Meta-analysis of flavonoids use into beef and dairy cattle diet: Performance, antioxidant status, ruminal fermentation, meat quality, and milk composition
- 1Departamento de Zootecnia, Universidad Autónoma Chapingo, Texcoco, Mexico
- 2División Académica de Ciencias Agropecuarias, Universidad Juárez Autónoma de Tabasco, Villahermosa, Mexico
- 3Departamento de Producción Agrícola y Animal, Universidad Autónoma Metropolitana—Xochimilco, Mexico City, Mexico
The objective of this study was to evaluate the effects of dietary supplementation with flavonoids (FLAs) on animal performance, diet digestibility, antioxidant status in blood serum, rumen parameters, meat quality, and milk composition in beef and dairy cattle through a meta-analysis. Thirty-six peer-reviewed publications were included in the data set. The weighted mean differences (WMD) between the FLAs treatments and the control treatment were used to assess the effect size. Dietary supplementation with FLAs decreased feed conversion ratio (WMD = −0.340 kg/kg; p = 0.050) and increased (p < 0.05) dry matter intake (WMD = 0.191 kg/d), dry matter digestibility (WMD = 15.283 g/kg of DM), and daily weight gain (WMD = 0.061 kg/d). In blood serum, FLAs supplementation decreased the serum concentration of malondialdehyde (WMD = −0.779 nmol/mL; p < 0.001) and increased (p < 0.01) the serum concentration of superoxide dismutase (WMD = 8.516 U/mL), glutathione peroxidase (WMD = 12.400 U/mL) and total antioxidant capacity (WMD = 0.771 U/mL). A higher ruminal propionate concentration (WMD = 0.926 mol/100 mol; p = 008) was observed in response to FLAs supplementation. In meat, the dietary inclusion of FLAs decreased (p < 0.05) shear force (WMD = −1.018 kgf/cm2), malondialdehyde content (WMD = −0.080 mg/kg of meat), and yellowness (WMD = −0.460). Supplementation with FLAs decreased milk somatic cell count (WMD = −0.251 ×103 cells/mL; p < 0.001) and increased (p < 0.01) milk production (WMD = 1.348 kg/d), milk protein content (WMD = 0.080/100 g) and milk fat content (WMD = 0.142/100 g). In conclusion, dietary supplementation with FLAs improves animal performance and nutrient digestibility in cattle. In addition, FLAs improve the antioxidant status in blood serum and the quality of meat and milk.
1. Introduction
As part of the strategies to satisfy the growing demand for meat and dairy products, it is necessary to increase effectiveness and productivity in bovine production systems (1). Dairy cows and beef cattle are frequently exposed to a wide variety of stressors, such as environmental (heat or cold stress), physiological (for example, rapid growth rate), and nutritional (presence of mycotoxins or oxidized fat in diets) (2, 3). All these factors promote the overproduction of reactive oxygen species, alter the redox balance and cause oxidative stress in animals (4). Oxidative stress is associated with a higher incidence of diseases (5, 6) and leads to diminished cattle productive and reproductive performance (3). According to Abuelo et al. (7), dietary supplementation with exogenous antioxidants such as vitamins and trace elements can reduce oxidative stress and improve cattle's health status and productive performance. However, in recent years, interest in using natural antioxidants (not from chemical synthesis) as alternatives to the synthetic antioxidants commonly used in animal feed has increased (8). Potential natural antioxidants include flavonoids (FLAs). The FLAs consist of two benzene rings joined by three carbon atoms to form an oxygenated heterocycle (9) and are present in a wide variety of plants (10).
It has been documented that FLAs possess diverse biological properties, such as antioxidant, anti-inflammatory, hepatoprotective, and antimicrobial (10). The effects of dietary inclusion of FLAs have been investigated mainly in broilers and laying hens (11–13). However, in ruminants, there is limited information on the effects of dietary supplementation with FLAs. In growing ruminants, dietary supplementation with FLAs results in the reduction of diarrhea's occurrence and severity. However, it is ineffective in improving animal metabolism and productive performance (14). On the other hand, in adult ruminants, there is evidence that dietary supplementation with FLAs increases the serum concentration of antioxidant enzymes, reduces lipid peroxidation, and improves total antioxidant capacity in blood serum (15). In cattle and goats, some parts of plants containing FLAs have been used to increase the productive performance and digestibility of consumed nutrients (16, 17). Previous studies (18, 19) have shown that, in adult cattle, FLAs supplementation reduces agonistic interactions and modifies the differential expression of genes involved in inflammation, regulation of feeding behavior, and animal behavior. Specifically, in the ruminal epithelium of beef cattle, Paniagua et al. (19) detected greater gene expression of two genes (free fatty acid receptor 3 and free fatty acid receptor 2) that improve the feeding pattern in beef cattle by increasing the time the animals spend consuming forage and concentrate (18). Likewise, in adult sheep and cattle, it has been reported that the dietary inclusion of FLAs has a positive impact on the composition of the rumen microbiome and the production of volatile fatty acids in the rumen (20, 21).
Particularly in beef cattle and dairy cows, some studies have evaluated the effects of dietary supplementation with FLAs on animal performance (19, 22), serum antioxidant status (23, 24), rumen fermentation and nutrient digestibility (16, 20), meat physicochemical characteristics (25, 26) and milk production and composition (15, 27). However, the results obtained so far are still inconsistent and controversial, probably due to the wide variability among these studies regarding the experimental periods, the doses, and the type of FLAs used (14). Therefore, it is necessary to identify and control this variability to develop products containing FLAs that can improve the antioxidant status, animal performance, and quality of beef and dairy cattle products.
In recent years, some review articles have been published (9, 14), mentioning that it is possible to use FLAs for the improvement of the antioxidant status in blood serum, health status, animal performance, and quality of food products derived from ruminants. However, these review articles neither focus only on beef cattle or dairy cows nor used a meta-analytic approach. Meta-analysis (MA) is a method that allows previously published results of a series of individual studies to be collected, combined, and statistically analyzed (28). Likewise, the MA helps identify sources of heterogeneity between studies (29). Therefore, there is a growing interest in the application of MA in the field of animal nutrition (30). However, the use of MA in research related to the inclusion of natural feed additives in ruminant diets is still limited (31). The hypothesis of this meta-analysis states that adding FLAs in beef and dairy cattle diets will benefit animal performance, antioxidant status, and rumen parameters without affecting the quality of products derived from these animals. Therefore, the objective of this study was to evaluate the effects of dietary supplementation with flavonoids FLAs on animal performance, diet digestibility, serum antioxidant status, rumen parameters, meat quality, and milk composition derived from beef and dairy cattle through a meta-analysis.
2. Materials and methods
2.1. Literature search and study selection
To reduce publication bias and ensure the quality of the meta-analysis, the present study was conducted following PRISMA guidelines (32), as shown in Figure 1. A systematic search for information was conducted using Web of Science, Scopus, PubMed, and ScienceDirect databases to identify previous studies evaluating the effects of dietary supplementation with FLAs on nutrient digestibility, animal performance, carcass characteristics, antioxidant status in blood serum, ruminal fermentation, as well as meat and milk quality in beef (Holstein, Simmental, Angus × Nellore, Jinjiang, Xianan, and native) and dairy cattle (Holstein). The keywords that were used in all the databases were the following: “flavonoids, beef cattle, growth performance, finishing steer, finishing bull, carcass, meat quality, dairy cattle, milk production, milk quality, digestibility, ruminal fermentation, antioxidant status”, and the main representatives of FLAs (33), such as “daidzein, naringin, puerarin, anthocyanin, and quercetin”. In all searches performed, results were restricted to studies published between January 2010 and November 2022. In total, 1,010 scientific publications were identified (Figure 1); however, duplicate publications found in more than one of the databases were excluded. After this, the remaining publications underwent a two-step selection process, as previously reported by other authors (34–36). First, based on the titles and abstracts of each publication, we excluded studies that were not conducted in beef cattle or dairy cows, studies that did not measure any of the variables of interest, in vitro experiments, studies that used animals experimentally infected, as well as simulation and review articles.
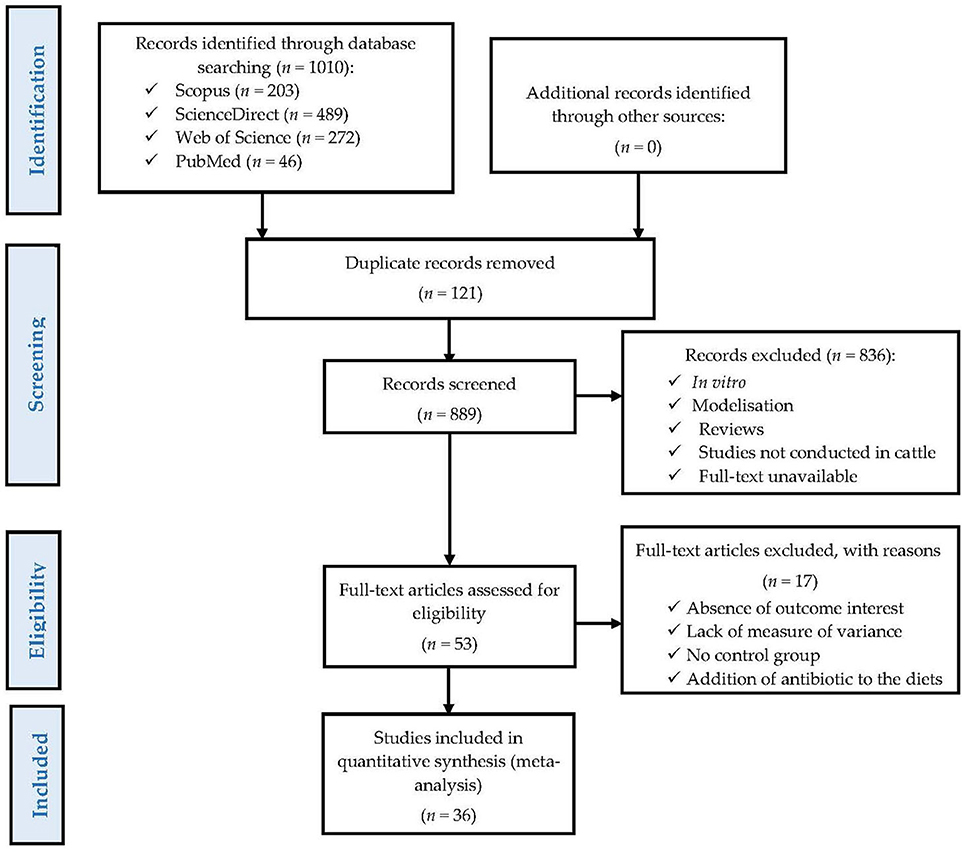
Figure 1. A PRISMA flow diagram detailing the literature search strategy and study selection for the meta-analysis.
Secondly, the articles analyzed had to meet some previously defined inclusion criteria to be included in the final database. In the present meta-analysis, the inclusion criteria used were similar to those previously reported by Dorantes-Iturbide et al. (35) and Orzuna-Orzuna et al. (36): (1) studies with beef cattle or dairy cows housed in confined conditions; (2) data on animal performance, nutrient digestibility, antioxidant status in blood serum, carcass characteristics, ruminal fermentation or quality of the derived products (meat or milk); (3) studies that had control and experimental treatments with similar diets, except for the presence of FLAs in the diets; (4) studies that reported the doses of FLAs used or had sufficient information to estimate the amount of FLAs included in the diets; (5) studies written and published in English and in peer-reviewed scientific journals; and (6) studies that reported the means of the control and FLA-supplemented treatments, the standard error or standard deviation, and the number of replicates.
2.2. Data extraction
After applying the inclusion criteria, only 36 peer-reviewed articles were included in the final database (Supplementary Table S1). Likewise, we only extracted quantitative data for response variables that were reported in at least three individual studies (31, 35, 36). Among the response variables included in the final database of this meta-analysis are the following: dry matter intake and nutrient digestibility (neutral detergent fiber, crude protein), daily weight gain, feed conversion ratio, carcass characteristics (carcass yield, backfat thickness), serum concentration of malondialdehyde and antioxidant enzymes (for example, superoxide dismutase), serum immunoglobulins (IgA, IgM, and IgG), rumen parameters (pH, ammonia nitrogen), physicochemical characteristics of the meat (pH, shear force, color), milk production, and milk composition (lactose, protein, and fat content).
Additionally, when available, the following complementary information was obtained from the selected publications: (1) author and year of publication; (2) period of supplementation with FLAs (days); (3) type of FLAs (for example, anthocyanin, daidzein); (4) method of inclusion of the FLAs (extract or naturally present in the diet); (5) amount of concentrate included in the diets (g/kg DM); (6) days in milk from dairy cows; (7) type of cattle (beef cattle or dairy cow); (8) nutritional composition of the diets used; and (9) country where the study was conducted.
Supplementary Table S1 shows the complete list of publications included in the final database of the present meta-analysis. The number of replicates, means, and standard deviations (SD) for the control and experimental treatments (supplemented with FLAs) were extracted from each of these publications. In all the publications in which the SD was not reported, SD was determined using the standard errors of the treatment means (SEM), by using the following equation (37): SD = SEM × √n, where n = number of repetitions.
2.3. Calculations and statistical analysis
Meta-analysis and meta-regression, as well as analyzes of subgroups, heterogeneity, and publication bias, were performed using the “metaphor” package (38), which is available in the statistical software R (version 4.1.2, R Core Team, Vienna, Austria). The effects of including FLAs in diets of beef cattle and dairy cows were evaluated using the weighted mean differences (WMD) between treatments supplemented with FLAs (diets with FLAs) and control treatments (diets without FLAs). For this, the means of the treatments were weighted by the inverse of the variance, according to the method for random effects models previously proposed by DerSimonian and Laird (39). In the present meta-analysis, the WMD was used because it allows interpretation of the results obtained in the original units of measurement (40). Additionally, with the PROC MEANS procedure of the statistical software SAS (41), descriptive statistics values were obtained for the continuous covariates level of concentrate in the diet, dose of FLAs, experimental period, and days in milk.
2.4. Heterogeneity and publication bias
The presence of heterogeneity between studies was identified with the chi-square (Q) test, in which a significance level of p ≤ 0.10 was used since this test has relatively low power (42). Additionally, to quantify the proportion of observed heterogeneity, we used the I2 statistic (29). For this test, I2 values <25% indicate that the degree of heterogeneity is low, I2 values between 25 and 50% indicate moderate heterogeneity, while I2 values >50% indicate high and significant heterogeneity (29, 43). On the other hand, to detect the presence of publication bias, the Egger regression asymmetry test (44) was applied, in which a significance level of p ≤ 0.05 was used. When publication bias was detected (p ≤ 0.05 in Egger's test), the “trim and fill” method of Duval and Tweedie (45) was applied to estimate the possible number of missing observations.
2.5. Meta-regression and subgroup analysis
Meta-regression analyses were performed on some of the variables evaluated to identify the presence of possible sources of heterogeneity. The criteria considered to apply meta-regression analysis were: (1) presence of significant heterogeneity (i.e., p ≤ 0.10 for Q or I2 > 50%); (2) p-value > 0.05 for Egger's test (44); and (3) response variables reported in 10 or more individual studies (46). For all meta-regression analyses, the method of moments of DerSimonian and Laird (39) was used, as it is well-established for estimating between-study variance. Subsequently, for the covariates analyzed that were significant with p ≤ 0.05, the WMD was evaluated through subgroup analysis. A subgroup assessment was not performed when an individual stratum has less than two effect sizes in the meta-analysis (35, 36). The type of FLAs (daidzein, anthocyanin, puerarin, naringin, quercetin, catechin, and blend), the method of supplementation with FLAs (extract or naturally present in an ingredient in the diet), and the type of cattle (beef cattle or dairy cow) were used as categorical covariates. On the other hand, the duration of the experimental period (days), the days in milk of the dairy cows, the content of concentrate in the diet (g/kg of DM), and the doses of FLAs were used as continuous covariates. When any categorical covariate (type of FLAs, type of bovine, and method of supplementation with FLAs) was found to be statistically significant (p ≤ 0.05), subgroup analysis was used to assess WMD (34, 35). Likewise, when the meta-regression was significant (p ≤ 0.05) for the continuous covariates, these were analyzed using the following subgroups: dietary dose of FLAs (≤600 and >600 mg/kg of DM), level of concentrate in the diet (≤400, 401–700, and >700 g/kg of DM), days in milk (≤100 and >100 days) and period of supplementation with FLAs (≤75 and >75 days).
3. Results
3.1. Study attributes
The studies included in this meta-analysis were conducted in eight different countries, mainly in China (44.4%), Spain (16.7%), Brazil (13.9%), and Japan (5.5%). Regarding the animal species (bovine), in 66.7% of the studies, beef cattle were used, and in the remaining studies (33.3%), dairy cows were used (Supplementary Table S1). Supplementary Table S2 shows that the doses of FLAs used were between 12 and 3,104 mg/kg DM. Dairy cows had between 7 and 164 days in milk and the experimental periods ranged between 24 and 168 days (Supplementary Table S2). Supplementary Table S1 shows seven different types of FLAs used in the present meta-analysis. Most of the studies used mixtures of FLAs (36.1%), daidzein (16.7%), anthocyanin (16.7%), and naringin (16.7%). Three other different types of FLAs were used in the remaining studies (13.8%). In addition, 61.1% of the studies used FLAs extracts, and 38.9% used plants or by-products naturally high in FLAs.
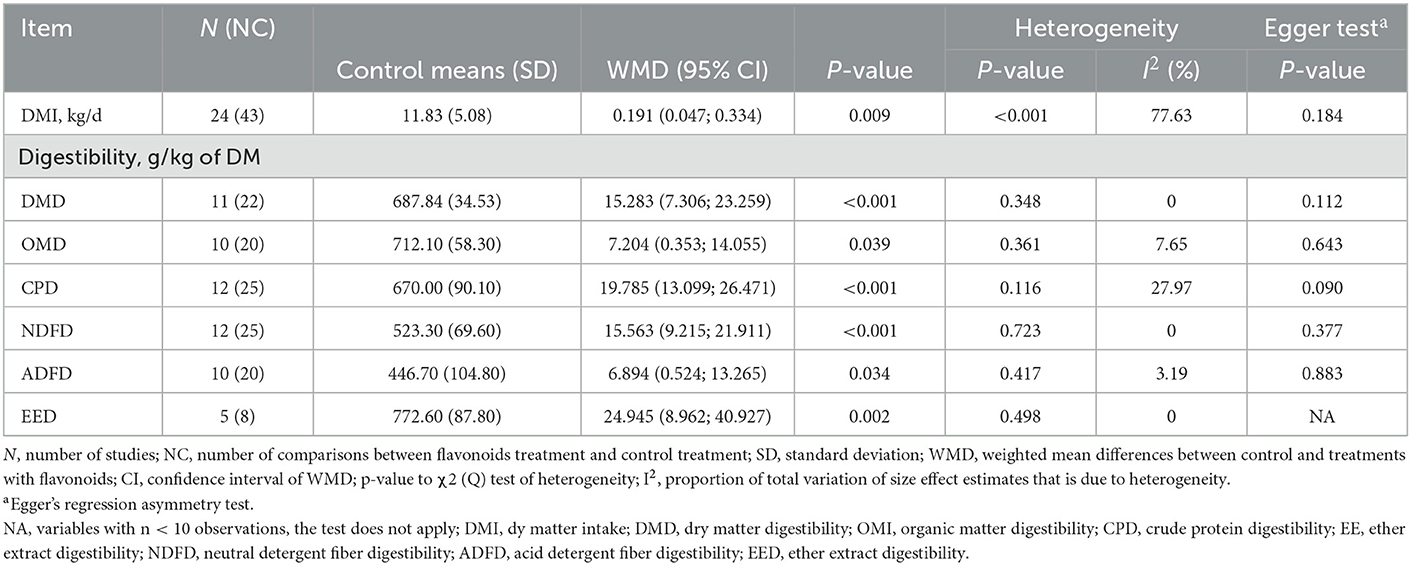
3.2. Dry matter intake and nutrient digestibility
Dry matter intake (DMI) increased in response to FLAs supplementation (Table 1). Likewise, dietary supplementation with FLAs increased (p < 0.05) dry matter digestibility (DMD), organic matter digestibility (OMD), crude protein digestibility (CPD), neutral detergent fiber digestibility (NDFD), acid detergent fiber digestibility (ADFD), and ether extract digestibility (EED).
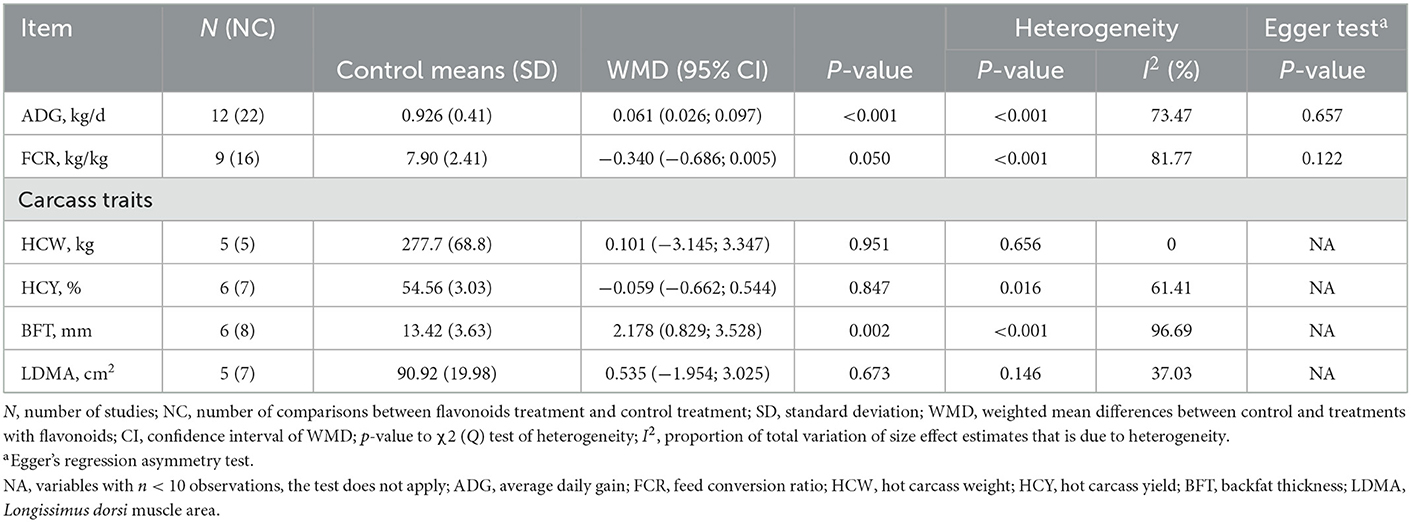
3.3. Growth performance and carcass traits
Table 2 shows that daily weight gain (ADG) and backfat thickness (BFT) increased in response to dietary supplementation with FLAs (p < 0.05). In contrast, the dietary inclusion of FLAs decreased the feed conversion ratio (FCR; p = 0.050). However, hot carcass weight (HCW), hot carcass yield (HCY), and Longissimus dorsi muscle area (LDMA) were not affected by FLAs supplementation (p > 0.05; Table 2).
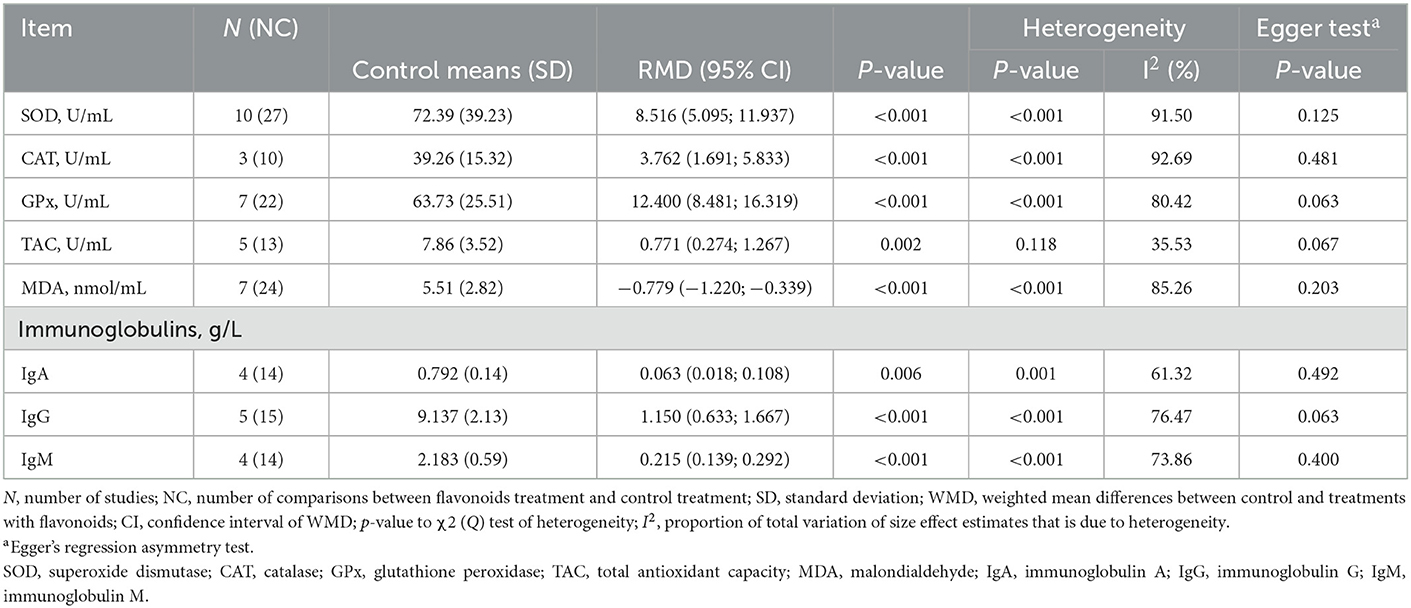
3.4. Antioxidant status and immune response
Table 3 shows that dietary supplementation with FLAs increased (p < 0.01) the serum concentration of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and total antioxidant capacity (TAC). In contrast, a lower (p < 0.001) serum concentration of malondialdehyde (MDA) was observed in animals supplemented with FLAs. On the other hand, the serum concentration of immunoglobulin A (IgA), immunoglobulin G (IgG), and immunoglobulin M (IgM) increased in response to dietary supplementation with FLAs (p < 0.01).
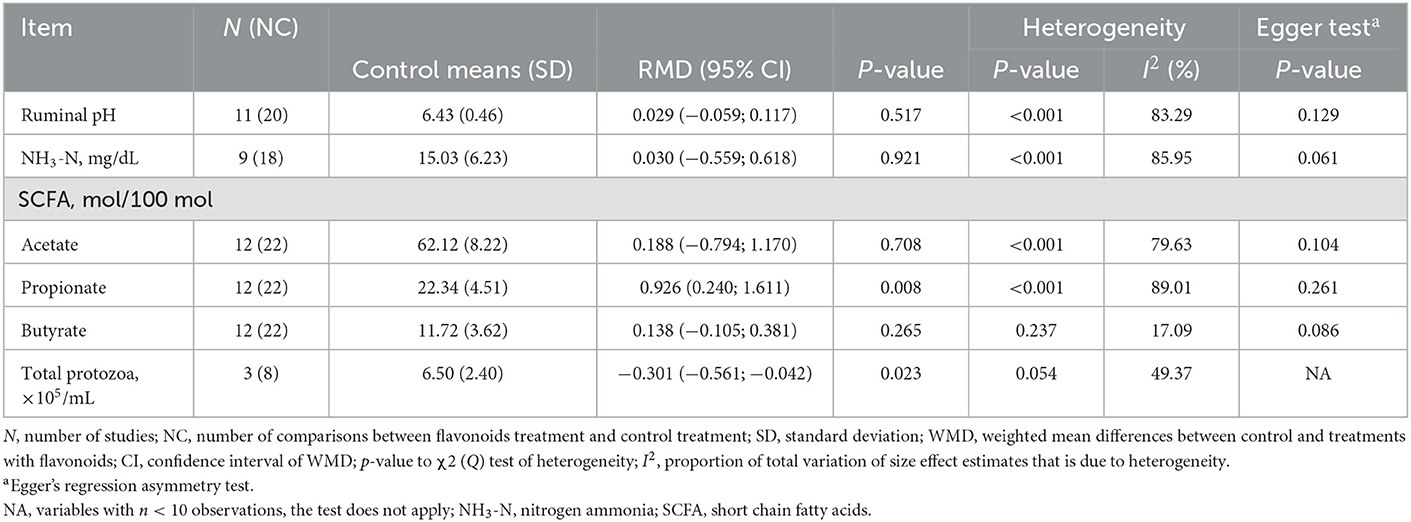
3.5. Rumen fermentation and protozoal count
Table 4 shows that the pH and the ruminal concentration of ammonia nitrogen (NH3-N), acetate, and butyrate were not affected by dietary supplementation with FLAs (p > 0.05). However, a higher (p = 0.008) rumen concentration of propionate and a lower (p = 0.023) concentration of total protozoa were observed in response to supplementation with FLAs.
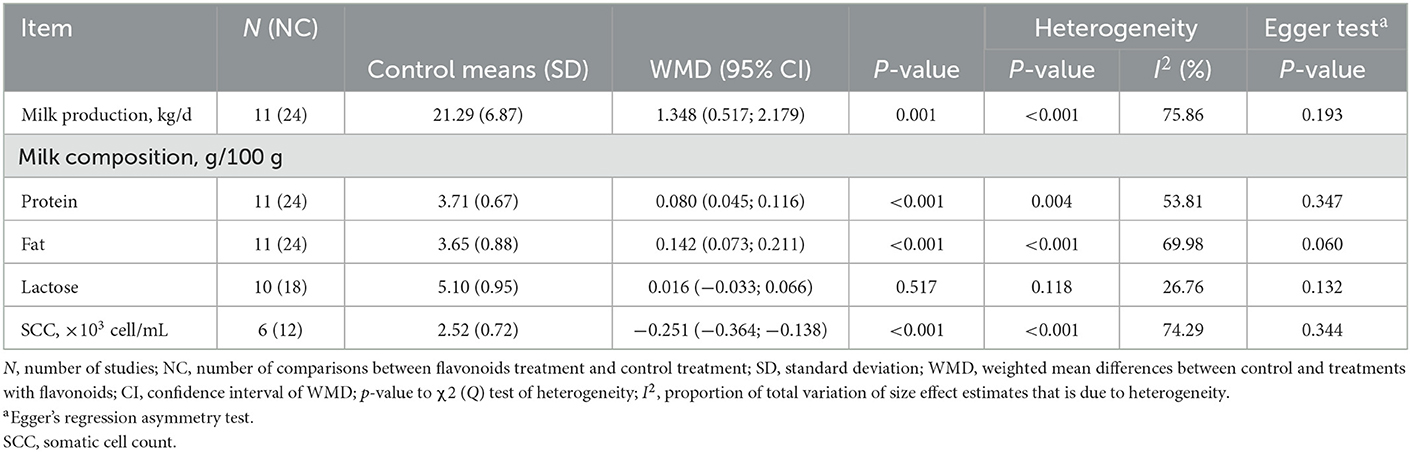
3.6. Meat quality
Dietary supplementation with FLAs did not affect (p > 0.05) pH, cooking loss (CL), lightness (L*), redness (a*), or meat protein, moisture, and ash content (Table 5). On the other hand, FLAs supplementation decreased (p < 0.05) the shear force (ShF), the malondialdehyde content (MDA), and the yellowness (b*) of the meat. However, meat's intramuscular fat content (IMF) increased in response to FLAs supplementation (p = 0.029).
3.7. Milk production and composition
Dietary supplementation with FLAs increased (p < 0.01) milk production and milk protein and fat content (Table 6). However, the lactose content in milk was not affected by FLAs supplementation (p > 0.05). In addition, lower milk somatic cell (SCC) counts were observed in response to dietary supplementation with FLAs (p < 0.001).
3.8. Meta-regression and publication bias
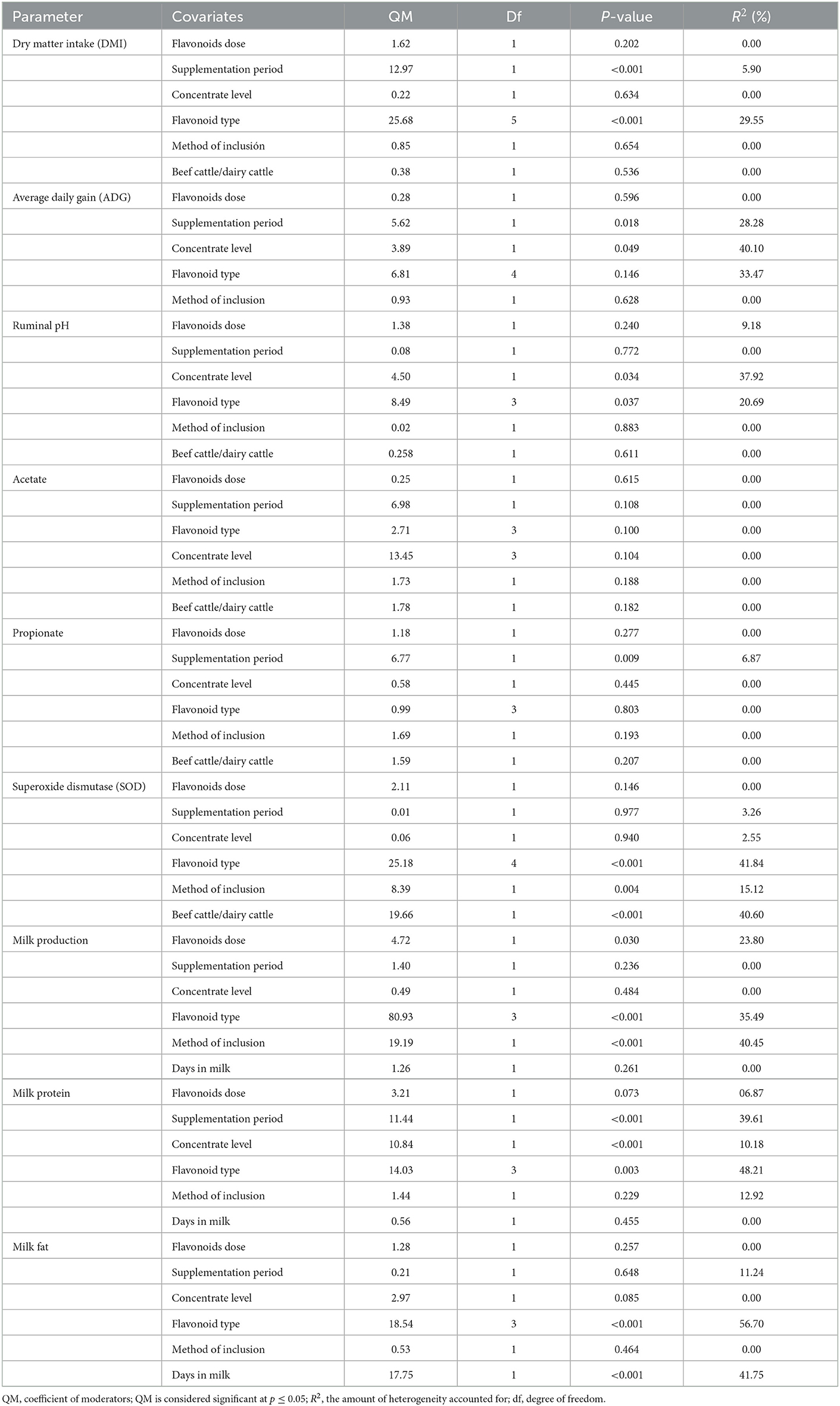
Tables 1–6 show no publication bias since the Egger regression asymmetry test was not significant (p > 0.05) for any of the variables evaluated. On the other hand, Tables 1–6 show that there was significant (p ≤ 0.10) heterogeneity (Q) for DMI, ADG, FCR, HCY, BFT, SOD, CAT, GPx, MDA in blood serum, IgA, IgG, IgM, rumen pH, NH3-N, acetate, propionate, total protozoa, meat pH, CL, ShF, L*, protein content, meat IMF and moisture, milk yield, and protein, fat, and SCC content in milk. However, to obtain reliable results, meta-regression analyses are only recommended when the variable of interest is reported in 10 or more studies (46). Consequently, meta-regression analyses were only performed for the following variables: DMI, ADG, rumen pH, acetate, propionate, SOD, milk yield, and milk protein and fat content.
Table 7 shows that the FLAs dose explained (p < 0.05) 23.80% of the observed heterogeneity for milk production. The supplementation period explained (p < 0.05) 5.90, 28.28, 6.87, and 39.61% of the heterogeneity observed for DMI, ADG, rumen propionate concentration, and milk protein content, respectively. On the other hand, the level of concentrate in the diet explained (p < 0.05) 40.10, 37.92, and 10.18% of the heterogeneity observed for ADG, ruminal pH, and milk protein content, respectively. The type of FLAs used explained (p < 0.05) between 20.69 and 56.70% of the observed heterogeneity for DMI, rumen pH, SOD, milk yield, milk protein, and milk fat content. Likewise, the FLAs inclusion method explained (p < 0.05) 15.12 and 40.45% of the observed heterogeneity for SOD and milk production, respectively. Bovine type explained (p < 0.001) 40.60% of the observed heterogeneity for SOD, and days in milk explained (p < 0.001) 41.75% of the observed heterogeneity for milk fat content. There was no significant relationship (p > 0.05) between the covariates used and the ruminal acetate concentration.
3.9. Subgroup analysis
DMI increased (WMD = 0.532 kg/d; p = 0.038) when dietary supplementation with FLAs lasted up to 75 days (Figure 2A). However, supplementation with FLAs for more than 75 days did not affect DMI (WMD = 0.015 kg/d; p = 0.805). Higher ADG (WMD = 0.093 kg/d; p < 0.001) was observed when cattle were supplemented with FLAs for periods up to 75 days (Figure 2B). However, when supplementation with FLAs lasted more than 75 days ADG was not affected (WMD = 0.017 kg/d; p = 0.206). Ruminal propionate concentration was increased (WMD = 1.962 mol/100 mol; p = 0.043) in animals supplemented with FLAs for up to 75 days (Figure 2C). However, ruminal propionate concentration was not affected when FLAs were offered for more than 75 days (WMD = 0.306 mol/100 mol; p = 0.486). The protein content in milk increased (p < 0.05) regardless of the period of supplementation with FLAs used (Figure 2D). However, the effect was greater when FLAs supplementation lasted longer than 75 days (WMD = 0.113/100 g) than when it lasted up to 75 days (WMD = 0.097/100 g).
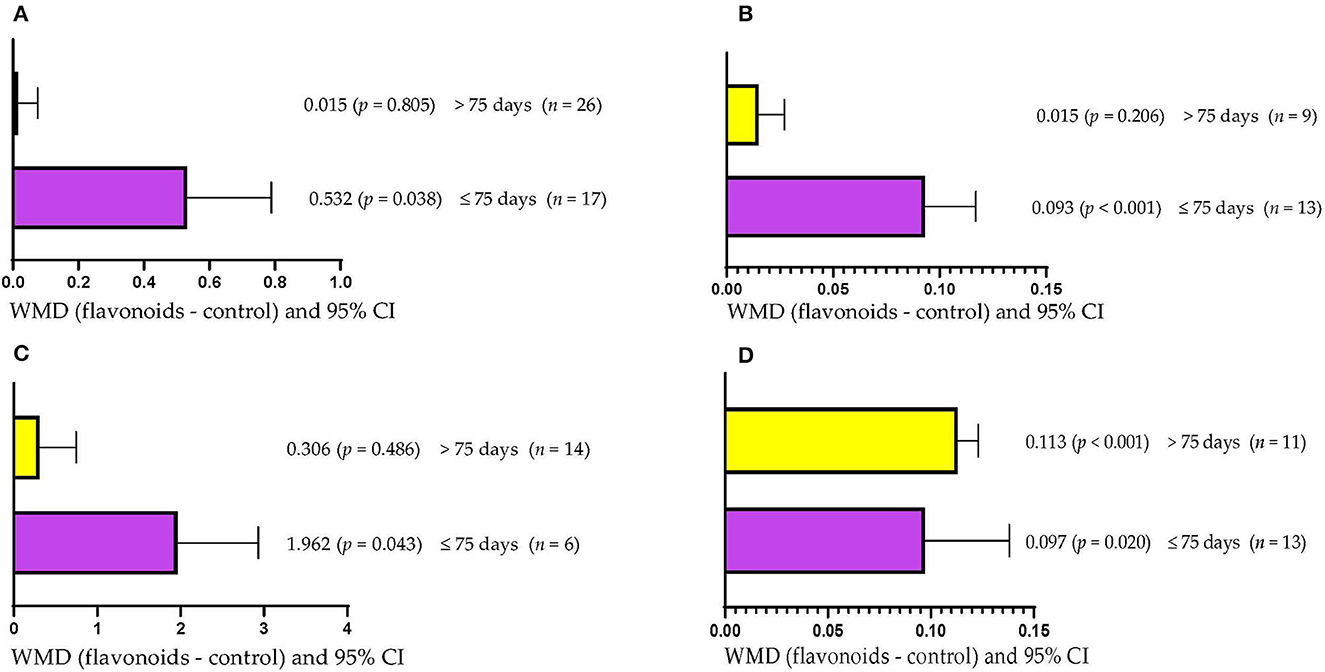
Figure 2. Subgroup analysis [subgroup = supplementation period (days)] of the effect of flavonoids on the diet of the cattle; WMDs, weighted mean differences between flavonoid treatments and control. (A) Dry matter intake (DMI), kg/d. (B) Average daily gain (ADG), kg/d. (C) Propionate, mol/100 mol. (D) Milk protein, g/100 g.
Figure 3A shows that ADG increased (WMD = 0.048 kg/d; p < 0.001) only when FLAs were included in high-concentrate diets (>700 g/kg DM). However, the inclusion of FLAs in diets with low ( ≤ 400 g/kg DM) or moderate (401–700 g/kg DM) concentrate levels did not affect ADG (p > 0.05). Rumen pH increased (WMD = 0.336; p < 0.001) when FLAs were supplemented in diets with more than 700 g/kg DM of concentrate (Figure 3B). However, the inclusion of FLAs in diets with low (≤400 g/kg DM) or moderate (401–700 g/kg DM) concentrate levels did not affect rumen pH. Figure 3C shows that the inclusion of FLAs in diets with 401–700 g/kg DM of concentrate increased the protein content in milk (WMD = 0.089/100 g; p < 0.001). However, milk protein content was not affected when FLAs were fed in low-concentrate diets (≤400 g/kg DM).
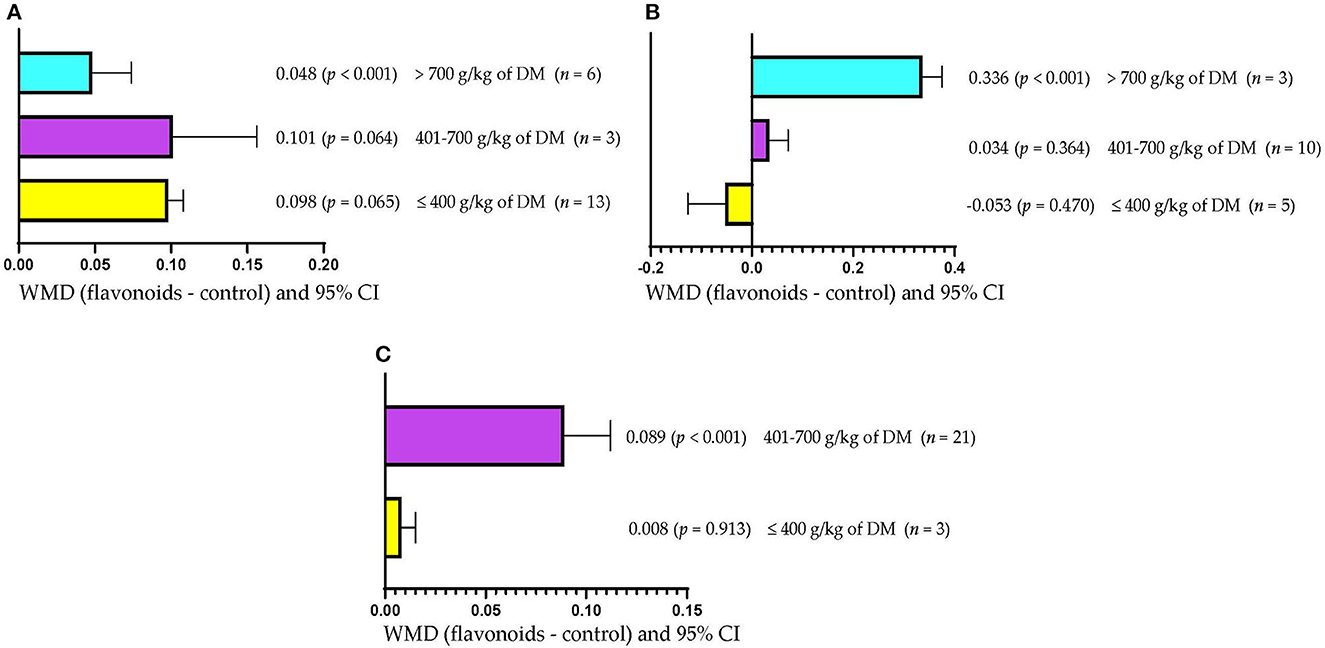
Figure 3. Subgroup analysis [subgroup = concentrate in diet (g/kg of DM)] of the effect of flavonoids on the diet of the cattle; WMDs, weighted mean differences between flavonoid treatments and control. (A) Average daily gain (ADG), kg/d. (B) Ruminal pH. (C) Milk protein, g/100 g.
Figure 4A shows that DMI increased (p < 0.05) when the type of FLAs used was daidzein (WMD = 0.500 kg/d), puerarin (WMD = 0.700 kg/d), and anthocyanin (WMD = 0.535 kg/d). However, DMI decreased when the type of FLAs used was naringin (WMD = −0.129 kg/d; p = 0.021) and was not affected when mixtures of FLAs were used (p > 0.05). Rumen pH increased (WMD = 0.071; p = 0.030) when FLAs mixtures were used (Figure 4B); however, it decreased when the FLAs used were daidzein (WMD = −0.350; p = 0.001) and anthocyanin (WMD = −0.100; p = 0.041). Likewise, when the type of FLAs used was naringin, the rumen pH was not affected (p > 0.05). Figure 4C shows that the serum concentration of SOD increased (p < 0.001) only when the FLAs used were daidzein (WMD = 9.373 U/mL) and puerarin (WMD = 19.733 U/mL). However, the serum SOD concentration was not affected when anthocyanin or FLA mixtures were used (p > 0.05). On the other hand, milk production increased (p < 0.001; Figure 4D) when mixtures of FLAs (WMD = 0.701 kg/d) and daidzein (WMD = 3.923 kg/d) were used; however, it decreased when the FLAs used were anthocyanins (WMD = −1.612 kg/d; p < 0.001). Figure 4E shows that milk protein content increased when mixtures of FLAs (WMD = 0.113/100 g; p < 0.001) and daidzein (WMD = 0.174/100 g; p = 0.044) were used. However, the protein content in milk was not affected when the FLAs used were anthocyanins (p > 0.05). Milk fat content increased (p < 0.01; Figure 4F) in response to supplementation with mixtures of FLAs (WMD = 0.106/100 g) and daidzein (WMD = 0.373/100 g); however, it was not affected by anthocyanin supplementation (p > 0.05).
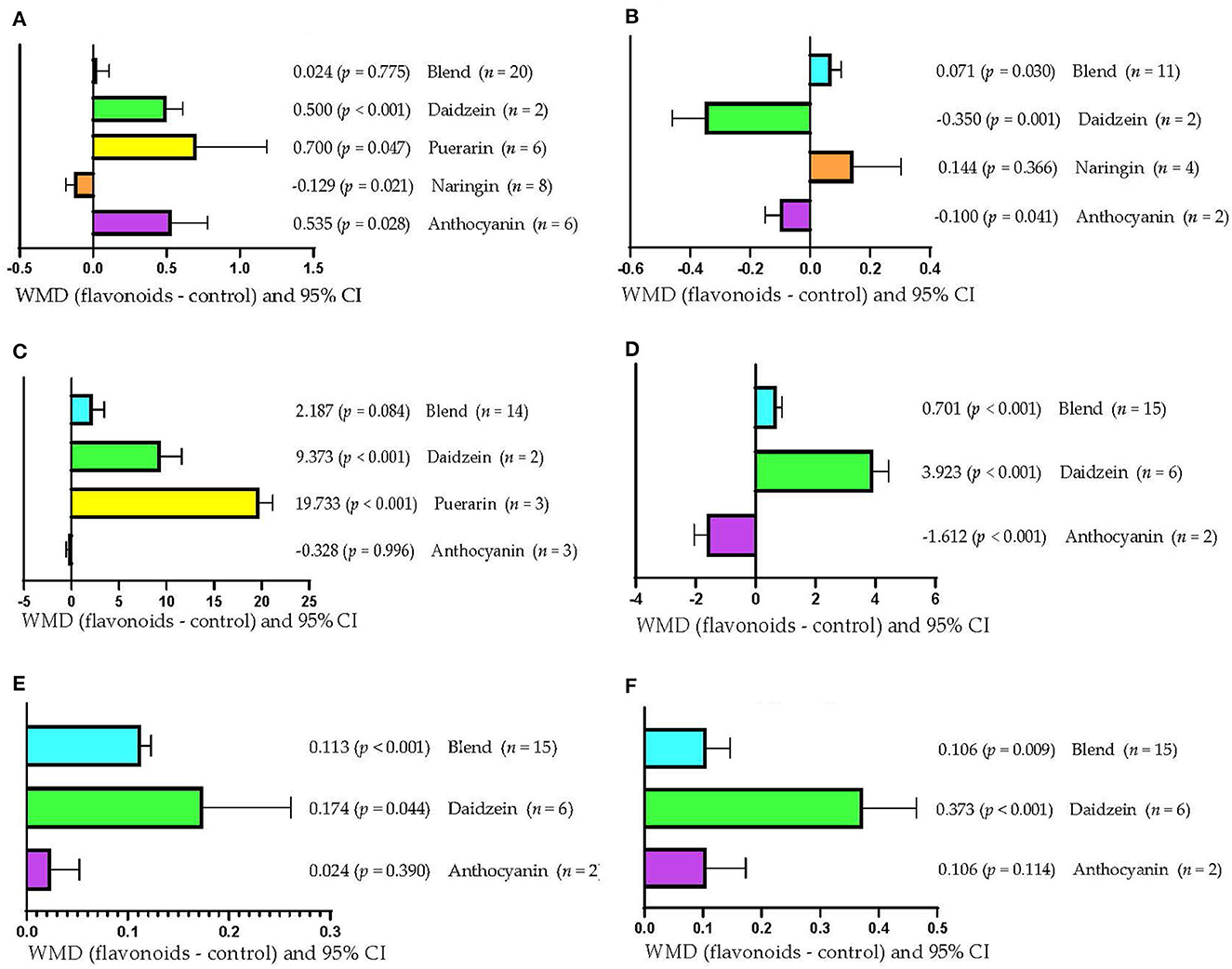
Figure 4. Subgroup analysis (subgroup = flavonoid type) of the effect of flavonoids on the diet of the cattle; WMDs, weighted mean differences between flavonoid treatments and control. (A) Dry matter intake (DMI), kg/d. (B) Ruminal pH. (C) Superoxide dismutase (SOD), U/mL. (D) Milk production, kg/d. (E) Milk protein, g/100 g. (F) Milk fat, g/100 g.
Serum SOD concentration increased (WMD =11.016 U/mL; p < 0.001) when FLAs extracts were added to diets (Figure 5A). However, when FLAs were supplied as part of the diet ingredients, serum SOD concentration was not affected (p > 0.05). Milk production increased (WMD = 2.748 kg/d; p < 0.001) in response to supplementation with FLAs extracts (Figure 5B). However, milk production was not affected (p > 0.05) when FLAs were supplied as part of the diet ingredients.
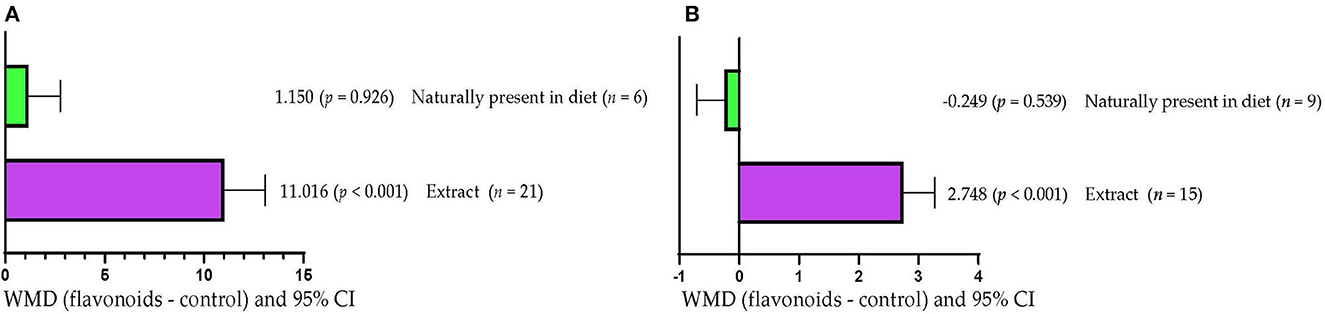
Figure 5. Subgroup analysis [subgroup = method of FLA's inclusion (extract or naturally present in the diet)] of the effect of flavonoids on the diet of the cattle; WMDs, weighted mean differences between flavonoid treatments and control. (A) Superoxide dismutase (SOD), U/mL. (B) Milk production, kg/d.
Figure 6A shows that milk production increased when FLAs doses ≤600 mg/kg DM were used (WMD = 1.774 kg/d; p < 0.001). However, milk production decreased (WMD = −1.209 kg/d; p = 0.002) when the FLAs doses used were >600 mg/kg DM. In addition, serum SOD concentration increased regardless of the type of bovine used (p < 0.001; Figure 6B). However, the effect was greater when FLAs were offered to beef cattle (WMD = 14.712 U/mL) than dairy cows (WMD = 3.615 U/mL). Likewise, milk fat content increased (WMD = 0.217/100 g; p < 0.001) when FLAs were offered to cattle that were longer than 100 days in milk (Figure 6C). However, in cattle that were up to 100 days in milk, FLA supplementation did not affect milk fat content (p > 0.05).
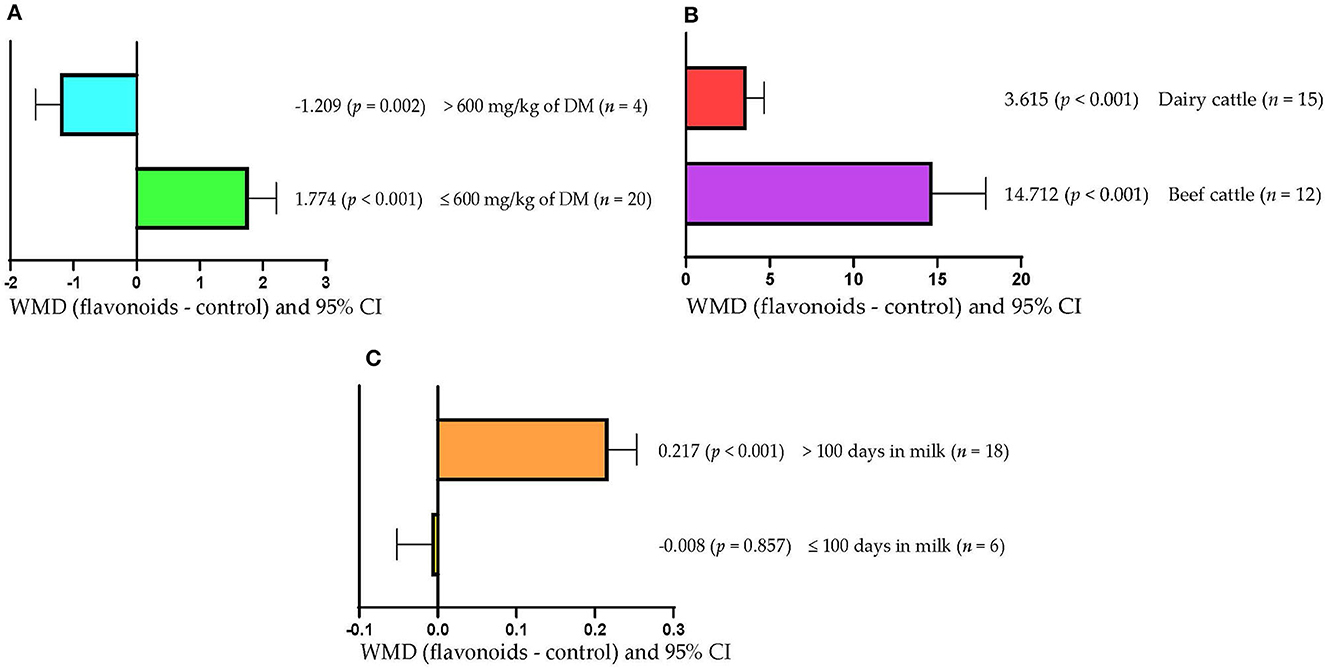
Figure 6. Subgroup analysis [subgroup = flavonoid dose (mg/kg of DM), type of cattle (beef cattle or dairy cattle), and days in milk] of the effect of flavonoids on the diet of the cattle; WMDs, weighted mean differences between flavonoid treatments and control. (A) Milk production, kg/d. (B) Superoxide dismutase (SOD), U/mL. (C) Mil fat, g/100 g.
4. Discussion
4.1. Dry matter intake and nutrient digestibility
It has been reported that the dietary inclusion of FLAs increases the relative abundance of ruminal bacteria involved in fiber degradation in adult sheep and cattle (20, 47). This effect could increase the rate of passage of feed particles in the rumen and result in higher DMI. In addition, in ruminants (yaks and sheep) dietary supplementation with FLAs increases the relative rumen abundance of the bacterial family Rikenelleceae (48, 49), which have a positive correlation with DMI in beef cattle (50). Therefore, similar effects of FLAs supplementation in the present meta-analysis partially explain the observed increase in DMI. On the other hand, in beef cattle, it has been reported that FLAs supplementation reduces the gene expression of bitter taste receptors (TAS2R, such as TAS2R7, TAS2R16, TAS2R38, and TAS2R39) in the rumen (18) and duodenal epithelium (51). This effect could decrease the release of anorexigenic molecules and increase DMI, since the activation of TAS2R triggers the release of anorexigenic molecules, such as cholecystokinin and peptide YY (52, 53). However, a subgroup analysis revealed that naringin supplementation decreased DMI. Naringin is part of the flavanones (a particular class of FLAs), which are abundant in citrus and impart a bitter taste (33). This effect could reduce the food's palatability and explain the lower DMI observed in response to naringin supplementation.
Previous studies (20, 47) have reported that, in adult ruminants (sheep and cattle), dietary supplementation with FLAs increases the relative abundance of ruminal bacteria of the genus Ruminococcus. Within this genus are the species Ruminococcus albus and R. flavefaciens, which play an important role in fiber degradation in the rumen (54). Kim et al. (55) observed that, under in vitro conditions, FLAs (catechins) increase the relative abundance of Fibrobacter succinogenes bacteria, which are also involved in fiber degradation in the rumen. Low doses (60 mg/kg body weight) of FLAs (mixtures of various types not reported) have been documented to increase the relative abundance of fungi in the rumen of dairy cows by up to 79% (56). According to Akin and Borneman (57), rumen fungi can completely penetrate the cell wall and produce large amounts of cellulases, hemicelluloses, and xylanases, which can increase cellulose degradation. In beef cattle, Niu et al. (58) observed that the dietary inclusion of plants with FLAs increased the relative abundance of rumen bacteria of the genus Succinivibrio, which have been positively correlated with NDFD, ADFD, and DMD in cattle (58). Furthermore, Zhao et al. (47) reported that, in growing lambs, supplementation with FLAs (anthocyanins) extracts decreases the relative abundance of ruminal microorganisms of the genus Prevotella, which have been negatively correlated with CPD in dairy cows (59). Thus, similar effects of FLAs supplementation in the present study partially explain the observed increases in CPD, NDFD, ADFD, DMD, and OMD.
4.2. Growth performance and carcass traits
In the present study, supplementation with FLAs increased DMI, CPD, NDFD, ADFD, EED, OMD, and DMD, which partially explains the higher ADG and lower FCR observed. In dairy cows, Zhan et al. (56) reported that dietary supplementation with FLAs increases the relative abundance of Tenericutes and Mollicutes rumen microorganisms, which have been positively correlated with ADG in finishing lambs (48). In growing lambs, FLAs supplementation reduces the relative ruminal abundance of the bacterial family Veillonellaceae (47), which has a negative correlation with ADG in sheep (60). Du et al. (48) reported that the dietary inclusion of plants containing FLAs increases the relative abundance of the Rikenellaceae microbial family in rumen fluid, which has a positive and negative correlation with ADG and FCR in beef cattle, respectively (50). Dorantes-Iturbide et al. (61) reported that, in finishing lambs, supplementation with low doses (1 g/kg DM) of polyherbal additives with FLAs increases up to 23% the efficiency of utilization of dietary energy for weight gain. Furthermore, supplementation with FLAs-rich plants increases muscle protein synthesis in lambs (62). Thus, similar effects of FLAs supplementation in the present study partially explain the observed increase and decrease for ADG and FCR, respectively.
In beef cattle, supplementation with FLAs (200 and 400 mg/kg DM) increases serum levels of IGF-1 (insulin-like growth factor 1) (24), which have a positive correlation (r within 0.61 and 0.67) with ADG in ruminants (63). In addition, in the present meta-analysis, higher serum concentrations of antioxidant enzymes (SOD, CAT, and GPx) and immunoglobulins (IgA, IgG, and IgM) were observed in response to FLAs supplementation. These effects could reduce oxidative stress and improve the health status of the animals, which could result in improved animal performance. On the other hand, a subgroup analysis revealed that ADG was significantly increased when FLAs were offered with high-concentrate diets (>700 g/kg DM). In beef cattle fed high-concentrate diets, FLAs supplementation increases the duodenal flux of microbial protein (64), which may increase metabolic amino acid availability and lead to higher ADG. In addition, previous studies (51, 65) have shown that the dietary inclusion of FLAs (400 mg/kg DM) improves the health of the rumen epithelium in beef cattle fed diets high in concentrate. This effect could result in increased absorption of volatile fatty acids and lead to increased ADG since the rumen epithelium contains papillae that serve as absorptive structures (66).
It has been documented that FLAs supplementation increases the number and diameter of muscle fibers in the ruminant Longissimus dorsi muscle (62, 67), which may result in increased LDMA. However, in the present study, FLAs supplementation did not affect LDMA. On the other hand, Liang et al. (25) reported that, in beef cattle, supplementation with FLAs (500 mg/kg DM) increases serum leptin concentration, which has been positively correlated with BFT in beef cattle (68). Consequently, similar effects of FLAs supplementation in the present study partially explain the higher BFT observed. In addition, FLAs promote adipogenesis in the subcutaneous adipose tissue of beef cattle through changes in the expression of several genes (delta like non-canonical notch ligand, insulin like growth factor binding protein 2, wnt family member 6, enhancer binding protein beta, DNA-binding protein inhibitor ID-3, sonic hedgehog protein, and family zinc finger 1) involved in adipogenesis differentiation of subcutaneous adipocytes (69).
4.3. Antioxidant status and immune response
According to Celi (5), the excessive accumulation of reactive oxygen species (ROS) causes oxidative stress in ruminants. Shi et al. (70) mentioned that FLAs can be used as natural antioxidants for cattle since they stimulate antioxidant enzymes and eliminate ROS. In the present study, supplementation with FLAs increased the serum levels of SOD, CAT, and GPx. These results suggest that FLAs reduce the oxidative stress caused by ROS in bovines since SOD, CAT, and GPx play an important role in converting ROS into other compounds that are less damaging to the tissues and cells of organisms (10). Furthermore, FLAs have been reported to induce activation of the transcription factor Nrf2 (71), which activates several antioxidant enzymes (10). Consequently, similar effects of FLAs consumption in the present meta-analysis partially explain the observed increases in SOD, CAT, and GPx.
In the present meta-analysis, FLAs supplementation increased TAC in beef and dairy cattle blood serum. This result suggests that the consumption of FLAs improves the total antioxidant status of bovines since TAC considers the total antioxidants present in the blood serum (5). Furthermore, Ghiselli et al. (72) mentions that serum TAC levels obtained after consuming products with antioxidants serve as indicators of the absorption and bioavailability of ingested antioxidants. Consequently, the higher TAC observed in the present study suggests that FLAs consumed by bovines may be absorbed and transferred to the bloodstream to act as blood antioxidants. Furthermore, it has been documented that TAC and ROS serum levels are negatively correlated (73). Therefore, the observed reduction of TAC in the present study suggests that FLAs supplementation decreases ROS in bovine blood serum. On the other hand, supplementation with FLAs decreased the serum concentration of MDA. This result suggests that the consumption of FLAs decreases lipid peroxidation in cattle blood because when lipid peroxidation is low, serum levels of MDA decrease (74).
According to Zhan et al. (75), immunoglobulins are a type of protein with chemical structures similar to antibodies, which participate in the regulation of immune responses. Therefore, obtaining information related to serum immunoglobulin concentrations in ruminants is important since it is an indicator of immunity against pathogenic microorganisms (76). Wolf et al. (77) mention that IgA inhibits the release of inflammatory cytokines, phagocytosis, and antibody-dependent cellular cytotoxicity. In addition, IgM and IgG act against infection since they participate in the phagocytic system and activate the complement system (24). In the present meta-analysis, FLAs supplementation increased serum IgA, IgG, and IgM concentrations, suggesting that FLAs improve immune competence in cattle. The mechanism of action of FLAs on serum immunoglobulin concentrations has not been studied in ruminants. However, FLAs have been documented to increase the expression of genes encoding IgA in mice (78). Likewise, various FLAs increase the number and activity of B1 and B2 lymphocytes (79), which secrete IgG and IgM (80, 81). Therefore, similar effects of FLAs consumption in the present meta-analysis would explain the observed increases in IgA, IgG, and IgM.
4.4. Ruminal fermentation
In the present meta-analysis, FLAs supplementation did not affect rumen pH. This result suggests that FLAs do not affect the stability of rumen functions in bovines since rumen pH is an important indicator of internal rumen homeostasis (36, 82). However, a subgroup analysis revealed that rumen pH increased when FLAs were offered in high-concentrate diets (>700 g/kg DM). Under in vitro conditions, FLAs decrease the concentration of lactate-producing bacteria (Streptococcus bovis) (83). In addition, in beef cattle fed high-concentrate diets, FLAs supplementation increases the abundance of lactate-consuming bacteria (Megasphera elsdenii and Selenomonas rumiantium) (64, 84). Similar effects of FLAs consumption in the present study could result in a lower rumen lactate concentration, which partially explains the increased rumen pH. On the other hand, the ruminal concentration of NH3-N is the primary nitrogenous substrate used by rumen bacteria for microbial protein synthesis (85). Therefore, the absence of changes observed in the present study for the ruminal concentration of NH3-N suggests that, in cattle, FLAs supplementation does not affect the synthesis of microbial protein in the rumen. Likewise, the absence of changes observed for NH3-N suggests that FLAs supplementation does not affect the balance between rumen ammonia release and uptake.
Balcells et al. (64) mentioned that FLAs supplementation improves rumen fermentation in cattle. In the present study, supplementation with FLAs increased the rumen concentration of propionate with no effect on the concentration of acetate and butyrate. It has been reported that FLAs supplementation decreases the relative abundance of the microbial families Succiniclasticum and Christensenellaceae (47), which negatively correlates with the rumen concentration of propionate in sheep (67). In addition, under in vitro conditions, FLAs increase the relative abundance of the microbial family Succinivibrionaceae (86), which positively correlates with the rumen concentration of propionate in beef cattle (87). Therefore, similar effects of FLAs consumption in the present meta-analysis partially explain the increased ruminal propionate concentration. Furthermore, the observed increase in propionate suggests that FLAs increase energy availability for growth and production in cattle, since ruminal propionate is the main precursor of gluconeogenesis in ruminants (88).
FLAs supplementation decreased the ruminal concentration of total protozoa. This effect could improve the utilization efficiency of the protein and energy consumed by bovines since the reduction of rumen protozoa leads to less rumen protein degradation (89) and decreases enteric methane emissions (90).
4.5. Meat quality
The supplementation with FLAs did not affect the meat's pH or CL. These results indicate that the FLAs do not affect the quality or the water-holding capacity (WHC) of beef since the pH and CL serve as indicators to evaluate the quality (91) and WHC of the meat (92), respectively. On the other hand, lower ShF and MDA were observed in beef cattle meat in response to FLAs supplementation. These results indicate that FLAs improve beef's tenderness and oxidative stability, as ShF and MDA are indicators of meat tenderness (93) and lipid peroxidation (94), respectively. The lower ShF could be related to the reduction in IMF observed in beef from bovines supplemented with FLAs, since there is a negative correlation (r = −0.54) between ShF and IMF in beef (95). In beef cattle, low doses (400 mg/kg DM) of FLAs have been reported to decrease skeletal muscle fiber diameter (67), which is positively correlated with ShF in beef (96). The reduction observed in the present study for lipid peroxidation of meat partially explains the lower ShF, as oxidation decreases post-mortem calpain activity and myofibrillar proteolysis, leading to higher ShF (97).
The reduction observed for MDA in meat indicates that FLAs supplementation improves beef's quality and shelf life because when oxidation reactions in meat increase, the quality, and shelf-life decrease (98). Previous studies (62, 99) have reported that FLAs supplementation increases the activity of SOD, CAT, and GPx in the Longissimus dorsi muscle of small ruminants. Therefore, similar effects of FLAs consumption in the present meta-analysis partially explain the lower MDA content observed. On the other hand, it is widely documented that meat color is a crucial factor that consumers consider when choosing fresh meat (93). L* and a* values are related to meat brightness and metmyoglobin content, respectively (93, 100). In the present meta-analysis, supplementation with FLAs did not affect L* and a* in meat, indicating that FLAs do not affect metmyoglobin formation or appearance in beef. Furthermore, the lower b* observed in response to FLAs supplementation is positive, as consumers expect to find low b* values in fresh meat (101).
FLAs supplementation did not affect the meat's protein, moisture, and ash content; however, the IMF increased. These results indicate that FLAs do not negatively affect the nutritional value of beef since the protein and ash content of the meat are related to its nutritional value (93, 102). In contrast, the higher IMF observed could be positive since IMF correlates positively with beef's tenderness and juiciness (103). In addition, some FLAs increase the adipogenesis of bovine preadipocytes (104), which participate in the deposition of IMF (105). In pigs, FLAs supplementation increases skeletal muscle PPARγ mRNA expression levels (106), which positively correlates with IMF (107). Similar effects of FLAs consumption in the present study partially explain the higher IMF observed.
4.6. Milk production and quality
It has been mentioned that increasing the utilization efficiency of ingested feed is necessary to improve milk production in ruminants (108). In the present study, FLAs supplementation increased DMD, OMD, CPD, NDFD, ADFD, and EED. These results indicate that FLAs increase the utilization efficiency of ingested feed and partially explain the higher milk production observed in response to FLAs supplementation. In addition, the higher milk production could be related to the increased ruminal propionate concentration observed since milk production in dairy cows increases curvilinearly in response to the supply of gluconeogenic precursors (109). In lactating buffaloes, it has been reported that supplementation with FLAs-rich plants increases serum somatotropin levels by up to 50% (110), which positively correlates with milk production in dairy cows (111). It has been documented that FLAs decrease the ruminal abundance of Clostridium microorganisms (112), which negatively correlates with milk production in bovines (113). In growing sheep, FLAs supplementation increases the relative abundance of the Ruminococcaceae microbial family (47), which positively correlates with milk production in dairy cows (113). Consequently, similar effects of FLAs consumption in the present meta-analysis partially explain the higher milk production observed.
Higher protein and fat content in milk was observed in response to FLAs supplementation. Under in vitro conditions, FLAs decrease the relative abundance of Clostridium (112) and Methanobrevibacter spp. (114), which negatively correlates with the percentage of milk protein in ruminants (115, 116). In beef cattle, FLAs supplementation increases the ruminal presence of the microbial family Succinivibrionaceae (57), which positively correlates with the protein content in milk from dairy cows (116). In the present study, the FLAs decreased the ruminal concentration of total protozoa, which negatively correlates with the fat content in the milk of small ruminants (117). In dairy cows, Kong et al. (59) detected that FLAs supplementation increases the relative rumen abundance of the microbial genus Butyrivibrio, which has a positive correlation with the fat percentage in dairy cows (116). In dairy goats, FLAs supplementation increases the expression of genes involved in milk fat synthesis, such as genes related to de novo fatty acid synthesis [acetyl-CoA carboxylase α (ACACA), fatty acid synthase (FASN), and stearoyl-CoA desaturase (SCD1)] and triglyceride synthesis [diacylglycerol Oacyltransferase 1 (DGAT1), diacylglycerol O-acyltransferase 2 (DGAT2), and glycerol-3-phosphate acyltransferase 1 (GPAM)] (118). Briefly, acetyl-CoA and malonyl-CoA are condensed under FASN catalysis, two carbon atoms are added to the carboxyl of the fatty acid, the ACACA gene limits the rate of the process, and the SCD1 gene catalyzes the synthesis of monounsaturated fatty acids (118). Likewise, the GPAM gene catalyzes the acyl group transfer from acyl-CoA to generate 1-acylglycerol-3-phosphate, while the GDAT1 and DGAT2 genes catalyze the formation of triglycerides with fatty acyl-CoA (118). Therefore, similar effects of FLAs consumption in the present study partially explain the increased milk fat and protein content observed. On the other hand, FLAs supplementation did not affect the lactose content in milk. This result was not expected since the FLAs increased the rumen concentration of propionate, which is the primary short-chain fatty acid required for lactose biosynthesis (108).
Tong et al. (119) mention that SCC is a widely used indicator to assess the health of the mammary gland and the quality of milk in bovines. For example, an increase in SCC is associated with intramammary infection and negatively affects raw milk quality (120). In the present meta-analysis, lower SCC was observed in response to FLAs supplementation, indicating that FLAs improve mammary gland health and milk quality in cattle. In dairy cows, FLAs supplementation decreases the presence of Staphylococcus bacteria in milk (121), which positively correlates with SCC in ruminant milk (122). In addition, IgA has been reported to be involved in the protection of mucous membranes, IgM is the first line of defense against infections, and IgG plays an important role in the immune response against infections (75, 121). In the present meta-analysis, IgG, IgA, and IgM serum levels increased in response to FLAs supplementation. Therefore, similar effects of FLAs consumption in the present study partially explain the lower SCC observed.
4.7. Limitations and strengths of the meta-analysis
The present meta-analysis was limited to research conducted only in beef cattle and dairy cows and may not apply to other ruminant species. In addition, high heterogeneity was detected in most of the response variables evaluated, which may represent a limitation in applying the global results obtained. However, this problem was diminished with the use of subgroup analysis, which allowed us to identify the specific conditions under which FLAs could be used successfully to improve different important parameters in beef cattle and dairy cows. Finally, this meta-analysis also establishes the steps for implementing future standardized experimental designs on the use of FLAs as growth promoters and natural antioxidants in beef cattle and dairy cows.
5. Conclusions
The results obtained in the present meta-analysis indicate that FLAs can be used as natural growth promoters in beef cattle and, at the same time, improve feed conversion. The best result for daily weight gain is obtained with FLAs supplementation periods up to 75 days and diets high in concentrate (>700 g/kg DM). Likewise, including FLAs in bovine diets improves dry matter intake and nutrient digestibility. The best dry matter intake is obtained with periods up to 75 days and when the FLAs used are puerarin, anthocyanin, and daidzein. Furthermore, supplementation with FLAs improves total antioxidant status and immune response in cattle by reducing serum concentration of malondialdehyde and increasing serum levels of antioxidant enzymes and immunoglobulins. The best results for serum concentration of superoxide dismutase are obtained with FLAs extracts and when the FLAs used are puerarin or daidzein. At the same time, FLAs supplementation improves meat quality by reducing shear force and malondialdehyde content. In addition, FLAs improve milk production and composition. The highest milk production is obtained when FLAs extracts are used, with daidzein or mixtures of FLAs, and low doses of FLAs (≤600 mg/kg DM). The best results for milk protein content are obtained with supplementation periods longer than 75 days, diets with moderate levels of concentrate (400–700 g/kg DM), and daidzein or mixtures of FLAs. Likewise, the best fat content in milk is achieved with daidzein or mixtures of FLAs and using cows with more than 100 days in milk. Finally, FLAs supplementation improves ruminal fermentation in cattle through increased ruminal propionate concentration and reduced total rumen protozoa. The best rumen propionate concentration is obtained with supplementation periods of up to 75 days.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
JO-O: conceptualization, methodology, data curation, formal analysis, investigation, visualization, writing—original draft preparation, and writing—review and editing. GD-I: methodology, data curation, formal analysis, validation, and writing—review and editing. AL-B: conceptualization, resources, writing—review and editing, supervision, project administration, resources, and funding acquisition. AC-C: methodology, investigation, data curation, and writing—review and editing. LM-R: data curation, supervision, and writing—review and editing. GM-M: software, supervision, and writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by Universidad Autónoma Chapingo.
Acknowledgments
JO-O was awarded a Conacyt scholarship during his Ph.D. studies in the Program of Animal Production at the Universidad Autónoma Chapingo.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1134925/full#supplementary-material
References
1. Kong F, Wang S, Dai D, Cao Z, Wang Y, Li S, et al. Preliminary investigation of the effects of rosemary extract supplementation on milk production and rumen fermentation in high-producing dairy cows. Antioxidants. (2022) 11:1715. doi: 10.3390/antiox11091715
2. Cronin GM, Rault, JL, Glatz PC. Lessons learned from past experience with intensive livestock management systems. Rev Sci Tech. (2014) 33:139–51. doi: 10.20506/rst.33.1.2256
3. Surai PF, Earle-Payne K. Antioxidant defences and redox homeostasis in animals. Antioxidants. (2022) 11:1012. doi: 10.3390/antiox11051012
4. Puppel K, Kapusta A, Kuczyńska B. The etiology of oxidative stress in the various species of animals a review. J Sci Food Agric. (2015) 95:2179–84. doi: 10.1002/jsfa.7015
5. Celi P. Oxidative stress in ruminants. In:Mandelker L, Vajdovich P, , editors. Studies on Veterinary Medicine. Totowa, NJ: Humana Press (2011). p. 191–231.
6. Lykkesfeldt J, Svendsen O. Oxidants and antioxidants in disease: oxidative stress in farm animals. Vet J. (2007) 173:502–11. doi: 10.1016/j.tvjl.2006.06.005
7. Abuelo A, Hernandez J, Benedito JL, Castillo C. The importance of the oxidative status of dairy cattle in the periparturient period: revisiting antioxidant supplementation. J Anim Physiol Anim Nutr. (2015) 99:1003–18. doi: 10.1111/jpn.12273
8. Majrashi KA. Effects of supplementing quails' (Coturnix japonica) diets with a blend of clove (Syzygium aromaticum) and black cumin (Nigella sativa) oils on growth performance and health aspects. Life. (2022) 12:1915. doi: 10.3390/life12111915
9. Gessner DK, Ringseis R, Eder K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J Anim Physiol Anim Nutr. (2016) 101:605–28. doi: 10.1111/jpn.12579
10. North MK, Dalle Zotte A, Hoffman LC. The use of dietary flavonoids in meat production: a review. Anim Feed Sci Technol. (2019) 257:114291. doi: 10.1016/j.anifeedsci.2019.114291
11. Kamboh AA, Leghari RA, Khan MA, Kaka U, Naseer M, Sazili AQ, et al. Flavonoids supplementation-an ideal approach to improve quality of poultry products. World's Poult Sci J. (2019) 75:115–26. doi: 10.1017/S0043933918000703
12. Prihambodo TR, Sholikin MM, Qomariyah N, Jayanegara A, Batubara I, Utomo DB. Effects of dietary flavonoids on performance blood constituents carcass composition and small intestinal morphology of broilers: a meta-analysis. Anim Biosci. (2021) 34:434–42. doi: 10.5713/ajas.20.0379
13. Iskender H, Yenice G, Dokumacioglu E, Kaynar O, Hayirli A, Kaya A. The effects of dietary flavonoid supplementation on the antioxidant status of laying hens. Braz J Poult Sci. (2016) 18:663–8. doi: 10.1590/1806-9061-2016-0356
14. Olagaray KE, Bradford BJ. Plant flavonoids to improve productivity of ruminants—A review. Anim Feed Sci Technol. (2019) 251:21–36. doi: 10.1016/j.anifeedsci.2019.02.004
15. Liu DY, He SJ, Jin EH, Liu SQ, Tang YG Li SH, Zhong LT. Effect of daidzein on production performance and serum antioxidative function in late lactation cows under heat stress. Livest Sci. (2013) 152:16–20. doi: 10.1016/j.livsci.2012.12.003
16. Tilahun M, Zhao L, Guo Z, Shen Y, Ma L, Callaway TR, et al. Amla (Phyllanthus emblica) fresh fruit as new feed source to enhance ruminal fermentation and milk production in lactating dairy cows. Anim Feed Sci Technol. (2022) 283:115160. doi: 10.1016/j.anifeedsci.2021.115160
17. Tian X, Li J, Luo Q, Wang X, Wang T, Zhou D, Xie L, Ban C, Lu Q. Effects of purple corn anthocyanin on growth performance meat quality muscle antioxidant status and fatty acid profiles in goats. Foods. (2022) 11:255. doi: 10.3390/foods11091255
18. Paniagua M, Crespo J, Aris A, Devant M. Citrus aurantium flavonoid extract improves concentrate efficiency animal behavior and reduces rumen inflammation of Holstein bulls fed high-concentrate diets. Anim Feed Sci Technol. (2019) 258:114304. doi: 10.1016/j.anifeedsci.2019.114304
19. Paniagua M, Crespo FJ, Arís A, Devant M. Effects of flavonoids extracted from Citrus aurantium on performance behavior and rumen gene expression in holstein bulls fed with high-concentrate diets in pellet form. Animals. (2021) 11:1387. doi: 10.3390/ani11051387
20. Liang H, Xu L, Zhao X, Bai J, Chen Z, Zhou S, et al. Effect of daidzein on fermentation parameters and bacterial community of finishing Xianan cattle. Ital J Anim Sci. (2018) 17:950–8. doi: 10.1080/1828051X.2018.1431965
21. Yanza YR, Szumacher-Strabel M, Lechniak D, Slusarczyk S, Kolodziejski P, Patra AK, et al. Dietary Coleus amboinicus Lour. decreases ruminal methanogenesis and biohydrogenation and improves meat quality and fatty acid composition in Longissimus thoracis muscle of lambs. J Animal Sci Biotechnol. (2022) 13:5. doi: 10.1186/s40104-021-00654-3
22. Totakul P, Viennasay B, Sommai S, Matra M, Infascelli F, Wanapat M. Chaya (Cnidoscolus aconitifolius Mill. Johnston) pellet supplementation improved rumen fermentation milk yield and milk composition of lactating dairy cows. Livest Sci. (2022) 262:104974. doi: 10.1016/j.livsci.2022.104974
23. Tilahun M, Zhao L, Sun L, Shen Y, Ma L, Callaway TR, et al. Fresh Phyllanthus emblica (Amla) fruit supplementation enhances milk fatty acid profiles and the antioxidant capacities of milk and blood in dairy cows. Antioxidants. (2022) 11:485. doi: 10.3390/antiox11030485
24. Zhao XH, Chen ZD, Zhou S, Song XZ, Ouyang KH, Pan K, et al. Effects of daidzein on performance serum metabolites nutrient digestibility and fecal bacterial community in bull calves. Anim Feed Sci Tech. (2017) 225:87–96. doi: 10.1016/j.anifeedsci.2017.01.014
25. Liang H, Zhao XH, Pan K, Xu LJ Yi ZH, Bai J, Qi XL, et al. Effects of daidzein on growth performance blood metabolites and meat quality of finishing Xianan beef cattle. J Agric Sci. (2019) 157:169–75. doi: 10.1017/S0021859619000340
26. Peng T, Shang H, Yang M, Li Y, Luo J, Qu M, et al. Puerarin improved growth performance and postmortem meat quality by regulating lipid metabolism of cattle under hot environment. Anim Sci J. (2021) 92:e13543. doi: 10.1111/asj.13543
27. Aguiar S, Cottica S, Boeing J, Samensari R, Santos G, Visentainer J, et al. Effect of feeding phenolic compounds from propolis extracts to dairy cows on milk production milk fatty acid composition and the antioxidant capacity of milk. Anim Feed Sci Technol. (2014) 193:148–54. doi: 10.1016/j.anifeedsci.2014.04.006
28. Weththasinghe P, Hansen JØ, Mydland LT, Øverland M. A systematic meta-analysis based review on black soldier fly (Hermetia illucens) as a novel protein source for salmonids. Rev Aquac. (2022) 14:938–56. doi: 10.1111/raq.12635
29. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
30. Sauvant D, Letourneau-Montminy M, Schmidely P, Boval M, Loncke C, Daniel J. Use and misuse of meta-analysis in Animal Science. Animal 2020 14 s207–s222. doi: 10.1017/S1751731120001688
31. Patra AK. Meta-analyses of effects of phytochemicals on digestibility and rumen fermentation characteristics associated with methanogenesis. J Sci Food Agric. (2010) 90:2700–8. doi: 10.1002/jsfa.4143
32. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
33. Serra V, Salvatori G, Pastorelli G. Dietary polyphenol supplementation in food producing animals: effects on the quality of derived products. Animals. (2021) 11:401. doi: 10.3390/ani11020401
34. Lean IJ, Golder HM, Grant TMD, Moate PJ. A meta-analysis of effects of dietary seaweed on beef and dairy cattle performance and methane yield. PLoS ONE. (2021) 16:e0249053. doi: 10.1371/journal.pone.0249053
35. Dorantes-Iturbide G, Orzuna-Orzuna JF, Lara-Bueno A, Mendoza-Martínez GD, Miranda-Romero LA, Lee-Rangel HA. Essential oils as a dietary additive for small ruminants: a meta-analysis on performance rumen parameters serum metabolites and product quality. Vet Sci. (2022) 9:475. doi: 10.3390/vetsci9090475
36. Orzuna-Orzuna JF, Dorantes-Iturbide G, Lara-Bueno A, Miranda-Romero LA, Mendoza-Martínez GD, Santiago-Figueroa I, et al. Meta-analysis of essential oils use for beef cattle feed: rumen fermentation blood metabolites meat quality performance and environmental and economic impact. Fermentation. (2022) 8:254. doi: 10.3390/fermentation8060254
37. Higgins J, Thomas J. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley and Sons Ltd. (2019). p. 143–76.
38. Viechtbauer W. Conducting meta-analysis in R with the metaphor package. J Stat Softw. (2010) 36:1–48. doi: 10.18637/jss.v036.i03
39. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
40. Appuhamy JRN, Strathe AB, Jayasundara S, Wagner-Riddle C, Dijkstra J, France J, et al. Anti-methanogenic effects of monensin in dairy and beef cattle: a meta-analysis. J Dairy Sci. (2013) 96:5161–73. doi: 10.3168/jds.2012-5923
41. SAS Institute Inc. Step-by-Step Programming With Base SASR Edition 9.4. Cary, NC: SAS Institute Inc. (2017).
42. Egger M, Smith GD, Altman DG. Systematic Reviews in Health Care. 2nd ed. London: MBJ Publishing Group (2001). p. 109–21.
43. Lean IJ, Thompson JM, Dunshea FR. A meta-analysis of zilpaterol and ractopamine effects on feedlot performance carcass traits and shear strength of meat in cattle. PLoS ONE. (2014) 9:e115904. doi: 10.1371/journal.pone.0115904
44. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
45. Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Amer Statist Assoc. (2000) 95:89–98. doi: 10.1080/01621459.2000.10473905
46. Littell JH, Corcoran J, Pillai V. Systematic Reviews and Meta-analysis. 1st ed. Oxford: Oxford University Press (2008). p. 111–32.
47. Zhao Y, Zhang Y, Khas E, Ao C, Bai C. Effects of Allium mongolicum Regel ethanol extract on three flavor-related rumen branched-chain fatty acids rumen fermentation and rumen bacteria in lambs. Front Microbiol. (2022) 13:978057. doi: 10.3389/fmicb.2022.978057
48. Du H, Erdene K, Chen S, Qi S, Bao Z, Zhao Y, Wang C, Zhao G, Ao C. Correlation of the rumen fluid microbiome and the average daily gain with a dietary supplementation of Allium mongolicum Regel extracts in sheep. J Anim Sci. (2019) 97:2865–77. doi: 10.1093/jas/skz139
49. Jiang C, Ding L, Dong Q, Wang X, Wei H, Hu C, et al. Effects of root extracts of three traditional Chinese herbs as dietary supplements on dry matter intake average daily gain rumen fermentation and ruminal microbiota in early weaned yak calves. Anim Feed Sci Technol. (2021) 278:115002. doi: 10.1016/j.anifeedsci.2021.115002
50. Yi X, Wu B, Ma J, Cui X, Deng Z, Hu S, et al. Effects of dietary capsaicin and Yucca schidigera extracts as feed additives on rumen fermentation and microflora of beef cattle fed with a moderate-energy diet. Fermentation. (2023) 9:30. doi: 10.3390/fermentation9010030
51. Paniagua M, Crespo JF, Arís A, Devant M. Supplementing Citrus aurantium flavonoid extract in high-fat finishing diets improves animal behavior and rumen health and modifies rumen and duodenum epithelium gene expression in holstein bulls. Animals. (2022) 12:1972. doi: 10.3390/ani12151972
52. Takay S, Yoshida R, Shigemura N, Ninomiya Y. Chemosensory Transduction. Cambridge, MA: Academic Press (2016). p. 299–317.
53. La Sala MS, La Hurtado MD, Brown AR, Bohórquez DV, Liddle RA, Herzog H, et al. Modulation of taste responsiveness by the satiation hormone peptide YY. FASEB J. (2013) 27:5022–33. doi: 10.1096/fj.13-228064
54. Koike S, Kobayashi Y. Fibrolytic rumen bacteria: their ecology and functions. Asian Aust J Anim Sci. (2009) 22:131–8. doi: 10.5713/ajas.2009.r.01
55. Kim ET, Guan LL, Lee SJ, Lee SM, Lee SS, Lee ID, et al. Effects of flavonoid-rich plant extracts on in vitro ruminal methanogenesis microbial populations and fermentation characteristics. Asian Aust J Anim Sci. (2015) 28:530–7. doi: 10.5713/ajas.14.0692
56. Zhan J, Liu M, Wu C, Su X, Zhan K, Zhao GQ. Effects of alfalfa flavonoids extract on the microbial flora of dairy cow rumen. Asian Aust J Anim Sci. (2017) 30:1261–9. doi: 10.5713/ajas.16.0839
57. Akin DE, Borneman WS. Role of rumen fungi in fiber degradation. J Dairy Sci. (1990) 73:3023–32. doi: 10.3168/jds.S0022-0302(90)78989-8
58. Niu Y, Meng Q, Li S, Ren L, Zhou B, Schonewille T, et al. Effects of diets supplemented with ensiled mulberry leaves and sun-dried mulberry fruit Pomace on the Ruminal bacterial and Archaeal community composition of finishing steers. PLoS ONE. (2016) 11:e0156836. doi: 10.1371/journal.pone.0156836
59. Kong F, Liu Y, Wang S, Zhang Y, Wang W, Yang H, et al. Nutrient digestibility microbial fermentation and response in bacterial composition to methionine dipeptide: an in vitro study. Biology. (2022) 11:93. doi: 10.3390/biology11010093
60. Zhang YK, Zhang XX Li FD, Li C, Li GZ, Zhang DY, Song QZ Li XL, et al. Characterization of the rumen microbiota and its relationship with residual feed intake in sheep. Animal. (2021) 15:100161. doi: 10.1016/j.animal.2020.100161
61. Dorantes-Iturbide G, Orzuna-Orzuna JF, Lara-Bueno A, Miranda-Romero LA, Mendoza-Martínez GD, Hernández-García PA. Effects of a polyherbal dietary additive on performance dietary energetics carcass traits and blood metabolites of finishing lambs. Metabolites. (2022) 12:413. doi: 10.3390/metabo12050413
62. Qin X, Zhang T, Cao Y, Deng B, Zhang J, Zhao J. Effects of dietary sea buckthorn pomace supplementation on skeletal muscle mass and meat quality in lambs. Meat Sci. (2020) 166:108141. doi: 10.1016/j.meatsci.2020.108141
63. Yang B, Le J, Wu P, Liu J, Guan LL, Wang J. Alfalfa intervention alters rumen microbial community development in Hu lambs during early life. Front Microbiol. (2018) 9:574. doi: 10.3389/fmicb.2018.00574
64. Balcells J, Aris A, Serrano A, Seradj A, Crespo J, Devant M. Effects of an extract of plant flavonoids (Bioflavex) on rumen fermentation and performance in heifers fed high-concentrate diets. J Anim Sci. (2012) 90:4975–84. doi: 10.2527/jas.2011-4955
65. Paniagua M, Crespo J, Bach A, Devant M. Effects of flavonoids extracted from Citrus aurantium on performance eating and animal behavior rumen health and carcass quality in Holstein bulls fed high-concentrate diets. Anim Feed Sci Technol. (2018) 246:114–26. doi: 10.1016/j.anifeedsci.2018.08.010
66. Graham C, Simmons N. Functional organization of the bovine rumen epithelium. Am J Physiol. (2005) 288:R173–81. doi: 10.1152/ajpregu.00425.2004
67. Li Y, Shang H, Zhao X, Qu M, Peng T, Guo B, et al. Radix Puerarin extract (Puerarin) could improve meat quality of heat-stressed beef cattle through changing muscle antioxidant ability and fiber characteristics. Front Vet Sci. (2021) 7:615086. doi: 10.3389/fvets.2020.615086
68. Geary TW, McFadin EL, MacNeil MD, Grings EE, Short RE, Funston RN, et al. Leptin as a predictor of carcass composition in beef cattle. J Anim Sci. (2003) 81:1–8. doi: 10.2527/2003.8111
69. Liang H, Xu L, Zhao X, Pan K, Yi Z, Bai J, et al. RNA-Seq analysis reveals the potential molecular mechanisms of daidzein on adipogenesis in subcutaneous adipose tissue of finishing Xianan beef cattle. J Anim Physiol Anim Nutr. (2020) 104:1–11. doi: 10.1111/jpn.13218
70. Shi L, Jin X, Xu Y, Xing Y, Yan S, Guo Y, et al. Effects of total flavonoids of Artemisia ordosica on growth performance oxidative stress and antioxidant status of lipopolysaccharide-challenged broilers. Antioxidants. (2022) 11:1985. doi: 10.3390/antiox11101985
71. Mendonca P, Soliman KFA. Flavonoids activation of the transcription factor Nrf2 as a hypothesis approach for the prevention and modulation of SARS-CoV-2 infection severity. Antioxidants. (2020) 9:659. doi: 10.3390/antiox9080659
72. Ghiselli A, Serafini M, Natella F, Scaccini C. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med. (2000) 29:1106–14. doi: 10.1016/S0891-5849(00)00394-4
73. Demirci-Çekiç S, Özkan G, Avan AN, Uzunboy S, Çapanoglu E, Apak R. Biomarkers of oxidative stress and antioxidant defense. J Pharm Biomed Anal. (2022) 209:114477. doi: 10.1016/j.jpba.2021.114477
74. Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem. (1997) 43:1209–14. doi: 10.1093/clinchem/43.7.1209
75. Zhan J, Liu M, Su X, Zhan K, Zhang C, Zhao G. Effects of alfalfa flavonoids on the production performance immune system and ruminal fermentation of dairy cows. Asian Aust J Anim Sci. (2017) 30:1416–24. doi: 10.5713/ajas.16.0579
76. Ingvartsen KL, Moyes K. Nutrition immune function and health of dairy cattle. Animal. (2013) 7:112–22. doi: 10.1017/S175173111200170X
77. Wolf HM, Fischer MB, Puhringer H, Samstag A, Vogel E, Eibl MM. Human serum IgA downregulates the release of inflammatory cytokines (tumor necrosis factor-alpha interleukin-6) in human monocytes. Blood. (1994) 83:1278–88. doi: 10.1182/blood.V83.5.1278.1278
78. Estruel-Amades S, Massot-Cladera M, Pérez-Cano FJ, Franch À, Castell M, Camps-Bossacoma M. Hesperidin effects on gut microbiota and gut-associated lymphoid tissue in healthy rats. Nutrients. (2019) 11:324. doi: 10.3390/nu11020324
79. Han L, Fu Q, Deng C, Luo L, Xiang T, Zhao H. Immunomodulatory potential of flavonoids for the treatment of autoimmune diseases and tumour. Scand J Immunol. (2022) 95:e13106. doi: 10.1111/sji.13106
80. Kageyama Y, Katayama N. Ontogeny of human B1 cells. Int J Hematol. (2020) 111:628–33. doi: 10.1007/s12185-019-02775-y
81. Chen L, Ishigami T, Doi H, Arakawa K, Tamura K. Gut microbiota and atherosclerosis: role of B cell for atherosclerosis focusing on the gut-immune-B2 cell axis. J Mol Med. (2020) 98:1235–44. doi: 10.1007/s00109-020-01936-5
82. Khonkhaeng B, Wanapat M, Wongtangtintharn S, Phesatcha K, Supapong C, Suntara C, et al. Tropical plant phytonutrient improves the use of insect protein for ruminant feed. Agriculture. (2022) 12:1628. doi: 10.3390/agriculture12101628
83. Seradj AR, Abecia L, Crespo J, Villalba D, Fondevila M, Balcells J. The effect of Bioflavex®and its pure flavonoid components on in vitro fermentation parameters and methane production in rumen fluid from steers given high concentrate diets. Anim Feed Sci Technol. (2014) 197:85–91. doi: 10.1016/j.anifeedsci.2014.08.013
84. Seradj AR, Gimeno A, Fondevila M, Crespo J, Armengol R, Balcells J. Effects of the citrus flavonoid extract bioflavex or its pure components on rumen fermentation of intensively reared beef steers. Anim Prod Sci. (2016) 58:553–60. doi: 10.1071/AN15146
85. Cherdthong A, Khonkhaeng B, Foiklang S, Wanapat M, Gunun N, Gunun P, et al. Effects of supplementation of Piper sarmentosum leaf powder on feed efficiency rumen ecology and rumen protozoal concentration in Thai native beef cattle. Animals. (2019) 9:130. doi: 10.3390/ani9040130
86. Bagheri VM, Klevenhusen F, Zebeli Q, Petri R. Scrophularia striata extract supports rumen fermentation and improves microbial diversity in vitro compared to monensin. Front Microbiol. (2018) 9:2164. doi: 10.3389/fmicb.2018.02164
87. McCabe MS, Cormican P, Keogh K, O'Connor A, O'Hara E, Palladino RA, et al. Illumina miseq phylogenetic amplicon sequencing shows a large reduction of an uncharacterised Succinivibrionaceae and an increase of the Methanobrevibacter gottschalkii clade in feed restricted cattle. PLoS ONE. (2015) 10:e0133234. doi: 10.1371/journal.pone.0133234
88. Wu QC, Wang WK, Zhang F, Li WJ, Wang YL, Lv LK, et al. Dietary cysteamine supplementation remarkably increased feed efficiency and shifted rumen fermentation toward glucogenic propionate production via enrichment of Prevotella in feedlot lambs. Microorganisms. (2022) 10:1105. doi: 10.3390/microorganisms10061105
89. Newbold CJ, de la Fuente G, Belanche A, Ramos-Morales E, McEwan NR. The role of ciliate protozoa in the rumen. Front Microbiol. (2015) 6:1313. doi: 10.3389/fmicb.2015.01313
90. Guyader J, Eugene M, Noziere P, Morgavi DP, Doreau M, Martin C. Influence of rumen protozoa on methane emission in ruminants: a meta-analysis approach. Animal. (2014) 8:1816–25. doi: 10.1017/S1751731114001852
91. Wang C, Matarneh S, Gerrard D, Tan J. Modelling of energy metabolism and analysis of pH variations in postmortem muscle. Meat Sci. (2021) 182:108634. doi: 10.1016/j.meatsci.2021.108634
92. Corazzin M, Del Bianco S, Bovolenta S, Piasentier E. More Than Beef Pork and Chicken—The Production Processing and Quality Traits of Other Sources of Meat for Human Diet. Cham: Springer International Publishing (2019). p. 119–65.
94. Amaral AB, da Silva MV, Lannes SCDS. Lipid oxidation in meat: mechanisms and protective factors—A review. Food Sci Technol. (2018) 38:1–15. doi: 10.1590/fst.32518
95. Liang R, Zhu H, Mao Y, Zhang Y, Zhu L, Cornforth D, et al. Tenderness and sensory attributes of the longissimus lumborum muscles with different quality grades from Chinese fattened yellow crossbred steers. Meat Sci. (2016) 112:52–7. doi: 10.1016/j.meatsci.2015.10.004
96. Seideman SC, Wheeler TL, Koohmaraie M. The influence of muscle fiber size on tenderness in A-maturity heifers. J Food Qual. (1988) 11:27–34. doi: 10.1111/j.1745-4557.1988.tb00862.x
97. Rowe LJ, Maddock KR, Lonergan SM, Huff-Lonergan E. Oxidative environments decrease tenderization of beef steaks through inactivation of μ-calpain1. J Anim Sci. (2004) 82:3254–66. doi: 10.2527/2004.82113254x
98. Pateiro M, Barba FJ, Domínguez R, Sant'Ana AS, Mousavi KA, Gavahian M, et al. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: a review. Food Res Int. (2018) 113:156–66. doi: 10.1016/j.foodres.2018.07.014
99. Mu C, Yang W, Wang P, Zhao J, Hao X, Zhang J. Effects of high-concentrate diet supplemented with grape seed proanthocyanidins on growth performance liver function meat quality and antioxidant activity in finishing lambs. Anim Feed Sci Technol. (2020) 266:114518. doi: 10.1016/j.anifeedsci.2020.114518
100. Ornaghi MG, Guerrero A, Vital ACP, de Souza KA, Passetti RAC, Mottin C, et al. Improvements in the quality of meat from beef cattle fed natural additives. Meat Sci. (2020) 163:108059. doi: 10.1016/j.meatsci.2020.108059
101. Orzuna-Orzuna JF, Dorantes-Iturbide G, Lara-Bueno A, Mendoza-Martínez GD, Miranda-Romero LA, López-Ordaz R, et al. Productive performance carcass traits and meat quality in finishing lambs supplemented with a polyherbal mixture. Agriculture. (2021) 11:942. doi: 10.3390/agriculture11100942
102. Sierra-Galicia MI, Rodríguez-de Lara R, Orzuna-Orzuna JF, Lara-Bueno A, García-Muñiz JG, Fallas-López M, et al. Supplying bee pollen and propolis to growing rabbits: effects on growth performance blood metabolites and meat quality. Life. (2022) 12:1987. doi: 10.3390/life12121987
103. Wood JD, Enser M, Fisher AV, Nute GR, Sheard PR, Richardson RI, et al. Fat deposition fatty acid composition and meat quality: a review. Meat Sci. (2008) 78:343–58. doi: 10.1016/j.meatsci.2007.07.019
104. Yun J, Yu Y, Zhou G, Luo X, Jin H, Zhao Y, et al. Effects of puerarin on the AKT signaling pathway in bovine preadipocyte differentiation. Asian Aust J Anim Sci. (2020) 33:4–11. doi: 10.5713/ajas.19.0004
105. Hocquette JF, Gondret F, Baéza E, Médale F, Jurie C, Pethick DW. Intramuscular fat content in meat-producing animals: development genetic and nutritional control and identification of putative markers. Animal. (2010) 4:303–19. doi: 10.1017/S1751731109991091
106. Liu Y, Xiao Y, Xie J, Peng Y, Li F, Chen C, et al. Dietary supplementation with flavonoids from mulberry leaves improves growth performance and meat quality and alters lipid metabolism of skeletal muscle in a chinese hybrid pig. Anim Feed Sci Techol. (2022) 285:115211. doi: 10.1016/j.anifeedsci.2022.115211
107. Cui J, Chen W, Liu J, Xu T, Zeng Y. Study on quantitative expression of PPARγ and ADRP in muscle and its association with intramuscular fat deposition of pig. Springerplus. (2016) 5:1501. doi: 10.1186/s40064-016-3187-0
108. Mierlita D, Santa A, Mierlita S, Daraban SV, Suteu M, Pop IM, et al. The effects of feeding milled rapeseed seeds with different forage: concentrate ratios in jersey dairy cows on milk production milk fatty acid composition and milk antioxidant capacity. Life. (2023) 13:46. doi: 10.3390/life13010046
109. Rigout S, Hurtaud C, Lemosquet S, Bach A, Rulquin H. Lactational effect of propionic acid and duodenal glucose in cows. J Dairy Sci. (2003) 86:243–53. doi: 10.3168/jds.S0022-0302(03)73603-0
110. Li M, Hassan F, Tang Z, Peng L, Liang X, Li L, et al. Mulberry leaf flavonoids improve milk production antioxidant and metabolic status in buffaloes during summer season. Front Vet Sci. (2020) 7:599. doi: 10.3389/fvets.2020.00599
111. Peel CJ, Bauman DE. Somatotropin and lactation. J Dairy Sci. (1987) 70:474–86. doi: 10.3168/jds.S0022-0302(87)80030-9
112. Sivakumaran S, Molan AL, Meagher LP, Kolb B, Foo LY, Lane GA, et al. Variation in antimicrobial action of proanthocyanidins from Dorycnium rectum against rumen bacteria. Phytochemistry. (2004) 65:2485–97. doi: 10.1016/j.phytochem.2004.08.046
113. Jami E, White BA, Mizrahi I. Potential role of the bovine rumen microbiome in modulating milk composition and feed efficiency. PLoS ONE. (2014) 9:e85423. doi: 10.1371/journal.pone.0085423
114. Eger M, Graz M, Riede S, Breves G. Application of MootralTM reduces methane production by altering the archaea community in the rumen simulation technique. Front Microbiol. (2018) 9:2094. doi: 10.3389/fmicb.2018.02094
115. Mavrommatis A, Skliros D, Flemetakis E, Tsiplakou E. Changes in the rumen bacteriome structure and enzymatic activities of goats in response to dietary supplementation with Schizochytrium spp. Microorganisms. (2021) 9:1528. doi: 10.3390/microorganisms9071528
116. Xue M, Sun H, Wu X, Guan LL, Liu J. Assessment of rumen microbiota from a large dairy cattle cohort reveals the pan and core bacteriomes contributing to varied phenotypes. Appl Environ Microbiol. (2018) 84:e00970–18. doi: 10.1128/AEM.00970-18
117. Mavrommatis A, Skliros D, Simoni M, Righi F, Flemetakis E, Tsiplakou E. Alterations in the rumen particle-associated microbiota of goats in response to dietary supplementation levels of Schizochytrium spp. Sustainability. (2021) 13:607. doi: 10.3390/su13020607
118. Zhao M, Lv D, Hu J, He Y, Wang Z, Liu X, et al. Hybrid Broussonetia papyrifera fermented feed can play a role through flavonoid extracts to increase milk production and milk fatty acid synthesis in dairy goats. Front Vet Sci. (2022) 9:794443. doi: 10.3389/fvets.2022.794443
119. Tong J, Zhang H, Zhang Y, Xiong B, Jiang L. Microbiome and metabolome analyses of milk from dairy cows with subclinical Streptococcus Agalactiae mastitis—potential biomarkers. Front Microbiol. (2019) 10:547. doi: 10.3389/fmicb.2019.02547
120. Sharma N, Singh NK, Bhadwal MS. Relationship of somatic cell count and mastitis: an overview. Asian Aust J Anim Sci. (2011) 24:429–38. doi: 10.5713/ajas.2011.10233
121. Hou K, Tong J, Zhang H, Gao S, Guo Y, Niu H, et al. Microbiome and metabolic changes in milk in response to artemisinin supplementation in dairy cows. AMB Express. (2020) 10:154. doi: 10.1186/s13568-020-01080-w
Keywords: oxidative stress, natural antioxidants, natural phytochemicals, immunity, digestibility
Citation: Orzuna-Orzuna JF, Dorantes-Iturbide G, Lara-Bueno A, Chay-Canul AJ, Miranda-Romero LA and Mendoza-Martínez GD (2023) Meta-analysis of flavonoids use into beef and dairy cattle diet: Performance, antioxidant status, ruminal fermentation, meat quality, and milk composition. Front. Vet. Sci. 10:1134925. doi: 10.3389/fvets.2023.1134925
Received: 31 December 2022; Accepted: 30 January 2023;
Published: 15 February 2023.
Edited by:
Yosra Ahmed Soltan, Alexandria University, EgyptReviewed by:
Panagiotis E. Simitzis, Agricultural University of Athens, GreeceNesrein M. Hashem, Alexandria University, Egypt
Naifeng Zhang, Institute of Feed Research (CAAS), China
Maghsoud Besharati, University of Tabriz, Iran
Copyright © 2023 Orzuna-Orzuna, Dorantes-Iturbide, Lara-Bueno, Chay-Canul, Miranda-Romero and Mendoza-Martínez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alejandro Lara-Bueno,  alarab_11@hotmail.com
alarab_11@hotmail.com
 José Felipe Orzuna-Orzuna
José Felipe Orzuna-Orzuna Griselda Dorantes-Iturbide1
Griselda Dorantes-Iturbide1  Alejandro Lara-Bueno
Alejandro Lara-Bueno Alfonso Juventino Chay-Canul
Alfonso Juventino Chay-Canul