Photobiomodulation Therapy in Oral Mucositis and Potentially Malignant Oral Lesions: A Therapy Towards the Future
Abstract
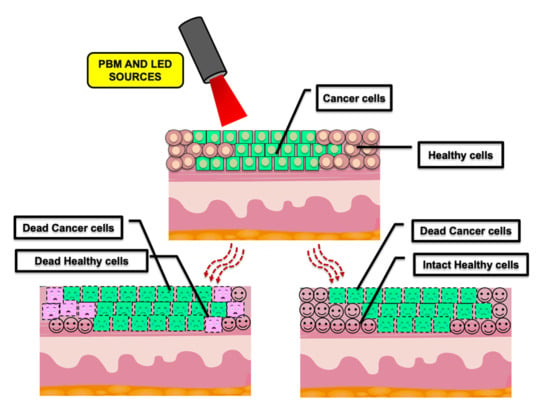
:1. Introduction
1.1. Photobiomodulation Mechanism of Action
- (1)
- How can PBMT be improved to identify the dose- and time-dependent conditions, and the laser therapeutic protocol, for down-regulating the cellular proliferation of suspected lesions and achieving optimal outcomes?
- (2)
- What is the effect of PBMT on tumour growth?
- (3)
- How can PBMT be a useful modality in enhancing a patient’s clinical outcome and improving their quality of life (QoL)?
- (4)
- How can PBMT be useful as a monotherapy, or as an adjunctive modality, in treating potential malignant lesions?
- (5)
- What are the benefits in, and necessary precautions for, utilising PBMT in potentially malignant oral lesions?
1.2. PBM-Influencing Factors for Treatment Optimisation
1.3. PBMT and Cancer
2. PBMT, Dose-Dependency and Its Correlation with Proliferation Rate in Head and Neck Squamous Cell Carcinoma (HNSCC)
Data Extracted from In Vitro Molecular and In Vivo Animal Studies
3. What Are the Significant Benefits of PBMT for H&N Oncology Patients?
OM Induced by Cancer Therapies (RT-CT)
Data Extracted from Clinical Studies
4. Benefits of PBMT in Potentially Malignant Oral Lesions Management
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karu, T.I. Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life 2010, 62, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Capaldi, R.A.; Malatesta, F.; Darley-Usmar, V.M. Structure of cytochrome c oxidase. Biochem. Biophys. Acta 1983, 726, 135–148. [Google Scholar] [CrossRef]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.Y.; Carroll, J.D.; Hamblin, M.R. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Freitas, L.F.; Hamblin, M.R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 1–37. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.Y.; Sharma, S.K.; Carroll, J.D.; Hamblin, M.R. Biphasic dose response in low level light therapy-an update. Dose Response 2011, 9, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I. Photobiological fundamentals of low power laser therapy. IEEE J. Quantum Electron. 1987, 23, 1703–1717. [Google Scholar] [CrossRef]
- Ansari, M.A.; Massudi, R.; Hejazi, M. Experimental and numerical study on simultaneous effects of scattering and absorption on fluorescence spectroscopy of a breast phantom. Opt. Laser Technol. 2009, 41, 746–750. [Google Scholar] [CrossRef]
- Wang, L.; Jacques, S.L.; Zheng, L. MCML-Monte Carlo modeling of light transport in multi-layered tissues. Comput. Methods Programs Biomed. 1995, 47, 131–146. [Google Scholar] [CrossRef]
- Arany, P.R. Craniofacial healing with photobiomodulation therapy: Insights and current challenges. J. Dent. Res. 2016, 95, 1–8. [Google Scholar] [CrossRef]
- Hanna, R.; Agas, D.; Benedicenti, S.; Laus, F.; Cuteri, V.; Lacava, G.; Sabbieti, M.G.; Amaroli, A. A comparative study between the effectiveness of 980 nm photobiomodulation delivered by hand-piece with gaussian vs. flat-top profiles on osteoblasts maturation. Front. Endocrinol. 2019, 10, 92. [Google Scholar] [CrossRef] [Green Version]
- Arany, P.R.; Cho, A.; Hunt, T.D.; Sidhu, G.; Shin, K.; Hahm, E.; Huang, G.X.; Weaver, J.; Chen, A.C.; Padwa, B.L.; et al. Photoactivation of endogenous latent transforming growth factor-beta1 directs dental stem cell differentiation for regeneration. Sci. Transl. Med. 2014, 6, 238–269. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Huang, Y.Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Photobiomodulation of human adipose-derived stem cells using 810 nm and 980 nm lasers operates via different mechanisms of action. Biochim. Biophys. Acta 2016, 1861, 441–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, M.; Guida, G.; Perlino, E.; Marra, E.; Quagliariello, E. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem. Biophys. Res. Commun. 1989, 163, 1428–1434. [Google Scholar] [CrossRef]
- Sonis, S.T.; Hashemi, S.; Epstein, J.B.; Nair, R.G.; Raber-Durlacher, J.E. Could the biological robustness of low level laser therapy (photobiomodulation) impact its use in the management of mucositis in head and neck cancer patients. Oral Oncol. 2016, 54, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Antunes, H.S.; Herchenhorn, D.; Small, I.A.; Araujo, C.M.M.; Viegas, C.M.P.; de Assis, R.G.; Dias, F.L.; Ferreira, C.G. Long-term survival of a randomized phase III trial of head and neck cancer patients receiving concurrent chemoradiation therapy with or with- out low-level laser therapy (LLLT) to prevent oral mucositis. Oral Oncol. 2017, 71, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Brandao, T.B.; Morais-Faria, K.; Ribeiro, A.C.P.; Rivera, C.; Salvajoli, J.V.; Lopes, M.A.; Epstein, J.B.; Arany, P.R.; de Castro, G., Jr.; Migliorati, C.A.; et al. Locally advanced oral squamous cell carcinoma patients treated with photobiomodulation for prevention of oral mucositis: Retrospective outcomes and safety analyses. Support. Care Cancer 2018, 26, 2417–2423. [Google Scholar] [CrossRef]
- Lopes, N.N.; Plapler, H.; Lalla, R.V.; Chavantes, M.C.; Yoshimura, E.M.; da Silva, M.A.; Alves, M.T. Effects of low-level laser therapy on collagen expression and neutrophil infiltrate in 5-fluorouracil-induced oral mucositis in hamsters. Lasers Surg. Med. 2010, 42, 546–552. [Google Scholar] [CrossRef]
- Silva, G.B.; Sacono, N.T.; Othon-Leite, A.F.; Mendonça, E.F.; Arantes, A.M.; Bariani, C.; Duarte, L.G.; Abreu, M.H.; Queiroz-Junior, C.M.; Silva, T.A.; et al. Effect of low-level laser therapy on inflammatory mediator release during chemotherapy-induced oral mucositis: A randomized preliminary study. Lasers Med. Sci. 2014, 30, 117–126. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Giudice, F.S.; Corrêa, L.; Pinto, D.S.; Hamblin, M.R.; de Sousa, S.C. Low-level laser therapy can produce increased aggressiveness of dysplastic and oral cancer cell lines by modulation of Akt/mTOR signaling pathway. J. Biophotonics 2013, 6, 839–847. [Google Scholar] [CrossRef]
- Zecha, J.A.; Raber-Durlacher, J.E.; Nair, R.G.; Epstein, J.B.; Elad, S.; Hamblin, M.R.; Barasch, A.; Migliorati, C.A.; Milstein, D.M.; Genot, M.T.; et al. Low-level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: Part 2: Proposed applications and treatment protocols. Support. Care Cancer 2016, 24, 2793–2805. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, T.E.; Morais, M.O.; Oton-Leite, A.F.; Valadares, M.C.; Batista, A.C.; Freitas, N.M.A.; Leles, C.R.; Mendonça, E.F. Effect of photobiomodulation on the severity of oral mucositis and molecular changes in head and neck cancer patients undergoing radiotherapy: A study protocol for a cost-effectiveness randomized clinical trial. Trials 2019, 20, 97. [Google Scholar]
- Schartinger, V.H.; Galvan, O.; Riechelmann, H.; Dudás, J. Differential responses of fibroblasts, non-neoplastic epithelial cells, and oral carcinoma cells to low-level laser therapy. Support. Care Cancer 2012, 20, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Robijns, J.; Censabella, S.; Bulens, P.; Maes, A.; Mebis, J. The use of low-level light therapy in supportive care for patients with breast cancer: Review of the literature. Lasers Med. Sci. 2017, 32, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Bamps, M.; Dok, R.; Nuyts, S. Low-level laser therapy stimulates proliferation in head and neck squamous cell carcinoma cells. Front. Oncol. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- González-Arriagada, W.A.; Ramos, L.M.A.; Andrade, M.A.C.; Lopes, M.A. Efficacy of low- level laser therapy as an auxiliary tool for management of acute side effects of head and neck radiotherapy. J. Cosmet. Laser Ther. 2018, 20, 117–122. [Google Scholar] [CrossRef]
- Schalch, T.D.; Fernandes, M.H.; Rodrigues, M.F.; Guimarães, D.M.; Nunes, F.D.; Rodrigues, J.C.; Garcia, M.P.; Ferrari, R.A.M.; Bussadori, S.K.; Fernandes, K. Photobiomodulation is associated with a decrease in cell viability and migration in oral squamous cell carcinoma. Lasers Med. Sci. 2019, 34, 629–636. [Google Scholar] [CrossRef]
- Moore, P.; Ridgway, T.D.; Higbee, R.G.; Howard, E.W.; Lucroy, M.D. Effect of wavelength on low intensity laser irradiation stimulated cell proliferation in vitro. Lasers Surg. Med. 2005, 36, 8–12. [Google Scholar] [CrossRef]
- El Batanouny, M.; Korraa, S.; Fekry, O. Mitogenic potential inducible by he: Ne laser in human lymphocytes in vitro. J. Photochem. Photobiol. B Biol. 2002, 68, 1–7. [Google Scholar] [CrossRef]
- Pinheiro, A.L.B.; Do Nascimento, S.C.; Vieira, A.L.B.; Rolim, A.B.; Da Silva, P.S.; Brugnera, A., Jr. Does LLLT stimulate laryngeal carcinoma cells? An in vitro study. Braz. Dent. J. 2002, 13, 109–112. [Google Scholar] [CrossRef] [Green Version]
- De Sanctis, V.; Bossi, P.; Sanguineti, G. Mucositis in head and neck cancer patients treated with radiotherapy and systemic therapies: Literature review and consensus statements. Crit. Rev. Hematol. Oncol. 2016, 100, 147–166. [Google Scholar] [CrossRef]
- Henriques, A.C.; Ginani, F.; Oliveira, R.M. Low-level laser therapy promotes proliferation and invasion of oral squamous cell carcinoma cells. Lasers Med. Sci. 2014, 29, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Tang, E.; Arany, P. Molecular pathway of near- infrared laser phototoxicity involves ATF-4 orchestrated ER stress. Sci. Rep. 2015, 5, 10581. [Google Scholar] [CrossRef]
- Myakishev-Rempel, M.; Stadler, I.; Brondon, P.; Axe, D.R.; Friedman, M.; Nardia, F.B.; Lanzafame, R. A preliminary study of the safety of red light phototherapy of tissues harboring cancer. Photomed. Laser Surg. 2012, 30, 551–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De C. Monteiro, J.S.; Pinheiro, A.N.B.; de Oliveira, S.C.; Aciole, G.T.; Sousa, J.A.; Canguss, M.C.; Dos Santos, J.N. Influence of laser phototherapy (660 nm) on the outcome of oral chemical carcinogenesis on the hamster cheek pouch model: Histological study. Photomed. Laser Surg. 2011, 29, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Guedes, C.D.; de Freitas Filho, S.A.; Faria, P.R.; Loyola, A.M.; Sabino-Silva, R.; Cardoso, S.V. Variation of energy in photobiomodulation for the control of radiotherapy-induced oral mucositis: A clinical study in head and neck cancer patients. Int. J. Dent. 2018, 2018, 4579279. [Google Scholar] [CrossRef]
- Takemoto, M.M.; Garcez, A.S.; Sperandio, M. High energy density LED-based photobiomodulation inhibits squamous cell carcinoma progression in co-cultures in vitro. J. Photochem. Photobiol. B 2019, 199, 111592. [Google Scholar] [CrossRef]
- Ottaviani, G.; Gobbo, M.; Sturnega, M.; Martinelli, V.; Mano, M.; Zanconati, F.; Bussani, R.; Perinetti, G.; Long, C.S.; Di Lenarda, R.; et al. Effect of class IV laser therapy on chemotherapy-induced oral mucositis: A clinical and experimental study. Am. J. Pathol. 2013, 183, 1747–1757. [Google Scholar] [CrossRef]
- Gobbo, M.; Verzegnassi, F.; Ronfani, L.; Zanon, D.; Melchionda, F.; Bagattoni, S.; Majorana, A.; Bardellini, E.; Mura, R.; Piras, A.; et al. Multicenter randomized, double-blind controlled trial to evaluate the efficacy of laser therapy for the treatment of severe oral mucositis induced by chemotherapy in children: laMPO RCT. Pediatr. Blood Cancer 2018. [Google Scholar] [CrossRef] [Green Version]
- Bensadoun, R.; Epstein, J.B. Photobiomodulation safety in cancer patients: In vivo data. Support. Care Cancer 2020, 28, 3003–3006. [Google Scholar] [CrossRef]
- Lalla, J. Evidence-based management of oral mucositis. JCO Oncol. Pract. 2020, 16, 111–112. [Google Scholar] [CrossRef]
- Multinational Association of Supportive Care in Cancer: MASCC/ISOO Mucositis Clinical Practice Guidelines, 2019. Available online: https://www.mascc.org/mucositis-guidelines (accessed on 10 July 2020).
- Bjordal, J.M.; Bensadoun, R.; Tunèr, J.; Frigo, L.; Gjerde, K.; Lopes- Martins, R.A. A systematic review with meta-analysis of the effect of low-level laser therapy (LLLT) in cancer therapy-induced oral mucositis. Support. Care Cancer 2011, 19, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, S.; Zamperlini-Netto, G.; Beyene, J.; Treister, N.S.; Sung, L. Effect of prophylactic low-level laser therapy on oral mucositis: A systematic review and meta-analysis. PLoS ONE 2014, 9, e2107418. [Google Scholar] [CrossRef] [PubMed]
- Oton-Leite, A.F.; de Castro, A.C.C.; Morais, M.O.; Pinezi, J.C.; Leles, C.R.; Mendonça, E.F. Effect of intraoral low-level laser therapy on quality of life of patients with head and neck cancer undergoing radiotherapy. Head Neck 2012, 34, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.N.; Plapler, H.; Chavantes, M.C.; Lalla, R.V.; Yoshimura, E.M.; Alves, M.T. Cyclooxygenase-2 and vascular endothelial growth factor expression in 5- fluorouracil-induced oral mucositis in hamsters: Evaluation of two low- intensity laser protocols. Support. Care Cancer 2009, 17, 1409–1415. [Google Scholar] [CrossRef]
- Migliorati, C.; Hewson, I.; Lalla, R.V.; Antunes, H.S.; Estilo, C.L.; Hodgson, B.; Lopes, N.N.; Scubert, M.M.; Bowen, J.; Elad, S. Systematic review of laser and other light therapy for the management of oral mucositis in cancer patients. Support. Care Cancer 2013, 21, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Bensadoun, R.J.; Nair, R.G. Low-level laser therapy in the prevention and treatment of cancer therapy induced mucositis: State of the art based on literature review and meta-analysis. Curr. Opin. Oncol. 2012, 24, 363–370. [Google Scholar] [CrossRef]
- Anschau, F.; Webster, J.; Capra, M.E.Z. Efficacy of low-level laser for treatment of cancer oral mucositis: A systematic review and meta-analysis. Lasers Med. Sci. 2019, 34, 1053–1062. [Google Scholar] [CrossRef]
- Carvalho, P.A.; Jaguar, G.C.; Pellizzon, A.C.; Prado, J.D.; Lopes, R.N.; Alves, F.A. Evaluation of low-level laser therapy in the prevention and treatment of radiation induced mucositis: A double-blind randomized study in head and neck cancer patients. Oral Oncol. 2011, 47, 1176–1181. [Google Scholar] [CrossRef] [Green Version]
- Arora, H.; Pai, K.M.; Maiya, A.; Vidyasagar, M.S.; Rajeev, A. Efficacy of He-Ne Laser in the prevention and treatment of radiotherapy-induced oral mucositis in oral cancer patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 180–186. [Google Scholar] [CrossRef]
- Chermetz, M.; Gobbo, M.; Ronfani, L.; Ottaviani, G.; Zanazzo, G.A.; Verzegnassi, F.; Treister, N.S.; Di Lenarda, R.; Biasotto, M.; Zacchigna, S. Class IV laser therapy as treatment for chemotherapy- induced oral mucositis in onco-haematological paediatric patients: A prospective study. Int. J. Paediatr. Dent. 2014, 24, 441–449. [Google Scholar] [CrossRef]
- Amadori, F.; Bardellini, E.; Conti, G.; Pedrini, N.; Schumacher, R.F.; Majorana, A. Low-level laser therapy for treatment of chemotherapy-induced oral mucositis in childhood: A randomized double-blind controlled study. Lasers Med. Sci. 2016, 31, 1231–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobbo, M.; Ottaviani, G.; Perinetti, G.; Ciriello, F.; Beorchia, A.; Giacca, M.; Di Lenarda, R.; Rupel, K.; Tirelli, G.; Zacchigna, S.; et al. Evaluation of nutritional status in head and neck radio- treated patients affected by oral mucositis: Efficacy of class IV laser therapy. Support. Care Cancer 2014, 22, 1851–1856. [Google Scholar] [CrossRef]
- Zecha, J.A.; Raber-Durlacher, J.E.; Nair, R.G.; Epstein, J.B.; Sonis, S.T.; Elad, S.; Hamblin, M.R.; Barasch, A.; Migliorati, C.A.; Milstein, D.M.; et al. Low-level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: Part 1: Mechanisms of action, dosimetric, and safety considerations. Support. Care Cancer 2016, 24, 2781–2792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zadik, Y.; Arany, P.R.; Fregnani, E.R.; Bossi, P.; Antunes, H.S.; Bensadoun, R.J.; Gueiros, L.A.; Majorana, A.; Nair, R.G.; Ranna, V.; et al. Systematic review of photobiomodulation for the management of oral mucositis in cancer patients and clinical practice guidelines. Support. Care Cancer 2019, 27, 3969–3983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, A.C.; Campos, L.; Brandao, T.B.; Cristofaro, M.; Eduardo, F.P.; Luiz, A.C.; Marques, M.M.; Eduardo, C.; Simões, A. Chemotherapy-induced oral mucositis: Effect of LED and laser phototherapy treatment protocols. Photomed. Laser Surg. 2014, 32, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.B.; Eduardo, F.P.; Zezell, D.M.; Bezinelli, L.M.; Shitara, P.P.; Correa, L. Effect of irradiation with red and infrared laser in the treatment of oral mucositis: A pilot study with patients undergoing chemotherapy with 5-FU. Lasers Med. Sci. 2012, 27, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, M.; Ottaviani, G.; Rupel, K.; Ciriello, F.; Beorchia, A.; Di Lenarda, R.; Zacchigna, S.; Biasotto, M. Same strategy for pitfalls of radiotherapy in different anatomical districts. Lasers Med. Sci. 2016, 31, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Trotti, A.; Bellm, L.A.; Epstein, J.B.; Frame, D.; Fuchs, H.J.; Gwede, C.K.; Komaroff, E.; Nalysnyk, L.; Zilberberg, M.D. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother. Oncol. 2003, 66, 253–262. [Google Scholar] [CrossRef]
- Ang, K.K.; Zhang, Q.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Sherman, E.J.; Weber, R.S.; Galvin, J.M.; Bonner, J.A.; Harris, J.; El-Naggar, A.K.; et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J. Clin. Oncol. 2014, 32, 2940–2950. [Google Scholar] [CrossRef]
- Brennan, M.T.; Spijkervet, F.K.; Elting, L.S. Systematic reviews and guidelines for oral complications of cancer therapies: Current challenges and future opportunities. Support. Care Cancer 2010, 18, 977–978. [Google Scholar] [CrossRef]
- El Mobadder, M.; Farhat, F.; El Mobadder, W.; Nammour, S. Photobiomodulation therapy in the treatment of oral mucositis, dysphagia, oral dryness, taste alteration, and burning mouth sensation due to cancer therapy: A case series. Int. J. Environ. Res. Public Health 2019, 16, 4505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortazavi, H.; Baharvand, M.; Mehdipour, M. Oral potentially malignant disorders: An overview of more than 20 entities. J. Dent. Res. Clin. Dent. Prospect. 2014, 8, 6–14. [Google Scholar]
- Fisher, S.E.; Frame, J.W. The effects of the carbon dioxide surgical laser on oral tissues. Br. J. Oral Maxillofac. Surg. 1984, 22, 414–425. [Google Scholar] [CrossRef]
- Wlodawsky, R.N.; Strauss, R.A. Intraoral laser surgery. Oral Maxillofac. Surg. Clin. N. Am. 2004, 16, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, K.S.; Raj, A.; Talukdar, J.S.; Kundu, M.; Arun, P.D.; Vijayan, S. Lasers in the management of oral pre-malignant lesions. Int. J. Sci. Stud. 2015, 3, 183–186. [Google Scholar]
- Jerjes, W.; Upile, T.; Hamdoon, Z.; Al-Khawalde, M.; Morcos, M.; Mosse, C.A.; Hopper, C. CO2 laser of oral dysplasia: Clinicopathological features of recurrence and malignant transformation. Lasers Med. Sci. 2012, 27, 169–179. [Google Scholar] [CrossRef]
- Arnaoutakis, D.; Bishop, J.; Westra, W.; Califano, J.A. Recurrence patterns and management of oral cavity premalignant lesions. Oral Oncol. 2013, 49, 814–817. [Google Scholar] [CrossRef]
- García-Pola, M.J.; González-Álvarez, L.; Garcia-Martin, G.M. Treatment of oral lichen planus. Systematic review and therapeutic guide. Med. Clin. 2017, 149, 351–362. [Google Scholar] [CrossRef]
- Jajarm, H.H.; Falaki, F.; Mahdavi, O. A comparative pilot study of low intensity laser versus topical corticosteroids in the treatment of erosive-atrophic oral lichen planus. Photomed. Laser Surg. 2011, 29, 421–425. [Google Scholar] [CrossRef]
- Cafaro, A.; Arduino, P.G.; Massolini, G.; Romagnoli, E.; Broccoletti, R. Clinical evaluation of the efficiency of low-level laser therapy for oral lichen planus: A prospective case series. Lasers Med. Sci. 2014, 29, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Al-Maweri, S.A.; Kalakonda, B.; Al-Soneidar, W.A.; Al-Shamiri, H.M.; Alakhali, M.S.; Alaizari, N. Efficacy of low-level laser therapy in management of symptomatic oral lichen planus: A systematic review. Lasers Med. Sci. 2017, 32, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Akram, Z.; Abduljabbar, T.; Vohra, F.; Javed, F. Efficacy of low-level laser therapy compared to steroid therapy in the treatment of oral lichen planus: A systematic review. J. Oral Pathol. Med. 2018, 47, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Kazancioglu, H.O.; Erisen, M. Comparison of low level laser therapy versus ozone therapy in the treatmen of oral lichen planus. Ann. Detmatol. 2015, 27, 485–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Biological | Clinical | |||
|---|---|---|---|---|
| Technical | Molecular | Cellular/tissue | Device | Delivery |
| Scale Kinetics Background | Target Regulation | Context | Wavelength Polarisation Coherence Fluence Irradiance Time Pulsing | Clinical treatment site Delivery method (fixed/moving) Depth of target Dose repetition Biomarkers Off-target (bystander) effects |
| Wavelength (nm) | Power Output (mW) | Spot Size (cm2) | Energy per Point (J) | Maximum Irradiation per Point (s) | Maximum Number of Irradiation Points | Minimal Sessions per Week During Cancer Treatment | Maximum Number of Days to Start PBMT, before Cancer Therapy (for Prevention) |
|---|---|---|---|---|---|---|---|
| Red (633–685) | 10–60 | 0.1–1.00 | 3 | 30 | 6 | 3 | 7 |
| Infrared (780–830) | 50–11 | 0.1–0.5 | 6 | 30 | 6 | 3 | 7 |
| Irradiation Site | Trigger Points | PBMT Protocol |
|---|---|---|
| Intra-oral | Bilaterally, four points to soft palate and onto oropharynx. | 635 nm, 3 J/cm2, 10 s exposure time on each point, 100 mW, continuous and contact mode. |
| Extra-oral | Lateral and ventral surfaces of the pharynx and larynx. Midline and lateral aspects of neck |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanna, R.; Dalvi, S.; Benedicenti, S.; Amaroli, A.; Sălăgean, T.; Pop, I.D.; Todea, D.; Bordea, I.R. Photobiomodulation Therapy in Oral Mucositis and Potentially Malignant Oral Lesions: A Therapy Towards the Future. Cancers 2020, 12, 1949. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12071949
Hanna R, Dalvi S, Benedicenti S, Amaroli A, Sălăgean T, Pop ID, Todea D, Bordea IR. Photobiomodulation Therapy in Oral Mucositis and Potentially Malignant Oral Lesions: A Therapy Towards the Future. Cancers. 2020; 12(7):1949. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12071949
Chicago/Turabian StyleHanna, Reem, Snehal Dalvi, Stefano Benedicenti, Andrea Amaroli, Tudor Sălăgean, Ioana Delia Pop, Doina Todea, and Ioana Roxana Bordea. 2020. "Photobiomodulation Therapy in Oral Mucositis and Potentially Malignant Oral Lesions: A Therapy Towards the Future" Cancers 12, no. 7: 1949. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12071949