Comparison between Two Types of Dental Unit Waterlines: How Evaluation of Microbiological Contamination Can Support Risk Containment
Abstract
:1. Introduction
2. Materials and Methods
2.1. DUWL Characteristics
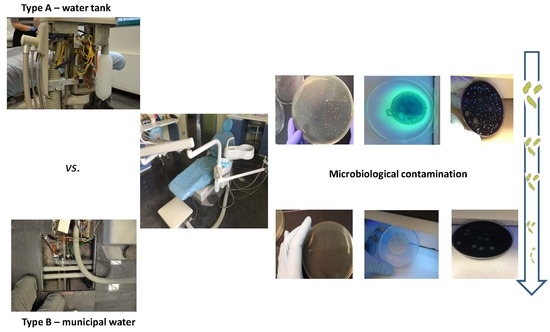
2.1.1. Type A
2.1.2. Type B
2.2. Water Sample Collection
2.3. Microbiological Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blake, G.C. The incidence and control of bacterial infection in dental spray reservoirs. Br. Dent. J. 1963, 115, 412–416. [Google Scholar]
- Rowland, B.M. Bacterial contamination of dental Unit waterlines: What is your dentist spraying into your mouth? Clin. Microbiol. Newsl. 2003, 25, 73–77. [Google Scholar] [CrossRef]
- Barbot, V.; Robert, A.; Rodier, M.H.; Imbert, C. Update on infectious risks associated with dental unit waterlines. FEMS Immunol. Med. Microbiol. 2012, 65, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Tuttlebee, C.M.; O’Donnell, M.J.; Keane, C.T.; Russell, R.J.; Sullivan, D.J.; Falkiner, F.; Coleman, D.C. Effective control of dental chair unit waterline biofilm and marked reduction of bacterial contamination of output water using two peroxide-based disinfectants. J. Hosp. Infect. 2002, 52, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.Y.; Fei, C.N.; Zhang, Y.; Zhang, W.; Liu, J.; Dong, J. Evaluation of bacterial contamination of dental unit waterlines and use of a newly designed measurement device to assess retraction of a dental chair unit. Int. Dent. J. 2016, 66, 208–214. [Google Scholar] [CrossRef]
- Putnins, E.E.; Di Giovanni, D.; Bhullar, A.S. Dental unit waterline contamination and its possible implications during periodontal surgery. J. Periodontol. 2001, 72, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Meiller, T.F.; Kelley, J.I.; Baqui, A.A.; DePaola, L.G. Disinfection of dental unit waterlines with an oral antiseptic. J. Clin. Dent. 2000, 11, 11–15. [Google Scholar] [PubMed]
- Pankhurst, C.L.; Johnson, N.W.; Woods, R.G. Microbial contamination of dental unit waterlines: The scientific argument. Int. Dent. J. 1998, 48, 359–368. [Google Scholar] [PubMed]
- Szymanska, J. Biofilm and dental unit waterlines. Ann. Agric. Environ. Med. 2003, 10, 151–157. [Google Scholar]
- Pankhurst, C.L.; Coulter, W.A. Do contaminated dental unit waterlines pose a risk of infection? J. Dent. 2007, 35, 712–720. [Google Scholar] [CrossRef]
- Fotedar, S.; Ganju, S. Microbial contamination of dental unit water lines in H.P. Government Dental College, Shimla. Saudi J. Dent. Res. 2015, 6, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Uzel, A.; Cogulu, D.; Oncag, O. Microbiological evaluation and antibiotic susceptibility of dental unit water systems in general dental practice. Int. J. Dent. Hyg. 2008, 6, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Ampornaramveth, R.S.; Akeatichod, N.; Lertnukkhid, J.; Songsang, N. Application of D-Amino Acids as Biofilm Dispersing Agent in Dental Unit Waterlines. Int. J. Dent. 2018, 2018, 9413925. [Google Scholar] [CrossRef] [PubMed]
- Pederson, E.D.; Stone, M.E.; Ragain, J.C.; Simecek, J.W. Waterline biofilm and the dental treatment facility: A review. Gen. Dent. 2002, 50, 190–195. [Google Scholar] [PubMed]
- Abdouchakour, F.; Dupont, C.; Grau, D.; Aujoulat, F.; Mournetas, P.; Marchandin, H.; Parer, S.; Gibert, P.; Valcarcel, J.; Jumas-Bilak, E. Pseudomonas aeruginosa and Achromobacter sp. clonal selection leads to successive waves of contamination of water in dental care units. Appl. Environ. Microbiol. 2015, 81, 7509–7524. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, J.; Sitkowska, J.; Dutkiewicz, J. Microbial contamination of dental unit waterlines. Ann. Agric. Environ. Med. 2008, 15, 173–179. [Google Scholar]
- O’Donnell, M.J.; Boyle, M.A.; Russell, R.J.; Coleman, D.C. Management of dental unit waterline biofilms in the 21st century. Future Microbiol. 2011, 6, 1209–1226. [Google Scholar] [CrossRef] [Green Version]
- Garg, S.K.; Mittal, S.; Kaur, P. Dental unit waterline management: Historical perspectives and current trends. J. Investig. Clin. Dent. 2012, 3, 247–252. [Google Scholar] [CrossRef]
- Coan, L.L.; Hughes, E.A.; Hudson, J.C.; Palenik, C.J. Sampling water from chemically cleaned dental units with detachable power scalers. Am. Dent. Hyg. Assoc. 2007, 81, 80. [Google Scholar]
- Lin, S.M.; Svoboda, K.K.H.; Giletto, A.; Seibert, J.; Puttaiah, R. Effects of hydrogen peroxide on dental unit biofilms and treatment water contamination. Eur. J. Dent. 2011, 5, 47–59. [Google Scholar]
- Ditommaso, S.; Giacomuzzi, M.; Ricciardi, E.; Garbuio, R.; Zotti, C.M. The role of chemical products at low doses in preventing the proliferation of bacteria in dental unit waterlines: The ICX® experience. J. Water Health 2018, 16, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Nante, N.; Ceriale, E.; Messina, G.; Lenzi, D.; Manzi, P. Effectiveness of ATP bioluminescence to assess hospital cleaning: A review. J. Prev. Med. Hyg. 2017, 58, 177–183. [Google Scholar]
- World Health Organization. Water Safety in Distribution Systems. 2014. Available online: http://www.who.int/water_sanitation_health/publications/Water_safety_distribution_systems_2014v1.pdf (accessed on 5 November 2018).
- USEPA (United States Environmental Protection Agency). Distribution System Indicators of Drinking Water Quality; Total Coliform Rule Issue Paper; United States Environmental Protection Agency, Office of Water, Office of Ground Water and Drinking Water: Washington, DC, USA, 2006. Available online: http://www.epa.gov/ogwdw/disinfection/tcr/pdfs/issuepaper_tcr_indicators.pdf (accessed on 5 November 2018).
- National Research Council. Drinking Water Distribution Systems: Assessing and Reducing Risks; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar] [CrossRef]
- William, G.; Kohn, A.S.; Collins, M.P.H.; Jennifer, L.; Cleveland, D.D.S.; Jennifer, A.; Harte, D.D.S.; Kathy, J.; Eklund, M.H.P.; Dolores, M.; et al. Centre for Disease Prevention and Control (CDC). Guidelines for Infection Control in Dental Health-Care Settings—2003. Recommendations and Reports; MMWR, 2003; Volume 52, pp. 1–76. Available online: http://www.cdc.gov/mmwr/PDF/ rr/rr5217.pdf (accessed on 5 November 2018).
- Health Technical Memorandum: Decontamination in Primary Care Dental Practices (HTM 01-05, 2013 Version). Department of Health and Social Care, GOV.UK. Available online: https://www.gov.uk/government/publications/decontamination-in-primary-care-dental-practices (accessed on 5 November 2018).
- Hwang, C.; Ling, F.; Andersen, G.L.; LeChevallier, M.W.; Liu, W.T. Microbial community dynamics of an urban drinking water distribution system subjected to phases of chloramination and chlorination treatments. Appl. Environ. Microbiol. 2012, 78, 7856–7865. [Google Scholar] [CrossRef] [PubMed]
- UNI EN ISO 6222:2001 Water Quality—Enumeration of Culturable Micro-Organisms—Colony Count by Inoculation in a Nutrient Agar Culture Medium. Available online: http://store.uni.com/catalogo/index.php/uni-en-iso-6222-2001.html (accessed on 5 November 2018).
- Decreto Legislativo 2 Febbraio 2001, n. 31, Attuazione Della Direttiva 98/83/CE Relativa Alla Qualità Delle Acque Destinate al Consumo Umano (G.U. n. 52 del 3 marzo 2001-s.o.n. 41). Available online: http://www.gazzettaufficiale.it/eli/id/2001/03/03/001G0074/sg (accessed on 5 November 2018).
- Ministerial Decree 14.06.2017. Implementation of Directive (EU) 2015/1787 Amending Annexes II and III of Directive 98/83/EC on the Quality of Water Intended for Human Consumption. Amendments to Annexes II and III of Legislative Decree 2 February 2001, n. 31. (17A05618) (GU General Series n.192 of 18-08-2017). 2017. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=3&ved=2ahUKEwis_Nm09ZTfAhUKw4sKHRPbAFAQFjACegQIAhAC&url=http%3A%2F%2Fwww.ceirsa.org%2Ffd.php%3Fpath%3D201710%2FDM_14_06_2017Acque_destinate_al_consumo_umano.pdf&usg=AOvVaw3eguX4JKnVJArmnYdE1oVD (accessed on 5 November 2018).
- UNI EN ISO 16266:2008—Water Quality—Detection and Enumeration of Pseudomonas aeruginosa—Method by Membrane Filtration. Available online: http://store.uni.com/catalogo/index.php/uni-en-iso-16266-2008.html?josso_back_to=http://store.uni.com/josso-security-check.php&josso_cmd=login_optional&josso_partnerapp_host=store.uni.com (accessed on 5 November 2018).
- ISO 11731:2017 Water Quality—Enumeration of Legionella. Available online: https://www.iso.org/standard/61782.html (accessed on 5 November 2018).
- Italian Health Ministry. Guidelines for Prevention and Control of Legionellosis. Approvate in Conferenza Stato-Regioni Seduta Del 7 Maggio 2015. Italy, 2015. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2362_allegato.pdf (accessed on 5 November 2018).
- Emilia-Romagna Region. Regional Guidelines for Surveillance and Control of Legionellosis. Delibera Della Giunta Regionale 12 Giugno 2017, N. 828. 2017. Available online: http://salute.regione.emilia-romagna.it/documentazione/leggi/regionali/dgr-2127-2016/dgr-n-828-2017-linee-guida-regionali-per-la-sorveglianza-e-il-controllo-della-legionellosi/view (accessed on 5 November 2018).
- World Health Organization. Guidelines for Drinking-Water Quality—4th ed. 2011. Available online: http://apps.who.int/iris/bitstream/10665/44584/1/9789241548151_eng.pdf (accessed on 5 November 2018).
- ADA. American Dental Association: Statement on Dental Unit Waterline. 2004. Available online: http://www.ada.org/ (accessed on 5 November 2018).
- Pasquarella, C.; Veronesi, L.; Castiglia, P.; Liguori, G.; Montagna, M.T.; Napoli, C.; Rizzetto, R.; Torre, I.; Masia, M.D.; Di onofrio, V.; et al. Siti workinggroup “Hygiene in Dentistry”. Italian multicentre study on microbial environmental contamination in dental clinics: A pilot study. Sci. Total Environ. 2010, 408, 4045–4051. [Google Scholar] [CrossRef] [PubMed]
- Sedlata Juraskova, E.; Sedlackova, H.; Janska, J.; Holy, O.; Lalova, I.; Matouskova, I. Legionella spp. in dental unit waterlines. Bratisl. Lek. Listy 2017, 118, 310–314. [Google Scholar] [CrossRef]
- Duda, S.; Baron, J.L.; Wagener, M.M.; Vidic, R.D.; Stout, J.E. Lack of correlation between Legionella colonization and microbial population quantification using heterotrophic plate count and adenosine triphosphate bioluminescence measurement. Environ. Monit. Assess. 2015, 187, 393. [Google Scholar] [CrossRef] [PubMed]
- Bristela, M.; Skolka, A.; Schmid-Schwap, M.; Piehslinger, E.; Indra, A.; Wewalka, G.; Stauffer, F. Testing for aerobic heterotrophic bacteria allows no prediction of contamination with potentially pathogenic bacteria in the output water of dental chair units. GMS Krankenhaushygiene Interdisziplinär 2012, 7, Doc12. [Google Scholar] [CrossRef]
- Ditommaso, S.; Giacomuzzi, M.; Ricciardi, E.; Zotti, C.M. Cultural and Molecular Evidence of Legionella spp. Colonization in Dental Unit Waterlines: Which Is the Best Method for Risk Assessment? Int. J. Environ. Res. Public Health 2016, 13, 211. [Google Scholar] [CrossRef]
- Wilson, R.; Dowling, R. Lung infections. 3. Pseudomonas aeruginosa and other related species. Thorax 1998, 53, 213–219. [Google Scholar]
- Kimura, S.; Tateda, K.; Ishii, Y.; Horikawa, M.; Miyairi, S.; Gotoh, N.; Ishiguro, M.; Yamaguchi, K. Pseudomonas aeruginosa Las quorum sensing autoinducer suppresses growth and biofilm production in Legionella species. Microbiology 2009, 155 Pt 6, 1934–1939. [Google Scholar] [CrossRef]
- Coleman, D.C.; O’Donnell, M.J.; Shore, A.C.; Russell, R.J. Biofilm problems in dental unit water systems and its practical control. J. Appl. Microbiol. 2009, 106, 1424–1437. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.L.; Fontana, S.; Pinci, F.; Fiumana, E.; Pedna, M.F.; Farolfi, P.; Bucci Sabattini, M.A.; Scaturro, M. Pneumonia associated with a dental unit waterline. Lancet 2012, 379, 684. [Google Scholar] [CrossRef]
- Rota, M.C.; Caporali, M.G.; Bella, A.; Ricci, M.L.; Napoli, C. Legionnaires’ disease in Italy: Results of the epidemiological surveillance from 2000 to 2011. Eurosurveillance 2013, 18, 20497. [Google Scholar] [CrossRef] [PubMed]



| Duwl Characteristics | Type A | Type B |
|---|---|---|
| Material | plastic-steel | plastic-steel |
| Water supply | storage tank/municipal water (button to switch the modality) | only municipal water supply |
| Anti-retraction valves | not present | present (changed monthly) |
| Storage capacity | 1 or 2 L | not present—external container for disinfection practice |
| Flushing | twice a day (every day at the beginning and at the end of the working day and after each patient) | twice a day (every day at the beginning and at the end of the working day and after each patient) |
| Disinfection product/ procedure in use | treatment with chemical agents based on effervescent tablets * | treatment with hydrogen peroxide/silver salts (3% v/v) approved and patented by the manufacturer |
| Timing of disinfection | weekly (at the end of each working day) after a period of inactivity (weekends and holidays) | weekly (at the end of each working day) and after a period of inactivity (weekends and holidays) |
| Type of disinfection | sanitization is manually performed (1 or 2 tablets/1L or 2 L tank) | the sanitization operation is controlled by an automatized pump system (continuous treatment) |
| Problem with disinfection | possible retrograde contamination (no water pressure) | use of continuous treatment can show modest effectiveness and select for resistant microorganisms |
| Risk assessment for water control | every 6 months and/or following noncompliant results | every 6 months and/or following noncompliant results |
| Microbiological Parameters | Statistical Parameters | Type A | Type B | Type A vs. Type B: p-value (One-Sided) |
|---|---|---|---|---|
| HPCs at 36 °C | % of positive samples (CDC 2003) | 38.1% | 0 | 0.002 |
| % of positive samples (D.lgs 31/2001) | 69.1% | 56.3% | ||
| Median, IQR | 1.95, 0.63 Log cfu/mL | 1.33, 0.41 Log cfu/mL | ||
| Range of the mean concentration (min-max) | 1.44–2.99 Log cfu/mL | 0.89–1.41 Log cfu/mL | ||
| Reference value: 500 cfu/mL (2.69 Log cfu/mL) Source of the reference value: CDC 2003 [26] Reference value: 20 cfu/mL (1.3 Log cfu/mL) Source of the reference value: Italian D.lgs 31/2001 [30,31] | ||||
| P. aeruginosa | % of positive samples | 77.4% | 25.0% | 0.004 |
| Median, IQR | 1.86, 0.73 Log cfu/100 mL | 0.31, 1.04 Log cfu/100 mL | ||
| Range of the mean concentration (min-max) | 0.92–2.58 Log cfu/100 mL | 0.00–1.17 Log cfu/100 mL | ||
| Reference value: 0 cfu/100 Ml Source of the reference value: Italian D.lgs 31/2001 [30,31] | ||||
| Legionella spp. | % of positive samples | 91.7% | 37.5% | 0.05 |
| Median, IQR | 2.50, 0.08 Log cfu/L | 2.29, 0.56 Log cfu/L | ||
| Range of the mean concentration (min-max) | 2.42–2.74 Log cfu/L | 1.80–2.52 Log cfu/L | ||
| Reference value: 100 cfu/L (2 Log cfu/L) Source of the reference values: Italian Guidelines 2015 [34] Emilia Romagna Guidelines, 2017 [35]. | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lizzadro, J.; Mazzotta, M.; Girolamini, L.; Dormi, A.; Pellati, T.; Cristino, S. Comparison between Two Types of Dental Unit Waterlines: How Evaluation of Microbiological Contamination Can Support Risk Containment. Int. J. Environ. Res. Public Health 2019, 16, 328. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16030328
Lizzadro J, Mazzotta M, Girolamini L, Dormi A, Pellati T, Cristino S. Comparison between Two Types of Dental Unit Waterlines: How Evaluation of Microbiological Contamination Can Support Risk Containment. International Journal of Environmental Research and Public Health. 2019; 16(3):328. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16030328
Chicago/Turabian StyleLizzadro, Jessica, Marta Mazzotta, Luna Girolamini, Ada Dormi, Tiziana Pellati, and Sandra Cristino. 2019. "Comparison between Two Types of Dental Unit Waterlines: How Evaluation of Microbiological Contamination Can Support Risk Containment" International Journal of Environmental Research and Public Health 16, no. 3: 328. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16030328