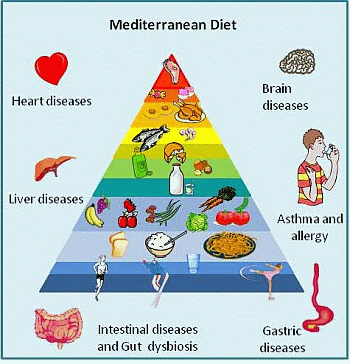
Mediterranean Diet and Health: Food Effects on Gut Microbiota and Disease Control
Abstract
:1. Introduction

2. MD and Diet Effects on Disease Risk

3. MD Influences Gut Microbiota Composition
4. Conclusions and Future Perspectives

Acknowledgments
Conflicts of Interest
References
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, S1402–S1406. [Google Scholar]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar]
- Estruch, R.; Salas-Salvado, J. Towards an even healthier Mediterranean diet. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1163–1166. [Google Scholar]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today—Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar]
- Lairon, D. Intervention studies on Mediterranean diet and cardiovascular risk. Mol. Nutr. Food Res. 2007, 51, 1209–1214. [Google Scholar]
- Ortega, R.M.; Palencia, A.; Lopez-Sobaler, A.M. Improvement of cholesterol levels and reduction of cardiovascular risk via the consumption of phytosterols. Br. J. Nutr. 2006, 96, S89–S93. [Google Scholar]
- Ninfali, P.; Mea, G.; Giorgini, S.; Rocchi, M.; Bacchiocca, M. Antioxidant capacity of vegetables, spices and dressings relevant to nutrition. Br. J. Nutr. 2005, 93, 257–266. [Google Scholar]
- Panico, A.M.; Cardile, V.; Garufi, F.; Puglia, C.; Bonina, F.; Ronsisvalle, G. Protective effect of Capparis spinosa on chondrocytes. Life Sci. 2005, 77, 2479–2488. [Google Scholar]
- Garritano, S.; Pinto, B.; Giachi, I.; Pistelli, L.; Reali, D. Assessment of estrogenic activity of flavonoids from Mediterranean plants using an in vitro short-term test. Phytomedicine 2005, 12, 143–147. [Google Scholar]
- Lopez-Miranda, J.; Perez-Jimenez, F.; Ros, E.; de Caterina, R.; Badimon, L.; Covas, M.I.; Escrich, E.; Ordovas, J.M.; Soriguer, F.; Abia, R.; et al. Olive oil and health: Summary of the II international conference on olive oil and health consensus report, Jaen and Cordoba (Spain) 2008. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 284–294. [Google Scholar]
- Jakobsen, M.U.; O’Reilly, E.J.; Heitmann, B.L.; Pereira, M.A.; Balter, K.; Fraser, G.E.; Goldbourt, U.; Hallmans, G.; Knekt, P.; Liu, S.; et al. Major types of dietary fat and risk of coronary heart disease: A pooled analysis of 11 cohort studies. Am. J. Clin. Nutr. 2009, 89, 1425–1432. [Google Scholar]
- Covas, M.I.; Nyyssonen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.J.F.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Baumler, H.; et al. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar]
- Bos, M.B.; de Vries, J.H.M.; Feskens, E.J.M.; van Dijk, S.J.; Hoelen, D.W.M.; Siebelink, E.; Heijligenberg, R.; de Groot, L.C.P.G.M. Effect of a high monounsaturated fatty acids diet and a Mediterranean diet on serum lipids and insulin sensitivity in adults with mild abdominal obesity. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 591–598. [Google Scholar]
- Serra-Majem, L.; Bes-Rastrollo, M.; Roman-Vinas, B.; Pfrimer, K.; Sanchez-Villegas, A.; Martinez-Gonzalez, M.A. Dietary patterns and nutritional adequacy in a Mediterranean country. Br. J. Nutr. 2009, 101, S21–S28. [Google Scholar]
- Grosso, G.; Pajak, A.; Marventano, S.; Castellano, S.; Galvano, F.; Bucolo, C.; Drago, F.; Caraci, F. Role of ω-3 fatty acids in the treatment of depressive disorders: A comprehensive meta-analysis of randomized clinical trials. PLoS One 2014, 9, e96905. [Google Scholar]
- Grosso, G.; Galvano, F.; Marventano, S.; Malaguarnera, M.; Bucolo, C.; Drago, F.; Caraci, F. ω-3 fatty acids and depression: Scientific evidence and biological mechanisms. Oxid. Med. Cell. Longev. 2014, 2014, 313570. [Google Scholar]
- Haas, P.; Machado, M.J.; Anton, A.A.; Silva, A.S.S.; de Francisco, A. Effectiveness of whole grain consumption in the prevention of colorectal cancer: Meta-analysis of cohort studies. Int. J. Food Sci. Nutr. 2009, 60, S6. [Google Scholar]
- Barclay, A.W.; Petocz, P.; McMillan-Price, J.; Flood, V.M.; Prvan, T.; Mitchell, P.; Brand-Miller, J.C. Glycemic index, glycemic load, and chronic disease risk—A meta-analysis of observational studies. Am. J. Clin. Nutr. 2008, 87, 627–637. [Google Scholar]
- Bartram, H.P.; Scheppach, W.; Gerlach, S.; Ruckdeschel, G.; Kelber, E.; Kasper, H. Does yogurt enriched with Bifidobacterium longum affect colonic microbiology and fecal metabolites in health subjects? Am. J. Clin. Nutr. 1994, 59, 428–432. [Google Scholar]
- Bibbins-Domingo, K.; Chertow, G.M.; Coxson, P.G.; Moran, A.; Lightwood, J.M.; Pletcher, M.J.; Goldman, L. Projected effect of dietary salt reductions on future cardiovascular disease. N. Engl. J. Med. 2010, 362, 590–599. [Google Scholar]
- Chiva-Blanch, G.; Arranz, S.; Lamuela-Raventos, R.M.; Estruch, R. Effects of wine, alcohol and polyphenols on cardiovascular disease risk factors: Evidences from human studies. Alcohol Alcohol. 2013, 48, 270–277. [Google Scholar]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar]
- Castro-Quezada, I.; Roman-Vinas, B.; Serra-Majem, L. The Mediterranean diet and nutritional adequacy: A review. Nutrients 2014, 6, 231–248. [Google Scholar]
- Ferguson, L.R. Potential value of nutrigenomics in Crohn’s disease. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 260–270. [Google Scholar]
- Mitrou, P.N.; Kipnis, V.; Thiebaut, A.C.M.; Reedy, J.; Subar, A.F.; Wirfalt, E.; Flood, A.; Mouw, T.; Hollenbeck, A.R.; Leitzmann, M.F.; et al. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: Results from the NIH-AARP diet and health study. Arch. Intern. Med. 2007, 167, 2461–2468. [Google Scholar]
- Couto, E.; Boffetta, P.; Lagiou, P.; Ferrari, P.; Buckland, G.; Overvad, K.; Dahm, C.C.; Tjonneland, A.; Olsen, A.; Clavel-Chapelon, F.; et al. Mediterranean dietary pattern and cancer risk in the EPIC cohort. Br. J. Cancer 2011, 104, 1493–1499. [Google Scholar]
- Cappellani, A.; Zanghì, A.; di Vita, M.; Cavallaro, A.; Piccolo, G.; Veroux, P.; Lo Menzo, E.; Cavallaro, V.; de Paoli, P.; Veroux, M.; et al. Strong correlation between diet and development of colorectal cancer. Front. Biosci. Landmark Ed. 2013, 18, 190–198. [Google Scholar] [CrossRef]
- Biondi, A.; Fisichella, R.; Fiorica, F.; Malaguarnera, M.; Basile, F. Food mutagen and gastrointestinal cancer. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1280–1282. [Google Scholar]
- Georgousopoulou, E.N.; Kastorini, C.M.; Milionis, H.J.; Ntziou, E.; Kostapanos, M.S.; Nikolaou, V.; Vemmos, K.N.; Goudevenos, J.A.; Panagiotakos, D.B. Association between Mediterranean diet and non-fatal cardiovascular events, in the context of anxiety and depression disorders: A case/case-control study. Hell. J. Cardiol. 2014, 55, 24–31. [Google Scholar]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health. BioFactors 2013, 39, 335–342. [Google Scholar]
- Salas-Salvado, J.; Bullo, M.; Estruch, R.; Ros, E.; Covas, M.-I.; Ibarrola-Jurado, N.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; et al. Prevention of diabetes with Mediterranean diets: A subgroup analysis of a randomized trial. Ann. Intern. Med. 2014, 160, 1–10. [Google Scholar]
- Marrazzo, G.; Barbagallo, I.; Galvano, F.; Malaguarnera, M.; Gazzolo, D.; Frigiola, A.; D’Orazio, N.; Li Volti, G. Role of dietary and endogenous antioxidants in diabetes. Crit. Rev. Food Sci. Nutr. 2014, 54, 1599–1616. [Google Scholar]
- Salamone, F.; Li Volti, G.; Titta, L.; Puzzo, L.; Barbagallo, I.; la Delia, F.; Zelber-Sagi, S.; Malaguarnera, M.; Pelicci, P.G.; Giorgio, M.; et al. Moro orange juice prevents fatty liver in mice. World J. Gastroenterol. 2012, 18, 3862–3868. [Google Scholar]
- Garcia-Marcos, L.; Castro-Rodriguez, J.A.; Weinmayr, G.; Panagiotakos, D.B.; Priftis, K.N.; Nagel, G. Influence of Mediterranean diet on asthma in children: A systematic review and meta-analysis. Pediatr. Allergy Immunol. 2013, 24, 330–338. [Google Scholar]
- Martinez-Gonzalez, M.A.; Bes-Rastrollo, M.; Serra-Majem, L.; Lairon, D.; Estruch, R.; Trichopoulou, A. Mediterranean food pattern and the primary prevention of chronic disease: Recent developments. Nutr. Rev. 2009, 67, S111–S116. [Google Scholar]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. Br. Med. J. 2008, 337, 673–675. [Google Scholar]
- La Vecchia, C.; Franceschi, S. Nutrition and gastric cancer. Can. J. Gastroenterol. 2000, 14, D51–D54. [Google Scholar]
- Pauwels, E.K.J. The protective effect of the Mediterranean diet: Focus on cancer and cardiovascular risk. Med. Princ. Pract. 2011, 20, 103–111. [Google Scholar]
- Praud, D.; Bertuccio, P.; Bosetti, C.; Turati, F.; Ferraroni, M.; la Vecchia, C. Adherence to the Mediterranean diet and gastric cancer risk in Italy. Int. J. Cancer 2013, 134, 2935–2941. [Google Scholar]
- Marmot, M.; Atinmo, T.; Byers, T.; Chen, J.; Hirohata, T.; Jackson, A.; James, W.; Kolonel, L.; Kumanyika, S.; Leitzmann, C.; et al. Food, nutrition, physical activity, and the prevention of cancer: A global perspective. World Cancer Res. Fund/Am. Inst. Cancer 2007, 46, 312–314. [Google Scholar]
- Gonzalez, C.A.; Riboli, E. Diet and cancer prevention: Contributions from the European prospective investigation into cancer and nutrition (EPIC) study. Eur. J. Cancer 2010, 46, 2555–2562. [Google Scholar] [CrossRef]
- Gonzalez, C.A.; Lujan-Barroso, L.; Bueno-de-Mesquita, H.B.; Jenab, M.; Duell, E.J.; Agudo, A.; Tjønneland, A.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; Touillaud, M.; et al. Fruit and vegetable intake and the risk of gastric adenocarcinoma: A reanalysis of the European prospective investigation into cancer and nutrition (EPIC-EURGAST) study after a longer follow-up. Int. J. Cancer 2012, 131, 2910–2919. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Tramacere, I.; Negri, E.; Pelucchi, C.; Bagnardi, V.; Rota, M.; Scotti, L.; Islami, F.; Corrao, G.; La Vecchia, C.; Boffetta, P. A meta-analysis on alcohol drinking and gastric cancer risk. Ann. Oncol. 2012, 23, 28–36. [Google Scholar] [CrossRef]
- Ziegler, R.G.; Hoover, R.N.; Pike, M.C.; Hildesheim, A.; Nomura, A.M.; West, D.W.; Wu-Williams, A.H.; Kolonel, L.N.; Horn-Ross, P.L.; Rosenthal, J.F.; et al. Migration patterns and breast cancer risk in Asian-American women. J. Natl. Cancer Inst. 1993, 85, 1819–1827. [Google Scholar] [CrossRef]
- Albuquerque, R.C.R.; Baltar, V.T.; Marchioni, D.M.L. Breast cancer and dietary patterns: A systematic review. Nutr. Rev. 2014, 72, 1–17. [Google Scholar] [CrossRef]
- Brennan, S.F.; Cantwell, M.M.; Cardwell, C.R.; Velentzis, L.S.; Woodside, J.V. Dietary patterns and breast cancer risk: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 91, 1294–1302. [Google Scholar] [CrossRef]
- Edefonti, V.; Randi, G.; la Vecchia, C.; Ferraroni, M.; Decarli, A. Dietary patterns and breast cancer: A review with focus on methodological issues. Nutr. Rev. 2009, 67, 297–314. [Google Scholar] [CrossRef]
- Torres-Sanchez, L.; Galvan-Portillo, M.; Wolff, M.S.; Lopez-Carrillo, L. Dietary consumption of phytochemicals and breast cancer risk in Mexican women. Public Health Nutr. 2009, 12, 825–831. [Google Scholar] [CrossRef]
- Lima, F.E.; Latorre, M.R.; Costa, M.J.C.; Fisberg, R.M. Diet and cancer in Northeast Brazil: Evaluation of eating habits and food group consumption in relation to breast cancer. Cad. Saude Pública 2008, 24, 820–828. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Stefanadis, C.; Toutouzas, P. The role of traditional mediterranean type of diet and lifestyle, in the development of acute coronary syndromes: Preliminary results from CARDIO 2000 study. Cent. Eur. J. Public Health 2002, 10, 11–15. [Google Scholar]
- Alvarez-Suarez, J.M.; Giampieri, F.; Tulipani, S.; Casoli, T.; di Stefano, G.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; Busco, F.; Quiles, J.L.; Cordero, M.D.; et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014, 25, 289–294. [Google Scholar]
- Kaliora, A.C.; Dedoussis, G.V.Z.; Schmidt, H. Dietary antioxidants in preventing atherogenesis. Atherosclerosis 2006, 187, 1–17. [Google Scholar]
- Dontas, A.S.; Zerefos, N.S.; Panagiotakos, D.B.; Vlachou, C.; Valis, D.A. Mediterranean diet and prevention of coronary heart disease in the elderly. Clin. Interv. Aging 2007, 2, 109–115. [Google Scholar]
- Yokoyama, Y.; Nishimura, K.; Barnard, N.D.; Takegami, M.; Watanabe, M.; Sekikawa, A.; Okamura, T.; Miyamoto, Y. Vegetarian diets and blood pressure: A meta-analysis. J. Am. Med. Assoc. Intern. Med. 2014, 174, 577–587. [Google Scholar]
- Kelpis, T.G.; Anastasiadis, K.; Nimatoudis, I.; Kelpi, M.G.; Hadjimiltiades, S.; Papakonstantinou, C. Prevalence of “distressed” personality in patients with coronary artery disease and its correlation with morbidity after coronary surgery. Hell. J. Cardiol. 2013, 54, 362–367. [Google Scholar]
- Vassallo, N.; Scerri, C. Mediterranean diet and dementia of the Alzheimer type. Curr. Aging Sci. 2013, 6, 150–162. [Google Scholar]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar]
- Feart, C.; Samieri, C.; Alles, B.; Barberger-Gateau, P. Potential benefits of adherence to the Mediterranean diet on cognitive health. Proc. Nutr. Soc. 2013, 72, 140–152. [Google Scholar]
- Dai, J.; Jones, D.P.; Goldberg, J.; Ziegler, T.R.; Bostick, R.M.; Wilson, P.W.; Manatunga, A.K.; Shallenberger, L.; Jones, L.; Vaccarino, V. Association between adherence to the Mediterranean diet and oxidative stress. Am. J. Clin. Nutr. 2008, 88, 1364–1370. [Google Scholar]
- Gaskins, A.J.; Rovner, A.J.; Mumford, S.L.; Yeung, E.; Browne, R.W.; Trevisan, M.; Perkins, N.J.; Wactawski-Wende, J.; Schisterman, E.F. Adherence to a Mediterranean diet and plasma concentrations of lipid peroxidation in premenopausal women. Am. J. Clin. Nutr. 2010, 92, 1461–1467. [Google Scholar]
- Feart, C.; Samieri, C.; Rondeau, V.; Amieva, H.; Portet, F.; Dartigues, J.-F.; Scarmeas, N.; Barberger-Gateau, P. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. J. Am. Med. Assoc. 2009, 302, 638–648. [Google Scholar]
- Scarmeas, N.; Luchsinger, J.A.; Stern, Y.; Gu, Y.; He, J.; DeCarli, C.; Brown, T.; Brickman, A.M. Mediterranean diet and magnetic resonance imaging-assessed cerebrovascular disease. Ann. Neurol. 2011, 69, 257–268. [Google Scholar]
- Caracciolo, B.; Xu, W.; Collins, S.; Fratiglioni, L. Cognitive decline, dietary factors and gut-brain interactions. Mech. Ageing Dev. 2013, 136–137, 59–69. [Google Scholar]
- Gorelick, P.B. Role of inflammation in cognitive impairment: Results of observational epidemiological studies and clinical trials. Ann. N.Y. Acad. Sci. 2010, 1207, 155–162. [Google Scholar]
- Panagiotakos, D.B.; Dimakopoulou, K.; Katsouyanni, K.; Bellander, T.; Grau, M.; Koenig, W.; Lanki, T.; Pistelli, R.; Schneider, A.; Peters, A. Mediterranean diet and inflammatory response in myocardial infarction survivors. Int. J. Epidemiol. 2009, 38, 856–866. [Google Scholar]
- Valls-Pedret, C.; Lamuela-Raventos, R.M.; Medina-Remon, A.; Quintana, M.; Corella, D.; Pinto, X.; Martinez-Gonzalez, M.A.; Estruch, R.; Ros, E. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J. Alzheimers Dis. 2012, 29, 773–782. [Google Scholar]
- Panagiotakos, D.B.; Kastorini, C.-M.; Pitsavos, C.; Stefanadis, C. The current Greek diet and the ω-6/ω-3 balance: The Mediterranean diet score is inversely associated with the ω-6/ω-3 ratio. World Rev. Nutr. Diet. 2011, 102, 53–56. [Google Scholar]
- Frisardi, V.; Panza, F.; Seripa, D.; Imbimbo, B.P.; Vendemiale, G.; Pilotto, A.; Solfrizzi, V. Nutraceutical properties of Mediterranean diet and cognitive decline: Possible underlying mechanisms. J. Alzheimers Dis. 2010, 22, 715–740. [Google Scholar]
- Wengreen, H.; Munger, R.G.; Cutler, A.; Quach, A.; Bowles, A.; Corcoran, C.; Tschanz, J.T.; Norton, M.C.; Welsh-Bohmer, K.A. Prospective study of dietary approaches to stop Hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: The Cache County study on memory, health and aging. Am. J. Clin. Nutr. 2013, 98, 1263–1271. [Google Scholar]
- Martinez-Gonzalez, M.A.; de la Fuente-Arrillaga, C.; Nunez-Cordoba, J.M.; Basterra-Gortari, F.J.; Beunza, J.J.; Vazquez, Z.; Benito, S.; Tortosa, A.; Bes-Rastrollo, M. Adherence to Mediterranean diet and risk of developing diabetes: Prospective cohort study. Br. Med. J. 2008, 336, 1348–1351. [Google Scholar]
- Esposito, K.; Maiorino, M.I.; di Palo, C.; Giugliano, D. Adherence to a Mediterranean diet and glycaemic control in Type 2 diabetes mellitus. Diabet. Med. J. Br. Diabet. Assoc. 2009, 26, 900–907. [Google Scholar]
- Salas-Salvado, J.; Bullo, M.; Babio, N.; Martinez-Gonzalez, M.A.; Ibarrola-Jurado, N.; Basora, J.; Estruch, R.; Covas, M.I.; Corella, D.; Aros, F.; et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: Results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011, 34, 14–19. [Google Scholar]
- Bantle, J.P.; Wylie-Rosett, J.; Albright, A.L.; Apovian, C.M.; Clark, N.G.; Franz, M.J.; Hoogwerf, B.J.; Lichtenstein, A.H.; Mayer-Davis, E.; Mooradian, A.D.; et al. Nutrition recommendations and interventions for diabetes: A position statement of the American Diabetes Association. Diabetes Care 2008, 31, S61–S78. [Google Scholar]
- Ramachandran, A.; Snehalatha, C.; Mary, S.; Mukesh, B.; Bhaskar, A.D.; Vijay, V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006, 49, 289–297. [Google Scholar]
- Kosaka, K.; Noda, M.; Kuzuya, T. Prevention of type 2 diabetes by lifestyle intervention: A Japanese trial in IGT males. Diabetes Res. Clin. Pract. 2005, 67, 152–162. [Google Scholar]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar]
- Tuomilehto, J.; Lindstrom, J.; Eriksson, J.G.; Valle, T.T.; Hamalainen, H.; Ilanne-Parikka, P.; Keinanen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar]
- Pan, X.R.; Li, G.W.; Hu, Y.H.; Wang, J.X.; Yang, W.Y.; An, Z.X.; Hu, Z.X.; Lin, J.; Xiao, J.Z.; Cao, H.B.; et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance—The Da Qing IGT and diabetes study. Diabetes Care 1997, 20, 537–544. [Google Scholar]
- Perez-Jimenez, F.; Alvarez de Cienfuegos, G.; Badimon, L.; Barja, G.; Battino, M.; Blanco, A.; Bonanome, A.; Colomer, R.; Corella-Piquer, D.; Covas, I.; et al. International conference on the healthy effect of virgin olive oil. Eur. J. Clin. Investig. 2005, 35, 421–424. [Google Scholar]
- Esposito, K.; Giugliano, D. Mediterranean diet and type 2 diabetes. Diabetes Metab. Res. Rev. 2014, 30, 34–40. [Google Scholar]
- Carter, P.; Gray, L.J.; Troughton, J.; Khunti, K.; Davies, M.J. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: Systematic review and meta-analysis. Br. Med. J. 2010, 341, c4229. [Google Scholar]
- Priebe, M.G.; van Binsbergen, J.J.; de Vos, R.; Vonk, R.J. Whole grain foods for the prevention of type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2008, 1, CD006061. [Google Scholar]
- De Koning, L.; Chiuve, S.E.; Fung, T.T.; Willett, W.C.; Rimm, E.B.; Hu, F.B. Diet-quality scores and the risk of type 2 diabetes in men. Diabetes Care 2011, 34, 1150–1156. [Google Scholar]
- Paniagua, J.A.; de la Sacristana, A.G.; Sanchez, E.; Romero, I.; Vidal-Puig, A.; Berral, F.J.; Escribano, A.; Moyano, M.J.; Perez-Martinez, P.; Lopez-Miranda, J.; et al. A MUFA-rich diet improves posprandial glucose, lipid and GLP-1 responses in insulin-resistant subjects. J. Am. Coll. Nutr. 2007, 26, 434–444. [Google Scholar]
- Ros, E. Dietary cis-monounsaturated fatty acids and metabolic control in type 2 diabetes. Am. J. Clin. Nutr. 2003, 78, S617–S625. [Google Scholar]
- Rocca, A.S.; LaGreca, J.; Kalitsky, J.; Brubaker, P.L. Monounsaturated fatty acid diets improve glycemic tolerance through increased secretion of glucagon-like peptide-1. Endocrinology 2001, 142, 1148–1155. [Google Scholar]
- Garg, A. High-monounsaturated-fat diets for patients with diabetes mellitus: A meta-analysis. Am. J. Clin. Nutr. 1998, 67, S577–S582. [Google Scholar]
- Perez-Jimenez, F.; Lopez-Miranda, J.; Mata, P. Protective effect of dietary monounsaturated fat on arteriosclerosis: Beyond cholesterol. Atherosclerosis 2002, 163, 385–398. [Google Scholar]
- Tortosa, A.; Bes-Rastrollo, M.; Sanchez-Villegas, A.; Basterra-Gortari, F.J.; Nunez-Cordoba, J.M.; Martinez-Gonzalez, M.A. Mediterranean diet inversely associated with the incidence of metabolic syndrome: The SUN prospective cohort. Diabetes Care 2007, 30, 2957–2959. [Google Scholar]
- Bach, A.; Serra-Majem, L.; Carrasco, J.L.; Roman, B.; Ngo, J.; Bertomeu, I.; Obrador, B. The use of indexes evaluating the adherence to the Mediterranean diet in epidemiological studies: A review. Public Health Nutr. 2006, 9, 132–146. [Google Scholar]
- Martinez-Gonzalez, M.A.; Lopez-Fontana, C.; Varo, J.J.; Sanchez-Villegas, A.; Martinez, J.A. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr. 2005, 8, 920–927. [Google Scholar]
- Devereux, G. The increase in the prevalence of asthma and allergy: Food for thought. Nat. Rev. Immunol. 2006, 6, 869–874. [Google Scholar]
- Chatzi, L.; Kogevinas, M. Prenatal and childhood Mediterranean diet and the development of asthma and allergies in children. Public Health Nutr. 2009, 12, 1629–1634. [Google Scholar]
- Arvaniti, F.; Priftis, K.N.; Panagiotakos, D.B. Dietary habits and asthma: A review. Allergy Asthma Proc. 2010, 31, e1–e10. [Google Scholar]
- Greene, L.S. Asthma, oxidant stress, and diet. Nutrition 1999, 15, 899–907. [Google Scholar]
- Torres-Borrego, J.; Moreno-Solis, G.; Molina-Teran, A.B. Diet for the prevention of asthma and allergies in early childhood: Much ado about something? Allergol. Immunopathol. 2012, 40, 244–252. [Google Scholar]
- Arvaniti, F.; Priftis, K.N.; Papadimitriou, A.; Papadopoulos, M.; Roma, E.; Kapsokefalou, M.; Anthracopoulos, M.B.; Panagiotakos, D.B. Adherence to the Mediterranean type of diet is associated with lower prevalence of asthma symptoms, among 10–12 years old children: The PANACEA study. Pediatr. Allergy Immunol. 2011, 22, 283–289. [Google Scholar]
- Castro-Rodriguez, J.A.; Garcia-Marcos, L.; Sanchez-Solis, M.; Perez-Fernandez, V.; Martinez-Torres, A.; Mallol, J. Olive oil during pregnancy is associated with reduced wheezing during the first year of life of the offspring. Pediatr. Pulmonol. 2010, 45, 395–402. [Google Scholar]
- Sewell, D.A.; Hammersley, V.S.; Devereux, G.; Robertson, A.; Stoddart, A.; Weir, C.; Worth, A.; Sheikh, A. Investigating the effectiveness of the Mediterranean diet in pregnant women for the primary prevention of asthma and allergy in high-risk infants: Protocol for a pilot randomised controlled trial. Trials 2013, 14, 173. [Google Scholar]
- Gdalevich, M.; Mimouni, D.; Mimouni, M. Breast-feeding and the risk of bronchial asthma in childhood: A systematic review with meta-analysis of prospective studies. J. Pediatr. 2001, 139, 261–266. [Google Scholar]
- Wright, A.L.; Holberg, C.J.; Taussig, L.M.; Martinez, F.D. Factors influencing the relation of infant feeding to asthma and recurrent wheeze in childhood. Thorax 2001, 56, 192–197. [Google Scholar]
- Forastiere, F.; Pistelli, R.; Sestini, P.; Fortes, C.; Renzoni, E.; Rusconi, F.; dell’Orco, V.; Ciccone, G.; Bisanti, L. Consumption of fresh fruit rich in vitamin C and wheezing symptoms in children. Thorax 2000, 55, 283–288. [Google Scholar] [CrossRef]
- Woods, R.K.; Walters, E.H.; Raven, J.M.; Wolfe, R.; Ireland, P.D.; Thien, F.C.K.; Abramson, M.J. Food and nutrient intakes and asthma risk in young adults. Am. J. Clin. Nutr. 2003, 78, 414–421. [Google Scholar]
- Chatzi, L.; Apostolaki, G.; Bibakis, I.; Skypala, I.; Bibaki-Liakou, V.; Tzanakis, N.; Kogevinas, M.; Cullinan, P. Protective effect of fruits, vegetables and the Mediterranean diet on asthma and allergies among children in Crete. Thorax 2007, 62, 677–683. [Google Scholar]
- Hodge, L.; Salome, C.M.; Peat, J.K.; Haby, M.M.; Xuan, W.; Woolcock, A.J. Consumption of oily fish and childhood asthma risk. Med. J. Aust. 1996, 164, 137–140. [Google Scholar]
- Shaheen, S.O.; Sterne, J.A.; Thompson, R.L.; Songhurst, C.E.; Margetts, B.M.; Burney, P.G. Dietary antioxidants and asthma in adults: Population-based case-control study. Am. J. Respir. Crit. Care Med. 2001, 164, 1823–1828. [Google Scholar] [CrossRef]
- Janson, C.; Anto, J.; Burney, P.; Chinn, S.; de Marco, R.; Heinrich, J.; Jarvis, D.; Kuenzli, N.; Leynaert, B.; Luczynska, C.; et al. The European Community Respiratory Health Survey: What are the main results so far? Eur. Respir. J. 2001, 18, 598–611. [Google Scholar] [CrossRef]
- Netuveli, G.; Hurwitz, B.; Sheikh, A. Lineages of language and the diagnosis of asthma. J. R. Soc. Med. 2007, 100, 19–24. [Google Scholar] [CrossRef]
- Lederberg, J. Infectious history. Science 2000, 288, 287–293. [Google Scholar]
- Ottman, N.; Smidt, H.; de Vos, W.M.; Belzer, C. The function of our microbiota: Who is out there and what do they do? Front. Cell. Infect. Microbiol. 2012. [Google Scholar] [CrossRef]
- Tremaroli, V.; Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Albenberg, L.G.; Lewis, J.D.; Wu, G.D. Food and the gut microbiota in inflammatory bowel diseases: A critical connection. Curr. Opin. Gastroenterol. 2012, 28, 314–320. [Google Scholar]
- Compare, D.; Coccoli, P.; Rocco, A.; Nardone, O.M.; de Maria, S.; Cartenì, M.; Nardone, G. Gut—Liver axis: The impact of gut microbiota on non alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 471–476. [Google Scholar]
- Weiss, R.; Dziura, J.; Burgert, T.S.; Tamborlane, W.V.; Taksali, S.E.; Yeckel, C.W.; Allen, K.; Lopes, M.; Savoye, M.; Morrison, J.; et al. obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 2004, 350, 2362–2374. [Google Scholar] [CrossRef]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609. [Google Scholar]
- Gareau, M.G.; Wine, E.; Rodrigues, D.M.; Cho, J.H.; Whary, M.T.; Philpott, D.J.; Macqueen, G.; Sherman, P.M. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 2011, 60, 307–317. [Google Scholar] [CrossRef]
- Hosseini, E.; Grootaert, C.; Verstraete, W.; van de Wiele, T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev. 2011, 69, 245–258. [Google Scholar] [CrossRef]
- Farrell, G.C.; van Rooyen, D.; Gan, L.; Chitturi, S. NASH is an inflammatory disorder: pathogenic, prognostic and therapeutic implications. Gut Liver 2012, 6, 149–171. [Google Scholar] [CrossRef]
- Henao-Mejia, J.; Elinav, E.; Strowig, T.; Flavell, R.A. Inflammasomes: Far beyond inflammation. Nat. Immunol. 2012, 13, 321–324. [Google Scholar] [CrossRef]
- Comito, D.; Cascio, A.; Romano, C. Microbiota biodiversity in inflammatory bowel disease. Ital. J. Pediatr. 2014. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Heuman, D.M.; Sanyal, A.J.; Hylemon, P.B.; Sterling, R.K.; Stravitz, R.T.; Fuchs, M.; Ridlon, J.M.; Daita, K.; Monteith, P.; et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One 2013, 8, e60042. [Google Scholar]
- Dhiman, R.K. Gut microbiota and hepatic encephalopathy. Metab. Brain Dis. 2013, 28, 321–326. [Google Scholar] [CrossRef]
- Bajaj, J.S. The role of microbiota in hepatic encephalopathy. Gut Microbes 2014. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Greco, F.; Barone, G.; Gargante, M.P.; Malaguarnera, M.; Toscano, M.A. Bifidobacterium longum with fructo-oligosaccharide (FOS) treatment in minimal hepatic encephalopathy: A randomized, double-blind, placebo-controlled study. Dig. Dis. Sci. 2007, 52, 3259–3265. [Google Scholar] [CrossRef]
- Sokol, H.; Seksik, P.; Furet, J.P.; Firmesse, O.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J.; Corthier, G.; Marteau, P.; Doré, J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189. [Google Scholar] [CrossRef]
- Camilleri, M. Peripheral mechanisms in irritable bowel syndrome. N. Engl. J. Med. 2012, 367, 1626–1635. [Google Scholar]
- Moayyedi, P.; Ford, A.C.; Talley, N.J.; Cremonini, F.; Foxx-Orenstein, A.E.; Brandt, L.J.; Quigley, E.M.M. The efficacy of probiotics in the treatment of irritable bowel syndrome: A systematic review. Gut 2010, 59, 325–332. [Google Scholar]
- Okada, H.; Kuhn, C.; Feillet, H.; Bach, J.F. The “hygiene hypothesis” for autoimmune and allergic diseases: An update. Clin. Exp. Immunol. 2010, 160, 1–9. [Google Scholar]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar]
- Malaguarnera, M.; Vacante, M.; Antic, T.; Giordano, M.; Chisari, G.; Acquaviva, R.; Mastrojeni, S.; Malaguarnera, G.; Mistretta, A.; Li Volti, G.; et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig. Dis. Sci. 2012, 57, 545–553. [Google Scholar]
- Uccello, M.; Malaguarnera, G.; Basile, F.; D’agata, V.; Malaguarnera, M.; Bertino, G.; Vacante, M.; Drago, F.; Biondi, A. Potential role of probiotics on colorectal cancer prevention. BMC Surg. 2012, 12, S35. [Google Scholar] [CrossRef]
- Del Chierico, F.; Gnani, D.; Vernocchi, P.; Petrucca, A.; Alisi, A.; Dallapiccola, B.; Nobili, V.; Lorenza, P. Meta-omic platforms to assist in the understanding of NAFLD gut microbiota alterations: Tools and applications. Int. J. Mol. Sci. 2014, 15, 684–711. [Google Scholar] [CrossRef]
- Del Chierico, F.; Vernocchi, P.; Bonizzi, L.; Carsetti, R.; Castellazzi, A.M.; Dallapiccola, B.; de Vos, W.; Guerzoni, M.E.; Manco, M.; Marseglia, G.L.; et al. Early-life gut microbiota under physiological and pathological conditions: The central role of combined meta-omics-based approaches. J. Proteomics 2012, 75, 4580–4587. [Google Scholar]
- Zhang, C.; Zhang, M.; Wang, S.; Han, R.; Cao, Y.; Hua, W.; Mao, Y.; Zhang, X.; Pang, X.; Wei, C.; et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010, 4, 232–241. [Google Scholar]
- De Filippo, C.; Cavalieri, D.; di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar]
- Muegge, B.D.; Kuczynski, J.; Knights, D.; Clemente, J.C.; Gonzalez, A.; Fontana, L.; Henrissat, B.; Knight, R.; Gordon, J.I. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science 2011, 332, 970–974. [Google Scholar]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef]
- Siddharth, J.; Holway, N.; Parkinson, S.J. A Western diet ecological module identified from the “humanized” mouse microbiota predicts diet in adults and formula feeding in children. PLoS One 2013, 8, e83689. [Google Scholar]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar]
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl. Environ. Microbiol. 2007, 73, 1073–1078. [Google Scholar] [CrossRef]
- Marlow, G.; Ellett, S.; Ferguson, I.R.; Zhu, S.; Karunasinghe, N.; Jesuthasan, A.C.; Han, D.Y.; Fraser, A.G.; Ferguson, L.R. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum. Genomics 2013. [Google Scholar] [CrossRef]
- Bisoendial, R.J.; Boekholdt, S.M.; Vergeer, M.; Stroes, E.S.G.; Kastelein, J.J.P. C-reactive protein is a mediator of cardiovascular disease. Eur. Heart J. 2010, 31, 2087–2091. [Google Scholar]
- Thomas, P.; Fenech, M. Cytokinesis-block micronucleus cytome assay in lymphocytes. Methods Mol. Biol. 2011, 682, 217–234. [Google Scholar]
- Nanau, R.M.; Neuman, M.G. Nutritional and probiotic supplementation in colitis models. Dig. Dis. Sci. 2012, 57, 2786–2810. [Google Scholar]
- Santoro, A.; Pini, E.; Scurti, M.; Palmas, G.; Berendsen, A.; Brzozowska, A.; Pietruszka, B.; Szczecinska, A.; Cano, N.; Meunier, N.; et al. The NU-AGE consortium combating inflammaging through a Mediterranean whole diet approach: The NU-AGE project’s conceptual framework and design. Mech. Ageing Dev. 2013, 136–137, 3–13. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Del Chierico, F.; Vernocchi, P.; Dallapiccola, B.; Putignani, L. Mediterranean Diet and Health: Food Effects on Gut Microbiota and Disease Control. Int. J. Mol. Sci. 2014, 15, 11678-11699. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms150711678
Del Chierico F, Vernocchi P, Dallapiccola B, Putignani L. Mediterranean Diet and Health: Food Effects on Gut Microbiota and Disease Control. International Journal of Molecular Sciences. 2014; 15(7):11678-11699. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms150711678
Chicago/Turabian StyleDel Chierico, Federica, Pamela Vernocchi, Bruno Dallapiccola, and Lorenza Putignani. 2014. "Mediterranean Diet and Health: Food Effects on Gut Microbiota and Disease Control" International Journal of Molecular Sciences 15, no. 7: 11678-11699. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms150711678