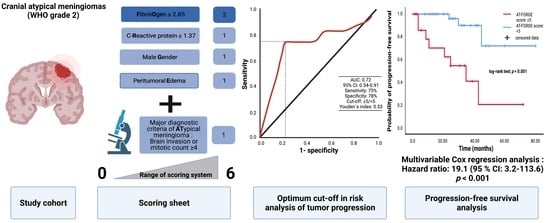
Combining FORGE Score and Histopathological Diagnostic Criteria of Atypical Meningioma Enables Risk Stratification of Tumor Progression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Characteristics
2.2. Data Recording
2.3. Histopathology
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Tumor Localization, Type of Treatment and Histopathological Characteristics
3.3. Value of Major Diagnostic Criteria for Atypical Meningioma on the Prediction of Recurrence
3.4. Comparison of Low vs. High AT-FORGE Score Groups
3.5. AT-FORGE Score in the Prediction of Progression-Free Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Hao, S.; Wu, Z.; Wang, L.; Jia, G.; Zhang, L.; Zhang, J. Treatment Response and Prognosis After Recurrence of Atypical Meningiomas. World Neurosurg. 2015, 84, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Durand, A.; Labrousse, F.; Jouvet, A.; Bauchet, L.; Kalamaridès, M.; Menei, P.; Deruty, R.; Moreau, J.J.; Fèvre-Montange, M.; Guyotat, J. WHO grade II and III meningiomas: A study of prognostic factors. J. Neuro Oncol. 2009, 95, 367–375. [Google Scholar] [CrossRef]
- Keric, N.; Kalasauskas, D.; Freyschlag, C.F.; Gempt, J.; Misch, M.; Poplawski, A.; Lange, N.; Ayyad, A.; Thomé, C.; Vajkoczy, P.; et al. Impact of postoperative radiotherapy on recurrence of primary intracranial atypical meningiomas. J. Neuro Oncol. 2020, 146, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Shakir, S.I.; Souhami, L.; Petrecca, K.; Mansure, J.J.; Singh, K.; Panet-Raymond, V.; Shenouda, G.; Al-Odaini, A.A.; Abdulkarim, B.; Guiot, M.-C. Prognostic factors for progression in atypical meningioma. J. Neurosurg. 2018, 129, 1240–1248. [Google Scholar] [CrossRef]
- Hwang, W.L.; Marciscano, A.E.; Niemierko, A.; Kim, D.W.; Stemmer-Rachamimov, A.O.; Curry, W.T.; Loeffler, J.S.; Oh, K.S.; Shih, H.A.; Larvie, M. Imaging and extent of surgical resection predict risk of meningioma recurrence better than WHO histopathological grade. Neuro Oncol. 2016, 18, 863–872. [Google Scholar] [CrossRef]
- Kalasauskas, D.; Kronfeld, A.; Renovanz, M.; Kurz, E.; Leukel, P.; Krenzlin, H.; Brockmann, M.A.; Sommer, C.J.; Ringel, F.; Keric, N. Identification of High-Risk Atypical Meningiomas According to Semantic and Radiomic Features. Cancers 2020, 12, 2942. [Google Scholar] [CrossRef]
- Barresi, V.; Lionti, S.; Caliri, S.; Caffo, M. Histopathological features to define atypical meningioma: What does really matter for prognosis? Brain Tumor Pathol. 2018, 35, 168–180. [Google Scholar] [CrossRef]
- Barrett, O.C.; Hackney, J.R.; McDonald, A.M.; Willey, C.D.; Bredel, M.; Fiveash, J.B. Pathologic Predictors of Local Recurrence in Atypical Meningiomas Following Gross Total Resection. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 453–459. [Google Scholar] [CrossRef]
- Chen, X.; Wang, G.; Zhang, J.; Zhang, G.; Lin, Y.; Lin, Z.; Gu, J.; Kang, D.; Ding, C. A Novel Scoring System Based on Pre-operative Routine Blood Test in Predicting Prognosis of Atypical Meningioma. Front Oncol. 2020, 10, 1705. [Google Scholar] [CrossRef]
- Vranic, A.; Popovic, M.; Cör, A.; Prestor, B.; Pizem, J. Mitotic Count, Brain Invasion, and Location Are Independent Predictors of Recurrence-Free Survival in Primary Atypical and Malignant Meningiomas: A Study of 86 Patients. Neurosurgery 2010, 67, 1124–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oya, S.; Kawai, K.; Nakatomi, H.; Saito, N. Significance of Simpson grading system in modern meningioma surgery: Integration of the grade with MIB-1 labeling index as a key to predict the recurrence of WHO Grade I meningiomas. J. Neurosurg. 2012, 117, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Kim, K.H.; Lee, E.H.; Lee, Y.M.; Lee, S.-H.; Kim, H.D.; Kim, Y.Z. Results of immunohistochemical staining for cell cycle regulators predict the recurrence of atypical meningiomas. J. Neurosurg. 2014, 121, 1189–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.; Song, S.Y.; Jiang, J.B.; Wang, T.J.; Yan, C.X. The prognostic role of Ki-67/MIB-1 in meningioma: A systematic review with meta-analysis. Medicine 2020, 99, e18644, discussion 44. [Google Scholar] [CrossRef] [PubMed]
- Roser, F.; Samii, M.; Ostertag, H.; Bellinzona, M. The Ki-67 proliferation antigen in meningiomas. Experience in 600 cases. Acta Neurochir. 2004, 146, 37. [Google Scholar] [CrossRef]
- Wach, J.; Lampmann, T.; Güresir, Á.; Schuss, P.; Vatter, H.; Herrlinger, U.; Becker, A.; Hölzel, M.; Toma, M.; Güresir, E. FORGE: A Novel Scoring System to Predict the MIB-1 Labeling Index in Intracranial Meningiomas. Cancers 2021, 13, 3643. [Google Scholar] [CrossRef]
- Antinheimo, J.; Haapasalo, H.; Haltia, M.; Tatagiba, M.; Thomas, S.; Brandis, A.; Sainio, M.; Carpen, O.; Samii, M.; Jääskeläinen, J. Proliferation potential and histological features in neurofibromatosis 2-associated and sporadic meningiomas. J. Neurosurg. 1997, 87, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Roser, F.; Nakamura, M.; Bellinzona, M.; Ritz, R.; Ostertag, H.; Tatagiba, M.S. Proliferation potential of spinal meningiomas. Eur. Spine J. 2006, 15, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jenkinson, M.D.; Sallabanda, K.; Houdart, E.; von Deimling, A.; Stavrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016, 17, e383–e391. [Google Scholar] [CrossRef] [Green Version]
- Henson, J.; Ulmer, S.; Harris, G. Brain Tumor Imaging in Clinical Trials. Am. J. Neuroradiol. 2008, 29, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.-W.; Kim, M.-S.; Kim, S.-W.; Chang, C.-H.; Kim, O.-L. Peritumoral Brain Edema in Meningiomas: Correlation of Radiologic and Pathologic Features. J. Korean Neurosurg. Soc. 2011, 49, 26–30. [Google Scholar] [CrossRef]
- Wach, J.; Apallas, S.; Schneider, M.; Güresir, A.; Schuss, P.; Herrlinger, U.; Vatter, H.; Güresir, E. Baseline Serum C-Reactive Protein and Plasma Fibrinogen-Based Score in the Prediction of Survival in Glioblastoma. Front. Oncol. 2021, 11, 653614. [Google Scholar] [CrossRef] [PubMed]
- Majores, M.; Schick, V.; Engels, G.; Fassunke, J.; Elger, C.E.; Schramm, J.; Blümcke, I.; Becker, A.J. Mutational and immunohistochemical analysis of ezrin-, radixin-, moesin (ERM) molecules in epilepsy-associated glioneuronal lesions. Acta Neuropathol. 2005, 110, 537–546. [Google Scholar] [CrossRef]
- Majores, M.; von Lehe, M.; Fassunke, J.; Schramm, J.; Becker, A.J.; Simon, M. Tumor recurrence and malignant progression of gangliogliomas. Cancer 2008, 113, 3355–3363. [Google Scholar] [CrossRef]
- Schneider, M.; Borger, V.; Güresir, Á.; Becker, A.; Vatter, H.; Schuss, P.; Güresir, E. High Mib-1-score correlates with new cranial nerve deficits after surgery for frontal skull base meningioma. Neurosurg. Rev. 2021, 44, 381–387. [Google Scholar] [CrossRef]
- Lemée, J.-M.; Corniola, M.V.; Meling, T.R. Benefits of re-do surgery for recurrent intracranial meningiomas. Sci. Rep. 2020, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its Associated Cutoff Point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef] [Green Version]
- Apra, C.; Peyre, M.; Kalamarides, M. Current treatment options for meningioma. Expert Rev. Neurother. 2018, 18, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Dundamadappa, S.K.; Thangasamy, S.; Flood, T.; Moser, R.; Smith, T.; Cauley, K.; Takhtani, D. Correlation of ap-parent diffusion coefficient with Ki-67 proliferation index in grading meningioma. Am. J. Roentgenol. 2014, 202, 1303–1308. [Google Scholar] [CrossRef]
- Pavelin, S.; Becic, K.; Forempoher, G.; Mrklic, I.; Pogorelic, Z.; Titlic, M.; Andelinovic, S. Expression of Ki-67 and p53 in men-ingiomas. Neoplasma 2013, 60, 480–485. [Google Scholar] [CrossRef] [Green Version]
- Behling, F.; Hempel, J.-M.; Schittenhelm, J. Brain Invasion in Meningioma—A Prognostic Potential Worth Exploring. Cancers 2021, 13, 3259. [Google Scholar] [CrossRef]
- Garcia-Segura, M.E.; Erickson, A.W.; Jairath, R.; Munoz, D.G.; Das, S. Necrosis and Brain Invasion Predict Radio-Resistance and Tumor Recurrence in Atypical Meningioma: A Retrospective Cohort Study. Neurosurgery 2020, 88, E42–E48. [Google Scholar] [CrossRef] [PubMed]
- Nakasu, S.; Nakasu, Y. Prognostic significance of brain invasion in meningiomas: Systematic review and meta-analysis. Brain Tumor Pathol. 2021, 38, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Domingo, R.A.; Tripathi, S.; Vivas-Buitrago, T.; Lu, V.M.; Chaichana, K.L.; Quiñones-Hinojosa, A. Mitotic Index and Pro-gression-Free Survival in Atypical Meningiomas. World Neurosurg. 2020, 142, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Olar, A.; Wani, K.M.; Sulman, E.P.; Mansouri, A.; Zadeh, G.; Wilson, C.D.; Demonte, F.; Fuller, G.; Aldape, K.D. Mitotic Index is an Independent Predictor of Recurrence-Free Survival in Meningioma. Brain Pathol. 2015, 25, 266–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, C.; Nicholas, M.K.; Engelhard, H.; Slavin, K.V.; Koshy, M. An analysis of prognostic factors associated with recurrence in the treatment of atypical meningiomas. Adv. Radiat. Oncol. 2016, 1, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Masalha, W.; Heiland, D.H.; Franco, P.; Delev, D.; Haaker, J.G.; Schnell, O.; Scheiwe, C.; Grauvogel, J. Atypical meningioma: Progression-free survival in 161 cases treated at our institution with surgery versus surgery and radiotherapy. J. Neuro Oncol. 2017, 136, 147–154. [Google Scholar] [CrossRef]
- Domenicucci, M.; Santoro, A.; D’Osvaldo, D.H.; Delfini, R.; Cantore, G.P.; Guidetti, B. Multiple intracranial meningiomas. J. Neurosurg. 1989, 70, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Koech, F.; Orege, J.; Ndiangui, F.; Macharia, B.; Mbaruku, N. Multiple Intracranial Meningiomas: A Review of the Literature and a Case Report. Case Rep. Surg. 2013, 2013, 131962. [Google Scholar] [CrossRef]
- Ramos-Fresnedo, A.; Domingo, R.A.; Vivas-Buitrago, T.; Lundy, L.; Trifiletti, D.M.; Jentoft, M.E.; Desai, A.B.; Quiñones-Hinojosa, A. Multiple meningiomas: Does quantity matter? a population-based survival analysis with underlined age and sex differences. J. Neuro Oncol. 2020, 149, 413–420. [Google Scholar] [CrossRef]
- Wong, L.Y.F.; Leung, R.Y.H.; Ong, K.L.; Cheung, B.M.Y. Plasma levels of fibrinogen and C-reactive protein are related to interleukin-6 gene −572C>G polymorphism in subjects with and without hypertension. J. Hum. Hypertens. 2007, 21, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Todo, T.; Adams, E.F.; Rafferty, B.; Fahlbusch, R.; Dingermann, T.; Werner, H. Secretion of interleukin-6 by human meningioma cells: Possible autocrine inhibitory regulation of neoplastic cell growth. J. Neurosurg. 1994, 81, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Maruo, N.; Morita, I.; Shirao, M.; Murota, S. IL-6 increases endothelial permeability in vitro. Endocrinology 1992, 131, 710–714. [Google Scholar] [CrossRef]
- Saija, A.; Princi, P.; Lanza, M.; Scalese, M.; Aramnejad, E.; De Sarro, A. Systemic cytokine administration can affect blood-brain barrier permeability in the rat. Life Sci. 1995, 56, 775–784. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ahsworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Devaraj, S.; Jialal, I. C-Reactive Protein Polarizes Human Macrophages to an M1 Phenotype and Inhibits Transformation to the M2 Phenotype. Arter. Thromb. Vasc. Biol. 2011, 31, 1397–1402. [Google Scholar] [CrossRef] [Green Version]
- Proctor, D.T.; Huang, J.; Lama, S.; Albakr, A.; Van Marle, G.; Sutherland, G.R. Tumor-associated macrophage infiltration in meningioma. Neuro Oncol. Adv. 2019, 1, vdz018. [Google Scholar] [CrossRef]
- Berhouma, M.; Jacquesson, T.; Jouanneau, E.; Cotton, F. Pathogenesis of peri-tumoral edema in intracranial meningiomas. Neurosurg. Rev. 2019, 42, 59–71. [Google Scholar] [CrossRef]
- Gadient, R.A.; Otten, U.H. Interleukin-6 (IL-6)—A molecule with both beneficial and destructive potentials. Prog. Neurobiol. 1997, 52, 379–390. [Google Scholar] [CrossRef]
- Boyle-Walsh, E.; Hashim, I.A.; Speirs, V.; Fraser, W.D.; White, M.C. Interleukin-6 (IL-6) production and cell growth of cultured human ameningiomas:-interactions with interleukin-1 beta (IL-1 beta) and interleukin-4 (IL-4) in vitro. Neurosci. Lett. 1994, 170, 129–132. [Google Scholar] [CrossRef]
- Jones, T.H.; Justice, S.K.; Timperley, W.R.; Royds, J.A. Effect of interleukin-1 and dexamethasone on interleukin-6 production and growth in human meningiomas. J. Pathol. 1997, 183, 460–468. [Google Scholar] [CrossRef]
- Kane, A.J.; Sughrue, M.E.; Rutkowski, M.J.; Shangari, G.; Fang, S.; McDermott, M.W.; Berger, M.S.; Parsa, A.T. Anatomic location is a risk factor for atypical and malignant meningiomas. Cancer 2011, 117, 1272–1278. [Google Scholar] [CrossRef] [Green Version]
- Escribano Mesa, J.A.; Alonso Morillejo, E.; Parron Carreño, T.; Huete Allut, A.; Narro Donate, J.M.; Mendez Román, P.; Contreras Jiménez, A.; Pedrero García, F.; Masegosa González, J. Risk of Recurrence in Operated Parasagittal Meningiomas: A Logistic Binary Regression Model. World Neurosurg. 2018, 110, e112–e118. [Google Scholar] [CrossRef]
- Mantle, R.E.; Lach, B.; Delgado, M.R.; Baeesa, S.; Bélanger, G. Predicting the probability of meningioma recurrence based on the quantity of peritumoral brain edema on computerized tomography scanning. J. Neurosurg. 1999, 91, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Simis, A.; Pires de Aguiar, P.H.; Leite, C.C.; Santana, P.A., Jr.; Rosemberg, S.; Teixeira, M.J. Peritumoral brain edema in benign meningiomas: Correlation with clinical, radiologic, and surgical factors and possible role on recurrence. Surg. Neurol. 2008, 70, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Ide, M.; Jimbo, M.; Yamamoto, M.; Umebara, Y.; Hagiwara, S.; Kubo, O. MIB-1 staining index and peritumoral brain edema of meningiomas. Cancer 1996, 78, 133–143. [Google Scholar] [CrossRef]
- Rogers, L.; Barani, I.; Chamberlain, M.; Kaley, T.; McDermott, M.; Raizer, J.; Schiff, D.; Weber, D.C.; Wen, P.Y.; Vogelbaum, M.A. Meningiomas: Knowledge base, treatment outcomes, and uncertainties. A RANO review. J. Neurosurg. 2015, 122, 4–23. [Google Scholar] [CrossRef] [Green Version]
- Hemmati, S.M.; Ghadjar, P.; Grün, A.; Badakhshi, H.; Zschaeck, S.; Senger, C.; Acker, G.; Misch, M.; Budach, V.; Kaul, D. Adjuvant radiotherapy improves progression-free survival in intracranial atypical meningioma. Radiat. Oncol. 2019, 14, 160. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Kang, H.-C.; Kim, I.H.; Park, S.-H.; Kim, D.G.; Park, C.-K.; Paek, S.H.; Jung, H.-W. The role of adjuvant radiotherapy in atypical meningioma. J. Neuro Oncol. 2013, 115, 241–247. [Google Scholar] [CrossRef]
- Aghi, M.K.; Carter, B.S.; Cosgrove, G.R.; Ojemann, R.G.; Amin-Hanjani, S.; Martuza, R.L.; Curry, W.T., Jr.; Barker, F.G., II. Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery 2009, 64, 56–60. [Google Scholar] [CrossRef]
- Mair, R.; Morris, K.; Scott, I.; Carroll, T.A. Radiotherapy for atypical meningiomas. J. Neurosurg. 2011, 115, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, D.; Bijmolt, S.; Veninga, T.; Rezvoy, N.; Villà, S.; Krengli, M.; Weber, D.C.; Baumert, B.G.; Canyilmaz, E.; Yalman, D.; et al. Atypical and Malignant Meningioma: Outcome and Prognostic Factors in 119 Irradiated Patients. A Multicenter, Retrospective Study of the Rare Cancer Network. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Goldbrunner, R.; Stavrinou, P.; Jenkinson, M.D.; Sahm, F.; Mawrin, C.; Weber, D.C.; Preusser, M.; Minniti, G.; Lund-Johansen, M.; Lefranc, F.; et al. EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol. 2021, noab150. [Google Scholar] [CrossRef] [PubMed]





| Median Age (IQR) (in y) | 63 (56–75) |
|---|---|
| Sex Female Male | 41 (57.7%) 30 (42.3%) |
| Median preoperative KPS (IQR) | 90 (80–100) |
| Tumor location | |
| Convexity | 27 (38.0%) |
| Falx | 18 (25.4%) |
| Sphenoid wing | 10 (14.1%) |
| Posterior fossa | 8 (11.3%) |
| Frontobasal | 7 (9.9%) |
| Others | 1 (1.4%) |
| Multiple meningiomas | 12 (16.9%) |
| Sinus invasion | 15 (21.1%) |
| Peritumoral edema | 46 (64.8%) |
| Simpson grade Simpson grade I&II Simpson grade ≥ III | 56 (78.9%) 15 (21.1%) |
| Brain invasion | 15 (21.1%) |
| High mitotic count (≥4) | 35 (49.3%) |
| Brain invasion and/or high mitotic count | 50 (70.4%) |
| Minor atypical criteria only | 21 (29.6%) |
| Median MIB-1 (IQR) | 5 (5–10) |
| Median mitotic count (IQR) | 3 (1–6) |
| Adjuvant radiotherapy | 3 (4.2%) |
| Variable | AT-FORGE Score: <5 (n = 49) | AT-FORGE Score: ≥5 (n = 22) | p-Value |
|---|---|---|---|
| Age (mean ± SD) | 62.5 ± 13.1 | 67.1 ± 14.3 | 0.19 |
| BMI (mean ± SD) | 27.2 ± 5.7 | 26.5 ± 2.7 | 0.49 |
| Preoperative KPS (mean ± SD) | 88.4 ± 12.8 | 82.7 ± 12.8 | 0.09 |
| Diabetes (yes/no) | 9/40 | 1/21 | 0.16 |
| Smoking (yes/no) | 14/32 | 8/14 | 0.99 |
| ASA intake (yes/no) | 9/40 | 6/16 | 0.53 |
| Platelet count (mean ± SD) | 252.5 ± 64.1 | 224.9 ± 49.69 | 0.08 |
| MPV (mean ± SD) | 10.9 ± 1.2 | 10.5 ± 0.7 | 0.15 |
| Location (Skull base/Non skull base) | 17/32 | 6/16 | 0.59 |
| Sinus invasion (present/absent) | 8/41 | 7/15 | 0.21 |
| Multiple meningiomas (present/absent) | 8/41 | 4/18 | 0.99 |
| Diffuse CD68+ macrophage infiltrates (available in 60 patients) | 27/15 | 9/9 | 0.39 |
| Simpson grade (≤II/>II) | 41/8 | 15/7 | 0.21 |
| Adjuvant radiotherapy | 1/48 | 2/20 | 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wach, J.; Lampmann, T.; Güresir, Á.; Vatter, H.; Becker, A.J.; Hölzel, M.; Toma, M.; Güresir, E. Combining FORGE Score and Histopathological Diagnostic Criteria of Atypical Meningioma Enables Risk Stratification of Tumor Progression. Diagnostics 2021, 11, 2011. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics11112011
Wach J, Lampmann T, Güresir Á, Vatter H, Becker AJ, Hölzel M, Toma M, Güresir E. Combining FORGE Score and Histopathological Diagnostic Criteria of Atypical Meningioma Enables Risk Stratification of Tumor Progression. Diagnostics. 2021; 11(11):2011. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics11112011
Chicago/Turabian StyleWach, Johannes, Tim Lampmann, Ági Güresir, Hartmut Vatter, Albert J. Becker, Michael Hölzel, Marieta Toma, and Erdem Güresir. 2021. "Combining FORGE Score and Histopathological Diagnostic Criteria of Atypical Meningioma Enables Risk Stratification of Tumor Progression" Diagnostics 11, no. 11: 2011. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics11112011