Effect of Compound Biochar Substrate on the Root Growth of Cucumber Plug Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Biochar
2.3. Preparation of the Substrates
2.4. Physicochemical Properties of the Substrates
2.5. Water Retention and Nitrogen Release of the Substrates
2.6. Cultivation and Growth of the Seedlings
2.7. Root System Analysis of the Seedlings
2.8. Data Analysis
3. Results and Discussion
3.1. Effect of Biochar on Physicochemical Properties of the Substrate
3.2. Determination of Water Retention and Nitrogen Release of the Substrates
3.3. Effect of Biochar on the Growth of the Seedlings
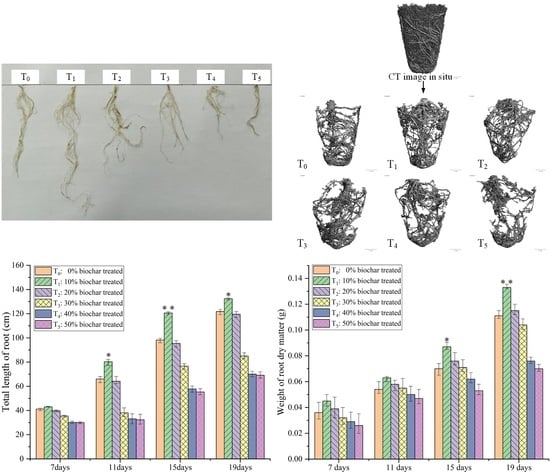
3.4. Growth Status on the Root System of the Seedlings
3.5. Growth Status on the Root System of the Seedlings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xia, G.; Han, C.; Guo, H.; Zhang, J.; Xu, Y. Research Status and Development Trend of Key Components of Automatic Transplanting Machine. J. Agric. Mech. Res. 2019, 41, 1–7. [Google Scholar]
- Yang, Y.; Ting, K.C.; Giacomelli, G.A. Factors affecting performance of sliding-needles gripper during robotic transplanting of seedlings. Trans. Asae 1991, 7, 493–498. [Google Scholar] [CrossRef]
- Hula, P.; Sindelr, R.; Trinkl, A. Verification of applicability of ABB robots for trans-planting seedlings in greenhouses. Res. Agric. Eng. 2008, 54, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Mao, H.; Hu, J.; Miao, X.; Tian, K.; Yang, X. Experiment on mechanical property of seedling pot for automatic transplanter. Trans. Csae 2015, 29, 24–26. [Google Scholar]
- Shaw, L.N. Changes needed to facilitate automatic field transplanting. Hort Technol. 1993, 3, 418–420. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Kumi, F.; Mao, H.; Hu, J. Design and tests of a multi-pin flexible seedling pick-up gripper for automatic transplanting. Appl. Eng. Agric. 2019, 35, 949–957. [Google Scholar] [CrossRef]
- Ting, K.C.; Giacomelli, G.A.; Shen, S.J. Robot workcell for transplanting of seedlings: PartⅡ-end-effector development. Trans. Asae 1990, 33, 1013–1017. [Google Scholar] [CrossRef]
- Petronela, N.; Elena, D.; Florin, C.; Elena, B. The biodegradability and mechanical strength of nutritive pots for vegetable planting based on lignocellulose composite materials. Bioresources 2010, 5, 1102–1113. [Google Scholar]
- Kumar, G.V.P.; Raheman, H. Identification of optimum combination of proportion of vermicompost in the soil-based potting mix and pot volume for the production of paper pot seedlings of vegetables. J. Plant. Nutr. 2012, 35, 1277–1289. [Google Scholar] [CrossRef]
- Min, B.; Ha, L.; Lee, J.; Choi, S.; Lee, S. The selection proper materials to develop specialized root substrate for working with bulbonion transplanter. Prot. Hortic. Plant. Fact. 2016, 25, 100–105. [Google Scholar] [CrossRef]
- Qu, P.; Cao, Y.; Wu, G.; Tang, W.; Xia, L. Preparation and properties of coir-based substrate bonded by modified urea formaldehyde reinsnfor seedlings. Bioresources 2018, 13, 4332–4345. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Z. Researches on biochar application technology. Eng. Sci. 2011, 13, 83–89. [Google Scholar]
- Lehmann, J.; Joseph, S. Biochar for environmental management: Science, technology and implementation. Sci. Technol. Earthscan 2015, 25, 15801–15811. [Google Scholar]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using poultry litter biochars as soil amendments. Aust. J. Soil Res. 2008, 46, 437–444. [Google Scholar] [CrossRef]
- Major, J.; Rondon, M.; Molina, D.; Riha, S.J.; Lehmann, J. Maize yield and nutrition during 4 years after biochar application to a colombian savanna oxisol. Plant. Soil 2010, 333, 117–128. [Google Scholar] [CrossRef]
- Zwieten, L.V.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Liang, B.L.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Neves, E.G. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef] [Green Version]
- Spokas, K.A.; Koskinen, W.C.; Baker, J.M.; Reicosky, D.C. Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a minnesota soil. Chemosphere 2006, 77, 574–581. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, P.; Chen, X.; Wang, Y.; Zhao, X. Effect of above-and below-ground interactions on maize/soybean intercropping advantage. Trans. Csam 2014, 45, 143–148. [Google Scholar]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal responses to biochar in soil—Concepts and mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Kimetu, J.M.; Lehmann, J. Stability and stabilisation of biochar and green manure in soil with different organic carbon contents. Aust. J. Soil Res. 2010, 48, 577. [Google Scholar] [CrossRef]
- Knoblauch, C.; Maarifat, A.A.; Pfeiffer, E.M.; Haefele, S.M. Degradability of black carbon and its impact on trace gas fluxes and carbon turnover in paddy soils. Soil Biol. Biochem. 2011, 43, 1768–1778. [Google Scholar] [CrossRef]
- Miao, X.; Mao, H.; Han, L.; Sun, H.; Yang, X. Analysis of Influencing Factors on Force of Picking Plug Seedlings and Pressure Resistance of Plug Seedlings. Trans. Csam 2013, 44, 27–32. [Google Scholar]
- Li, F.; Li, M.; Liu, J.; Hu, Y.; Zhang, Q.; Zhao, H. Effect of Biochar on Fungal Abundance of Rhizosphere Soil and Cucumber Root Growth in Greenhouse. Trans. Csam 2017, 48, 265–271. [Google Scholar]
- Tracy, S.R.; Black, C.R.; Roberts, J.A.; Mooney, S.J. Exploring the interacting effect of soil texture and bulk density on root system development in tomato (solanum lycopersicum L.). Environ. Exp. Bot. 2013, 91, 38–47. [Google Scholar] [CrossRef]
- Zhao, H. Growth habit and fertilizer requirement of the cucumber. Farmers Consult. 2018, 24, 37. [Google Scholar]
- Song, B.; Sun, R.; Liang, H.; Hu, Y.; Peng, P.; She, D. Effects of Lignin and Biochar Addition on Soil Nitrogen and Phosphorus Nutrients and Water Loss. J. Soil Water Conserv. 2019, 33, 227–232, 241. [Google Scholar]
- Bao, G.; Xu, Y.; Yang, L.; Ya, M.; Qian, J. Research and popularization of instrumental method for determination of total nitrogen in soy sauce. J. Light Ind. 2018, 34, 107–108, 172. [Google Scholar]
- Wen, W.; Guo, X.; Zhao, C.; Wang, C.; Xiao, B. Crop roots configuration and visualization: A review. Sci. Agric. Sin. 2015, 48, 436–448. [Google Scholar]
- Liu, Y.; Mao, H.; Han, L.; Xu, J.; Ma, G. Plug damage detection and parameter optimization of picking up cucumber seedlings from tray cells based on Micro-CT. Trans. Csae 2018, 34, 27–34. [Google Scholar]
- Dai, X.; Sun, W.; Fan, Q.; Luo, P.; He, J. Physicochemical property of mixed substrates with agricultural and forestry wastes and comprehensive evaluation of their effect on growth of Camellia oleifera seedlings. J. Plant Resour. Environ. 2016, 25, 54–61. [Google Scholar]
- Zhang, S.; Yu, H.; Jiang, W. Seedling effects of corncob and bagasse composting substrates in cucumber. Trans. Csae 2015, 31, 236–242. [Google Scholar]
- Kang, H.; Zhang, Q.; Tang, J. Research Advances on Growth Media. Chin. J. Soil Sci. 2005, 36, 124–127. [Google Scholar]
- Yang, E.; Meng, J.; Hu, H.; Cheng, D.; Zhu, C.; Chen, W. Effects of organic molecules from biochar-extracted liquor on the growth of rice seedlings. Ecotoxicol. Environ. Saf. 2019, 170, 338–345. [Google Scholar]
- Zhang, Q.; Xu, H.; Ren, X.; Lu, W.; Liu, H.; Zhou, L.; Cai, H.; Yi, W. Preparation and properties of agroforestry wastes biochar/high density polyethylene composites. Acta Mater. Compos. Sin. 2020, 1–9. [Google Scholar] [CrossRef]






| Samples | Bulk Density/(g·cm−3) | Aeration Porosity/% | Water-Holding Porosity/% | Whole Porosity/% | Void Ratio | pH Value | EC/(mS·cm−1) |
|---|---|---|---|---|---|---|---|
| T0 | 0.243 a | 43.79 e | 18.89 e | 62.67 f | 0.431 c | 6.02 f | 0.752 f |
| T1 | 0.215 b | 50.39 d | 21.94 d | 72.34 e | 0.435 c | 6.23 e | 0.959 e |
| T2 | 0.2 c | 51.65 c | 23.57 c | 75.23 d | 0.456 b | 6.45 d | 1.008 d |
| T3 | 0.185 d | 52.64 b | 24.18 c | 76.82 c | 0.459 b | 6.71 c | 1.047 c |
| T4 | 0.171 e | 53.33 b | 25.03 b | 78.36 b | 0.469 b | 7.02 b | 1.128 b |
| T5 | 0.159 f | 55.41 a | 27.82 a | 83.24 a | 0.502 a | 7.29 a | 1.175 a |
| Parameters of Root Systems | Time (Days) | Samples | |||||
|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | T5 | ||
| Total length (cm) | 7 | 41.214 | 47.006 | 39.773 | 35.409 | 30.244 | 28.012 |
| 11 | 66.030 | 80.251 | 64.187 | 38.065 | 33.063 | 32.319 | |
| 15 | 98.238 | 120.555 | 95.546 | 76.725 | 57.811 | 55.360 | |
| 19 | 121.803 | 142.415 | 119.612 | 85.005 | 70.067 | 69.128 | |
| Surface area (cm2) | 7 | 4.817 | 5.938 | 4.797 | 4.547 | 3.598 | 3.388 |
| 11 | 8.773 | 10.006 | 8.365 | 6.839 | 4.486 | 3.957 | |
| 15 | 15.462 | 19.018 | 14.024 | 10.757 | 7.323 | 6.953 | |
| 19 | 20.010 | 24.857 | 19.638 | 13.850 | 8.712 | 8.696 | |
| Average diameter (mm) | 7 | 0.447 | 0.503 | 0.436 | 0.381 | 0.357 | 0.344 |
| 11 | 0.506 | 0.594 | 0.480 | 0.426 | 0.405 | 0.386 | |
| 15 | 0.581 | 0.699 | 0.559 | 0.489 | 0.431 | 0.417 | |
| 19 | 0.656 | 0.771 | 0.629 | 0.537 | 0.455 | 0.424 | |
| Total volume (cm3) | 7 | 0.061 | 0.0795 | 0.0605 | 0.046 | 0.0345 | 0.033 |
| 11 | 0.105 | 0.158 | 0.102 | 0.089 | 0.077 | 0.075 | |
| 15 | 0.203 | 0.24 | 0.206 | 0.154 | 0.129 | 0.126 | |
| 19 | 0.254 | 0.295 | 0.241 | 0.205 | 0.168 | 0.157 | |
| Total tips | 7 | 226 | 249 | 226 | 184 | 163 | 154 |
| 11 | 409 | 534 | 364 | 327 | 187 | 182 | |
| 15 | 791 | 1030 | 730 | 619 | 461 | 430 | |
| 19 | 904 | 1139 | 851 | 736 | 611 | 551 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, G.; Mao, H.; Bu, Q.; Han, L.; Shabbir, A.; Gao, F. Effect of Compound Biochar Substrate on the Root Growth of Cucumber Plug Seedlings. Agronomy 2020, 10, 1080. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy10081080
Ma G, Mao H, Bu Q, Han L, Shabbir A, Gao F. Effect of Compound Biochar Substrate on the Root Growth of Cucumber Plug Seedlings. Agronomy. 2020; 10(8):1080. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy10081080
Chicago/Turabian StyleMa, Guoxin, Hanping Mao, Quan Bu, Luhua Han, Abdul Shabbir, and Feng Gao. 2020. "Effect of Compound Biochar Substrate on the Root Growth of Cucumber Plug Seedlings" Agronomy 10, no. 8: 1080. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy10081080