Experimental Neospora caninum Infection in Pregnant Cattle: Different Outcomes Between Inoculation With Tachyzoites and Oocysts
- 1Department of Anatomy, Pathology and Veterinary Clinics, School of Veterinary Medicine and Animal Science, Federal University of Bahia, Salvador, Brazil
- 2School of Animal and Veterinary Sciences, The University of Adelaide, Adelaide, SA, Australia
Neospora caninum is a globally distributed abortifacient protozoan of cattle. Experimental infections with N. caninum in cattle have provided valuable information on host-parasite interaction and immunopathogenesis. Experimental infection of pregnant cows has been reported in about 20 articles, with most studies using cultured parasite tachyzoites as the inoculum. Only three experimental studies have been conducted in pregnant cows using the parasite's oocysts which are shed by dogs, in large part because transmission experiments using oocysts take more time and are more complex and expensive than experiments using tachyzoites. In this minireview, we discuss differences between N. caninum tachyzoites and oocysts as inocula for experimental infection of pregnant cows, as well as the route animals are inoculated.
Introduction
Neospora caninum is a cyst-forming coccidian parasite that was initially observed in dogs with neuromuscular disorders (1, 2) and was named in 1988 after a retrospective work using formalin-fixed paraffin embedded canine tissues (3). In the same year of its classification, N. caninum was isolated from dogs and propagated in cell culture (4). Even before the naming of N. caninum in 1988, the parasite had been identified by two research groups in calves with encephalomyelitis (5, 6); those authors pointed out that the parasite was distinct from Toxoplasma gondii and Sarcocystis sp., but they did not name it. Within a few years of its primary description, N. caninum was identified as a common cause of bovine abortion worldwide (7–10).
Three natural methods of transmission of N. caninum are believed to occur in cattle: (1) ingestion of sporulated oocysts of the parasite (horizontal transmission); (2) transplacental infection (vertical transmission) from a previously infected dam to its offspring (11); or (3) horizontal transmission to a pregnant dam followed by vertical transmission to its fetus (combined horizontal and vertical transmission). Useful technical terms to distinguish these three transmission methods are: (1) horizontal; (2) endogenous transplacental; and (3) exogenous transplacental transmission (12).
Dogs (13) and several other canids (14–16) are definitive hosts of N. caninum, shedding oocysts of the parasite in feces after consuming tissues of N. caninum-infected mice or cattle in experiments (17). Domestic dogs are the most widely distributed definitive host and are the major source of horizontal transmission to cattle worldwide.
Studies on experimental bovine neosporosis have been conducted using animals inoculated with two stages of N. caninum: tachyzoites or oocysts. In the majority of the studies, the inocula consisted of tachyzoites propagated in cell culture. Tachyzoites have been inoculated in cows by different routes, such as intravenous (IV), subcutaneous (SC), intramuscular (IM), intra-uterine, and conjunctival (18–21). Sporulated oocysts were employed as inoculum for pregnant cows in only three studies and the cows were inoculated orally (22–24). This minireview compares the outcome of transplacental infection, abortion or fetal death following experimental administration of N. caninum to cattle using tachyzoites or oocysts.
Experimental Infection in Cattle With N. caninum Tachyzoites
In previous studies, prior to identification of the oocyst stage shed by dogs, N. caninum tachyzoites were inoculated in cattle and provided valuable data on the immunopathogenesis of bovine neosporosis (19, 25). The rationale for using tachyzoites on experimental infection in pregnant cows is to mimic the propagation of the parasite by two different modes: (1) conversion of bradyzoites to tachyzoites derived from recrudescence of a latent infection; (2) conversion of sporozoites to tachyzoites following ingestion of sporulated oocysts (26). A number of additional studies have inoculated tachyzoites into pregnant cows, and 14 of them are partially summarized in Table 1.
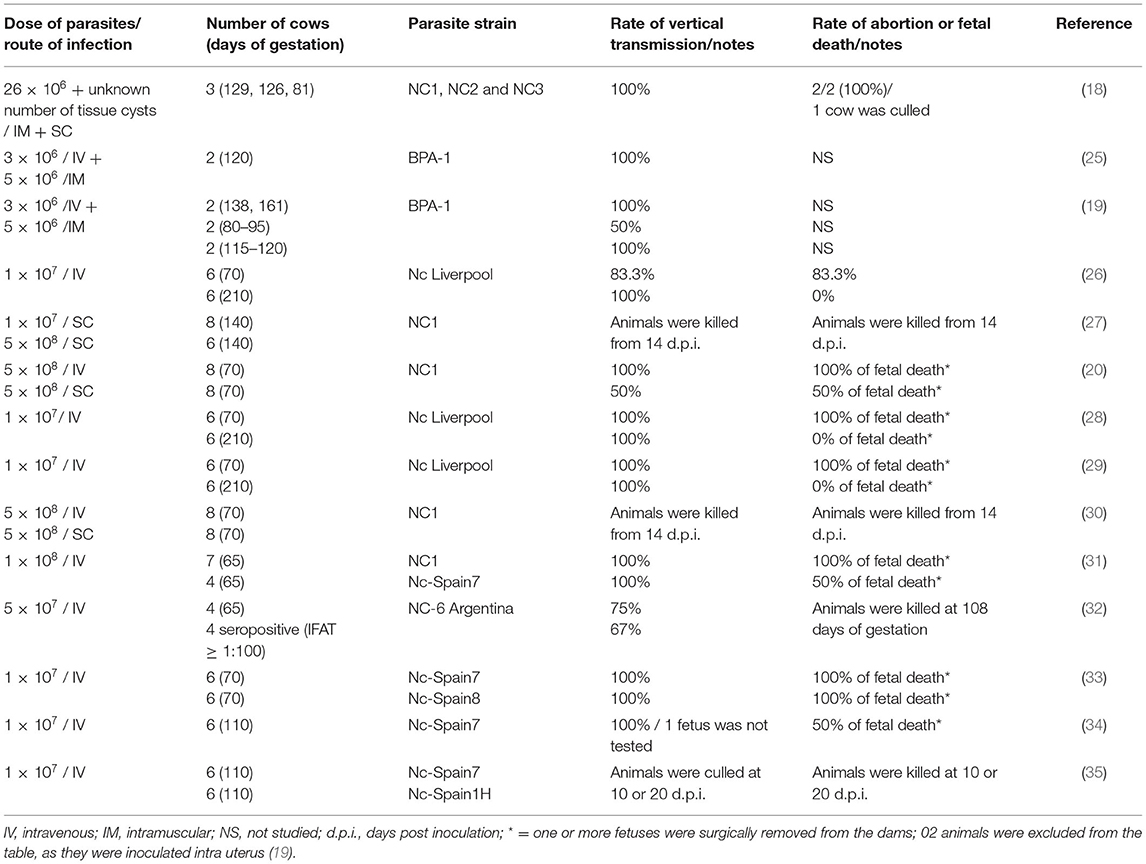
Table 1. Experimental infection of pregnant cattle with Neospora caninum tachyzoites or tissue cysts (a single report).
Transplacental infection is consistently achieved by IV injection of N. caninum tachyzoites in pregnant cows, even when inoculated in the first trimester and regardless whether the cows are seronegative or seropositive at the time of tachyzoite administration. Intravenous inoculation of high numbers of tachyzoites (107 to 5 × 108) in seronegative cows at 65 or 70 days of gestation induced transplacental infection in almost 100% of animals, and this caused abortion in more than 80% (20, 26, 28, 29, 32, 33, 36). Likewise, IV administration of 5 × 107 tachyzoites to seropositive cows on the 65th day of gestation was associated with transplacental infection in all animals, however that study was terminated after 43 days so it was not possible to definitively conclude whether there would have been an effect on abortion (32).
When pregnant cows are SC inoculated, the spread of tachyzoites is slower in comparison with the IV route, and the innate immune-response of the animals seems to alleviate the rapid propagation of the parasite and the occurrence of fetal lesions (30). A study was performed with cows infected IV or SC at 70 days of gestation with 5 × 108 tachyzoites; 50% of the cows that were SC inoculated presented fetal death, whereas fetal death or abortions were observed in all of the IV inoculated animals (30).
The performance of N. caninum strains with high or low-to-moderate virulence (as determined in vitro and in mouse bioassays) was evaluated evaluated in pregnant cows (33). The animals were inoculated IV at 70 days of pregnancy with 107 tachyzoites of each strain. Fetal death was observed in all inoculated animals, although it occurred more rapidly in those inoculated with the most virulent strain (33).
In other relevant studies, pregnant cows were inoculated with tachyzoites at 110 or 210 days of gestation. In cows inoculated IV with 107 tachyzoites at 110 days of gestation, transplacental infection also occurred in 100% (n = 6) of the tested animals (34); however, abortions were observed in only 50% (3/6), contrasting with the 100% of abortions detected in cows inoculated at 70 days of gestation. In cows inoculated IV with 107 tachyzoites of the NC-Liverpool strain at 210 days of gestation, transplacental infection occurred in all animals, but no fetal death was observed (28, 29); the authors stated that the immunocompetence of the fetus at 210 days plays a critical role in its survival.
Experimental N. caninum infection by the intra-uterine route was conducted in a study (19), but is not further considered in this review which focusses on transplacental transmission. In another study, N. caninum tachyzoites were inoculated by the conjunctival (2 cows) or IV route (2 cows) with 2.5 × 108 tachyzoites at 161 days of gestation; no transplacental infection occurred in cows inoculated by the conjunctival route, in contrast with those IV inoculated, which had transplacental transmission. In three studies, pregnant cows were inoculated with N. caninum tachyzoites using combined IM and IV routes (18, 19, 25); as the IM route was not attempted by itself in any study, outcomes attributable to IM inoculation could not be clearly defined for the purposes of this review.
Experimental Infection in Cattle With N. caninum Oocysts
Bovine abortion outbreaks due to neosporosis continue to be a serious economic problem. Exposure of cattle to oocysts shed by dogs or wild definitive hosts may cause point source exposure of pregnant cows and the occurrence of abortion storms (37–40).
Identification of the domestic dog as a definitive host of N. caninum and experimental production of oocysts (13) allowed investigation of exogenous transplacental transmission (i.e., horizontal exposure with vertical transmission) and associated immune responses in cattle infected with N. caninum oocysts. The rationale for inoculating oocysts in cattle by the oral route is to simulate ingestion of the parasite in food or water (23).
Experimental infections with N. caninum oocysts in cattle were first conducted in calves (41). Oral inoculation of oocysts in seven calves induced infection in all animals, which developed N. caninum specific IgG antibodies starting at 2 weeks post inoculation (p.i.). Detectable antibody titers, as well as lymphocyte proliferation response to N. caninum, persisted until the moment calves were euthanized, ~2.5 months p.i. (41).
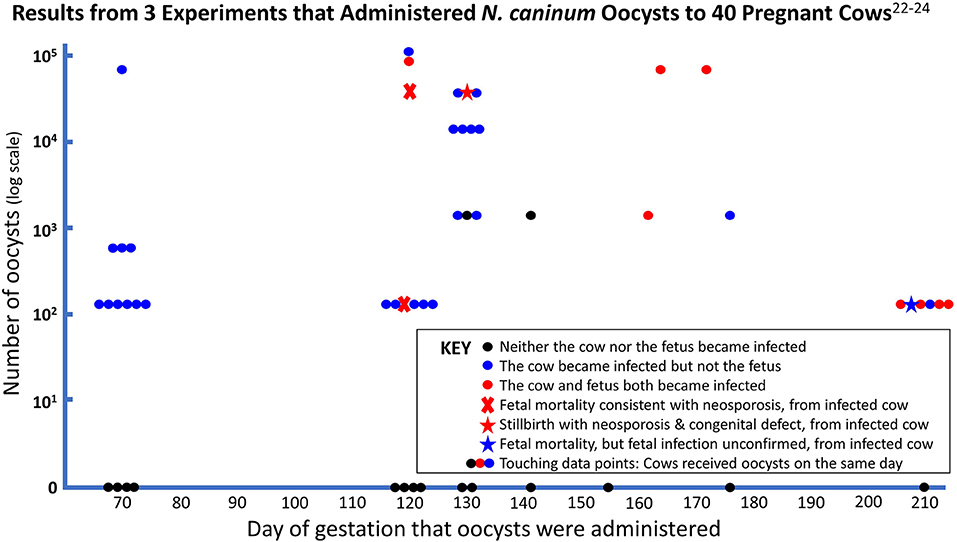
In the first experimental inoculation of N. caninum oocysts in pregnant cows, three seronegative cows were orally inoculated with 600 oocysts at 70 days of gestation (22); although infection was confirmed in the inoculated cows, transplacental transmission did not occur. The three cows developed detectable antibody titers starting at 3 weeks p.i. and delivered non-infected calves. In a second study, 19 seronegative pregnant cows were orally inoculated with variable numbers of N. caninum oocysts (1,500–115,000) and 17 cows developed IgG antibodies to the parasite (23). Among the 19 inoculated cows, only one received oocysts (70,000) at 70 days of gestation. The remaining cows (n = 18) received oocysts at 120–176 days of gestation. After inoculation of oocysts, 10 of 11 cows that became infected and did not transmit infection to their fetuses, had decreasing antibody titers after an apex that occurred between 14 and 42 days p.i. In those cows that presented transplacental infection (n = 6), antibody titers continued to increase until delivery of infected calves or at the time of euthanasia. The risk of transplacental infection in the inoculated animals increased with larger doses of oocysts and later than 160 days of gestation. Two of four cows that were infected with 41,000 oocysts at 120–130 days of gestation had N. caninum abortion or stillbirth, however death of the stillborn calf was conservatively not attributed to neosporosis, despite having a high antibody titer and typical histological lesions, because it also had an unrelated congenital defect. Seven other cows administered lower doses of oocysts on gestational day 130 did not have transplacental transmission.
A third study was conducted using 18 pregnant cows that received oocysts by the oral route at three different moments of pregnancy (70, 120 or 210 days) (24). Although 40,000 oocysts were administered to each animal, a bioassay indicated that only 127 of the oocysts were viable in each dose (author's note: the shipment of oocysts had prolonged delay of delivery with thawed ice packs). Transplacental infection did not occur in six cows inoculated at 70 days of pregnancy. Abortion was observed in one of the six cows inoculated at 120 days of gestation, while the remaining five cows did not present transplacental infection. Four of six calves from cows administered oocysts at 210 days of gestation had subclinical congenital infections. Seven of the cows that became infected after oocyst inoculation were selected for rebreeding; these seven cows gave birth to non-infected calves in the following pregnancy.
Results of all three studies that used oocysts in pregnant cows are summarized in Figure 1.
Tachyzoites vs. Oocysts in Experimental Infection of Pregnant Cows
Under natural conditions, it is difficult to precisely predict the outcome of transplacental infection by N. caninum in cattle. A single serological screening cannot accurately identify all infected or non-infected cows (31). Experimental infections of pregnant cows with N. caninum tachyzoites or oocysts may answer numerous questions, including the outcomes of vertical transmission and fetal lesions or fetal death. Moreover, a bovine model for neosporosis that mimics natural challenge of the parasite is needed to accurately predict the efficacy of potential vaccines.
In the majority of experimental infections in cows, the most frequent times of gestation selected for these studies were 70, 110 and 210 days, which represent the late first, early second, and third trimesters of pregnancy. In most reports, cows were inoculated by the IV route with high numbers of tachyzoites. In only three studies, cows were orally administered N. caninum oocysts, and the viability of the oocysts in one of those studies was very low (127 per 40,000), apparently affected by international shipping conditions.
As described above, pregnant cows infected IV at 70 days of gestation with high numbers of N. caninum tachyzoites (≥ 107) consistently exhibited transplacental infection and fetal death (20, 26, 28–30). In contrast, in three experiments, no cow orally inoculated at 70 days of gestation with up to 70,000 oocysts transmitted the infection to the developing fetus (22–24). These findings are strong evidence that transplacental transmission of horizontally acquired infection is unlikely to occur in the first trimester of gestation, but when the transmission barrier is overcome by IV inoculation of tachyzoites, then the first trimester fetus is highly susceptible to fatal infection.
Cows infected IV with tachyzoites (≥ 107) at the second trimester of gestation (110 days) consistently presented transplacental infection, but a lower rate of fetal death (about 50%) when compared with cows at 70 days of gestation. When oocysts were administered to 9 cows at 120 days of gestation (127–41,000 oocysts), two neosporosis abortions (22%) were reported among the tested cows (23, 24), as well as a stillbirth with diagnostic complications. Twenty-two percent is well within the range of the abortion incidence of several naturally-occurring neosporosis abortion outbreaks (23), despite six of the nine cows having only received 127 viable oocysts. Abortions occurred 39–44 days after inoculation, corresponding with abortion at about 5 months of gestation, which is similar to the average time for naturally occurring neosporosis abortions, reported to be 5.4 months (42).
In the third trimester, both IV inoculation of tachyzoites and oral administration of oocysts led to high rates of transplacental transmission and the birth of congenitally infected but clinically healthy calves. Intravenous inoculation of millions of tachyzoites caused vertical transmission of the parasite in all animals, whereas administration of oocysts later than 160 days, mostly using very low doses (127 viable oocysts), induced subclinical congenital infection in 7 of 10 offspring (70%).
Viewed together, these experiments indicate that transplacental transmission is rare when naïve cows consume oocysts in the first trimester when the fetus is otherwise defenseless, and that transplacental transmission is highly efficient when oocysts are consumed in the third trimester when the fetal immune system is able to withstand the infectious challenge. There appears to be a transitional period, later than 70 days but earlier than 160 days, when transplacental infection occurs in a proportion of pregnancies and is likely to be fatal when it happens, leading to abortion about 6 weeks later. The precision of this observation needs to be improved by experimenting with greater numbers of pregnant cows administered higher doses of N. caninum oocysts within this susceptible period. The gestational pattern of neosporosis susceptibility and consequences in cattle appears to be similar to congenital toxoplasmosis in humans (43).
Future Directions
Despite the difficulty of conducting experimental N. caninum infections in cattle, future studies should focus on mimicking, as much as possible, the natural route of infection. Unfortunately, experimental production of N. caninum oocysts adds greatly to the complexity, duration, and cost of already expensive bovine gestational experiments. Establishing networks to find, collect, and share N. caninum oocysts from naturally infected dogs might provide an alternative method of sourcing oocysts for use in bovine gestational experiments. Due to the sporadic excretion of N. caninum oocysts under natural conditions, experimental infection in dogs by feeding them tissues from naturally or experimentally infected animals may be considered; in these bioassays, local strains of the parasite should be used, and the dogs, whenever possible, should be offered for donation after conclusion of the experiment, instead of euthanized, to alleviate ethical concerns of using dogs.
Author Contributions
LG drafted the manuscript. Both authors contributed to the article and approved the submitted version.
Funding
LG was recipient of a productivity fellowship by Conselho Nacional de Desenvolvimento Científico e Tecnológico (Process number: 311051/2019-7) from Brazil.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hilali M, Lindberg R, Waller T, Wallin B. Enigmatic cyst-forming sporozoon in the spinal cord of a dog. Acta Vet Scand. (1986) 27:623–5. doi: 10.1186/BF03548142
2. Bjerkas I, Mohn SF, Presthus J. Unidentified cyst-forming sporozoon causing encephalomyelitis and myositis in dogs. Z Parasitenkd. (1984) 70:271–4. doi: 10.1007/BF00942230
3. Dubey JP, Carpenter JL, Speer CA, Topper MJ, Uggla A. Newly recognized fatal protozoan disease of dogs. J Am Vet Med Assoc. (1988) 192:1269–85.
4. Dubey JP, Hattel AL, Lindsay DS, Topper MJ. Neonatal Neospora caninum infection in dogs: isolation of the causative agent and experimental transmission. J Am Vet Med Assoc. (1988) 193:1259–63.
5. O'Toole D, Jeffrey M. Congenital sporozoan encephalomyelitis in a calf. Vet Rec. (1987) 121:563–6.
6. Parish SM, Maag-Miller L, Besser TE, Weidner JP, McElwain T, Knowles DP, et al. Myelitis associated with protozoal infection in newborn calves. J Am Vet Med Assoc. (1987) 191:1599–600.
7. Thilsted JP, Dubey JP. Neosporosis-like abortions in a herd of dairy cattle. J Vet Diagn Invest. (1989) 1:205–9. doi: 10.1177/104063878900100301
8. Anderson M, Barr BC, Conrad PA, Thurmond M, Picanso J, Dubey JP. Bovine protozoal abortions in california. Bov Pract. (1991). 102–4.
9. Thorton RN, Thompson EJ, Dubey JP. Neospora abortion in New Zealand cattle. N Z Vet J. (1991) 39:129–33. doi: 10.1080/00480169.1991.35679
10. Wouda W, van den Ingh TS, van Knapen F, Sluyter FJ, Koeman JP, Dubey JP. [Neospora abortion in cattle in the Netherlands]. Tijdschr Diergeneeskund. (1992) 117:599–602.
11. Goodswen SJ, Kennedy PJ, Ellis JT. A review of the infection, genetics, and evolution of Neospora caninum: from the past to the present. Infect Genet Evol. (2013) 13:133–50. doi: 10.1016/j.meegid.2012.08.012
12. Trees AJ, Williams DJ. Endogenous and exogenous transplacental infection in Neospora caninum and Toxoplasma gondii. Trends Parasitol. (2005) 21:558–61. doi: 10.1016/j.pt.2005.09.005
13. McAllister MM, Dubey JP, Lindsay DS, Jolley WR, Wills RA, McGuire AM. Dogs are definitive hosts of Neospora caninum. Int J Parasitol. (1998) 28:1473–8. doi: 10.1016/S0020-7519(98)00138-6
14. Gondim LFP, McAllister MM, Pitt WC, Zemlicka DE. Coyotes (Canis Latrans) are definitive hosts of Neospora caninum. Int J Parasitol. (2004) 34:159–61. doi: 10.1016/j.ijpara.2004.01.001
15. King JS, Slapeta J, Jenkins DJ, Al-Qassab SE, Ellis JT, Windsor PA. Australian dingoes are definitive hosts of Neospora caninum. Int J Parasitol. (2010) 40:945–50. doi: 10.1016/j.ijpara.2010.01.008
16. Dubey JP, Jenkins MC, Rajendran C, Miska K, Ferreira LR, Martins J, et al. Gray wolf (Canis lupus) is a natural definitive host for Neospora caninum. Vet Parasitol. (2011) 181:382–7. doi: 10.1016/j.vetpar.2011.05.018
17. Gondim LFP, Gao L, McAllister MM. Improved production of Neospora caninum oocysts, cyclical oral transmission between dogs and cattle, and in vitro isolation from oocysts. J Parasitol. (2002) 88:1159–63. doi: 10.1645/0022-3395(2002)088[1159:IPONCO]2.0.CO;2
18. Dubey JP, Lindsay DS, Anderson ML, Davis SW, Shen SK. Induced transplacental transmission of Neospora caninum in cattle. J Am Vet Med Assoc. (1992) 201:709–13.
19. Barr BC, Rowe JD, Sverlow KW, BonDurant RH, Ardans AA, Oliver MN, et al. Experimental reproduction of bovine fetal Neospora infection and death with a bovine Neospora isolate. J Vet Diagn Invest. (1994) 6:207–15. doi: 10.1177/104063879400600212
20. Macaldowie C, Maley SW, Wright S, Bartley P, Esteban-Redondo I, Buxton D, et al. Placental pathology associated with fetal death in cattle inoculated with Neospora caninum by two different routes in early pregnancy. J Comp Pathol. (2004) 131:142–56. doi: 10.1016/j.jcpa.2004.02.005
21. Moore DP, Alvarez-Garcia G, Chiapparrone ML, Regidor-Cerrillo J, Lischinsky LH, de Yaniz MG, et al. Neospora caninum tachyzoites inoculated by the conjunctival route are not vertically transmitted in pregnant cattle: a descriptive study. Vet Parasitol. (2014) 199:1–7. doi: 10.1016/j.vetpar.2013.10.006
22. Trees AJ, McAllister MM, Guy CS, McGarry JW, Smith RF, Williams DJ. Neospora caninum: oocyst challenge of pregnant cows. Vet Parasitol. (2002) 109:147–54. doi: 10.1016/S0304-4017(02)00234-0
23. Gondim LFP, McAllister MM, Anderson-Sprecher RC, Bjorkman C, Lock TF, Firkins LD, et al. Transplacental transmission and abortion in cows administered Neospora caninum oocysts. J Parasitol. (2004) 90:1394–400. doi: 10.1645/GE-359R
24. McCann CM, McAllister MM, Gondim LF, Smith RF, Cripps PJ, Kipar A, et al. Neospora caninum in Cattle: experimental infection with oocysts can result in exogenous transplacental infection, but not endogenous transplacental infection in the subsequent pregnancy. Int J Parasitol. (2007) 37:1631–9. doi: 10.1016/j.ijpara.2007.05.012
25. Conrad PA, Sverlow K, Anderson M, Rowe J, BonDurant R, Tuter G, et al. Detection of serum antibody responses in cattle with natural or experimental Neospora infections. J Vet Diagn Invest. (1993) 5:572–8. doi: 10.1177/104063879300500412
26. Williams DJ, Guy CS, McGarry JW, Guy F, Tasker L, Smith RF, et al. Neospora caninum-associated abortion in cattle: the time of experimentally-induced parasitaemia during gestation determines foetal survival. Parasitology. (2000) 121:347–58. doi: 10.1017/S0031182099006587
27. Maley SW, Buxton D, Rae AG, Wright SE, Schock A, Bartley PM, et al. The pathogenesis of neosporosis in pregnant cattle: Inoculation at mid-gestation. J Comp Pathol. (2003) 129:186–95. doi: 10.1016/S0021-9975(03)00032-X
28. Gibney EH, Kipar A, Rosbottom A, Guy CS, Smith RF, Hetzel U, et al. The extent of parasite-associated necrosis in the placenta and foetal tissues of cattle following Neospora caninum infection in early and late gestation correlates with foetal death. Int J Parasitol. (2008) 38:579–88. doi: 10.1016/j.ijpara.2007.09.015
29. Rosbottom A, Gibney EH, Guy CS, Kipar A, Smith RF, Kaiser P, et al. Upregulation of cytokines is detected in the placentas of cattle infected with Neospora caninum and is more marked early in gestation when fetal death is observed. Infect Immun. (2008) 76:2352–61. doi: 10.1128/IAI.01780-06
30. Bartley PM, Wright SE, Maley SW, Macaldowie CN, Nath M, Hamilton CM, et al. Maternal and foetal immune responses of cattle following an experimental challenge with Neospora caninum at day 70 of gestation. Vet Res. (2012) 43:38. doi: 10.1186/1297-9716-43-38
31. Dijkstra T, Barkema HW, Eysker M, Beiboer ML, Wouda W. Evaluation of a single serological screening of dairy herds for Neospora caninum antibodies. Vet Parasitol. (2003) 110:161–9. doi: 10.1016/S0304-4017(02)00323-0
32. Bacigalupe D, Basso W, Caspe SG, More G, Lischinsky L, Gos ML, et al. Neospora caninum Nc-6 argentina induces fetopathy in both serologically positive and negative experimentally inoculated pregnant dams. Parasitol Res. (2013) 112:2585–92. doi: 10.1007/s00436-013-3424-1
33. Regidor-Cerrillo J, Arranz-Solis D, Benavides J, Gomez-Bautista M, Castro-Hermida JA, Mezo M, et al. Neospora caninum infection during early pregnancy in cattle: how the isolate influences infection dynamics, clinical outcome and peripheral and local immune responses. Vet Res. (2014) 45:10. doi: 10.1186/1297-9716-45-10
34. Almeria S, Serrano-Perez B, Darwich L, Domingo M, Mur-Novales R, Regidor-Cerrillo J, et al. Foetal death in naive heifers inoculated with Neospora caninum isolate Nc-Spain7 at 110 days of pregnancy. Exp Parasitol. (2016) 168:62–9. doi: 10.1016/j.exppara.2016.06.009
35. Garcia-Sanchez M, Jimenez-Pelayo L, Vazquez P, Horcajo P, Regidor-Cerrillo J, Jimenez-Melendez A, et al. Maternal and foetal cellular immune responses in dams infected with high- and low- virulence isolates of Neospora caninum at mid-gestation. Frot Cell Infect Microbiol. (2021) 11:684670. doi: 10.3389/fcimb.2021.684670
36. Caspe SG, Moore DP, Leunda MR, Cano DB, Lischinsky L, Regidor-Cerrillo J, et al. The Neospora caninum-spain 7 isolate induces placental damage, fetal death and abortion in cattle when inoculated in early gestation. Vet Parasitol. (2012) 189:171–81. doi: 10.1016/j.vetpar.2012.04.034
37. Perotta JH, Freitas BB, Marcom NN, Pescador CA, Pereira CC, Locatelli-Dittrich R, et al. An abortion storm in dairy cattle associated with neosporosis in Southern Brazil. Rev Bras Parasitol Vet. (2021) 30:e001821. doi: 10.1590/s1984-29612021045
38. Melendez P, Ilha M, Woldemeskel M, Graham J, Coarsey M, Baughman D, et al. An Outbreak of Neospora caninum abortion in a dairy herd from the state of Georgia, United States. Vet Med Sci. (2021) 7:141–7. doi: 10.1002/vms3.346
39. McAllister MM, Huffman EM, Hietala SK, Conrad PA, Anderson ML, Salman MD. Evidence suggesting a point source exposure in an outbreak of bovine abortion due to neosporosis. J Vet Diagn Invest. (1996) 8:355–7. doi: 10.1177/104063879600800313
40. McAllister MM, Bjorkman C, Anderson-Sprecher R, Rogers DG. Evidence of point-source exposure to Neospora caninum and protective immunity in a herd of beef cows. J Am Vet Med Assoc. (2000) 217:881–7. doi: 10.2460/javma.2000.217.881
41. De Marez T, Liddell S, Dubey JP, Jenkins MC, Gasbarre L. Oral infection of calves with Neospora caninum oocysts from dogs: humoral and cellular immune responses. Int J Parasitol. (1999) 29:1647–57. doi: 10.1016/S0020-7519(99)00154-X
42. Anderson ML, Blanchard PC, Barr BC, Dubey JP, Hoffman RL, Conrad PA. Neospora-like protozoan infection as a major cause of abortion in California dairy cattle. J Am Vet Med Assoc. (1991) 198:241–4.
Keywords: neosporosis, bovine, transplacental transmission, abortion, experimental
Citation: Gondim LFP and McAllister MM (2022) Experimental Neospora caninum Infection in Pregnant Cattle: Different Outcomes Between Inoculation With Tachyzoites and Oocysts. Front. Vet. Sci. 9:911015. doi: 10.3389/fvets.2022.911015
Received: 01 April 2022; Accepted: 27 April 2022;
Published: 17 May 2022.
Edited by:
Dadin Prando Moore, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaReviewed by:
Carlos Robello, Universidad de la República, UruguayLucia Maria Campero, Instituto de Innovación Para la Producción Agropecuaria y el Desarrollo Sostenible (IPADS), Argentina
Copyright © 2022 Gondim and McAllister. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luís F. Pita Gondim, pita@ufba.br
†These authors have contributed equally to this work
 Luís F. Pita Gondim
Luís F. Pita Gondim Milton M. McAllister
Milton M. McAllister