Colorimetric and Real-Time Loop-Mediated Isothermal Amplification (LAMP) for Detection of Loa loa DNA in Human Blood Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
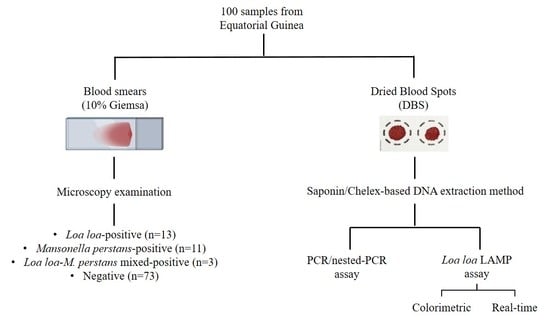
2.2. Samples Obtaining and Selection
2.3. DNA Extraction from Dried Blood Spots
2.4. Molecular Analysis
2.4.1. PCR/Nested-PCR Assay
2.4.2. Colorimetric LAMP Assay
2.4.3. Real-Time LAMP Assay
2.5. Statistics
3. Results
3.1. Application of Molecular Methods on Dried Blood Samples
3.1.1. PCR/Nested-PCR Assay
3.1.2. Colorimetric LAMP Assay
3.1.3. Real-Time LAMP Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boussinesq, M. Loiasis. Ann. Trop. Med. Parasitol. 2006, 8, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Metzger, W.G.; Mordmüller, B. Loa loa-does it deserve to be neglected? Lancet Infect. Dis. 2014, 14, 353–357. [Google Scholar] [CrossRef]
- Gobbi, F.; Bottieau, E.; Bouchaud, O.; Buonfrate, D.; Salvador, F.; Rojo-Marcos, G.; Rodari, P.; Clerinx, J.; Treviño, B.; Herrera-Ávila, J.P.; et al. Comparison of different drug regimens for the treatment of loiasis—A TropNet retrospective study. PLoS Negl. Trop. Dis. 2018, 12, e0006917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyebe, S.; Sabbagh, A.; Pion, S.D.; Nana-Djeunga, H.C.; Kamgno, J.; Boussinesq, M.; Chesnais, C.B. Familial aggregation and heritability of loa loa microfilaremia. Clin. Infect. Dis. 2018, 66, 751–757. [Google Scholar] [CrossRef]
- Expanded Special Project for Elimination of NTDs. Available online: https://espen.afro.who.int/ (accessed on 23 November 2021).
- Mathison, B.A.; Couturier, M.R.; Pritt, B.S. Diagnostic identification and differentiation of microfilariae. J. Clin. Microbiol. 2019, 57, e00706-19. [Google Scholar] [CrossRef] [Green Version]
- Ojurongbe, O.; Akindele, A.A.; Adeleke, M.A.; Oyedeji, M.O.; Adedokun, S.A.; Ojo, J.F.; Akinleye, C.A.; Bolaji, O.S.; Adefioye, O.A.; Adeyeba, O.A. Co-endemicity of Loiasis and Onchocerciasis in Rain Forest Communities in Southwestern Nigeria. PLoS Negl. Trop. Dis. 2015, 9, e0003633. [Google Scholar] [CrossRef]
- Simonsen, P.E.; Onapa, A.W.; Asio, S.M. Mansonella perstans filariasis in Africa. Acta Trop. 2011, 120, S109–S120. [Google Scholar] [CrossRef]
- Ta-Tang, T.-H.; Crainey, J.; Post, R.J.; Luz, S.L.; Rubio, J. Mansonellosis: Current perspectives. Res. Rep. Trop. Med. 2018, 9, 9–24. [Google Scholar] [CrossRef] [Green Version]
- Puente, S.; Lago, M.; Subirats, M.; Sanz-Esteban, I.; Arsuaga, M.; Vicente, B.; Alonso-Sardon, M.; Belhassen-Garcia, M.; Muro, A. Imported Mansonella perstans infection in Spain. Infect. Dis. Poverty 2020, 9, 105. [Google Scholar] [CrossRef]
- D’Ambrosio, M.V.; Bakalar, M.; Bennuru, S.; Reber, C.; Skandarajah, A.; Nilsson, L.; Switz, N.; Kamgno, J.; Pion, S.; Boussinesq, M.; et al. Point-of-care quantification of blood-borne filarial parasites with a mobile phone microscope. Sci. Transl. Med. 2015, 7, 286re4. [Google Scholar] [CrossRef] [Green Version]
- Kamgno, J.; Pion, S.D.; Chesnais, C.B.; Bakalar, M.H.; D’Ambrosio, M.V.; Mackenzie, C.D.; Nana-Djeunga, H.C.; Gounoue-Kamkumo, R.; Njitchouang, G.-R.; Nwane, P.; et al. A Test-and-Not-Treat Strategy for Onchocerciasis in Loa loa–Endemic Areas. N. Engl. J. Med. 2017, 377, 2044–2052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emukah, E.; Rakers, L.J.; Kahansim, B.; Miri, E.S.; Nwoke, B.E.B.; Griswold, E.; Saka, Y.; Anagbogu, I.; Davies, E.; Ityonzughul, C.; et al. In Southern Nigeria Loa loa Blood Microfilaria Density is Very Low even in Areas with High Prevalence of Loiasis: Results of a Survey Using the New LoaScope Technology. Am. J. Trop. Med. Hyg. 2018, 99, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Pion, S.D.; Nana-Djeunga, H.; Niamsi-Emalio, Y.; Chesnais, C.B.; Deléglise, H.; Mackenzie, C.; Stolk, W.; Fletcher, D.A.; Klion, A.D.; Nutman, T.B.; et al. Implications for annual retesting after a test-and-not-treat strategy for onchocerciasis elimination in areas co-endemic with Loa loa infection: An observational cohort study. Lancet Infect. Dis. 2020, 20, 102–109. [Google Scholar] [CrossRef]
- Johnson, O.; Giorgi, E.; Fronterrè, C.; Amoah, B.; Atsame, J.; Ella, S.N.; Biamonte, M.; Ogoussan, K.; Hundley, L.; Gass, K.; et al. Geostatistical modelling enables efficient safety assessment for mass drug administration with ivermectin in Loa loa endemic areas through a combined antibody and LoaScope testing strategy for elimination of onchocerciasis. PLoS Negl. Trop. Dis. 2022, 16, e0010189. [Google Scholar] [CrossRef]
- Burbelo, P.D.; Ramanathan, R.; Klion, A.D.; Iadarola, M.J.; Nutman, T.B. Rapid, novel, specific, high-throughput assay for diagnosis of Loa loa infection. J. Clin. Microbiol. 2008, 46, 2298–2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanji, S.; Amvongo-Adjia, N.; Koudou, B.; Njouendou, A.J.; Chounna Ndongmo, P.W.; Kengne-Ouafo, J.A.; Datchoua-Poutcheu, F.R.; Fovennso, B.A.; Tayong, D.B.; Fombad, F.F.; et al. Cross-Reactivity of Filariais ICT Cards in Areas of Contrasting Endemicity of Loa loa and Mansonella perstans in Cameroon: Implications for Shrinking of the Lymphatic Filariasis Map in the Central African Region. PLoS Negl. Trop. Dis. 2015, 9, e0004184. [Google Scholar] [CrossRef] [Green Version]
- Pedram, B.; Pasquetto, V.; Drame, P.M.; Ji, Y.; Gonzalez-Moa, M.J.; Baldwin, R.K.; Nutman, T.B.; Biamonte, M.A. A novel rapid test for detecting antibody responses to Loa loa infections. PLoS Negl. Trop. Dis. 2017, 11, e0005741. [Google Scholar] [CrossRef] [Green Version]
- Touré, F.S.; Bain, O.; Nerrienet, E.; Millet, P.; Wahl, G.; Toure, Y.; Doumbo, O.; Nicolas, L.; Georges, A.J.; McReynolds, L.A.; et al. Detection of Loa loa-specific DNA in blood from occult-infected individuals. Exp. Parasitol. 1997, 86, 163–170. [Google Scholar] [CrossRef]
- Fink, D.L.; Kamgno, J.; Nutman, T.B. Rapid molecular assays for specific detection and quantitation of Loa loa microfilaremia. PLoS Negl. Trop. Dis. 2011, 5, e1299. [Google Scholar] [CrossRef]
- Ta-Tang, T.H.; Moya, L.; Nguema, J.; Aparicio, P.; Miguel-Oteo, M.; Cenzual, G.; Canorea, I.; Lanza, M.; Benito, A.; Crainey, J.L.; et al. Geographical distribution and species identification of human filariasis and onchocerciasis in Bioko Island, Equatorial Guinea. Acta Trop. 2018, 180, 12–17. [Google Scholar] [CrossRef]
- Alhassan, A.; Li, Z.; Poole, C.B.; Carlow, C.K.S. Expanding the MDx toolbox for filarial diagnosis and surveillance. Trends Parasitol. 2015, 31, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Notomi, T.; Mori, Y.; Tomita, N.; Kanda, H. Loop-mediated isothermal amplification (LAMP): Principle, features, and future prospects. J. Microbiol. 2015, 53, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Nagamine, K.; Tomita, N.; Notomi, T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem. Biophys. Res. Commun. 2001, 289, 150–154. [Google Scholar] [CrossRef]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Mvoulouga, P.O.; Akue, J.P.; Abán, J.L.; Santiago, B.V.; Sánchez, M.C.; Muro, A. Development of a highly sensitive Loop-Mediated Isothermal Amplification (LAMP) method for the detection of Loa loa. PLoS ONE 2014, 9, e94664. [Google Scholar] [CrossRef]
- Drame, P.M.; Fink, D.L.; Kamgno, J.; Herrick, J.A.; Nutman, T.B. Loop-mediated isothermal amplification for rapid and semiquantitative detection of loa loa infection. J. Clin. Microbiol. 2014, 52, 2071–2077. [Google Scholar] [CrossRef] [Green Version]
- Poole, C.B.; Ettwiller, L.; Tanner, N.A.; Evans, T.C.; Wanji, S.; Carlow, C.K.S. Genome filtering for new DNA biomarkers of Loa loa infection suitable for loop-mediated isothermal amplification. PLoS ONE 2015, 10, e0139286. [Google Scholar] [CrossRef]
- Takagi, H.; Itoh, M.; Kasai, S.; Yahathugoda, T.C.; Weerasooriya, M.V.; Kimura, E. Development of loop-mediated isothermal amplification method for detecting Wuchereria bancrofti DNA in human blood and vector mosquitoes. Parasitol. Int. 2011, 60, 493–497. [Google Scholar] [CrossRef]
- Poole, C.B.; Tanner, N.A.; Zhang, Y.; Evans, T.C.; Carlow, C.K.S. Diagnosis of Brugian Filariasis by Loop-Mediated Isothermal Amplification. PLoS Negl. Trop. Dis. 2012, 6, e1948. [Google Scholar] [CrossRef]
- Alhassan, A.; Makepeace, B.L.; Lacourse, E.J.; Osei-Atweneboana, M.Y.; Carlow, C.K.S. A simple isothermal DNA amplification method to screen black flies for Onchocerca volvulus infection. PLoS ONE 2014, 9, 118323. [Google Scholar] [CrossRef] [Green Version]
- Lagatie, O.; Merino, M.; Batsa Debrah, L.; Debrah, A.Y.; Stuyver, L.J. An isothermal DNA amplification method for detection of Onchocerca volvulus infection in skin biopsies. Parasites Vectors 2016, 9, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, C.B.; Sinha, A.; Ettwiller, L.; Apone, L.; McKay, K.; Panchapakesa, V.; Lima, N.F.; Ferreira, M.U.; Wanji, S.; Carlow, C.K.S. In Silico Identification of Novel Biomarkers and Development of New Rapid Diagnostic Tests for the Filarial Parasites Mansonella perstans and Mansonella ozzardi. Sci. Rep. 2019, 9, 10275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smit, P.W.; Elliott, I.; Peeling, R.W.; Mabey, D.; Newton, P.N. Review article: An overview of the clinical use of filter paper in the diagnosis of tropical diseases. Am. J. Trop. Med. Hyg. 2014, 90, 195–210. [Google Scholar] [CrossRef]
- Simon, N.; Shallat, J.; Williams Wietzikoski, C.; Harrington, W.E. Optimization of Chelex 100 resin-based extraction of genomic DNA from dried blood spots. Biol. Methods Protoc. 2020, 5, bpaa009. [Google Scholar] [CrossRef] [PubMed]
- Bereczky, S.; Mårtensson, A.; Gil, J.P.; Färnert, A. Short report: Rapid DNA extraction from archive blood spots on filter paper for genotyping of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 2005, 72, 249–251. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, B.; Xu, W.; González, I.J.; Polley, S.D.; Bell, D.; Shakely, D.; Msellem, M.I.; Björkman, A.; Mårtensson, A. Loop mediated isothermal amplification (LAMP) accurately detects malaria DNA from filter paper blood samples of low density parasitaemias. PLoS ONE 2014, 9, e103905. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.P.; Komaki-Yasuda, K.; Iwagami, M.; Kawai, S.; Kano, S. Combination of PURE-DNA extraction and LAMP-DNA amplification methods for accurate malaria diagnosis on dried blood spots 11 Medical and Health Sciences 1108 Medical Microbiology. Malar. J. 2018, 17, 373. [Google Scholar] [CrossRef] [Green Version]
- Willard, J.M.; Lee, D.A.; Holland, M.M. Recovery of DNA for PCR amplification from blood and forensic samples using a chelating resin. Methods Mol. Biol. 1998, 98, 9–18. [Google Scholar] [CrossRef]
- Rubio, J.M.; Post, R.J.; Van Leeuwen, W.M.D.; Henry, M.C.; Lindergard, G.; Hommel, M. Alternative polymerase chain reaction method to identify Plasmodium species in human blood samples: The semi-nested multiplex malaria PCR (SnM-PCR). Trans. R. Soc. Trop. Med. Hyg. 2002, 96, S199. [Google Scholar] [CrossRef]
- Hwang, J.; Jaroensuk, J.; Leimanis, M.L.; Russell, B.; McGready, R.; Day, N.; Snounou, G.; Nosten, F.; Imwong, M. Long-term storage limits PCR-based analyses of malaria parasites in archival dried blood spots. Malar. J. 2012, 11, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, A.; Baidjoe, A.; Rosenthal, P.J.; Dorsey, G.; Bousema, T.; Greenhouse, B. The effect of storage and extraction methods on amplification of plasmodium falciparum DNA from dried blood spots. Am. J. Trop. Med. Hyg. 2015, 92, 922–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touré, F.S.; Kassambara, L.; Williams, T.; Millet, P.; Bain, O.; Georges, A.J.; Egwang, T.G. Human occult loiasis: Improvement in diagnostic sensitivity by the use of a nested polymerase chain reaction. Am. J. Trop. Med. Hyg. 1998, 59, 144–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eberhard, M.L.; Lammie, P.J. Laboratory diagnosis of filariasis. Clin. Lab. Med. 1991, 4, 977–1010. [Google Scholar] [CrossRef]
- Plowe, C.V.; Djimde, A.; Bouare, M.; Doumbo, O.; Wellems, T.E. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: Polymerase chain reaction methods for surveillance in Africa. Am. J. Trop. Med. Hyg. 1995, 52, 565–568. [Google Scholar] [CrossRef]
- Blas, I.; Ruíz-Zarzuela, I.; Vallejo, A. WinEpi: Working in Epidemiology. An Online Epidemiological Tool. ISVEE 11: Proceedings of the 11th Symposium of the International Society for Veterinary Epi-Demiology and Economics, Cairns (Australia). Theme 4-Tools & Training for Epidemiologists: Poste. Available online: http://winepi.net/ (accessed on 4 May 2021).
- Walther, M.; Muller, R. Diagnosis of human filariases (except onchocerciasis). Adv. Parasitol. 2003, 53, 149–193. [Google Scholar]
- Walsh, P.S.; Metzger, D.A.; Higuchi, R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 2013, 54, 506–513. [Google Scholar] [CrossRef] [Green Version]
- Chaorattanakawee, S.; Natalang, O.; Hananantachai, H.; Nacher, M.; Brockman, A.; Krudsood, S.; Looareesuwan, S.; Patarapotikul, J. Storage duration and polymerase chain reaction detection of Plasmodium falciparum from blood spots on filter paper. Am. J. Trop. Med. Hyg. 2003, 69, 42–44. [Google Scholar] [CrossRef]
- Hsiang, M.S.; Lin, M.; Dokomajilar, C.; Kemere, J.; Pilcher, C.D.; Dorsey, G.; Greenhouse, B. PCR-based pooling of dried blood spots for detection of malaria parasites: Optimization and application to a cohort of Ugandan children. J. Clin. Microbiol. 2010, 48, 3539–3543. [Google Scholar] [CrossRef] [Green Version]
- Bouyou Akotet, M.K.; Owono-Medang, M.; Mawili-Mboumba, D.P.; Moussavou-Boussougou, M.N.; Nzenze Afène, S.; Kendjo, E.; Kombila, M. The relationship between microfilaraemic and amicrofilaraemic loiasis involving co-infection with Mansonella perstans and clinical symptoms in an exposed population from Gabon. J. Helminthol. 2016, 90, 469–475. [Google Scholar] [CrossRef]
- Whittaker, C.; Walker, M.; Pion, S.D.S.; Chesnais, C.B.; Boussinesq, M.; Basáñez, M.G. The Population Biology and Transmission Dynamics of Loa loa. Trends Parasitol. 2018, 34, 335–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Quyen, T.L.; Hung, T.Q.; Chin, W.H.; Wolff, A.; Bang, D.D. A lab-on-a-chip system with integrated sample preparation and loop-mediated isothermal amplification for rapid and quantitative detection of Salmonella spp. in food samples. Lab Chip 2015, 15, 1898–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanji, S.; Eyong, E.E.J.; Tendongfor, N.; Ngwa, C.J.; Esuka, E.N.; Kengne-Ouafo, A.J.; Datchoua-Poutcheu, F.R.; Enyong, P.; Agnew, D.; Eversole, R.R.; et al. Ivermectin treatment of Loa loa hyper-microfilaraemic baboons (Papio anubis): Assessment of microfilarial load reduction, haematological and biochemical parameters and histopathological changes following treatment. PLoS Negl. Trop. Dis. 2017, 11, e0005576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amambo, G.N.; Abong, R.A.; Fombad, F.F.; Njouendou, A.J.; Nietcho, F.; Beng, A.A.; Ritter, M.; Esum, M.E.; Deribe, K.; Cho, J.F.; et al. Validation of Loop Mediated Isothermal Amplification for the Detection of Loa loa Infection in Chrysops sp in Experimental and Natural Field Conditions. Parasit. Vectors 2020, 14, 19. [Google Scholar] [CrossRef]
- Gandasegui, J.; Fernández-Soto, P.; Dacal, E.; Rodríguez, E.; Saugar, J.M.; Yepes, E.; Aznar-Ruiz-de-Alegría, M.L.; Espasa, M.; Ninda, A.; Bocanegra, C.; et al. Field and laboratory comparative evaluation of a LAMP assay for the diagnosis of urogenital schistosomiasis in Cubal, Central Angola. Trop. Med. Int. Health 2018, 23, 992–1001. [Google Scholar] [CrossRef]
- Cevallos, W.; Fernández-Soto, P.; Calvopiña, M.; Buendía-Sánchez, M.; López-Abán, J.; Vicente, B.; Muro, A. Diagnosis of amphimeriasis by LAMPhimerus assay in human stool samples long-Term storage onto filter paper. PLoS ONE 2018, 13, e0192637. [Google Scholar] [CrossRef] [Green Version]




| Sample Groups | Parasitological Finding | Sample Number | mf/mL | PCR/ Nested-PCR | Colorimetric LAMP | Real-Time LAMP |
|---|---|---|---|---|---|---|
| G1 (n = 13) | Loa loa | 18 | 1100 | + | + | + |
| 19 | 300 | + | + | + | ||
| 21 | 500 | + | + | + | ||
| 32 | 2200 | + | - | - | ||
| 39 | 3600 | + | + | + | ||
| 48 | 12,200 | + | + | + | ||
| 51 | 400 | - | + | + | ||
| 53 | 500 | - | + | + | ||
| 62 | 2000 | + | + | + | ||
| 69 | 11,600 | + | + | + | ||
| 75 | 450 | - | + | + | ||
| 76 | 5600 | + | + | + | ||
| 82 | 1900 | + | + | + | ||
| G2 (n = 11) | Mansonella perstans | 24 | 200 | - | + | - |
| 44 | 600 | - | - | - | ||
| 49 | 800 | - | + | - | ||
| 50 | 100 | - | - | - | ||
| 52 | 100 | - | + | + | ||
| 54 | 1300 | - | - | - | ||
| 77 | 3200 | - | - | - | ||
| 78 | 100 | - | - | - | ||
| 79 | 400 | - | + | + | ||
| 83 | 1000 | - | - | - | ||
| 91 | 1000 | - | + | + | ||
| G3 (n = 3) | Loa loa/M. perstans | 55 | 200/200 | - | + | + |
| 70 | 200/200 | + | + | + | ||
| 81 | 6000/1500 | + | + | + | ||
| G4 (n = 73) | No findings | 20 | - | + | - | |
| 42 | - | + | - | |||
| 43 | - | + | + | |||
| 45 | - | + | + | |||
| 47 | - | + | + | |||
| 68 | - | + | - | |||
| 74 | - | + | - | |||
| Remaining nos. up to 100 | - | −66 | −70 |
| Colorimetric LAMP | Real-Time LAMP | PCR/Nested-PCR | |
|---|---|---|---|
| Sensitivity (95% CI) | 94.1% (81.9–105.6%) | 94.1% (82.9–105.3%) | 75.0% (53.8–96.2%) |
| Specificity (95% CI) | 87.5% (78.5–93.3%) | 93.3% (88.2–98.5%) | 100.0% (100.0–100.0%) |
| PPV (95% CI) | 57.1% (36.8–74.3%) | 72.7 % (54.1–91.3%) | 100.0% (100.0–100.0%) |
| NPV (95% CI) | 98.8% (96.0–101.3%) | 98.8% (96.5–101.1%) | 95.5% (91.1–99.8%) |
| Kappa (95% CI) | 62.2% (43.6–80.7%) ** | 76.9% (57.6–96.2%) ** | 83.4% (67.5–99.3%) *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Febrer-Sendra, B.; Fernández-Soto, P.; Crego-Vicente, B.; Diego, J.G.-B.; Ta-Tang, T.-H.; Berzosa, P.; Nguema, R.; Ncogo, P.; Romay-Barja, M.; Herrador, Z.; et al. Colorimetric and Real-Time Loop-Mediated Isothermal Amplification (LAMP) for Detection of Loa loa DNA in Human Blood Samples. Diagnostics 2022, 12, 1079. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics12051079
Febrer-Sendra B, Fernández-Soto P, Crego-Vicente B, Diego JG-B, Ta-Tang T-H, Berzosa P, Nguema R, Ncogo P, Romay-Barja M, Herrador Z, et al. Colorimetric and Real-Time Loop-Mediated Isothermal Amplification (LAMP) for Detection of Loa loa DNA in Human Blood Samples. Diagnostics. 2022; 12(5):1079. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics12051079
Chicago/Turabian StyleFebrer-Sendra, Begoña, Pedro Fernández-Soto, Beatriz Crego-Vicente, Juan García-Bernalt Diego, Thuy-Huong Ta-Tang, Pedro Berzosa, Rufino Nguema, Policarpo Ncogo, María Romay-Barja, Zaida Herrador, and et al. 2022. "Colorimetric and Real-Time Loop-Mediated Isothermal Amplification (LAMP) for Detection of Loa loa DNA in Human Blood Samples" Diagnostics 12, no. 5: 1079. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics12051079