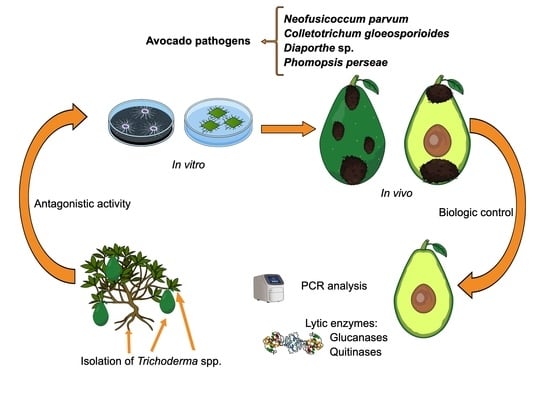
Isolation and Characterization of Trichoderma spp. for Antagonistic Activity against Avocado (Persea americana Mill) Fruit Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Phytopathogens
2.2. Isolation of Trichoderma spp. from Avocado Orchards
2.3. Characterization and Identification of Trichoderma spp. Strains Isolated
2.3.1. Morphological and Microscopical Characterization
2.3.2. Molecular Identification
2.4. In Vitro Evaluation of the Antagonistic Capacity of Trichoderma spp.
2.5. In Vivo Evaluation of the Antagonistic Capacity of Trichoderma spp.
2.6. Crude Extracellular Extract of Trichoderma spp.
2.7. Reducing Sugars Content
2.8. Chitinase and Glucanase Activities
2.8.1. Chitinase Activity
2.8.2. Glucanase Activity
2.9. Statistical Analysis
3. Results
3.1. Isolation and Identification of Trichoderma spp. Strains
3.2. In Vitro Evaluation of the Antagonistic Capacity of Trichoderma spp.
3.3. In Vivo Evaluation of the Antagonistic Capacity of Trichoderma spp.
3.4. Lytic Activity (Chitinase and Glucanase) of Trichoderma harzianum
3.4.1. Reducing Sugars
3.4.2. Total Enzyme Activity of Chitinase and Glucanase
4. Discussion
4.1. Isolation and Identification of Trichoderma spp. Strains
4.2. In Vitro Evaluation of the Antagonistic Capacity of Trichoderma spp.
4.3. In Vivo Evaluation of the Antagonistic Capacity of Trichoderma spp.
4.4. Lytic Activity (Chitinase and Glucanase) of Trichoderma harzianum
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Domingues, M.V.P.F.; Moura, K.E.D.; Salomão, D.; Elias, L.M.; Patricio, F.R.A. Effect of temperature on mycelial growth of Trichoderma, Sclerotinia minor and S. sclerotiorum, as well as on mycoparasitism. Summa Phytopathol. 2016, 42, 222–227. [Google Scholar] [CrossRef] [Green Version]
- Ab Rahman, S.F.S.; Singh, E.; Pieterse, C.M.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, M.J. The Value of Plant Disease Early-Warning Systems: A Case Study of USDA’s Soybean Rust Coordinated Framework; USDA Economic Research Service: Washington, DC, USA, 2006.
- Vinchira-Villarraga, D.M.; Moreno-Sarmiento, N. Control biológico: Camino a la agricultura moderna. Rev. Colomb. Biotecnol. 2019, 21, 2–5. [Google Scholar] [CrossRef]
- Awad, N.E.; Kassem, H.A.; Hamed, M.A.; El-Feky, A.M.; Elnaggar, M.A.; Mahmoud, K.; Ali, M.A. Isolation and characterization of the bioactive metabolites from the soil derived fungus Trichoderma viride. Mycology 2018, 9, 70–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NawRocka, J.; Szczech, M.; MałolepSza, U. Trichoderma atroviride enhances phenolic synthesis and cucumber protection against Rhizoctonia solani. Plant Prot. Sci. 2017, 54, 17–23. [Google Scholar]
- Debode, J.; De Tender, C.; Cremelie, P.; Lee, A.S.; Kyndt, T.; Muylle, H.; De Swaef, T.; Vandecasteele, B. Trichoderma-inoculated miscanthus straw can replace peat in strawberry cultivation, with beneficial effects on disease control. Front. Plant Sci. 2018, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.V.; Herrmann-Andrade, A.M.; Calabrese, C.D.; Bello, F.; Vázquez, D.; Musumeci, M.A. Effectiveness of Trichoderma strains isolated from the rhizosphere of citrus tree to control Alternaria alternata, Colletotrichum gloeosporioides and Penicillium digitatum A21 resistant to pyrimethanil in post-harvest oranges (Citrus sinensis L.(Osbeck)). J. Appl. Microbiol. 2020, 129, 712–727. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, R.; Duan, Y.; Jiang, W.; Chen, X.; Shen, X.; Yin, C.; Mao, Z. The endophytic strain Trichoderma asperellum 6S-2: An efficient biocontrol agent against apple replant disease in China and a potential plant-growth-promoting fungus. J. Fungi 2021, 7, 1050. [Google Scholar] [CrossRef]
- Wong, C.K.F.; Zulperi, D.; Saidi, N.B.; Vadamalai, G. A consortium of Pseudomonas aeruginosa and Trichoderma harzianum for improving growth and induced biochemical changes in Fusarium wilt infected bananas. Trop. Life Sci. Res. 2021, 32, 23. [Google Scholar] [CrossRef]
- Grano-Maldonado, M.I.; Ramos-Payan, R.; Rivera-Chaparro, F.; Aguilar-Medina, M.; Romero-Quintana, J.G.; Rodríguez-Santiago, A.; Nieves-Soto, M. First molecular characterization of Colletotrichum sp. and Fusarium sp. isolated from mangrove in Mexico and the antagonist effect of Trichoderma harzianum as an effective biocontrol agent. Plant Pathol. J. 2021, 37, 465. [Google Scholar] [CrossRef] [PubMed]
- Intana, W.; Kheawleng, S.; Sunpapao, A. Trichoderma asperellum T76-14 released volatile organic compounds against postharvest fruit rot in muskmelons (Cucumis melo) caused by Fusarium incarnatum. J. Fungi 2021, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Liming, X.; Xueliang, S. High-yield cellulase production by Trichoderma reesei ZU-02 on corn cob residue. Bioresour. Technol. 2004, 91, 259–262. [Google Scholar] [CrossRef]
- Tapia Rodríguez, A.; Ramírez Dávila, J.F.; Salgado Siclán, M.L.; Castañeda Vildózola, Á.; Maldonado Zamora, F.I.; Díaz, A.V.L. Distribución espacial de antracnosis (Colletotrichum gloeosporioides Penz) en aguacate en el Estado de México, México. Rev. Argent. De Microbiol. 2020, 52, 72–81. [Google Scholar] [CrossRef] [PubMed]
- SIAP Servicio de Información Agroalimentaria y Pesquera. México. Available online: https://www.gob.mx/siap (accessed on 23 October 2020).
- Twizeyimana, M.; Förster, H.; McDonald, V.; Wang, D.; Adaskaveg, J.; Eskalen, A. Identification and pathogenicity of fungal pathogens associated with stem-end rot of avocado in California. Plant Dis. 2013, 97, 1580–1584. [Google Scholar] [CrossRef] [PubMed]
- Moral, J.; Agusti-Brisach, C.; Perez-Rodriguez, M.; Xavier, C.; Raya, M.C.; Rhouma, A.; Trapero, A. Identification of fungal species associated with branch dieback of olive and resistance of table cultivars to Neofusicoccum mediterraneum and Botryosphaeria dothidea. Plant Dis. 2017, 101, 306–316. [Google Scholar] [CrossRef] [Green Version]
- Herrera-González, J.A.; Bautista-Baños, S.; Salazar-García, S.; Gutiérrez-Martínez, P. Situación actual del manejo poscosecha y de enfermedades fungosas del aguacate ‘Hass’ para exportación en Michoacán. Rev. Mex. De Cienc. Agrícolas 2020, 11, 1647–1660. [Google Scholar] [CrossRef]
- Companioni González, B.; Domínguez Arizmendi, G.; García Velasco, R. Trichoderma: Su potencial en el desarrollo sostenible de la agricultura. Biotecnol. Veg. 2019, 19, 237–248. [Google Scholar]
- Ceja Torres, L.F.; Téliz Ortiz, D.; Osada Kawasoe, S.; Morales García, J.L. Etiología, distribución e incidencia del cancro del aguacate Persea americana Mill. En cuatro municipios del estado de Michoacán, México. Rev. Mex. Fitopatol. 2000, 18, 79–86. [Google Scholar]
- Samuels, G.J.; Suarez, C.; Solis, K.; Holmes, K.A.; Thomas, S.E.; Ismaiel, A.; Evans, H.C. Trichoderma theobromicola and T. paucisporum: Two new species isolated from cacao in South America. Mycol. Res. 2006, 110, 381–392. [Google Scholar] [CrossRef]
- Harman, G.E.; Kubicek, C.P. Trichoderma and Gliocladium; CRC Press: Boca Raton, FL, USA, 2002; Volume 1. [Google Scholar]
- Cenis, J. Rapid extraction of fungal DNA for PCR amplification. Nucleic Acids Res. 1992, 20, 2380. [Google Scholar] [CrossRef]
- Chakraborty, B.; Chakraborty, U.; Saha, A.; Dey, P.; Sunar, K. Molecular characterization of Trichoderma viride and Trichoderma harzianum isolated from soils of North Bengal based on rDNA markers and analysis of their PCR-RAPD Profiles. J. Biotechnol. Biochem. 2010, 5, 55–61. [Google Scholar]
- Komon-Zelazowska, M.; Bissett, J.; Zafari, D.; Hatvani, L.; Manczinger, L.; Woo, S.; Lorito, M.; Kredics, L.; Kubicek, C.P.; Druzhinina, I.S. Genetically closely related but phenotypically divergent Trichoderma species cause green mold disease in oyster mushroom farms worldwide. Appl. Environ. Microbiol. 2007, 73, 7415–7426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibarra-Medina, V.A.; Ferrera-Cerrato, R.; Alarcón, A.; Lara-Hernández, M.E.; Valdez-Carrasco, J.M. Aislamiento y selección de cepas de Trichoderma antagonistas a Sclerotinia sclerotiorum y Sclerotinia minor. Rev. Mex. De Micol. 2010, 31, 53–63. [Google Scholar]
- Janisiewicz, W. Biocontrol of postharvest diseases of apples with antagonist mixtures. Phytopathology 1988, 78, 194–198. [Google Scholar] [CrossRef]
- López-López, M.E.; Del-Toro-Sánchez, C.L.; Ochoa-Ascencio, S.; Aguilar-López, J.A.; Martínez-Cruz, O.; Madrigal-Pulido, J.A.; Robles-García, M.A.; Bernal-Mercado, A.T.; Ávila-Novoa, M.G.; Guerrero-Medina, P.J.; et al. Antagonism Trichoderma spp. strains isolated from Tanaxuri, Michoacan, Mexico against pathogens of avocado (Persea americana Mill). Biotecnia, 2022; in press. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Ting, A.S.Y.; Chai, J.Y. Chitinase and β-1, 3-glucanase activities of Trichoderma harzianum in response towards pathogenic and non-pathogenic isolates: Early indications of compatibility in consortium. Biocatalysis 2015, 4, 109–113. [Google Scholar] [CrossRef]
- Kumar, K.; Amaresan, N.; Bhagat, S.; Madhuri, K.; Srivastava, R. Isolation and characterization of Trichoderma spp. for antagonistic activity against root rot and foliar pathogens. Indian J. Microbiol. 2012, 52, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.G.; Schmoll, M.; Herrera-Estrella, A.; Upadhyay, R.; Druzhinina, I.; Tuohy, M. Biotechnology and Biology of Trichoderma; Newnes; Elsevier: Cambridge, MA, USA, 2014. [Google Scholar] [CrossRef]
- Andrade-Hoyos, P.; Luna-Cruz, A.; Osorio-Hernández, E.; Molina-Gayosso, E.; Landero-Valenzuela, N.; Barrales-Cureño, H.J. Antagonismo de Trichoderma spp. vs hongos asociados a la marchitez de chile. Rev. Mex. De Cienc. Agrícolas 2019, 10, 1259–1272. [Google Scholar] [CrossRef] [Green Version]
- Sanabria Velázquez, A.D. Evaluación de aislados de Trichoderma spp. nativos del Paraguay para el control de Colletotrichum spp. causante de la antracnosis en frutilla. Investig. Agrar. 2020, 22, 53–62. [Google Scholar] [CrossRef]
- Rivera-Méndez, W.; Brenes-Madriz, J.; Zúñiga-Vega, C. Efectos de la aplicación de Trichoderma asperellum y su filtrado en el crecimiento de almácigos de cebolla (Allium cepa). Rev. Tecnol. En Marcha 2018, 31, 98–105. [Google Scholar] [CrossRef]
- Chaverri, P.; Branco-Rocha, F.; Jaklitsch, W.; Gazis, R.; Degenkolb, T.; Samuels, G.J. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia 2015, 107, 558–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- du Plessis, I.L.; Druzhinina, I.S.; Atanasova, L.; Yarden, O.; Jacobs, K. The diversity of Trichoderma species from soil in South Africa, with five new additions. Mycologia 2018, 110, 559–583. [Google Scholar] [CrossRef]
- Jang, S.; Kwon, S.L.; Lee, H.; Jang, Y.; Park, M.S.; Lim, Y.W.; Kim, C.; Kim, J.-J. New report of three unrecorded species in Trichoderma harzianum species complex in Korea. Mycobiology 2018, 46, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawaz, K.; Shahid, A.A.; Bengyella, L.; Subhani, M.N.; Ali, M.; Anwar, W.; Iftikhar, S.; Ali, S.W. Diversity of Trichoderma species in chili rhizosphere that promote vigor and antagonism against virulent Phytophthora capsici. Sci. Hortic. 2018, 239, 242–252. [Google Scholar] [CrossRef]
- Hernández, A.; Jiménez, M.; Arcia, A.; Ulacio, D.; Méndez, N. Caracterización molecular de doce aislamientos de Trichoderma spp. mediante RAPD y rADN-ITS. Bioagro 2013, 25, 167–174. [Google Scholar]
- Rios Velasco, C.; Caro Cisneros, J.M.; Berlanga Reyes, D.I.; Ruiz Cisneros, M.F.; Ornelas Paz, J.J.; Salas Marina, M.Á.; Guerrero Prieto, V. Identification and antagonistic activity in vitro of Bacillus spp. and Trichoderma spp. isolates against common phytopathogenic fungi. Rev. Mex. Fitopatol. 2016, 34, 85–99. [Google Scholar] [CrossRef]
- Lücking, R.; Aime, M.C.; Robbertse, B.; Miller, A.N.; Aoki, T.; Ariyawansa, H.A.; Cardinali, G.; Crous, P.W.; Druzhinina, I.S.; Geiser, D.M. Fungal taxonomy and sequence-based nomenclature. Nat. Microbiol. 2021, 6, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Delgadillo, R.; Valdés-Rodríguez, S.E.; Olalde-Portugal, V.; Abraham-Juárez, R.; García-Hernández, J.L. Efecto del pH y temperatura sobre el crecimiento y actividad antagónica de Bacillus subtilis sobre Rhizoctonia solani. Rev. Mex. Fitopatol. 2018, 36, 256–275. [Google Scholar] [CrossRef] [Green Version]
- Ronnie-Gakegne, E.; Martínez-Coca, B. Antibiosis y efecto de pH-temperatura sobre el antagonismo de cepas de Trichoderma asperellum frente a Alternaria solani. Rev. De Protección Veg. 2018, 33, 1–9. [Google Scholar]
- Komarek, A.M.; Drogue, S.; Chenoune, R.; Hawkins, J.; Msangi, S.; Belhouchette, H.; Flichman, G. Agricultural household effects of fertilizer price changes for smallholder farmers in central Malawi. Agric. Syst. 2017, 154, 168–178. [Google Scholar] [CrossRef]
- Chen, J.-L.; Sun, S.-Z.; Miao, C.-P.; Wu, K.; Chen, Y.-W.; Xu, L.-H.; Guan, H.-L.; Zhao, L.-X. Endophytic Trichoderma gamsii YIM PH30019: A promising biocontrol agent with hyperosmolar, mycoparasitism, and antagonistic activities of induced volatile organic compounds on root-rot pathogenic fungi of Panax notoginseng. J. Ginseng Res. 2016, 40, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Cao, X.; Ma, X.; Guo, M.; Liu, C.; Yan, L.; Bian, Y. Diversity and effect of Trichoderma spp. associated with green mold disease on Lentinula edodes in China. Microbiologyopen 2016, 5, 709–718. [Google Scholar] [CrossRef] [Green Version]
- Sánchez Hernández, L.; Arias Mota, R.M.; Rosique Gil, J.E.; Pacheco Figueroa, C.J. Diversidad del género Trichoderma (Hypocraceae) en un Área Natural Protegida en Tabasco, México. Acta Bot. Mex. 2018, 123, 167–182. [Google Scholar] [CrossRef]
- Gajera, H.; Vakharia, D. Molecular and biochemical characterization of Trichoderma isolates inhibiting a phytopathogenic fungi Aspergillus niger Van Tieghem. Physiol. Mol. Plant Pathol. 2010, 74, 274–282. [Google Scholar] [CrossRef]
- Sosa, D.; Pérez Martínez, S.; Molina, S.; Demey, J.; Gómez, K.; Domínguez, D.; Istúriz, M.; Rumbos, R.; Parra, D. Diversidad genética de Trichoderma spp. en Venezuela, determinada mediante análisis combinado ITS-AFLP. Rev. De Protección Veg. 2014, 29, 42–51. [Google Scholar]
- García-Núñez, H.G.; Martínez-Campos, Á.R.; Hermosa-Prieto, M.R.; Monte-Vázquez, E.; Aguilar-Ortigoza, C.J.; González-Esquivel, C.E. Morphological and molecular characterization of native isolates of Trichoderma and its potential biocontrol against Phytophthora infestans. Rev. Mex. Fitopatol. 2017, 35, 58–79. [Google Scholar]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbagh, S.; Roudini, M.; Panjehkeh, N. Systemic resistance induced by Trichoderma harzianum and Glomus mossea on cucumber damping-off disease caused by Phytophthora melonis. Arch. Phytopathol. Plant Prot. 2017, 50, 375–388. [Google Scholar] [CrossRef]
- Martínez Coca, B. Bases científico-metodológicas para la selección, caracterización y uso de aislamientos de Trichoderma como agente de control biológico del tizón de la vaina (Rhizoctonia solani KÜHN) en arroz. An. Acad. Cienc. Cuba 2017, 7, 1–7. [Google Scholar]
- Martínez-Scott, M. Evaluación de aislados nativos de Trichoderma sp. para el control de hongos fitopatógenos del suelo en tomate. Rev. De Cienc. Nat. Y Agropecu. 2016, 3, 32–42. [Google Scholar]
- Sánchez-García, B.M.; Espinosa-Huerta, E.; Villordo-Pineda, E.; Rodríguez-Guerra, R.; Mora-Avilés, M.A. Identificación molecular y evaluación antagónica in vitro de cepas nativas de Trichoderma spp. sobre hongos fitopatógenos de raíz en frijol (Phaseolus vulgaris L.) cv. Montcalm. Agrociencia 2017, 51, 63–79. [Google Scholar]
- Pérez, A.A.; Pérez, M.A.; Coca, B.M.; Rollhaiser, I.N.; Blengini, C.M. Selección de aislamientos de Trichoderma spp. in vitro como potenciales biofungicidas para el control de Rhizoctonia solani Kühn en la papa. Agriscientia 2020, 37, 21–30. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Horwitz, B.A.; Herrera-Estrella, A.; Schmoll, M.; Kenerley, C.M. Trichoderma research in the genome era. Annu. Rev. Phytopathol. 2013, 51, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Safari Motlagh, M.R.; Samimi, Z. Evaluation of Trichoderma spp., as biological agents in some of plant pathogens. Ann. Biol. Res. 2013, 4, 173–179. [Google Scholar]
- Krauss, U.; Hidalgo, E.; Bateman, R.; Adonijah, V.; Arroyo, C.; García, J.; Crozier, J.; Brown, N.A.; ten Hoopen, G.M.; Holmes, K.A. Improving the formulation and timing of application of endophytic biocontrol and chemical agents against frosty pod rot (Moniliophthora roreri) in cocoa (Theobroma cacao). Biol. Control 2010, 54, 230–240. [Google Scholar] [CrossRef]
- Villamil Carvajal, J.E.; Viteri Rosero, S.E.; Villegas Orozco, W.L. Aplicación de antagonistas microbianos para el control biológico de Moniliophthora roreri Cif & Par en Theobroma cacao L. bajo condiciones de campo. Rev. Fac. Nac. De Agron. Medellín 2015, 68, 7441–7450. [Google Scholar]
- Latorre, B.A.; Torres, R.; Silva, T.; Elfar, K. Evaluación de fungicidas y agentes biocontroladores como protectores de heridas contra la cancrosis del tallo (Neofusicoccum parvum) del arándano. Cienc. E Investig. Agrar. 2013, 40, 547–557. [Google Scholar] [CrossRef] [Green Version]
- Gramaje, D.; Agustí-Brisach, C.; Pérez-Sierra, A.; Moralejo, E.; Olmo, D.; Mostert, L.; Damm, U.; Armengol, J. Fungal trunk pathogens associated with wood decay of almond trees on Mallorca (Spain). Pers. Mol. Phylogeny Evol. Fungi 2012, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pérez Márquez, A.; Vidal Aguiar, Y.; Mulkay Vitón, T. Contenido de fenoles totales en frutos de mango’Super Haden’dañados por antracnosis y tratados en poscosecha. Cultiv. Trop. 2016, 37, 71–77. [Google Scholar]
- Mattiuz, B.-H.; Ducamp-Collin, M.-N.; Mattiuz, C.F.M.; Vigneault, C.; Marques, K.M.; Sagoua, W.; Montet, D. Effect of propolis on postharvest control of anthracnose and quality parameters of ‘Kent’mango. Sci. Hortic. 2015, 184, 160–168. [Google Scholar] [CrossRef]
- Ochoa, S. Enfermedades y desordenes fisiológicos más comunes del fruto de aguacate en postcosecha. In Guía Ilustrada para Técnicos y Empacadores, 1st ed.; APEAM, AC: Uruapan, Mexico, 2014. [Google Scholar]
- Ruiz-Cisneros, M.F.; Ornelas-Paz, J.D.J.; Olivas-Orozco, G.I.; Acosta-Muñiz, C.H.; Sepúlveda-Ahumada, D.R.; Pérez-Corral, D.A.; Rios-Velasco, C.; Salas-Marina, M.Á.; Fernández-Pavía, S.P. Efecto de Trichoderma spp. y hongos fitopatógenos sobre el crecimiento vegetal y calidad del fruto de jitomate. Rev. Mex. Fitopatol. 2018, 36, 444–456. [Google Scholar] [CrossRef] [Green Version]
- Shafique, H.A.; Sultana, V.; Ehteshamul-Haque, S.; Athar, M. Management of soil-borne diseases of organic vegetables. J. Plant Prot. Res. 2016, 56, 221–230. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Di Mattia, E.; El-Nakhel, C.; Cardarelli, M. Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J. Sci. Food Agric. 2015, 95, 1706–1715. [Google Scholar] [CrossRef]
- Martínez, B.; Obret, Y.; Pérez, S.; Reyes, Y. Antagonismo in vitro de cepas de Trichoderma spp. frente a Sarocladium oryzae (Sawada) W. Gams & D. Hawksworth. Rev. De Protección Veg. 2014, 29, 106–111. [Google Scholar]
- Grahovac, J.; Rončević, Z.; Tadijan, I.; Jokić, A.; Dodić, J. Optimization of media for antimicrobial compounds production by Bacillus subtilis. Acta Aliment. 2015, 44, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Michrina, J.; Michalikova, A.; Rohacik, T.; Kulichova, R. Antibiosis as a possible mechanism of antagonistic action of Trichoderma harzianum against Fusarium culmorum. Ochr. Rostl. UZPI 1996, 31, 177–184. [Google Scholar]
- Essalmani, H.; Lahlou, H. In vitro antagonistic activity of some microorganisms towards Fusarium oxysporum f. sp. lentis (french). Cryptogam. Mycol. 2002, 23, 221–234. [Google Scholar]
- Dodd, S.; Hill, R.; Stewart, A. Control of Athelia rolfsii disease on lentil seedlings using 6-pentyl-α-pyrone. Soil Biol. Biochem. 2000, 32, 1033–1034. [Google Scholar] [CrossRef]
- Petrișor, C.; Paica, A.; Constantinescu, F. Temperature and pH influence on antagonistic potential of Trichoderma sp. strains against Rhizoctonia solani. Sci. Papers Ser. B Hortic. 2016, 60, 275–278. [Google Scholar]
- González, I.; Infante, D.; Martínez, B.; Arias, Y.; González, N.; Miranda, I.; Peteira, B. Inducción de quitinasas y glucanasas en cepas de Trichoderma spp. promisorias como agentes para el control biológico. Biotecnol. Apl. 2012, 29, 12–16. [Google Scholar]
- Hartl, L.; Zach, S.; Seidl-Seiboth, V. Fungal chitinases: Diversity, mechanistic properties and biotechnological potential. Appl. Microbiol. Biotechnol. 2012, 93, 533–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Espejo, C.N.; Mamani-Mamani, M.M.; Chávez-Lizárraga, G.A.; Álvarez-Aliaga, M.T. Evaluación de la actividad enzimática del Trichoderma inhamatum (BOL-12 QD) como posible biocontrolador. J. Selva Andin. Res. Soc. 2016, 7, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Radjacommare, R.; Venkatesan, S.; Samiyappan, R. Biological control of phytopathogenic fungi of vanilla through lytic action of Trichoderma species and Pseudomonas fluorescens. Arch. Phytopathol. Plant Prot. 2010, 43, 1–17. [Google Scholar] [CrossRef]
- Yan, R.; Hou, J.; Ding, D.; Guan, W.; Wang, C.; Wu, Z.; Li, M. In vitro antifungal activity and mechanism of action of chitinase against four plant pathogenic fungi. J. Basic Microbiol. 2008, 48, 293–301. [Google Scholar] [CrossRef]







| Sample Code | Sampling Location (Orchard) | City | State | Coordinates | m.a.s.l. 1 | Season | Particular Characteristics |
|---|---|---|---|---|---|---|---|
| TSMICH1 | Rancho Las Ayacatas | Uruapan (Jucutacato) | Michoacan | Latitude (19°22′39″ N) Longitude (103°55′13″ W) | 2186 | Fall–winter | Avocado variety Hass. Crop age: 30 to 35 years. Use of chemicals. |
| TSMICH2 | |||||||
| TSMICH3 | |||||||
| TSMICH4 | |||||||
| TSMICH5 | |||||||
| TSMICH6 | |||||||
| TSMICH7 | |||||||
| TSMICH8 | Rancho El Durazno | Uruapan (Jucutacato) | Michoacan | Latitude (19°4′52″ N) Longitude (102°20′38″ W) | 2329 | Fall–winter | Avocado variety Hass. Crop age: 30 to 35 years. Use of chemicals. |
| TSMICH9 | |||||||
| TSMICH10 | |||||||
| TSMICH11 | Rancho Villa de las Flores | Uruapan (Jucutacato) | Michoacan | Latitude (19°4′52″ N) Longitude (102°20′38″ W) | 2329 | Fall–winter | Avocado variety Hass. Crop age: 30 to 35 years. Use of chemicals. |
| TSMICH12 | |||||||
| TSMICH13 | |||||||
| TSMICH14 | |||||||
| TSMICH15 | |||||||
| TSMICH16 | |||||||
| TSMICH17 | |||||||
| TSMICH18 | |||||||
| TSMICH19 | |||||||
| TSMICH20 | |||||||
| TSMICH21 | |||||||
| TSMICH22 | |||||||
| TRJAL23 | Rancho Las Palmas | San Francisco de Asis, Location of Atotonilco, El Alto | Jalisco | Latitude (20°65′17″ N) Longitude (103°25′46″ W) | 1963 | Spring–summer | Avocado varieties Hass and Fuerte. Crop age: 25 to 30 years. 100% organic. Interspersed with fruit trees such as citrus, papaya, walnut, vine, peach, among others. |
| TRJAL24 | |||||||
| TRJAL25 |
| Sample Code | Sampling Material | Morphological Characteristic | |||
|---|---|---|---|---|---|
| Radial Growth | Mycelial Color | Presence of Diffusible Pigments | Concentric Rings | ||
| TSMICH1 | Soil | 4.1 cm in 120 h | Green | No | Yes |
| TSMICH2 | Soil | 4.8 cm in 120 h | Green | No | Yes |
| TSMICH3 | Root | 4.5 cm in 120 h | Green | Yes | No |
| TSMICH4 | Soil | 4.7 cm in 120 h | White | Yes | No |
| TSMICH5 | Root | 4.7 cm in 120 h | Green | Yes | Yes |
| TSMICH6 | Soil | 4.8 cm in 120 h | White-yellow | Yes | Yes |
| TSMICH7 | Root | 5 cm in 96 h | Green and white | Yes | Yes but undefined |
| TSMICH8 | Root | 4.9 cm in 96 h | White | No | Yes but undefined |
| TSMICH9 | Root | 4.8 cm in 96 h | White and beige | No | No |
| TSMICH10 | Soil | 4.8 cm in 96 h | Beige | Yes | Yes but undefined |
| TSMICH11 | Soil | 4.6 cm in 120 h | Green-beige | No | No |
| TSMICH12 | Soil | 4.8 cm in 120 h | Green | No | Yes but undefined |
| TSMICH13 | Soil | 4.8 cm in 120 h | White | Yes | No |
| TSMICH14 | Soil | 4.7 cm in 120 h | Beige | Yes | No |
| TSMICH15 | Soil | 4.8 cm in 96 h | Beige | Yes | No |
| TSMICH16 | Soil | 4.7 cm in 120 h | White | No | Yes |
| TSMICH17 | Soil | 4.6 cm in 120 h | Green | Yes | Yes |
| TSMICH18 | Soil | 4.6 cm in 120 h | White | Yes | Yes |
| TSMICH19 | Root | 4.5 cm in 120 h | Green and white | No | Yes but undefined |
| TSMICH20 | Soil | 4.7 cm in 120 h | Green | No | Yes |
| TSMICH21 | Soil | 4.5 cm in 120 h | Yellow | No | No |
| TSMICH22 | Soil | 4.7 cm in 120 h | Green and white | Yes, but little presence | Yes |
| TRJAL23 | Root | 4.9 cm in 120 h | Green | No | Yes |
| TRJAL24 | Root | 5 cm in 96 h | Green | No | Yes |
| TRJAL25 | Root | 4.9 cm in 120 h | Green | No | Yes |
| Strain | Best Hit | ITS Region | Translation Elongation Factor Tef 1-α | ||
|---|---|---|---|---|---|
| % Identity | GenBank Accession Number | % Identity | GenBank Accession Number | ||
| TSMICH7 | Trichoderma harzianum | 99.81 | ON407089 | 100 | ON423618 |
| TSMICH8 | Trichoderma harzianum | 97.70 | ON407088 | 99.31 | ON423617 |
| TSMICH9 | Trichoderma harzianum | 99.62 | ON407090 | 99.09 | ON423619 |
| TSMICH10 | Trichoderma harzianum | 98.90 | ON407087 | 99.65 | ON423616 |
| TSMICH15 | Trichoderma harzianum | 99.63 | ON407086 | 99.82 | ON423615 |
| TRJAL25 | Trichoderma harzianum | 98.56 | ON407091 | 98.65 | ON423620 |
| Antagonistic Strains Trichoderma | Diameter of Hyphae(µm) | Pathogen | Diameter of Hyphae of the Phytopathogen (µm) | Confrontation Trichoderma/Phytopathogen Diameter of Hyphae (µm) | Diameter Differential after Confrontation (µm) * | ||
|---|---|---|---|---|---|---|---|
| Trichoderma | Phytopathogen | Trichoderma | Phytopathogen | ||||
| TSMICH7 | 2.85 ± 0.49 b | N. parvum | 4.92 ± 1.41 ab | 4.34 ± 0.59 b | 4.95 ± 0.74 a | +1.49 | +0.03 |
| C. gloeosporioides | 5.96 ± 1.06 a | 4.50 ± 0.44 b | 4.36 ± 0.69 ab | +1.65 | −1.60 | ||
| Diaporthe sp. | 3.58 ± 0.53 c | 4.95 ± 1.40 b | 3.59 ± 0.59 b | +2.10 | +0.002 | ||
| P. perseae | 5.62 ± 2.08 a | 3.90 ± 0.41 c | 3.89 ± 0.42 b | +1.05 | −1.73 | ||
| TSMICH8 | 2.08 ± 0.81 c | N. parvum | 4.92 ± 1.41 ab | 4.97 ± 1.05 b | 3.80 ± 0.69 b | +2.89 | −1.11 |
| C. gloeosporioides | 5.96 ± 1.06 a | 5.72 ± 1.44 a | 3.49 ± 0.92 bc | +3.63 | −2.46 *** | ||
| Diaporthe sp. | 3.58 ± 0.53 c | 3.47 ± 0.77 bc | 3.88 ± 1.24 b | +1.38 | +0.29 | ||
| P. perseae | 5.62 ± 2.08 a | 3.54 ± 0.92 c | 4.45 ± 0.83 a | +1.45 | −1.17 | ||
| TSMICH9 | 3.34 ± 0.59 a | N. parvum | 4.92 ± 1.41 ab | 5.17 ± 1.16 ab | 3.67 ± 1.21 b | +1.80 | −1.24 |
| C. gloeosporioides | 5.96 ± 1.06 a | 4.44 ± 1.23 b | 3.55 ± 0.67 b | +1.10 | −2.40 | ||
| Diaporthe sp. | 3.58 ± 0.53 c | 4.91 ± 0.38 b | 4.43 ± 1.37 a | +1.56 | +0.85 | ||
| P. perseae | 5.62 ± 2.08 a | 4.63 ± 0.52 b | 3.92 ± 0.68 b | +1.29 | −1.70 | ||
| TSMICH10 | 3.48 ± 0.20 a | N. parvum | 4.92 ± 1.41 ab | 3.78 ± 0.72 bc | 4.63 ± 0.65 a | +0.29 | −0.28 |
| C. gloeosporioides | 5.96 ± 1.06 a | 2.95 ± 0.32 cd | 3.21 ± 0.71 bc | −0.52 | −2.74 | ||
| Diaporthe sp. | 3.58 ± 0.53 c | 3.24 ± 0.59 c | 2.69 ± 0.49 c | −0.24 | −0.88 | ||
| P. perseae | 5.62 ± 2.08 a | 2.89 ± 0.30 d | 2.58 ± 0.70 c | −0.59 | −3.04 ** | ||
| TSMICH15 | 3.56 ± 0.86 a | N. parvum | 4.92 ± 1.41 ab | 4.19 ± 0.45 b | 4.20 ± 1.34 ab | +0.63 | −0.71 |
| C. gloeosporioides | 5.96 ± 1.06 a | 3.24 ± 0.60 c | 3.42 ± 0.96 bc | −0.31 | −2.53 | ||
| Diaporthe sp. | 3.58 ± 0.53 c | 4.99 ± 0.68 b | 4.69 ± 0.82 a | +1.43 | +1.10 | ||
| P. perseae | 5.62 ± 2.08 a | 5.90 ± 1.53 a | 2.58 ± 0.70 c | +2.34 | −3.04 | ||
| TRJAL25 | 2.97 ± 0.24 ab | N. parvum | 4.92 ± 1.41 ab | 4.32 ± 0.76 b | 4.34 ± 0.77 ab | +2.97 | −0.57 |
| C. gloeosporioides | 5.96 ± 1.06 a | 2.28 ± 0.42 d | 3.13 ± 0.72 bc | −0.69 | −2.83 | ||
| Diaporthe sp. | 3.58 ± 0.53 c | 3.92 ± 0.43 bc | 4.43 ± 0.64 a | +0.94 | +0.84 | ||
| P. perseae | 5.62 ± 2.08 a | 4.35 ± 0.69 b | 2.58 ± 0.70 c | +1.37 | −3.04 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-López, M.E.; Del-Toro-Sánchez, C.L.; Gutiérrez-Lomelí, M.; Ochoa-Ascencio, S.; Aguilar-López, J.A.; Robles-García, M.A.; Plascencia-Jatomea, M.; Bernal-Mercado, A.T.; Martínez-Cruz, O.; Ávila-Novoa, M.G.; et al. Isolation and Characterization of Trichoderma spp. for Antagonistic Activity against Avocado (Persea americana Mill) Fruit Pathogens. Horticulturae 2022, 8, 714. https://0-doi-org.brum.beds.ac.uk/10.3390/horticulturae8080714
López-López ME, Del-Toro-Sánchez CL, Gutiérrez-Lomelí M, Ochoa-Ascencio S, Aguilar-López JA, Robles-García MA, Plascencia-Jatomea M, Bernal-Mercado AT, Martínez-Cruz O, Ávila-Novoa MG, et al. Isolation and Characterization of Trichoderma spp. for Antagonistic Activity against Avocado (Persea americana Mill) Fruit Pathogens. Horticulturae. 2022; 8(8):714. https://0-doi-org.brum.beds.ac.uk/10.3390/horticulturae8080714
Chicago/Turabian StyleLópez-López, María Estela, Carmen Lizette Del-Toro-Sánchez, Melesio Gutiérrez-Lomelí, Salvador Ochoa-Ascencio, José Antonio Aguilar-López, Miguel Angel Robles-García, Maribel Plascencia-Jatomea, Ariadna Thalia Bernal-Mercado, Oliviert Martínez-Cruz, María Guadalupe Ávila-Novoa, and et al. 2022. "Isolation and Characterization of Trichoderma spp. for Antagonistic Activity against Avocado (Persea americana Mill) Fruit Pathogens" Horticulturae 8, no. 8: 714. https://0-doi-org.brum.beds.ac.uk/10.3390/horticulturae8080714