Antioxidants in Alzheimer’s Disease: Current Therapeutic Significance and Future Prospects
Abstract
:Simple Summary
Abstract
1. Introduction
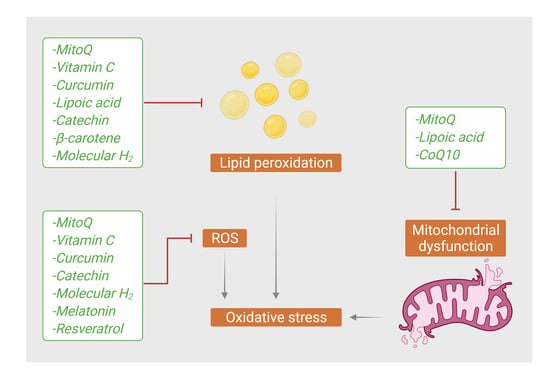
2. Oxidative Stress and Alzheimer’s Disease
3. Oxidative Stress Biomarkers in Blood Cells
4. Antioxidants
5. Role of Antioxidant-Rich Diet in Alzheimer’s Disease
6. Role of Antioxidants in Alzheimer’s Disease
6.1. Vitamin E
6.2. Glutathione
6.3. Molecular Hydrogen
6.4. Monoamine Oxidase-b Inhibitor
6.5. Melatonin
6.6. Ascorbyl Palmitate
6.7. Curcumin
6.8. Coenzyme Q and SK-PC-B70M
6.9. Estrogen, Astaxanthin, and Quercetin
6.10. Lipoic Acid
6.11. Resveratrol
6.12. MitoQ
6.13. Catechins
6.14. Silibinin
6.15. Palmatine
6.16. Serotonin
6.17. Gintonin
7. Role of Other Nutrients in Alzheimer’s Disease
8. Limitations and Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Dementia. 21 September 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 22 December 2021).
- National Institute on Aging. Alzheimer’s Disease Fact Sheet. 22 May 2019. Available online: https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet (accessed on 21 December 2021).
- Sanabria-Castro, A.; Alvarado-Echeverría, I.; Monge-Bonilla, C. Molecular pathogenesis of Alzheimer’s disease: An update. Ann. Neurosci. 2017, 24, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.A.; Parker, T.D.; Cash, D.M.; Macpherson, K.; Donnachie, E.; Murray-Smith, H.; Barnes, A.; Barker, S.; Beasley, D.G.; Bras, J.; et al. Study protocol: Insight 46—A neuroscience sub-study of the MRC National Survey of Health and Development. BMC Neurol. 2017, 17, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, M.; Sharma, A.K. Cu and Zn interactions with Aβ peptides: Consequence of coordination on aggregation and formation of neurotoxic soluble Aβ oligomers. Metallomics 2019, 11, 64–84. [Google Scholar] [CrossRef]
- Korol, T.Y.; Kostyuk, E.P.; Kostyuk, P.G. Disruption of calcium homeostasis in Alzheimer’s disease. Neurophysiology 2008, 40, 385–392. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Smith, M.A.; Peggy, L.; Richey, H.; Sayre, L.M.; Beckman, J.S.; Perry, G. Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J. Neurosci. 1997, 17, 2653–2657. [Google Scholar] [CrossRef] [Green Version]
- Kalaria, R.N. Microglia and Alzheimer’s disease. Curr. Opin. Hematol. 1999, 6, 15. [Google Scholar] [CrossRef]
- Hempen, B.; Brion, J.-P. Reduction of acetylated α-tubulin immunoreactivity in neurofibrillary tangle-bearing neurons in Alzheimer’s disease. J. Neuropathol. Exp. Neurol. 1996, 55, 964–972. [Google Scholar] [CrossRef] [Green Version]
- Kandiah, N.; Ong, P.A.; Yuda, T.; Ng, L.; Mamun, K.; Merchant, R.A.; Chen, C.; Dominguez, J.; Marasigan, S.; Ampil, E.; et al. Treatment of dementia and mild cognitive impairment with or without cerebrovascular disease: Expert consensus on the use of Ginkgo biloba extract, EGb 761®. CNS Neurosci. Ther. 2019, 25, 288–298. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Domenico, S.; Boulestin, H.; Campbell, F.M.; Williams, L.M. The role of dietary advanced glycation end products in metabolic dysfunction. Mol. Nutr. Food Res. 2021, 65, 1900934. [Google Scholar]
- Wiramon, R.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B. Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar]
- Irit, L.; Ricny, J.; Atrakchi-Baranes, D.; Shemesh, C.; Kravitz, E.; Liraz-Zaltsman, S.; Maksin-Matveev, A.; Cooper, I.; Leibowitz, A.; Uribarri, J.; et al. High dietary advanced glycation end products are associated with poorer spatial learning and accelerated Aβ deposition in an Alzheimer mouse model. Aging Cell 2016, 15, 309–316. [Google Scholar]
- Piras, S.; Furfaro, A.L.; Domenicotti, C.; Traverso, N.; Marinari, U.M.; Pronzato, M.A.; Nitti, M. RAGE expression and ROS generation in neurons: Differentiation versus damage. Oxidative Med. Cell. Longev. 2016, 2016, 9348651. [Google Scholar] [CrossRef] [Green Version]
- Leclerc, E.; Sturchler, E.; Vetter, S.W. The S100B/RAGE axis in Alzheimer’s disease. Cardiovasc. Psychiatry Neurol. 2010, 2010, 539581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Zhang, H.; Schmidt, A.M.; Zhang, C. AGE/RAGE produces endothelial dysfunction in coronary arterioles in type 2 diabetic mice. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H491–H498. [Google Scholar] [CrossRef] [Green Version]
- Resende, R.; Moreira, P.I.; Proença, T.; Deshpande, A.; Busciglio, J.; Pereira, C.; Oliveira, C.R. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic. Biol. Med. 2008, 44, 2051–2057. [Google Scholar] [CrossRef] [Green Version]
- Hartl, D.; Schuldt, V.; Forler, S.; Zabel, C.; Klose, J.; Rohe, M. Presymptomatic alterations in energy metabolism and oxidative stress in the APP23 mouse model of Alzheimer disease. J. Proteome Res. 2012, 11, 3295–3304. [Google Scholar] [CrossRef]
- Arimon, M.; Takeda, S.; Post, K.L.; Svirsky, S.; Hyman, B.T.; Berezovska, O. Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiol. Dis. 2015, 84, 109–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoltowska, M.K.; Berezovska, O. Dynamic nature of presenilin1/γ-secretase: Implication for Alzheimer’s disease pathogenesis. Mol. Neurobiol. 2018, 55, 2275–2284. [Google Scholar] [CrossRef] [PubMed]
- Gwon, A.-R.; Park, J.-S.; Arumugam, T.V.; Kwon, Y.-K.; Chan, S.L.; Kim, S.H.; Baik, S.H.; Yang, S.; Yun, Y.K.; Choi, Y.; et al. Oxidative lipid modification of nicastrin enhances amyloidogenic γ-secretase activity in Alzheimer’s disease. Aging Cell 2012, 11, 559–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, C.; Chan, H.; Ashok, I.; Krishnamoorthi, S.K.; Li, M.; Li, H.; Wong, M.S. Amyloid-β oligomer targeted theranostic probes for in vivo NIR imaging and inhibition of self-aggregation and amyloid-β induced ROS generation. Talanta 2021, 224, 121830. [Google Scholar] [CrossRef] [PubMed]
- Leuner, K.; Schütt, T.; Kurz, C.; Eckert, S.H.; Schiller, C.; Occhipinti, A.; Mai, S.; Jendrach, M.; Eckert, G.P.; Kruse, S.E.; et al. Mitochondrion-derived reactive oxygen species lead to enhanced amyloid beta formation. Antioxid. Redox Signal. 2012, 16, 1421–1433. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Cui, J.; Lee, J.C.M.; Ding, S.; Chalimoniuk, M.; Simonyi, A.; Sun, A.Y.; Gu, Z.; Weisman, G.A.; Wood, W.G.; et al. Prolonged exposure of cortical neurons to oligomeric amyloid-β impairs NMDA receptor function via NADPH oxidase-mediated ROS production: Protective effect of green tea (-)-epigallocatechin-3-gallate. ASN Neuro 2011, 3, AN20100025. [Google Scholar]
- Shelat Phullara, B.; Chalimoniuk, M.; Wang, J.; Strosznajder, J.B.; Lee, J.C.; Sun, A.Y.; Simonyi, A.; Sun, G.Y. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J. Neurochem. 2008, 106, 45–55. [Google Scholar] [CrossRef]
- Faridis, S.; Chang, A.; Hernandez, C.; Pautler, R.G.; Sweatt, J.D.; Klann, E. NADPH oxidase mediates β-amyloid peptide-induced activation of ERK in hippocampal organotypic cultures. Mol. Brain 2009, 2, 1–10. [Google Scholar]
- Bilkei-Gorzo, A. Genetic mouse models of brain ageing and Alzheimer’s disease. Pharmacol. Ther. 2014, 142, 244–257. [Google Scholar] [CrossRef]
- Robinson, R.A.; Lange, M.B.; Sultana, R.; Galvan, V.; Fombonne, J.; Gorostiza, O.; Bredesen, D.E. Differential expression and redox proteomics analyses of an Alzheimer disease transgenic mouse model: Effects of the amyloid-β peptide of amyloid precursor protein. Neuroscience 2011, 177, 207–222. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Galvan, V.; Lange, M.B.; Tang, H.; Sowell, R.A.; Spilman, P.; Fombonne, J.; Gorostiza, O.; Zhang, J.; Sultana, R.; et al. In vivo oxidative stress in brain of Alzheimer disease transgenic mice: Requirement for methionine 35 in amyloid β-peptide of APP. Free Radic. Biol. Med. 2010, 48, 136–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojsiat, J.; Laskowska-Kaszub, K.; Mietelska-Porowska, A.; Wojda, U. Search for Alzheimer’s disease biomarkers in blood cells: Hypotheses-driven approach. Biomark. Med. 2017, 11, 917–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angiulli, F.; Conti, E.; Zoia, C.P.; da Re, F.; Appollonio, I.; Ferrarese, C.; Tremolizzo, L. Blood-based biomarkers of neuroinflammation in Alzheimer’s disease: A central role for periphery? Diagnostics 2021, 11, 1525. [Google Scholar] [CrossRef] [PubMed]
- Wojda, U. Alzheimer’s disease lymphocytes: Potential for biomarkers? Biomark. Med. 2016, 10, 1–4. [Google Scholar] [CrossRef]
- Wojsiat, J.; Prandelli, C.; Laskowska-Kaszub, K.; Martín-Requero, A.; Wojda, U. Oxidative stress and aberrant cell cycle in Alzheimer’s disease lymphocytes: Diagnostic prospects. J. Alzheimer’s Dis. 2015, 46, 329–350. [Google Scholar] [CrossRef]
- Khan, T.K.; Alkon, D.L. Peripheral biomarkers of Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 44, 729–744. [Google Scholar] [CrossRef] [Green Version]
- Leutner, S.; Schindowski, K.; Frölich, L.; Maurer, K.; Kratzsch, T.; Eckert, A.; Müller, W.E. Enhanced ROS-generation in lymphocytes from Alzheimer’s patients. Pharmacopsychiatry 2005, 38, 312–315. [Google Scholar] [CrossRef] [Green Version]
- Vignini, A.; Nanetti, L.; Moroni, C.; Tanase, L.; Bartolini, M.; Luzzi, S.; Mazzanti, L. Modifications of platelet from Alzheimer disease patients: A possible relation between membrane properties and NO metabolites. Neurobiol. Aging 2007, 28, 987–994. [Google Scholar] [CrossRef]
- Calabrese, V.; Sultana, R.; Scapagnini, G.; Guagliano, E.; Sapienza, M.; Bella, R.; Kanski, J.; Pennisi, G.; Mancuso, C.; Stella, A.M.G.; et al. Nitrosative stress, cellular stress response, and thiol homeostasis in patients with Alzheimer’s disease. Antioxid. Redox Signal. 2006, 8, 1975–1986. [Google Scholar] [CrossRef] [Green Version]
- Skoumalova, A.; Ivica, J.; Šantorová, P.; Topinkova, E.; Wilhelm, J. The lipid peroxidation products as possible markers of Alzheimer’s disease in blood. Exp. Gerontol. 2011, 46, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Buizza, L.; Cenini, G.; Lanni, C.; Ferrari-Toninelli, G.; Prandelli, C.; Govoni, S.; Szybinska, A. Conformational altered p53 as an early marker of oxidative stress in Alzheimer’s disease. PLoS ONE 2012, 7, e29789. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, A.; Perry, G.; Aliev, G.; Hirai, K.; Takeda, A.; Balraj, E.K.; Jones, P.K.; Ghanbari, H.; Wataya, T.; Shimohama, S.; et al. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001, 60, 759–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunomura, A.; Castellani, R.J.; Zhu, X.; Moreira, P.I.; Perry, G.; Smith, M.A. Involvement of oxidative stress in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006, 65, 631–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadioglu, E.; Sardas, S.; Aslan, S.; Isik, E.; Karakaya, A.E. Detection of oxidative DNA damage in lymphocytes of patients with Alzheimer’s disease. Biomarkers 2004, 9, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Mórocz, M.; Kálmán, J.; Juhász, A.; Sinkó, I.; McGlynn, A.P.; Downes, C.S.; Janka, Z.; Raskó, I. Elevated levels of oxidative DNA damage in lymphocytes from patients with Alzheimer’s disease. Neurobiol. Aging 2002, 23, S0197–S4580. [Google Scholar]
- Velagapudi, R.; El-Bakoush, A.; Olajide, O.A. Activation of nrf2 pathway contributes to neuroprotection by the dietary flavonoid tiliroside. Mol. Neurobiol. 2018, 55, 8103–8123. [Google Scholar] [CrossRef] [Green Version]
- Irshad, M.; Chaudhuri, P.S. Oxidant-Antioxidant System: Role and Significance in Human Body. 2002. Available online: http://nopr.niscair.res.in/handle/123456789/23569 (accessed on 15 December 2021).
- Hill, M.F. Emerging role for antioxidant therapy in protection against diabetic cardiac complications: Experimental and clinical evidence for utilization of classic and new antioxidants. Curr. Cardiol. Rev. 2008, 4, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, A.; Chatterjee, A.; Ghosal, S.; Bhattacharya, S.K. Antioxidant Activity of Active Tannoid Principles of Emblica Officinalis (Amla). 1999. Available online: http://nopr.niscair.res.in/handle/123456789/19103 (accessed on 13 December 2021).
- Veurink, G.; Perry, G.; Singh, S.K. Role of antioxidants and a nutrient rich diet in Alzheimer’s disease. Open Biol. 2020, 10, 200084. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X. Antioxidant therapies for Alzheimer’s disease. Oxidative Med. Cell. Longev. 2012, 2012, 472932. [Google Scholar] [CrossRef] [Green Version]
- Suárez, S.; Mu, T.; Sun, H.; Añón, M.C. Antioxidant activity, nutritional, and phenolic composition of sweet potato leaves as affected by harvesting period. Int. J. Food Prop. 2020, 23, 178–188. [Google Scholar] [CrossRef]
- Orsavová, J.; Hlaváčová, I.; Mlček, J.; Snopek, L.; Mišurcová, L. Contribution of phenolic compounds, ascorbic acid and vitamin E to antioxidant activity of currant (Ribes L.) and gooseberry (Ribes uva-crispa L.) fruits. Food Chem. 2019, 284, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Lopa, S.S.; Al-Amin, M.; Hasan, M.; Ahammed, M.; Islam, K.M.; Alam, A.H.M.; Tanaka, T.; Sadik, M. Phytochemical analysis and cholinesterase inhibitory and antioxidant activities of Enhydra fluctuans relevant in the management of Alzheimer’s disease. Int. J. Food Sci. 2021, 2021, 8862025. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.E.; Ahmed, S.A.; Amer, S.A.; Al-Gabri, N.A.; Ahmed, A.I.; Abdel-Warith, A.W.A.; Younis, E.S.M.; Metwally, A.E. Influence of vitamin C feed supplementation on the growth, antioxidant activity, immune status, tissue histomorphology, and disease resistance in Nile tilapia, Oreochromis niloticus. Aquac. Rep. 2020, 18, 100545. [Google Scholar] [CrossRef]
- Ouknin, M.; Aghraz, A.; Chibane, M.; Boumezzourh, A.; Costa, J.; Majidi, L. Enzyme inhibitory, antioxidant activity and phytochemical analysis of essential oil from cultivated Rosmarinus officinalis. J. Food Meas. Charact. 2021, 15, 3782–3790. [Google Scholar] [CrossRef]
- Boccardi, V.; Arosio, B.; Cari, L.; Bastiani, P.; Scamosci, M.; Casati, M.; Ferri, E.; Bertagnoli, L.; Ciccone, S.; Rossi, P.D.; et al. Beta-carotene, telomerase activity and Alzheimer’s disease in old age subjects. Eur. J. Nutr. 2019, 59, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Mielech, A.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. Vitamins in Alzheimer’s disease—Review of the latest reports. Nutritients 2020, 12, 3458. [Google Scholar] [CrossRef] [PubMed]
- Alam, J. Vitamins: A nutritional intervention to modulate the Alzheimer’s disease progression. Nutr. Neurosci. 2020, 1–18. [Google Scholar] [CrossRef]
- Gavazza, M.; Marmunti, M.; Leaden, P.; Zeinsteger, P.; Palacios, A. Alpha-lipoic acid: Antioxidant activity against non-enzymatic peroxidation of rat kidney and liver mitochondria. J. Clin. Biomed. Investig. 2021, 1, 1–4. [Google Scholar] [CrossRef]
- Pei, X.; Hu, F.; Luo, F.; Huang, X.; Li, X.; Xing, S.; Long, D. The neuroprotective effects of alpha-lipoic acid on an experimental model of Alzheimer’s disease in PC12 cells. J. Appl. Toxicol. 2021, 42, 284–295. [Google Scholar] [CrossRef]
- Wear, D.; Vegh, C.; Sandhu, J.K.; Sikorska, M.; Cohen, J.; Pandey, S. Ubisol-Q10, a nanomicellar and water-dispersible formulation of coenzyme-Q10 as a potential treatment for Alzheimer’s and Parkinson’s disease. Antioxidants 2021, 10, 764. [Google Scholar] [CrossRef]
- Attia, H.; Albuhayri, S.; Alaraidh, S.; Alotaibi, A.; Yacoub, H.; Mohamad, R.; Al-Amin, M. Biotin, coenzyme Q10, and their combination ameliorate aluminium chloride-induced Alzheimer’s disease via attenuating neuroinflammation and improving brain insulin signaling. J. Biochem. Mol. Toxicol. 2020, 34, e22519. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.F.; Chang, W.H.; Black, R.M.; Liu, J.R.; Sompol, P.; Chen, Y.; Wei, H.; Zhao, Q.; Cheng, I.H. Crude caffeine reduces memory impairment and amyloid β1–42 levels in an Alzheimer’s mouse model. Food Chem. 2012, 135, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Londzin, P.; Zamora, M.; Kąkol, B.; Taborek, A.; Folwarczna, J. Potential of caffeine in Alzheimer’s disease—A review of experimental studies. Nutritients 2021, 13, 537. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, C.; Zhang, D.; Yuan, M.; Chen, C.-H.; Li, M. Synergic effects of berberine and curcumin on improving cognitive function in an Alzheimer’s disease mouse model. Neurochem. Res. 2020, 45, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Rubab, M.; Tyagi, A.; Oh, D.-H. Curcumin and its derivatives as theranostic agents in Alzheimer’s disease: The implication of nanotechnology. Int. J. Mol. Sci. 2021, 22, 196. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H. Berberine neuroprotection and antioxidant activity. Oxidative stress diet. Antioxid. Neurol. Dis. 2020, 199–216. [Google Scholar] [CrossRef]
- Tarabasz, D.; Kukula-Koch, W. Palmatine: A review of pharmacological properties and pharmacokinetics. Phyther. Res. 2020, 34, 33–50. [Google Scholar] [CrossRef]
- Chaves, S.K.M.; Afzal, M.I.; Islam, M.T.; Hameed, A.; Da Mata, A.M.O.F.; da Silva Araújo, L.; Ali, S.W.; Rolim, H.M.L.; De Medeiros, M.D.G.F.; Costa, E.V.; et al. Palmatine antioxidant and anti-acetylcholinesterase activities: A pre-clinical assessment. Cell. Mol. Biol. 2020, 66, 54–59. [Google Scholar] [CrossRef]
- Qajari, N.M.; Khonakdar-Tarsi, A. Short communication silibinin and neurological diseases. J. Neurobiol. Physiol. 2021, 3, 8–9. [Google Scholar]
- Bai, D.; Jin, G.; Yin, S.; Zou, D.; Zhu, Q.; Yang, Z.; Liu, X.; Ren, L.; Sun, Y.; Gan, S. Antioxidative and anti-apoptotic roles of silibinin in reversing learning and memory deficits in APP/PS1 mice. Neurochem. Res. 2017, 42, 3439–3445. [Google Scholar] [CrossRef]
- Zhao, L.J.; Liu, W.; Xiong, S.H.; Tang, J.; Lou, Z.H.; Xie, M.X.; Xia, B.H.; Lin, L.M.; Liao, D.F. Determination of total flavonoids contents and antioxidant activity of Ginkgo biloba leaf by near-infrared reflectance method. Int. J. Anal. Chem. 2018, 2018, 8195784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and antioxidant effect of Ginkgo biloba extract against AD and other neurological disorders. Neurotherapeuthics 2019, 16, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Pachón-Angona, I.; Refouvelet, B.; Andrýs, R.; Martin, H.; Luzet, V.; Iriepa, I.; Moraleda, I.; Diez-Iriepa, D.; Oset-Gasque, M.J.; Marco-Contelles, J.; et al. Donepezil + chromone + melatonin hybrids as promising agents for Alzheimer’s disease therapy. J. Enzym. Inh. Med. Chem. 2019, 34, 479–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.F.; Wang, N.; Chen, R.; Li, S.; Roy, J.; Uddin, M.G.; Li, Z.; Lim, L.W.; Song, Y.Q. Exploring the multifunctional role of melatonin in regulating autophagy and sleep to mitigate Alzheimer’s disease neuropathology. Ageing Res. Rev. 2021, 67, 101304. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Yan, Y.; He, X.Y.; Yang, H.; Liang, B.; Wang, J.; He, Y.; Ding, Y.; Yu, H. Effects of resveratrol on the mechanisms of antioxidants and estrogen in Alzheimer’s disease. BioMed. Res. Int. 2019, 2019, 8983752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guglielmotto, M.; Manassero, G.; Vasciaveo, V.; Venezia, M.; Tabaton, M.; Tamagno, E. Estrogens inhibit amyloid-β-mediated paired helical filament-like conformation of tau through antioxidant activity and miRNA 218 regulation in hTau mice. J. Alzheimer’s Dis. 2020, 77, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Guglielmotto, M. Estrogens still represent an attractive therapeutic approach for Alzheimer’s disease. Neural Regen. Res. 2022, 17, 93. [Google Scholar] [CrossRef]
- Feizipour, S.; Sobhani, S.; Mehrafza, S.; Gholami, M.; Motaghinejad, M.; Motevalian, M.; Safari, S.; Davoudizadeh, R. Selegiline acts as neuroprotective agent against methamphetamine-prompted mood and cognitive related behavior and neurotoxicity in rats: Involvement of CREB/BDNF and Akt/GSK3 signal pathways. Iran. J. Basic Med. Sci. 2020, 23, 606. [Google Scholar] [CrossRef]
- Cai, M.; Yang, E.J. Effect of combined electroacupuncture and selegiline treatment in Alzheimer’s disease: An animal model. Front. Pharmacol. 2020, 11, 2048. [Google Scholar] [CrossRef]
- Gomes, B.A.Q.; Silva, J.P.; Romeiro, C.F.; Dos Santos, S.M.; Rodrigues, C.A.; Goncalves, P.R.; Sakai, J.T.; Mendes, P.F.; Varela, E.L.; Monteiro, M.C. Neuroprotective mechanisms of resveratrol in Alzheimer’s disease: Role of SIRT1. Oxidative Med. Cell. Longev. 2018, 2018, 8152373. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Chang, J.H.; Barroso, E.; Espina, M.; Kühne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B.; et al. Dual-drug loaded nanoparticles of epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s disease mice model. J. Control. Release 2019, 301, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Ide, K.; Matsuoka, N.; Yamada, H.; Furushima, D.; Kawakami, K. Effects of tea catechins on Alzheimer’s disease: Recent updates and perspectives. Molecules 2018, 23, 2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olajide, O.A.; Bhatia, H.S.; de Oliveira, A.C.; Wright, C.W.; Fiebich, B.L. Inhibition of neuroinflammation in LPS-activated microglia by cryptolepine. Evid. Based Complementary Altern. Med. 2013, 2013, 459723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynish, W.; Andrieu, S.; Nourhashemi, F.; Vellas, B. Nutritional factors and Alzheimer’s disease. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M675–M680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, F.C.; Shukitt-Hale, B.; Joseph, J.A. Nutritional intervention in brain aging. In Inflammation in the Pathogenesis of Chronic Diseases; Springer: Dordrecht, The Netherlands, 2007; pp. 299–318. [Google Scholar] [CrossRef]
- Burgener, S.C.; Buettner, L.; Buckwalter, K.C.; Beattie, E.; Bossen, A.L.; Fick, D.M.; Schreiner, A. Evidence supporting nutritional interventions for persons in early stage Alzheimer’s disease (AD). J. Nutr. Health Aging 2008, 12, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Khalsa, D.S.; Perry, G. The four pillars of Alzheimer’s prevention. In Cerebrum: The Dana Forum on Brain Science; Dana Foundation: New York, NY, USA, 2017; Volume 2017. [Google Scholar]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Antioxidant activities, phenolic profiles, and organic acid contents of fruit vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef] [Green Version]
- Nazıroğlu, M.; Güler, M.; Özgül, C.; Saydam, G.; Küçükayaz, M.; Sözbir, E. Apple cider vinegar modulates serum lipid profile, erythrocyte, kidney, and liver membrane oxidative stress in ovariectomized mice fed high cholesterol. J. Membr. Biol. 2014, 247, 667–673. [Google Scholar] [CrossRef]
- Frassetto, L.; Morris, R.C., Jr.; Sellmeyer, D.E.; Todd, K.; Sebastian, A. Diet, evolution and aging. Eur. J. Nutr. 2001, 40, 200–213. [Google Scholar] [CrossRef]
- Sebastian, A.; Frassetto, L.A.; Sellmeyer, D.E.; Morris, R.C., Jr. The evolution-informed optimal dietary potassium intake of human beings greatly exceeds current and recommended intakes. Semin. Nephrol. 2006, 26, 447–453. [Google Scholar] [CrossRef]
- Mathew, B.C.; Biju, R.S. Neuroprotective effects of garlic a review. Libyan J. Med. 2008, 3, 23–33. [Google Scholar]
- Arslan, J.; Jamshed, H.; Qureshi, H. Early detection and prevention of Alzheimer’s disease: Role of oxidative markers and natural antioxidants. Front. Aging Neurosci. 2020, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Lloret, A.; Esteve, D.; Monllor, P.; Cervera-Ferri, A.; Lloret, A. The effectiveness of vitamin E treatment in Alzheimer’s disease. Int. J. Mol. Sci. 2019, 20, 879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliev, G.; Priyadarshini, M.; Reddy, V.P.; Grieg, N.H.; Kaminsky, Y.; Cacabelos, R.; Zamyatnin, J. Oxidative stress mediated mitochondrial and vascular lesions as markers in the pathogenesis of Alzheimer disease. Curr. Med. Chem. 2014, 21, 2208–2217. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.P.; de Castro, A.A.; Soares, F.V.; da Cunha, E.F.; Ramalho, T.C. Future therapeutic perspectives into the alzheimer’s disease targeting the oxidative stress hypothesis. Molecules 2019, 24, 4410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, Y.B.; Praticò, D. Vitamin E in aging, dementia, and Alzheimer’s disease. Biofactors 2012, 38, 90–97. [Google Scholar] [CrossRef]
- Rigotti, A. Absorption, transport, and tissue delivery of vitamin E. Mol. Asp. Med. 2007, 28, 423–436. [Google Scholar] [CrossRef]
- Zhang, S.M.; Hernan, M.A.; Chen, H.; Spiegelman, D.; Willett, W.C.; Ascherio, A. Intakes of vitamins E and C, carotenoids, vitamin supplements, and PD risk. Neurology 2002, 59, 1161–1169. [Google Scholar] [CrossRef]
- Fata, G.L.; Weber, P.; Mohajeri, M.H. Effects of vitamin E on cognitive performance during ageing and in Alzheimer’s disease. Nutrients 2014, 6, 5453–5472. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Chen, X.; Liu, Y.; Shu, Y.; Chen, T.; Xu, L.; Li, M.; Guan, X. Do low-serum vitamin E levels increase the risk of Alzheimer disease in older people? Evidence from a meta-analysis of case-control studies. Int. J. Geriatr. Psychiatry 2018, 33, e257–e263. [Google Scholar] [CrossRef]
- Marklund, S.L. Properties of extracellular superoxide dismutase from human lung. Biochem. J. 1984, 220, 269–272. [Google Scholar] [CrossRef]
- Cowan, C.M.; Sealey, M.A.; Mudher, A. Suppression of tau-induced phenotypes by vitamin E demonstrates the dissociation of oxidative stress and phosphorylation in mechanisms of tau toxicity. J. Neurochem. 2021, 157, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Iijima-Ando, K.; Iijima, K. Transgenic Drosophila models of Alzheimer’s disease and tauopathies Brain Struct. Funct. 2010, 214, 245–262. [Google Scholar] [CrossRef] [Green Version]
- Casati, M.; Boccardi, V.; Ferri, E.; Bertagnoli, L.; Bastiani, P.; Ciccone, S.; Mansi, M.; Scamosci, M.; Rossi, P.D.; Mecocci, P.; et al. Vitamin E and Alzheimer’s disease: The mediating role of cellular aging. Aging Clin. Exp. Res. 2020, 32, 459–464. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Castegna, A.; Pocernich, C.B.; Drake, J.; Scapagnini, G.; Calabrese, V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J. Nutr. Biochem. 2002, 13, 444–461. [Google Scholar] [CrossRef]
- Dringen, R.; Brandmann, M.; Hohnholt, M.C.; Blumrich, E.M. Glutathione-dependent detoxification processes in astrocytes. Neurochem. Res. 2015, 40, 2570–2582. [Google Scholar] [CrossRef]
- Sears, M.E. 2013 Chelation: Harnessing and enhancing heavy metal detoxification—A review. Sci. World J. 2013, 2013, 219840. [Google Scholar] [CrossRef] [Green Version]
- Montserrat, M.; de Gregorio, E.; de Dios, C.; Roca-Agujetas, V.; Cucarull, B.; Tutusaus, A.; Morales, A.; Colell, A. Mitochondrial glutathione: Recent insights and role in disease. Antioxidants 2020, 9, 909. [Google Scholar]
- Barbero-Camps, E.; Fernández, A.; Martínez, L.; Fernández-Checa, J.C.; Colell, A. APP/PS1 mice overexpressing SREBP-2 exhibit combined Aβ accumulation and tau pathology underlying Alzheimer’s disease. Hum. Mol. Genet. 2013, 22, 3460–3476. [Google Scholar] [CrossRef]
- Mariet, A.; Zou, F.; Chai, H.S.; Younkin, C.S.; Miles, R.; Nair, A.A.; Crook, J.E.; Pankratz, V.S.; Carrasquillo, M.M.; Rowley, C.N.; et al. Glutathione S-transferase omega genes in Alzheimer and Parkinson disease risk, age-at-diagnosis and brain gene expression: An association study with mechanistic implications. Mol. Neurodegener. 2012, 7, 1–12. [Google Scholar]
- Slezák, J.; Kura, B.; Frimmel, K.; Zálešák, M.; Ravingerová, T.; Viczenczová, C.; Tribulová, N. Preventive and therapeutic application of molecular hydrogen in situations with excessive production of free radicals. Physiol. Res. 2016, 65 (Suppl. S1), S11–S28. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Klichko, V.I.; Safonov, V.L.; Safonov, M.Y.; Radyuk, S.N. Supplementation with hydrogen-producing composition confers beneficial effects on physiology and life span in Drosophila. Heliyon 2019, 5, e01679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, T. Monoamine oxidase-B inhibitors in the treatment of Alzheimers disease. Neurobiol. Aging 2000, 21, 343–348. [Google Scholar] [CrossRef]
- Sano, M.; Ernesto, C.; Thomas, R.G.; Klauber, M.R.; Schafer, K.; Grundman, M.; Schneider, L.S. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. N. Engl. J. Med. 1997, 336, 1216–1222. [Google Scholar] [CrossRef] [Green Version]
- Tuppo, E.E.; Forman, L.J. Free radical oxidative damage and Alzheimer’s disease. J. Am. Osteopath. Assoc. 2001, 101 (Suppl. S121), 11S–15S. [Google Scholar]
- Ross, D.; Mendiratta, S.; Qu, Z.C.; Cobb, C.E.; May, J.M. Ascorbate 6-palmitate protects human erythrocytes from oxidative damage. Free Radic. Biol. Med. 1999, 26, 81–89. [Google Scholar] [CrossRef]
- Nishida, S. Metabolic effects of melatonin on odative stress and dbetes mellitus. Endocrine 2005, 27, 131–135. [Google Scholar] [CrossRef]
- Fusco, D.; Colloca, G.; Monaco, M.R.L.; Cesari, M. Effects of antioxidant supplementation on the aging process. Clin. Interv. Aging 2007, 2, 377. [Google Scholar]
- Deng, Y.Q.; Xu, G.G.; Duan, P.; Zhang, Q.; Wang, J.Z. Effects of melatonin on wortmannin-induced tau hyperphosphorylation. Acta Pharmacol. Sin. 2005, 26, 519–526. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.Z.; Wang, Z.F. Role of melatonin in Alzheimer-like neurodegeneration. Acta Pharmacol. Sin. 2006, 27, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.J.; Wang, J.Z. Alzheimer-like tau phosphorylation induced by wortmannin in vivo and its attenuation by melatonin. Acta Pharmacol. Sin. 2002, 23, 183. [Google Scholar]
- Wang, D.L.; Ling, Z.Q.; Cao, F.Y.; Zhu, L.Q.; Wang, J.Z. Melatonin attenuates isoproterenol-induced protein kinase A overactivation and tau hyperphosphorylation in rat brain. J. Pineal Res. 2004, 37, 11–16. [Google Scholar] [CrossRef]
- Wang, X.C.; Zhang, J.; Yu, X.; Han, L.; Zhou, Z.T.; Zhang, Y.; Wang, J.Z. Prevention of isoproterenol-induced tau hyperphosphorylation by melatonin in the rat. Sheng Li Xue Bao Acta Physiol. Sin. 2005, 57, 7–12. [Google Scholar]
- Zhou, J.; Zhang, S.; Zhao, X.; Wei, T. Melatonin impairs NADPH oxidase assembly and decreases superoxide anion production in microglia exposed to amyloid-β1–42. J. Pineal Res. 2008, 45, 157–165. [Google Scholar] [CrossRef]
- Fakhri, S.; Yosifova Aneva, I.; Farzaei, M.H.; Sobarzo-Sánchez, E. The neuroprotective effects of astaxanthin: Therapeutic targets and clinical perspective. Moleluces 2019, 24, 2640. [Google Scholar] [CrossRef] [Green Version]
- Pokorski, M.; Marczak, M.; Dymecka, A.; Suchocki, P. Ascorbyl palmitate as a carrier of ascorbate into neural tissues. J. Biomed. Sci. 2003, 10, 193–198. [Google Scholar] [CrossRef]
- Frank, B.; Gupta, S. A review of antioxidants and Alzheimer’s disease. Ann. Clin. Psychiatry 2005, 17, 269–286. [Google Scholar] [CrossRef]
- Reddy, P.H.; Manczak, M.; Yin, X.; Grady, M.C.; Mitchell, A.; Tonk, S.; Kuruva, C.S.; Bhatti, J.S.; Kandimalla, R.; Vijayan, M.; et al. Protective effects of Indian spice curcumin against amyloid-β in Alzheimer’s disease. J. Alzheimers Dis. 2018, 61, 843–866. [Google Scholar] [CrossRef]
- Wei, Q.Y.; Chen, W.F.; Zhou, B.; Yang, L.; Liu, Z.L. Inhibition of lipid peroxidation and protein oxidation in rat liver mitochondria by curcumin and its analogues. Biochim. Biophys. Acta 2006, 1760, 70–77. [Google Scholar] [CrossRef]
- Baum, L.; Ng, A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer’s disease animal models. J. Alzheimers Dis. 2004, 6, 367–377. [Google Scholar] [CrossRef]
- Nishinaka, T.; Ichijo, Y.; Ito, M.; Kimura, M.; Katsuyama, M.; Iwata, K.; Miura, T.; Terada, T.; Yabe-Nishimura, C. Curcumin activates human glutathione S-transferase P1 expression through antioxidant response element. Toxicol. Lett. 2007, 170, 238–247. [Google Scholar] [CrossRef]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef]
- Serafini, M.M.; Catanzaro, M.; Rosini, M.; Racchi, M.; Lanni, C. Curcumin in Alzheimer’s disease: Can we think to new strategies and perspectives for this molecule? Pharmacol. Res. 2017, 124, 146–155. [Google Scholar] [CrossRef]
- Baschiera, E.; Sorrentino, U.; Calderan, C.; Desbats, M.A.; Salviati, L. The multiple roles of coenzyme Q in cellular homeostasis and their relevance for the pathogenesis of coenzyme Q deficiency. Free Radic. Biol. Med. 2021, 166, 277–286. [Google Scholar] [CrossRef]
- Seo, J.S.; Kim, T.K.; Leem, Y.H.; Lee, K.W.; Park, S.K.; Baek, I.S.; Han, P.L. SK-PC-B70M confers anti-oxidant activity and reduces Aβ levels in the brain of Tg2576 mice. Brain Res. 2009, 1261, 100–108. [Google Scholar] [CrossRef]
- Mulnard, R.A.; Cotman, C.W.; Kawas, C.; van Dyck, C.H.; Sano, M.; Doody, R.; Grundman, M. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: A randomized controlled trial. JAMA 2000, 283, 1007–1015. [Google Scholar] [CrossRef]
- Trivic, T.; Vojnovic, M.; Drid, P.; Ostojic, S.M. Drinking hydrogen-rich water for 4 weeks positively affects serum antioxidant enzymes in healthy men: A pilot study. Curr. Top. Nutraceutical Res. 2017, 15, 45–48. [Google Scholar]
- Shah, M.; Mahfuzur, R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Gong, N.; Zhao, J.; Yu, Z.; Gu, F.; Chen, J.; Dong, W. Powerful beneficial effects of benfotiamine on cognitive impairment and β-amyloid deposition in amyloid precursor protein/presenilin-1 transgenic mice. Brain 2010, 133, 1342–1351. [Google Scholar] [CrossRef]
- Gonzales, M.M.; Garbarino, V.R.; Marques Zilli, E.; Petersen, R.C.; Kirkland, J.L.; Tchkonia, T.; Musi, N.; Seshadri, S.; Craft, S.; Miranda, E. Orr senolytic therapy to modulate the progression of Alzheimer’s disease (SToMP-AD): A pilot clinical trial. J. Prev. Alzheimer’s Dis. 2021, 1–8. [Google Scholar] [CrossRef]
- Siedlak, S.L.; Casadesus, G.; Webber, K.M.; Pappolla, M.A.; Atwood, C.S.; Smith, M.A.; Perry, G. Chronic antioxidant therapy reduces oxidative stress in a mouse model of Alzheimer’s disease. Free Radic. Res. 2009, 43, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Raina, A.K.; Perry, G.; Smith, M.A. Alzheimer’s disease: The two-hit hypothesis. Lancet Neurol. 2004, 3, 219–226. [Google Scholar] [CrossRef]
- Quinn, J.F.; Bussiere, J.R.; Hammond, R.S.; Montine, T.J.; Henson, E.; Jones, R.E.; Stackman, R.W., Jr. Chronic dietary alpha-lipoic acid reduces deficits in hippocampal memory of aged Tg2576 mice. Neurobiol. Aging 2007, 28, 213–225. [Google Scholar] [CrossRef]
- Sajjad, N.; Wani, A.; Hassan, S.; Ali, R.; Hamid, R.; Akbar, S.; Bhat, E. Interplay of antioxidants in Alzheimer’s disease. J. Transl. Sci. 2019, 5, 1–11. [Google Scholar]
- Farr, S.A.; Price, T.O.; Banks, W.A.; Ercal, N.; Morley, J.E. Effect of alpha-lipoic acid on memory, oxidation, and lifespan in SAMP8 mice. J. Alzheimer’s Dis. 2012, 32, 447–455. [Google Scholar] [CrossRef]
- Banks William, A.; Elizabeth, M. Rhea the blood–brain barrier, oxidative stress, and insulin resistance. Antioxidants 2021, 10, 1695. [Google Scholar] [CrossRef]
- Singh, S.K.; Srikrishna, S.; Castellani, R.J.; Perry, G. Antioxidants in the prevention and treatment of Alzheimer’s disease. In Nutritional Antioxidant Therapies: Treatments and Perspectives; Springer: Cham, Switzerland, 2017; pp. 523–553. [Google Scholar]
- Arbo, B.D.; André-Miral, C.; Nasre-Nasser, R.G.; Schimith, L.E.; Santos, M.G.; Costa-Silva, D.; Muccillo-Baisch, A.L.; Hort, M.A. Resveratrol Derivatives as Potential Treatments for Alzheimer’s and Parkinson’s Disease. Front. Aging Neurosci. 2020, 12, 103. [Google Scholar] [CrossRef]
- McManus, M.J.; Murphy, M.P.; Franklin, J.L. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2011, 31, 15703–15715. [Google Scholar] [CrossRef] [Green Version]
- James, A.M.; Sharpley, M.S.; Manas, A.R.; Frerman, F.E.; Hirst, J.; Smith, R.A.; Murphy, M.P. Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J. Biol. Chem. 2007, 282, 14708–14718. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.P.; Smith, R.A. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 629–656. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhang, D.; Whiteman, M.; Armstrong, J.S. Is antioxidant potential of the mitochondrial targeted ubiquinone derivative MitoQ conserved in cells lacking mtDNA? Antioxid. Redox Signal. 2008, 10, 651–660. [Google Scholar] [CrossRef]
- Ng, L.F.; Gruber, J.; Cheah, I.K.; Goo, C.K.; Cheong, W.F.; Shui, G.; Halliwell, B. The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radic. Biol. Med. 2014, 71, 390–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manczak, M.; Mao, P.; Calkins, M.J.; Cornea, A.; Reddy, A.P.; Murphy, M.P.; Szeto, H.H.; Park, B.; Reddy, P.H. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J. Alzheimer’s Dis. JAD 2010, 20 (Suppl. S2), S609–S631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.T.; Jung, C.H.; Lee, S.R.; Bae, J.H.; Baek, W.L.; Suh, M.H.; Park, J.; Park, C.W.; Suh, S.I. The green tea polyphenol (−)-epigallocatechin gallate attenuates β-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci. 2001, 70, 603–614. [Google Scholar] [CrossRef]
- Haque, A.M.; Hashimoto, M.; Katakura, M.; Hara, Y.; Shido, O. Green tea catechins prevent cognitive deficits caused by Abeta1-40 in rats. J. Nutr. Biochem. 2008, 19, 619–626. [Google Scholar] [CrossRef]
- Kaur, T.; Pathak, C.M.; Pandhi, P.; Khanduja, K.L. Effects of green tea extract on learning, memory, behavior and acetylcholinesterase activity in young and old male rats. Brain Cogn. 2008, 67, 25–30. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Hasegawa, H.; Okuda, S.; Muraki, T.; Uruno, T.; Kubota, K. Ameliorative effects of tea catechins on active oxygen-related nerve cell injuries. J. Pharmacol. Exp. Ther. 1995, 274, 602–608. [Google Scholar]
- Duan, S.; Guan, X.; Lin, R.; Liu, X.; Yan, Y.; Lin, R.; Zhang, T.; Chen, X.; Huang, J.; Sun, X.; et al. Silibinin inhibits acetylcholinesterase activity and amyloid β peptide aggregation: A dual-target drug for the treatment of Alzheimer’s disease. Neurobiol. Aging 2015, 36, 1792–1807. [Google Scholar] [CrossRef]
- Yin, F.; Liu, J.; Ji, X.; Wang, Y.; Zidichouski, J.; Zhang, J. Silibinin: A novel inhibitor of Aβ aggregation. Neurochem. Int. 2011, 58, 399–403. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Cui, L.; Liu, B.; Liu, W.; Hayashi, T.; Mizuno, K.; Hattori, S.; Ushiki-Kaku, Y.; Onodera, S.; Ikejima, T. Silibinin ameliorates STZ-induced impairment of memory and learning by up-regulating insulin signaling pathway and attenuating apoptosis. Physiol. Behav. 2020, 213, 112689. [Google Scholar] [CrossRef]
- Jia, W.; Su, Q.; Cheng, Q.; Peng, Q.; Qiao, A.; Luo, X.; Zhang, J.; Wang, Y. Neuroprotective Effects of Palmatine via the Enhancement of Antioxidant Defense and Small Heat Shock Protein Expression in Aβ-Transgenic Caenorhabditis elegans. Oxidative Med. Cell. Longev. 2021, 2021, 9966223. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Hong, J.; Hu, C.; Huang, C.; Gao, J.; Huang, J.; Wang, D.; Geng, Q.; Dong, Y. Palmatine protects against cerebral ischemia/reperfusion injury by activation of the AMPK/Nrf2 pathway. Oxidative Med. Cell. Longev. 2021, 2021, 6660193. [Google Scholar] [CrossRef]
- Mak, S.; Luk, W.W.; Cui, W.; Hu, S.; Tsim, K.W.; Han, Y. Synergistic inhibition on acetylcholinesterase by the combination of berberine and palmatine originally isolated from Chinese medicinal herbs. J. Mol. Neurosci. 2014, 53, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhan, C.; Zou, Y.; Qian, Z.; Wei, G.; Zhang, Q. Serotonin and Melatonin Show Different Modes of Action on Aβ42 Protofibril Destabilization. ACS Chem. Neurosci. 2021, 12, 799–809. [Google Scholar] [CrossRef]
- Geldenhuys, W.J.; Van der Schyf, C.J. Role of serotonin in Alzheimer’s disease. CNS Drugs 2011, 25, 765–781. [Google Scholar] [CrossRef]
- Aaldijk, E.; Vermeiren, Y. The role of serotonin within the microbiota-gut-brain axis in the development of Alzheimer’s disease: A narrative review. Ageing Res. Rev. 2022, 75, 101556. [Google Scholar] [CrossRef]
- Ikram, M.; Jo, M.G.; Park, T.J.; Kim, M.W.; Khan, I.; Jo, M.H.; Kim, M.O. Oral administration of gintonin protects THE Brains of mice against Aβ-induced Alzheimer disease pathology: Antioxidant and anti-inflammatory effects. Oxidative Med. Cell. Longev. 2021, 2021, 6635552. [Google Scholar] [CrossRef]
- Jakaria, M.; Azam, S.; Go, E.A.; Uddin, M.S.; Jo, S.H.; Choi, D.K. Biological evidence of gintonin efficacy in memory disorders. Pharmacol. Res. 2021, 163, 105221. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, R.; Nam, S.M.; Kim, D.G.; Cho, I.H.; Kim, H.C.; Cho, Y.; Rhim, H.; Nah, S.Y. Ginseng gintonin, aging societies, and geriatric brain diseases. Integr. Med. Res. 2021, 10, 100450. [Google Scholar] [CrossRef]
- Jang, M.; Choi, S.H.; Choi, J.H.; Oh, J.; Lee, R.M.; Lee, N.E.; Cho, Y.J.; Rhim, H.; Kim, H.C.; Cho, I.H.; et al. Ginseng gintonin attenuates the disruptions of brain microvascular permeability and microvascular endothelium junctional proteins in an APPswe/PSEN-1 double-transgenic mouse model of Alzheimer’s disease. Exp. Ther. Med. 2021, 21, 1. [Google Scholar] [CrossRef]
- Firdaus, Z.; Tryambak, D. Singh an insight in pathophysiological mechanism of Alzheimer’s disease and its management using plant natural products. Mini Rev. Med. Chem. 2021, 21, 35–57. [Google Scholar] [CrossRef] [PubMed]
- He, F.Q.; Qiu, B.Y.; Li, T.K.; Xie, Q.; Cui, D.J.; Huang, X.L.; Gan, H.T. Tetrandrine suppresses amyloid-β-induced inflammatory cytokines by inhibiting NF-κB pathway in murine BV2 microglial cells. Int. Immunopharmacol. 2011, 11, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, S.U. Flavonoids for treatment of Alzheimer’s disease: An up to date review. EXCLI J. 2021, 20, 495–502. [Google Scholar]
- Wang, J.A.; Tong, M.L.; Zhao, B.; Zhu, G.; Xi, D.H.; Yang, J.P. Parthenolide ameliorates intracerebral hemorrhage-induced brain injury in rats. Phytother. Res. PTR 2020, 34, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Qiang, W.; Cai, W.; Yang, Q.; Yang, L.; Dai, Y.; Zhao, Z.; Yin, J.; Li, Y.; Li, Q.; Wang, Y.; et al. Artemisinin B improves learning and memory impairment in AD dementia mice by suppressing neuroinflammation. Neuroscience 2018, 395, 1–12. [Google Scholar] [CrossRef]
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.E.; Nofal, S.; Abdel-Mottaleb, Y.; Badary, O.A. Thymoquinone alleviates the experimentally induced Alzheimer’s disease inflammation by modulation of TLRs signaling. Hum. Exp. Toxicol. 2018, 37, 1092–1104. [Google Scholar] [CrossRef]
- Fang, F.; Chen, X.; Huang, T.; Lue, L.F.; Luddy, J.S.; Yan, S.S. Multi-faced neuroprotective effects of Ginsenoside Rg1 in an Alzheimer mouse model. Biochim. Biophys. Acta 2012, 1822, 286–292. [Google Scholar] [CrossRef] [Green Version]
- Karakani, A.M.; Riazi, G.; Mahmood Ghaffari, S.; Ahmadian, S.; Mokhtari, F.; Jalili Firuzi, M.; Zahra Bathaie, S. Inhibitory effect of corcin on aggregation of 1N/4R human tau protein in vitro. Iran. J. Basic Med Sci. 2015, 18, 485–492. [Google Scholar]
- Azimi, A.; Ghaffari, S.M.; Riazi, G.H.; Arab, S.S.; Tavakol, M.M.; Pooyan, S. α-Cyperone of Cyperus rotundus is an effective candidate for reduction of inflammation by destabilization of microtubule fibers in brain. J. Ethnopharmacol. 2016, 194, 219–227. [Google Scholar] [CrossRef]
- Gong, H.; He, Z.; Peng, A.; Zhang, X.; Cheng, B.; Sun, Y.; Zheng, L.; Huang, K. Effects of several quinones on insulin aggregation. Sci. Rep. 2014, 4, 5648. [Google Scholar] [CrossRef] [Green Version]
- Keshavarz, M.; Farrokhi, M.R.; Amiri, A. Caffeine neuroprotective mechanism against β-amyloid neurotoxicity in SHSY5Y cell line: Involvement of adenosine, ryanodine, and N-methyl-D-aspartate receptors. Adv. Pharm. Bull. 2017, 7, 579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Alcântara Almeida, I.; Dorvigny, B.M.; Tavares, L.S.; Santana, L.N.; Lima-Filho, J.V. Anti-inflammatory activity of caffeine (1, 3, 7-trimethylxanthine) after experimental challenge with virulent Listeria monocytogenes in Swiss mice. Int. Immunopharmacol. 2021, 100, 108090. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.L.; Arantes, L.P.; da Silveira, T.L.; Zamberlan, D.C.; Cordeiro, L.M.; Obetine, F.B.B.; da Silva, A.F.; da Cruz, I.B.M.; Soares, F.A.A.; Riva de Oliveria, P. Ilex paraguariensis extract provides increased resistance against oxidative stress and protection against Amyloid beta-induced toxicity compared to caffeine in Caenorhabditis elegans. Nutr. Neurosci. 2021, 24, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Solfrizzi, V.; Barulli, M.R.; Bonfiglio, C.; Guerra, V.; Osella, A.; Seripa, D.; Sabbà, C.; Pilotto, A.; Logroscino, G. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: A systematic review. J. Nutr. Health Aging 2015, 19, 313–328. [Google Scholar] [CrossRef]
- Taheri, P.; Yaghmaei, P.; Tehrani, H.S.; Ebrahim-Habibi, A. Effects of eugenol on alzheimer’s disease-like manifestations in insulin-and Aβ-induced rat models. Neurophysiology 2019, 51, 114–119. [Google Scholar] [CrossRef]
- Studzinski, C.M.; Li, F.; Bruce-Keller, A.J.; Fernandez-Kim, S.O.; Zhang, L.; Weidner, A.M.; Markesbery, W.R.; Murphy, M.P.; Keller, J.N. Effects of short-term Western diet on cerebral oxidative stress and diabetes related factors in APP× PS1 knock-in mice. J. Neurochem. 2009, 108, 860–866. [Google Scholar] [CrossRef] [Green Version]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Manly, J.J.; Schupf, N.; Luchsinger, J.A. Mediterranean diet and mild cognitive impairment. Arch. Neurol. 2009, 66, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Vardarajan, B.N.; Barral, S.; Jaworski, J.; Beecham, G.W.; Blue, E.; Tosto, G.; Reyes-Dumeyer, D.; Medrano, M.; Lantigua, R.; Naj, A.; et al. Whole genome sequencing of Caribbean Hispanic families with late-onset Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2018, 5, 406–417. [Google Scholar] [CrossRef] [Green Version]
- Nair, S.; Rocha-Ferreira, E.; Fleiss, B.; Nijboer, C.H.; Gressens, P.; Mallard, C.; Hagberg, H. Neuroprotection offered by mesenchymal stem cells in perinatal brain injury: Role of mitochondria, inflammation, and reactive oxygen species. J. Neurochem. 2021, 158, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Se Thoe, E.; Fauzi, A.; Tang, Y.Q.; Chamyuang, S.; Chia, A.Y.Y. A review on advances of treatment modalities for Alzheimer’s disease. Life Sci. 2021, 276, 119129. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Terzo, S.; Mulè, F. Natural compounds as beneficial antioxidant agents in neurodegenerative disorders: A focus on Alzheimer’s disease. Antioxidants 2019, 8, 608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabel, M.; Nackenoff, A.; Kirsch, W.M.; Harrison, F.E.; Perry, G.; Schrag, M. Markers of oxidative damage to lipids, nucleic acids and proteins and antioxidant enzymes activities in Alzheimer’s disease brain: A meta-analysis in human pathological specimens. Free Radic. Biol. Med. 2018, 115, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Karim, N.; Khan, H.; Khan, I.; Guo, O.; Sobarzo-Sánchez, E.; Rastrelli, L.; Kamal, M.A. An increasing role of polyphenols as novel therapeutics for Alzheimer’s: A review. Med. Chem. 2019, 16, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Pasinetti, G.M.; Wang, J.; Ho, L.; Zhao, W.; Dubner, L. Roles of resveratrol and other grape-derived polyphenols in Alzheimer’s disease prevention and treatment. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 1202–1208. [Google Scholar] [CrossRef] [Green Version]
- Pinchuk, I.; Shoval, H.; Dotan, Y.; Lichtenberg, D. Evaluation of antioxidants: Scope, limitations and relevance of assays. Chem. Phys. Lipids 2012, 165, 638–647. [Google Scholar] [CrossRef]
- Martinelli, C.; Pucci, C.; Battaglini, M.; Marino, A.; Ciofani, G. Antioxidants and nanotechnology: Promises and limits of potentially disruptive approaches in the treatment of central nervous system diseases. Adv. Healthc. Mater. 2020, 9, 1901589. [Google Scholar] [CrossRef]


| Drug Name (Generic/Brand) | Manufacturer | Drug Type | Drug Use | Mechanism | Side Effects |
|---|---|---|---|---|---|
| Aducanumab Aduhelm™ | Biogen, Eisai Co. Ltd., Tokyo, Japan | Disease-modifying immunotherapy | Alzheimer’s disease (MCI or mild dementia) | Removes abnormal Aβ helping to reduce the number of plaques in the brain | Amyloid-related imaging abnormalities (ARIA), which can lead to fluid buildup or bleeding in the brain; headache, dizziness, falls, diarrhea, confusion |
| Donepezil Aricept® | Eisai Inc. and Pfizer Inc., New York, NY, USA | Cholinesterase inhibitor | Mild, moderate, and severe symptoms of AD | Prevents the breakdown of acetylcholine in the brain | Nausea, vomiting, diarrhea, muscle cramps, fatigue, weight loss, loss of appetite, and increased frequency of bowel movements. |
| Rivastigmine Exelon® | Novartis, Basel, Switzerland | Cholinesterase inhibitor | Mild to moderate symptoms of AD | Prevents acetylcholine and butyrylcholine from being degraded in the brain | Nausea, vomiting, diarrhea, weight loss, indigestion, muscle weakness |
| Galantamine Razadyne® | Ortho-McNeil Neurologics, Johnson & Johnson, Titusville, NJ, USA. | Cholinesterase inhibitor | Mild to moderate symptoms of AD | Prevents the breakdown of acetylcholine and stimulates nicotinic receptors to release more acetylcholine into the brain | Nausea, vomiting, diarrhea, decreased appetite, dizziness, headache |
| Memantine Namenda® | Allergan plc, Dublin, Ireland | N-methyl D-aspartate (NMDA) antagonist | Moderate to severe symptoms of AD | Blocks the toxic effects associated with excess glutamate and regulates glutamate activation | Dizziness, headache, diarrhea, constipation, confusion |
| Memantine + Donepezil Namzaric® | Actavis and Adamas Pharmaceuticals, Dublin, Ireland | NMDA antagonist and cholinesterase inhibitor | Moderate to severe symptoms of AD | Blocks the toxic effects associated with excess glutamate and prevents the breakdown of acetylcholine in the brain | Nausea, vomiting, loss of appetite, increased frequency of bowel movements, headache, constipation, confusion, and dizziness |
| Antioxidant | Chemical Structure | Functions | References |
|---|---|---|---|
| Vitamin E |  |
| [54,55] |
| Vitamin C |  |
| [56,57] |
| β carotene |  |
| [58,59] |
| Vitamin B12 |  |
| [60,61] |
| α-lipoic acid |  |
| [62,63] |
| CoQ10 |  |
| [64,65] |
| Caffeine |  |
| [66,67] |
| Curcumin |  |
| [68,69] |
| Berberine |  |
| [69,70] |
| Palmatine |  |
| [71,72] |
| Silibinin |  |
| [73,74] |
| Quercetin |  |
| [75,76] |
| Melatonin |  |
| [77,78] |
| Estrogen |  |
| [78,79,80,81] |
| Selegiline |  |
| [82,83] |
| Resveratrol |  |
| [84,85] |
| Tea polyphenols-(−)- epicatechin (EC) (−)-epicatechin-3-gallate (ECG) (−)-epigallocatechin (EGC) (−) epigallocatechin-3- gallate (EGCG) |  |
| [86,87] |
| Sl. No. | NCT Number | Conditions | Interventions | Outcome Measures | Phases |
|---|---|---|---|---|---|
| 1 | NCT00117403 (https://clinicaltrials.gov/show/NCT00117403, accessed on 20 December 2021) | AD | Drugs: Vitamin E, Vitamin C, and Alpha-lipoic Acid Drug: Coenzyme Q Drug: Placebo capsules Drug: Placebo wafers | Effect on CSF biomarkers related to oxidative damage change in plasma and CSF concentrations of Aβ42 and Aβ40 | Phase 1 |
| 2 | NCT00090402 (https://clinicaltrials.gov/show/NCT00090402, accessed on 20 December 2021) | AD Oxidative Stress Dementia Hyperlipidemia Inflammation | Dietary Supplement: Fish Oil Dietary Supplement: Lipoic Acid Other: Fish Oil Placebo Other: Lipoic Acid Placebo | F2-isoprostane Level: Urine F2-Isoprostanes Change in Mini-Mental State Exam (MMSE) Score From Baseline to 12 Months Change in Activities of Daily Living/Instrumental Activities of Daily Living (ADL/IADL) Scores From Baseline to 12 Months | Phase 1 Phase 2 |
| 3 | NCT00678431 (https://clinicaltrials.gov/show/NCT00678431, accessed on 20 December 2021) | AD | Dietary Supplement: Resveratrol with Glucose and Malate Dietary Supplement: Placebo | Alzheimer’s Disease Assessment Scale (ADAScog) CGIC | Phase 3 |
| 4 | NCT00000173 (https://clinicaltrials.gov/show/NCT00000173, accessed on 20 December 2021) | AD | Drug: Donepezil Drug: Vitamin E | Phase 3 | |
| 5 | NCT00951834 (https://clinicaltrials.gov/show/NCT00951834, accessed on 20 December 2021) | AD | Drug: Epigallocatechin-Gallate Drug: Placebo | ADAS-COG (Score 0–70) (Baseline to treatment) Safety and tolerability of the verum MMSE (Score 0–30) after 18 months compared to baseline Time to hospitalization and Time to death related to AD Brain atrophy assessed by brain MRI Baseline-ADAS-COG and Baseline-MMSE as covariates CIBIC+ and WHO-QOL-Bref Trail Making Test and MVGT | Phase 2 Phase 3 |
| 6 | NCT01707719 (https://clinicaltrials.gov/show/NCT01707719, accessed on 20 December 2021) | AD Oxidative-Stress Adrenocortical-hyperfunction | Malondialdehyde assay Relationship between urinary excretion of cortisol and levels of malondialdehyde | ||
| 7 | NCT00628017 (https://clinicaltrials.gov/show/NCT00628017, accessed on 20 December 2021) | AD Mild Cognitive Impairment | Dietary Supplement: omega-3 polyunsaturated fatty acids (EPA+DHA) | The Clinician’s Interview-Based Impression of Change Scale (CIBIC-plus) The cognitive portion of the Alzheimer’s Disease Assessment Scale (ADAS-cog) Mini-Mental Status Examination (MMSE) score 17-item Hamilton Depression Scale (HDRS) 18-adverse events | Not Applicable |
| 8 | NCT00099710 (https://clinicaltrials.gov/show/NCT00099710, accessed on 20 December 2021) | AD | Dietary Supplement: Curcumin C3 Complex | Side effect checklist Oxidative damage Inflammation/gliosis A-beta levels Tau levels Total plasma cholesterol, LDL and HDL; ApoE Plasma curcumin and metabolites Cognitive and behavioral measures | Phase 2 |
| 9 | NCT00597376 (https://clinicaltrials.gov/show/NCT00597376, accessed on 20 December 2021) | Subjective Memory Loss in Older Persons | Other: Cerefolin NAC (a medical food) Other: Cerefolin NAC placebo | Six-month blood levels of Homocysteine, Glutathione, and the Ratio of Aβ42 to Aβ40 (as a Percent of Baseline Levels) After Daily Intake of Cerefolin NAC Plus a Multivitamin Versus a Multivitamin Only Tolerability of Cerefolin NAC and a Multivitamin Versus a Multivitamin Only Six Month Levels of Inflammation and Oxidative Stress Markers (as a Percent of Baseline Levels) After Daily Treatment with Cerefolin NAC and a Multivitamin or a Multivitamin Only | Not Applicable |
| 10 | NCT00940589 (https://clinicaltrials.gov/show/NCT00940589, accessed on 20 December 2021) | AD Sleep Disorder | Drug: Circadin Drug: Placebo | Change From Baseline to 24 Weeks in ADAS-cog Change From Baseline to 24 Weeks in iADL Change From Baseline to 24 Weeks in MMSE | Phase 2 |
| 11 | NCT00000171 (https://clinicaltrials.gov/show/NCT00000171, accessed on 20 December 2021) | AD Dyssomnias | Drug: Melatonin | Phase 3 | |
| 12 | NCT01058941 (https://clinicaltrials.gov/show/NCT01058941, accessed on 20 December 2021) | AD | Drug: Lipoic acid and fish oil concentrate Drug: Placebo | Change From Baseline in Activities of Daily Living (ADL) at 18 Months Change From Baseline in Alzheimer’s Disease Assessment Scale—Cognitive Subscale (ADAS-cog) at 18 Months | Phase 1 Phase 2 |
| 13 | NCT01370954 (https://clinicaltrials.gov/show/NCT01370954, accessed on 20 December 2021) | Early Memory Loss MCI AD VaD | Other: CerefolinNAC® | To determine if CerefolinNAC® affects a subject’s quality of life as measured by the Quality of Life-Alzheimer’s Disease Scale (QOL-AD) To determine overall patient satisfaction with CerefolinNAC® using a 9-point satisfaction scale | |
| 14 | NCT01504854 (https://clinicaltrials.gov/show/NCT01504854, accessed on 20 December 2021) | AD | Drug: Resveratrol Drug: Placebo | Number of Adverse Events Change From Baseline in Volumetric Magnetic Resonance Imaging (MRI) Change in Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL) Comparison of the Response to Treatment of Resveratrol Based on ApoE Genotype | Phase 2 |
| 15 | NCT00040378 (https://clinicaltrials.gov/show/NCT00040378, accessed on 20 December 2021) | AD | Drug: alphatocopherol Drug: Selenium Drug: Placebo replacement for vitamin E Drug: Placebo replacement for Selenium | Incidence of dementia (including Alzheimer’s disease) | |
| 16 | NCT00235716 (https://clinicaltrials.gov/show/NCT00235716, accessed on 20 December 2021) | AD | Drug: dl-alpha-tocopherol Drug: Memantine Drug: Placebo | Alzheimer’s Disease Cooperative Study/Activities of Daily Living (ADCS/ADL) Inventory Change From Baseline Mini-Mental State Examination Change From Baseline Alzheimer’s Disease Assessment Scale—Cognitive (ADAS-cog) Change From Baseline Neuropsychiatric Inventory Change From Baseline Caregiver Activity Survey Change From Baseline Dependence Scale: Time to Event Analysis (Increase of One Dependence Level) | Phase 3 |
| 17 | NCT01716637 (https://clinicaltrials.gov/show/NCT01716637, accessed on 20 December 2021) | AD | Biological: Etanercept Dietary Supplement: Curcum.Luteol.Theaflav.Lip.Acid, FishOil, Quercet., Resveratr. | The difference in effects of treatment for 6 weeks with etanercept + nutritional supplements versus nutritional supplements alone on the Mini-Mental Status Examination (MMSE) score; The difference in the effects of treatment for six weeks with etanercept + nutritional supplements versus nutritional supplements alone on the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-cog) score; The difference in the effects of treatment for six weeks with etanercept + nutritional supplements versus nutritional supplements alone on the Montreal Cognitive Assessment (MoCA) score | Phase 1 |
| 18 | NCT00692510 (https://clinicaltrials.gov/show/NCT00692510, accessed on 20 December 2021) | AD | Drug: AZD3480 Drug: Placebo Drug: Cocktail mix (Caffeine, Bupropion, Rosiglitazone, Omeprazole, Midazolam, Bilirubin) | PK variables Safety variables (adverse events, blood pressure, pulse, safety lab) | Phase 1 |
| 19 | NCT01594346 (https://clinicaltrials.gov/show/NCT01594346, accessed on 20 December 2021) | AD DS | Drug: Alpha-Tocopherol Drug: Sugar Pill | The Brief Praxis Test The Fuld Object Memory Test New Dot Test Orientation Test Vocabulary Test Behavior and Function Clinical Global Impression Incident Dementia | Phase 3 |
| 20 | NCT01780974 (https://clinicaltrials.gov/show/NCT01780974, accessed on 20 December 2021) | Treated Hypertension | Drug: Lipoic Acid plus Omega-3 Fatty Acids Drug: Placebo | Trails Making Test Part B (Executive Function) White Matter Hyperintensity Volume (Brain MRI) | Phase 1 Phase 2 |
| 21 | NCT01699711 (https://clinicaltrials.gov/show/NCT01699711, accessed on 20 December 2021) | DS | Dietary Supplement: Epigallocatechin-3-gallate (EGCG) | Change in Cognitive Evaluation and Amyloidosis Biomarker Treatment compliance Change in Biomarkers of lipid oxidation and DYRK1A activity biomarkers COMT val158met genetic polymorphism (catechol methyl transferase) (Taqman) Change in AST (SGOT-serum glutamic oxaloacetic transaminase-) and ALT (SGPT-Serum Glutamic Pyruvate Transaminase-) (Pentra Autoanalyzer, and ELISA Mercodia for LDLox) Change in Body Composition by electrical impedance (TANITA-MC-180) Changes in Neurophysiology | Phase 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pritam, P.; Deka, R.; Bhardwaj, A.; Srivastava, R.; Kumar, D.; Jha, A.K.; Jha, N.K.; Villa, C.; Jha, S.K. Antioxidants in Alzheimer’s Disease: Current Therapeutic Significance and Future Prospects. Biology 2022, 11, 212. https://0-doi-org.brum.beds.ac.uk/10.3390/biology11020212
Pritam P, Deka R, Bhardwaj A, Srivastava R, Kumar D, Jha AK, Jha NK, Villa C, Jha SK. Antioxidants in Alzheimer’s Disease: Current Therapeutic Significance and Future Prospects. Biology. 2022; 11(2):212. https://0-doi-org.brum.beds.ac.uk/10.3390/biology11020212
Chicago/Turabian StylePritam, Pingal, Rahul Deka, Anuradha Bhardwaj, Rashi Srivastava, Dhruv Kumar, Abhimanyu Kumar Jha, Niraj Kumar Jha, Chiara Villa, and Saurabh Kumar Jha. 2022. "Antioxidants in Alzheimer’s Disease: Current Therapeutic Significance and Future Prospects" Biology 11, no. 2: 212. https://0-doi-org.brum.beds.ac.uk/10.3390/biology11020212