25). The most recent reports from Latin America demonstrate the presence of the new CPV2a in Ecuador (28), Columbia (22), Brazil (7), and the Caribbean (29).
The small sample size from three cities is a main limitation of this study. Hence, the results may not be representative of the genetic and antigenic diversity of CPV2 in the whole country. However, our results confirm the presence of two variants, CPV2a and CPV2c, in Chile. The CPV2c variant is the predominant variant in Chillan city, but the low number of samples in Los Andes and Santiago may not represent the real diversity. In spite of these limitations, the results confirm that CVP2c is widespread in Chile in a range of 470 km. Further studies are required to confirm the predominance of CPV2c across the country as well as to confirm the absence of CPV2b, which was not identified in this study. We only analyzed partial genomes, but in general, all studies target the VP2 because it is the major antigenic protein.
In conclusion, this is the first genetic characterization of the CPV2 in Chilean dog populations the results of which confirm the widespread occurrence of the CPV2c variant. Future investigations particularly molecular epidemiological studies should be conducted to understand the impact of this virus on domestic and wild canine populations.
Data Availability Statement
The data for this manuscript is uploaded to the NCBI GenBank (accession numbers MN389736–MN389748).
Ethics Statement
The animal study was reviewed and approved by Comité de Bioetica de la Facultad de Ciencias Veterinarias, Universidad de Concepcion. Written informed consent for participation was not obtained from the owners because the animals were not identified individually.
Author Contributions
CC, VN, RO, SG, and RP wrote the manuscript. VN, PA, and SC collected samples. PA, N-AZ, AS, VN, SG, RP, YP, DS, and RO performed the data analysis and data interpretation. RP and RO were in charge of the study design.
Funding
This study was partly funded by project VRID Inicio, UdeC number 217.152.024-1.0 to RO, the Programa Fondecyt de Iniciación No. 11170877, and the Programa de Investigación Asociativa from the Comisión Nacional de Investigación Científica y Tecnológica, project CONICYT-PIA Anillo ACT 1408 to VN.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the staff of FISENLAB for all their support in the initial processing sampling, especially to Pedro Rojas. We thank the Molecular Diagnostic Laboratory of Department of Animal Pathology, especially to Álvaro Ruiz, for all their support in providing their facilities. We thank Dr. Francisco Espina Lopez for providing clinical cases.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2020.00194/full#supplementary-material
References
1. Cotmore SF, Agbandje-McKenna M, Chiorini JA, Mukha D V, Pintel DJ, Qiu J, et al. The family parvoviridae. Arch Virol. (2014) 159:1239–47. doi: 10.1007/s00705-013-1914-1
2. Reed AP, Jones E V, Miller TJ. Nucleotide sequence and genome organization of canine parvovirus. J Virol. (1988) 62:266–76.
3. Decaro N, Buonavoglia C. Canine parvovirus–a review of epidemiological and diagnostic aspects, with emphasis on type 2c. Vet Microbiol. (2012) 155:1–12. doi: 10.1016/j.vetmic.2011.09.007
4. Parrish CR. Host range relationships and the evolution of canine parvovirus. Vet Microbiol. (1999) 69:29–40.
5. Grecco S, Iraola G, Decaro N, Alfieri A, Alfieri A, Gallo Calderón M, et al. Inter- and intracontinental migrations and local differentiation have shaped the contemporary epidemiological landscape of canine parvovirus in South America. Virus Evol. (2018) 4:vey011. doi: 10.1093/ve/vey011
6. Guo L, Yang S, Chen S, Zhang Z, Wang C, Hou R, et al. Identification of canine parvovirus with the Q370R point mutation in the VP2 gene from a giant panda (Ailuropoda melanoleuca). Virol J. (2013) 10:163. doi: 10.1186/1743-422X-10-163
7. de Oliveira PSB, Cargnelutti JF, Masuda EK, Weiblen R, Flores EF. New variants of canine parvovirus in dogs in southern Brazil. Arch Virol. (2019) 164:1361–9. doi: 10.1007/s00705-019-04198-w
8. Ohshima T, Hisaka M, Kawakami K, Kishi M, Tohya Y, Mochizuki M. Chronological analysis of canine parvovirus type 2 isolates in Japan. J Vet Med Sci. (2008) 70:769–75. doi: 10.1292/jvms.70.769
9. Martella V, Cavalli A, Decaro N, Elia G, Desario C, Campolo M, et al. Immunogenicity of an intranasally administered modified live canine parvovirus type 2b vaccine in pups with maternally derived antibodies. Clin Diagn Lab Immunol. (2005) 12:1243–5. doi: 10.1128/CDLI.12.10.1243-1245.2005
10. Acosta-Jamett G, Cunningham AA, Bronsvoort BM, Cleaveland S. Serosurvey of canine distemper virus and canine parvovirus in wild canids and domestic dogs at the rural interface in the Coquimbo region, chile. Eur J Wildl Res. (2015) 61:329–32. doi: 10.1007/s10344-014-0886-0
11. Acosta-Jamett G, Surot D, Cortés M, Marambio V, Valenzuela C, Vallverdu A, et al. Epidemiology of canine distemper and canine parvovirus in domestic dogs in urban and rural areas of the Araucanía region in chile. Vet Microbiol. (2015) 178:260–4. doi: 10.1016/j.vetmic.2015.05.012
12. Truyen U, Schunck B, Kraft W. Short communication a simple touch-down polymerase chain reaction for the detection of canine parvovirus and feline panleukopenia virus in feces. J Virol Methods. (1995) 55:427–433.
13. Buonavoglia C, Martella V, Pratella A, Tempesta M, Cavalli A, Buonavoglia D, et al. Evidence for evolution of canine parvovirus type 2 in Italy. J Gen Virol. (2001) 82:3021–5. doi: 10.1099/0022-1317-82-12-3021
14. Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. (2016) 33:msw054. doi: 10.1093/molbev/msw054
15. Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, et al. Mrbayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol. (2012) 61:539–42. doi: 10.1093/sysbio/sys029
16. Darriba D, Taboada GL, Doallo R, Posada D. JModelTest 2: more models, new heuristics and parallel computing. Nat Methods. (2012) 9:772. doi: 10.1038/nmeth.2109
17. Rambaut A. FigTree v111: Tree Figure Drawing Tool. Available online at: http://tree.bio.ed.ac.uk/software/figtree (accessed March 21, 2019).
18. Geng Y, Guo D, Li C, Wang E, Wei S, Wang Z, et al. Co-Circulation of the rare CPV-2c with unique Gln370Arg substitution, new CPV-2b with unique Thr440Ala substitution, and new CPV-2a with high prevalence and variation in Heilongjiang province, Northeast China. PLoS ONE. (2015) 10:e0137288. doi: 10.1371/journal.pone.0137288
19. Calderón MG, Romanutti C, D'Antuono A, Keller L, Mattion N, La Torre J. Evolution of canine parvovirus in Argentina between years 2003 and 2010: CPV2c has become the predominant variant affecting the domestic dog population. Virus Res. (2011) 157:106–10. doi: 10.1016/j.virusres.2011.02.015
20. Calderón MG, Romanutti C, Wilda M, D'Antuono A, Keller L, Giacomodonato MN, et al. Resurgence of canine parvovirus 2a strain in the domestic dog population from Argentina. J Virol Methods. (2015) 222:145–9. doi: 10.1016/j.jviromet.2015.06.012
21. Quino Quispe R, Luna Espinoza L, Rímac Beltrán R, Rosadio Alcántara R, Maturrano Hernández L. Canine parvovirus types 2a and 2c detection from dogs with suspected parvoviral enteritis in Peru. Virusdisease. (2018) 29:109–12. doi: 10.1007/s13337-018-0425-9
22. Duque-García Y, Echeverri-Zuluaga M, Trejos-Suarez J, Ruiz-Saenz J. Prevalence and molecular epidemiology of Canine parvovirus 2 in diarrheic dogs in Colombia, South America: a possible new CPV-2a is emerging? Vet Microbiol. (2017) 201:56–61. doi: 10.1016/j.vetmic.2016.12.039
23. Charoenkul K, Tangwangvivat R, Janetanakit T, Boonyapisitsopa S, Bunpapong N, Chaiyawong S, et al. Emergence of canine parvovirus type 2c in domestic dogs and cats from Thailand. Transbound Emerg Dis. (2019) 66:1518–28. doi: 10.1111/tbed.13177
24. Mira F, Purpari G, Di Bella S, Colaianni ML, Schirò G, Chiaramonte G, et al. Spreading of canine parvovirus type 2c mutants of Asian origin in southern Italy. Transbound Emerg Dis. (2019) 66:2297–304. doi: 10.1111/tbed.13283
25. Rez RP, Calleros L, Marandino A, Sarute N, Iraola G, Grecco S, et al. Phylogenetic and genome-wide deep-sequencing analyses of Canine parvovirus reveal co-infection with field variants and emergence of a recent recombinant strain. PLoS ONE. (2014) 9:e111779. doi: 10.1371/journal.pone.0111779
26. Pinto LD, Streck AF, Gonçalves KR, Souza CK, Corbellini ÂO, Corbellini LG, et al. Typing of canine parvovirus strains circulating in Brazil between 2008 and 2010. Virus Res. (2012) 165:29–33. doi: 10.1016/j.virusres.2012.01.001
27. Fontana DS, Rocha PRD, Cruz RAS, Lopes LL, Melo ALT, Silveira MM, et al. A phylogenetic study of canine parvovirus type 2c in midwestern Brazil. Pesqui Vet Bras. (2013) 33:214–8. doi: 10.1590/S0100-736X2013000200013
28. De la Torre D, Mafla E, Puga B, Erazo L, Astolfi-Ferreira C, Ferreira AP. Molecular characterization of canine parvovirus variants (CPV-2a, CPV-2b, and CPV-2c) based on the VP2 gene in affected domestic dogs in ecuador. Vet World. (2018) 11:480–7. doi: 10.14202/vetworld.2018.480-487
29. Navarro R, Nair R, Peda A, Aung MS, Ashwinie GS, Gallagher CA, et al. Molecular characterization of canine parvovirus and canine enteric coronavirus in diarrheic dogs on the island of St. Kitts: first report from the Caribbean region. Virus Res. (2017) 240:154–60. doi: 10.1016/j.virusres.2017.08.008
Keywords: canine parvovirus type 2, CPV2c, Chile, genetic characterization, South America
Citation: Castillo C, Neira V, Aniñir P, Grecco S, Pérez R, Panzera Y, Zegpi N-A, Sandoval A, Sandoval D, Cofre S and Ortega R (2020) First Molecular Identification of Canine Parvovirus Type 2 (CPV2) in Chile Reveals High Occurrence of CPV2c Antigenic Variant. Front. Vet. Sci. 7:194. doi: 10.3389/fvets.2020.00194
Received: 20 October 2019; Accepted: 24 March 2020;
Published: 05 May 2020.
Edited by:
Armanda Bastos, University of Pretoria, South AfricaReviewed by:
Souvik Ghosh, Ross University School of Veterinary Medicine, Saint Kitts and NevisFernando Bauermann Oklahoma State University, United States
Copyright © 2020 Castillo, Neira, Aniñir, Grecco, Pérez, Panzera, Zegpi, Sandoval, Sandoval, Cofre and Ortega. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Victor Neira, vneiraram@gmail.com; Rene Ortega, reortega@udec.cl
 Cristobal Castillo
Cristobal Castillo Victor Neira
Victor Neira Pamela Aniñir
Pamela Aniñir Sofia Grecco
Sofia Grecco Ruben Pérez
Ruben Pérez Yanina Panzera3
Yanina Panzera3