Recently Discovered Adipokines and Cardio-Metabolic Comorbidities in Childhood Obesity
Abstract
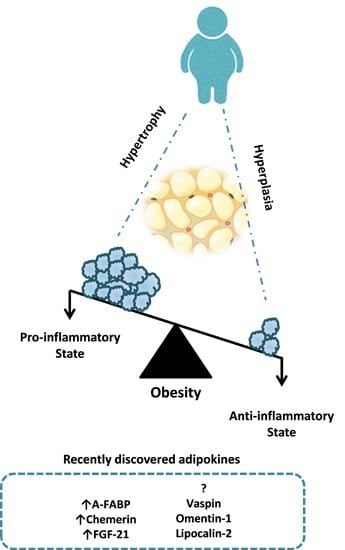
:1. Introduction
| Adipokine | Author [Ref.] | Population and Design | Main Findings | ||
|---|---|---|---|---|---|
| A-FABP | Corripio et al. [5] | At baseline: 73 obese vs. 47 normal-weight children aged 6–10 years; At 2 years: 31 obese patients lost weight; prospective cohort interventional study (2 years). | At baseline: 35.2 ± 14.6 vs. 10.4 ± 4.54 ng/mL (p < 0.001); At 2 years: in weigh losers from 37.3 ± 18.4 at baseline to 24.4 ± 13.9 ng/mL at 2 years (p < 0.001) | ||
| Choi et al. [6] | At baseline: 48 overweight vs. 111 normal-weight children aged 9 years. At 3 years: 55 overweight vs. 104 normal-weight children of whom 10 boys developed MetS; prospective cohort observational study (3 years). | At baseline: 23.6 ± 8.2 vs. 12.8 ± 5.1 μg/L (p < 0.05); At 3 years: 25.9 ± 10.5 in those who developed MetS vs. 15.6 ± 7.4 μg/L (p < 0.001) | |||
| Yun et al. [7] | 161 children aged 9 years: 80 boys: 12 overweight vs. 25 at overweight vs. 43 normal weight and 81 girls: 13 overweight vs. 23 overweight vs. 43 normal-weight; cross-sectional observational study. | In boys: 22.3 ± 8.7 vs. 14.4 ± 5.2 vs. 8.5 ± 3.7 ng/mL; In girls: 24.4 ± 8.7 vs. 16.0 ± 7.9 vs. 7.8 ± 4.3 ng/mL (p < 0.01 for both) | |||
| Krzystek-Korpacka et al. [8] | At baseline: 87 obese vs. 27 overweight vs. 31 normal-weight children aged 10–17 years; At 1 year: 84 patients under weight loss program and/or metformin treatment; prospective cohort interventional study (1 year). | Mean (95 CI%): 48.2 (44.5–51.9) at baseline vs. 33.2 (30.1–36.3) μg/L at 1 year (p < 0.001) | |||
| Reinehr et al. [9] | At baseline: 30 obese vs. 10 normal-weight children aged 8–15 years; At 1 year: 10 lost weight; prospective cohort interventional study (1 year). | At baseline: increased A-FABP levels in obese children (data not reported, p = 0.009). Median and (IQR) at 1 year: in weigh losers from 41 (31–49) at to 29 (20–37) μg/L at 1 year (p < 0.01) | |||
| Schipper et al. [10] | 60 obese vs. 30 normal-weight children aged 6–16 years; cross-sectional observational study. | Median (IQR): 24.0 (21.5–27.0) vs. 23.6 (20.5–27.9) ng/mL (p = NS) | |||
| Reyman et al. [11] | 36 25(OH)D-deficient vs. 28 (in)sufficient obese children aged 6–16 years and 28 obese vs. 27 normal-weight 25(OH)D (in)sufficient children aged 6–16 years; cross-sectional observational study. | Median (IQR): 23.0 (20.9–26.4) vs. 25.7 (22.6–27.3) vs. 22.8 (20.4–27.6) ng/mL (p = NS) | |||
| Chemerin | Schipper et al. [10] | 60 obese vs. 30 normal-weight children aged 6–16 years; cross-sectional observational study. | Median (IRQ): 3.0 ± 0.5 vs. 2.8 ± 0.4 µg/mL (p < 0.05) | ||
| Reyman et al. [11] | 36 25(OH)D-deficient vs. 28 (in)sufficient obese children aged 6–16 years and 28 obese vs. 27 normal-weight 25(OH)D (in)sufficient children aged 6–16 years; cross-sectional observational study. | Median (IQR): 3.13 (2.74–3.47) vs. 2.87 µg/mL (2.50–3.11) (p < 0.05);2.87 (2.50–3.11) vs. 2.80 µg/mL (2.48–3.00) (p = NS) | |||
| Landgraf et al. [12] | 105 obese vs. 69 normal-weight children aged 7–18 years; cross-sectional observational study. | 117.82 ± 26.4 vs. 89.8 ± 16.1 ng/mL (p < 0.001) | |||
| FGF-21 | Reinehr et al. [13] | At baseline: 60 obese vs. 40 normal-weight children aged 12–15 years; At 1 year: obese children with decreased SDS-BMI; prospective cohort interventional study (1 year). | Median (IQR): 195 (114–347) vs. 56 (33–122) pg/mL (p < 0.001); In weight loser from 206 (98–406) at baseline to 1 year 139 pg/mL (66–307) at 1 year (p = 0.038) | ||
| Giannini et al. [14] | 79 obese with high hepatic fat fraction (HFF% ≥ 5.5%) vs. 107 obese with HFF% < 5.5% vs. 31 normal-weight with HFF% < 5.5% adolescents aged 14–17 years; cross-sectional observational study. | 277 ± 21 vs. 135 ± 8 vs. 99 ± 12 pg/mL (p < 0.001) | |||
| Lipocalin-2 | Corripio et al. [5] | At baseline: 73 obese vs. 47 normal-weight children aged 6–10 years; At 2 years: 31 weight losers; prospective cohort interventional study (2 years). | At baseline: 50.7 ± 18.4 vs. 28.0 ± 7.75 ng/mL (p < 0.001). At 2 years: in weight losers from 48.4 ± 18.4 to 50.7 ± 23.4 ng/mL (p = 0.875) | ||
| Akelma et al. [15] | 33 obese vs. 34 normal-weight children aged 9–14 years; cross-sectional observational study. | 103.72 ± 41.26 vs. 94.03 ± 49.61 ng/mL (p = NS) | |||
| Kanaka-Gantenbein et al. [16] | 20 morbidly obese vs. 20 obese vs. 20 overweight vs. 20 normal-weight girls aged 9–16 years; cross-sectional observational study. | Mean (SD): 16.3 (3.7) vs. 12.2 (1.7) vs. 17.5 (3.1) vs. 23.3 (4.6) μg/L; (p < 0.05 between obese and normal-weight) | |||
| Omentin-1 | Schipper et al. [10] | 60 obese vs. 30 normal-weight children aged 6–16 years; cross-sectional observational study. | Median (IQR): 4.0 (3.5–4.5) vs. 3.8 (3.3–4.4) pg/mL (p = 0.032) | ||
| Reyman et al. [11] | 36 25(OH)D-deficient vs. 28 (in)sufficient obese children aged 6–16 years and 28 obese vs. 27 normal-weight 25(OH)D (in)sufficient children aged 6–16 years; cross-sectional observational study. | Median (IQR): 4.06 (3.43–4.55) vs. 3.81 (3.32–4.53) vs. 3.79 (3.33–4.40) pg/mL | |||
| Catli et al. [17] | 49 obese vs. 30 normal-weight children aged 6–14 years; cross-sectional observational study. | 24.3 ± 9.8 vs. 29.0 ± 6.7 ng/mL (p = 022) | |||
| Vaspin | Ko et al. [18] | 82 overweight vs. 86 normal-weight boys aged 9 years and 86 overweight vs. 90 normal-weight girls aged 9 years; cross-sectional observational study. | 0.33 ± 0.59 vs. 0.18 ± 0.26 ng/mL (p < 0.05); 0.28 ± 0.52 vs. 0.17 ± 0.13 ng/mL (p < 0.05) | ||
| Suleymanoglu et al. [19] | 33 obese vs. 36 normal-weight children aged 11–16 years; cross-sectional observational study. | 0.64 ± 0.3 vs. 0.42 ± 0.24 μg/L (p = 0.002) | |||
| Korner et al. [20] | 67 obese vs. 65 normal-weight aged 7–19 years; cross-sectional observational study. | 0.55 ± 0.06 vs. 0.92 ± 0.14 ng /mL (p = 0.013) | |||
| Martos Moreno et al. [21] | 100 obese vs. 42 normal-weight children aged 5–13 years; prospective cohort interventional study (duration not reported). | At baseline 0.34 ± 0.34 vs. 0.34 ± 0.33 ng/mL (p = NS); no significant change after either 1 or 2 SDS BMI reduction | |||
2. Adipose Tissue and Inflammation in Childhood Obesity

2.1. Adipocyte-Fatty Acid-Binding Protein
2.2. Chemerin
2.3. Fibroblastic Growth Factor-21
2.4. Lipocalin-2

2.5. Omentin-1
2.6. Vaspin
3. Conclusions
Abbreviations
| A-FABP | adipocytes fatty acids binding protein |
| BAT | brown adipose tissue |
| BMI | body mass index |
| BP | blood pressure |
| FFA | free fatty acids |
| FGF-21 | fibroblast growth factor-21 |
| HDL-c | high density lipoprotein-cholesterol |
| HOMA-IR | homeostasis model assessment—insulin resistance |
| Hs-CRP | high sensitivity C-reactive protein |
| IR | insulin resistance |
| ISI | insulin sensitivity index |
| LDL-c | low density lipoprotein-cholesterol |
| MetS | metabolic syndrome |
| NAFLD | non-alcoholic fatty liver |
| OGTT | Oral glucose tolerance test |
| SAT | subcutaneous adipose tissue |
| T2DM | type II diabetes |
| TAG | triacylglycerols |
| VAT | visceral adipose tissue |
| WAT | white adipose tissue |
| WC | waist circumference |
Author Contributions
Conflicts of Interest
References
- Cali, A.M.; Caprio, S. Obesity in children and adolescents. J. Clin. Endocrinol. Metab. 2008, 93, S31–S36. [Google Scholar] [CrossRef] [PubMed]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, K.A.; Lidell, M.E.; Orava, J.; Heglind, M.; Westergren, R.; Niemi, T.; Taittonen, M.; Laine, J.; Savisto, N.-J.; Enerba ck, S.; et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009, 360, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Balagopal, P.B.; de Ferranti, S.D.; Cook, S.; Daniels, S.R.; Gidding, S.S.; Hayman, L.L.; McCrindle, B.W.; Mietus-Snyder, M.L.; Steinberger, J. Nontraditional risk factors and biomarkers for cardiovascular disease: Mechanistic, research, and clinical considerations for youth—A scientific statement from the American Heart Association. Circulation 2011, 123, 2749–2769. [Google Scholar] [CrossRef] [PubMed]
- Corripio, R.; Gonzalez-Clemente, J.M.; Perez-Sanchez, J.; Naf, S.; Gallart, L.; Nosas, R.; Vendrell, J.; Caixàs, A. Weight loss in prepubertal obese children is associated with a decrease in adipocyte fatty-acid-binding protein without changes in lipocalin-2: A 2-year longitudinal study. Eur. J. Endocrinol. 2010, 163, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Yannakoulia, M.; Park, M.S.; Cho, G.J.; Kim, J.H.; Lee, S.H.; Hwang, T.G.; Yang, S.J.; Kim, S.M.; Yoo, H.J.; et al. Serum adipocyte fatty acid-binding protein, retinol-binding protein 4, and adiponectin concentrations in relation to the development of the metabolic syndrome in korean boys: A 3-year prospective cohort study. Am. J. Clin. Nutr. 2011, 93, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.E.; Kim, S.M.; Choi, K.M.; Park, H.S. Association between adipocyte fatty acid-binding protein levels and childhood obesity in korean children. Metabolism 2009, 58, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Krzystek-Korpacka, M.; Patryn, E.; Bednarz-Misa, I.; Mierzchala, M.; Hotowy, K.; Czapinska, E.; Kustzeba-Wojcicka, I.; Gamian, A.; Noczynska, A. Circulating adipocyte fatty acid-binding protein, juvenile obesity, and metabolic syndrome. J. Pediatr. Endocrinol. Metable 2011, 24, 921–928. [Google Scholar]
- Reinehr, T.; Stoffel-Wagner, B.; Roth, C.L. Adipocyte fatty acid-binding protein in obese children before and after weight loss. Metabolism 2007, 56, 1735–1741. [Google Scholar] [CrossRef]
- Schipper, H.S.; Nuboer, R.; Prop, S.; van den Ham, H.J.; de Boer, F.K.; Kesmir, C.; Mombers, I.M.; van Bekkum, K.A.; Woudstra, J.; Kieft, J.H. Systemic inflammation in childhood obesity: Circulating inflammatory mediators and activated CD14++ monocytes. Diabetologia 2012, 55, 2800–2810. [Google Scholar] [CrossRef] [PubMed]
- Reyman, M.; Verrijn Stuart, A.A.; van Summeren, M.; Rakhshandehroo, M.; Nuboer, R.; de Boer, F.K.; van den Ham, H.J.; Kalkhoven, E.; Prakken, B.; Schipper, H.S. Vitamin D deficiency in childhood obesity is associated with high levels of circulating inflammatory mediators, and low insulin sensitivity. Int. J. Obes. (Lond.) 2014, 38, 46–52. [Google Scholar] [CrossRef]
- Landgraf, K.; Friebe, D.; Ullrich, T.; Kratzsch, J.; Dittrich, K.; Herberth, G.; Adams, V.; Kiess, W.; Erbs, S.; Korner, A. Chemerin as a mediator between obesity and vascular inflammation in children. J. Clin. Endocrinol. Metable 2012, 97, E556–E564. [Google Scholar] [CrossRef]
- Zhang, X.; Yeung, D.C.; Karpisek, M.; Stejskal, D.; Zhou, Z.G.; Liu, F.; Wong, R.L.; Chow, W.S.; Tso, A.W.; Lam, K.S.L.; et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008, 5, 1246–1253. [Google Scholar] [CrossRef]
- Reinehr, T.; Woelfle, J.; Wunsch, R.; Roth, C.L. Fibroblast growth factor 21 (FGF-21) and its relation to obesity, metabolic syndrome, and nonalcoholic fatty liver in children: A longitudinal analysis. J. Clin. Endocrinol. Metable 2012, 97, 2143–2150. [Google Scholar] [CrossRef]
- Akelma, A.Z.; Abaci, A.; Ozdemir, O.; Celik, A.; Avci, Z.; Razi, C.H.; Hizli, S.; Akin, O. The association of serum lipocalin-2 levels with metabolic and clinical parameters in obese children: A pilot study. J. Pediatr. Endocrinol. Metable 2012, 525, 525–528. [Google Scholar]
- Kanaka-Gantenbein, C.; Margeli, A.; Pervanidou, P.; Sakka, S.; Mastorakos, G.; Chrousos, G.P.; Papassotiriou, I. Retinol-binding protein 4 and lipocalin-2 in childhood and adolescent obesity: When children are not just “small adults”. Clin. Chem. 2008, 54, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Catli, G.; Anik, A.; Abaci, A.; Kume, T.; Bober, E. Low omentin-1 levels are related with clinical and metabolic parameters in obese children. Exp. Clin. Endocrinol. Diabetes 2013, 121, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.J.; Lee, M.; Park, H.S.; Han, K.; Cho, G.J.; Hwang, T.G.; Kim, J.H.; Lee, S.H.; Lee, H.Y.; Kim, S.M. Elevated vaspin and leptin levels are associated with obesity in prepubertal Korean children. Endocr. J. 2013, 60, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Suleymanoglu, S.; Tascilar, E.; Pirgon, O.; Tapan, S.; Meral, C.; Abaci, A. Vaspin and its correlation with insulin sensitivity indices in obese children. Diabetes Res. Clin. Pract. 2009, 84, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Korner, A.; Neef, M.; Friebe, D.; Erbs, S.; Kratzsch, J.; Dittrich, K.; Bluher, S.; kapellen, T.M.; Kovacs, P.; Stumvoll, M.; et al. Vaspin is related to gender, puberty and deteriorating insulin sensitivity in children. Int. J. Obes. (Lond.) 2011, 35, 578–586. [Google Scholar] [CrossRef]
- Martos-Moreno, G.A.; Kratzsch, J.; Korner, A.; Barrios, V.; Hawkins, F.; Kiess, W.; Argente, J. Serum visfatin and vaspin levels in prepubertal children: Effect of obesity and weight loss after behavior modifications on their secretion and relationship with glucose metabolism. Int. J. Obes. (Lond.) 2011, 35, 1355–1362. [Google Scholar] [CrossRef]
- Martos-Moreno, G.A.; Barrios, V.; Chowen, J.A.; Argente, J. Adipokines in childhood obesity. Vitam. Horm. 2013, 91, 107–142. [Google Scholar] [PubMed]
- Manco, M.; Putignani, L.; Bottazzo, G.F. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr. Rev. 2010, 31, 817–844. [Google Scholar] [CrossRef] [PubMed]
- Taveras, E.M.; Rifas-Shiman, S.L.; Belfort, M.B.; Kleinman, K.P.; Oken, E.; Gillman, M.W. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics 2009, 123, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Rolland-Cachera, M.F.; Deheeger, M.; Bellisle, F.; Sempé, M.; Guilloud-Bataille, M.; Patois, E. Adiposity rebound in children: a simple indicator for predicting obesity. Am. J. Clin. Nutr. 1984, 39, 129–135. [Google Scholar] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Drubach, L.A.; Palmer, E.L., 3rd.; Connolly, L.P.; Baker, A.; Zurakowski, D.; Cypess, A.M. Pediatric brown adipose tissue: detection, epidemiology, and differences from adults. J. Pediatr. 2011, 159, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, J.; Cereijo, R.; Villarroya, F. An endocrine role for brown adipose tissue? Am. J. Physiol. Endocrinol. Metable 2013, 305, E567–E572. [Google Scholar] [CrossRef]
- Daniels, S.R.; Arnett, D.K.; Eckel, R.H.; Gidding, S.S.; Hayman, L.L.; Kumanyika, S.; Robinson, T.N.; Scott, B.J.; St Jeor, S.; Williams, C.L. Overweight in children and adolescents. Pathophysiology, consequences, prevention, and treatment. Circulation 2005, 111, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Taksali, S.E.; Caprio, S.; Dziura, J.; Dufour, S.; Cali, A.M.; Goodman, T.R.; Papademetris, X.; Burgert, T.S.; Pierpont, B.M.; Savoye, A.A.; et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: A determinant of an adverse metabolic phenotype. Diabetes 2008, 57, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Manco, M.; Bottazzo, G.; DeVito, R.; Marcellini, M.; Mingrone, G.; Nobili, V. Nonalcoholic fatty liver disease in children. J. Am. Coll. Nutr. 2008, 27, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Manco, M.; Morandi, A.; Marigliano, M.; Rigotti, F.; Manfredi, R.; Maffeis, C. Epicardial fat, abdominal adiposity and insulin resistance in obese pre-pubertal and early pubertal children. Atherosclerosis 2013, 226, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Fucho, R.; Gorgun, C.Z.; Tuncman, G.; Cao, H.; Hotamisligil, G.S. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J. Clin. Investig. 2008, 118, 2640–2650. [Google Scholar] [PubMed]
- Rolph, M.S.; Young, T.R.; Shum, B.O.; Gorgun, C.Z.; Schmitz-Peiffer, C.; Ramshaw, I.A.; Hotamisligil, G.S.; Mackay, C.R. Regulation of dendritic cell function and T cell priming by the fatty acid-binding protein AP2. J. Immunol. 2006, 177, 7794–7801. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Wang, Y.; Xu, J.Y.; Stejskal, D.; Tam, S.; Zhang, J.; Wat, N.M.; Wong, W.K.; Lam, K.S. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin. Chem. 2006, 52, 405–413. [Google Scholar] [CrossRef]
- Cabre, A.; Lazaro, I.; Girona, J.; Manzanares, J.M.; Marimon, F.; Plana, N.; Heras, M.; Masana, L. Fatty acid binding protein 4 is increased in metabolic syndrome and with thiazolidinedione treatment in diabetic patients. Atherosclerosis 2007, 195, e150–e158. [Google Scholar] [CrossRef] [PubMed]
- Milner, K.L.; van der Poorten, D.; Xu, A.; Bugianesi, E.; Kench, J.G.; Lam, K.S.; Chisholm, D.J.; George, J. Adipocyte fatty acid binding protein levels relate to inflammation and fibrosis in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1926–1934. [Google Scholar] [CrossRef] [PubMed]
- Kralisch, S.; Fasshauer, M. Adipocyte fatty acid binding protein: A novel adipokine involved in the pathogenesis of metabolic and vascular disease? Diabetologia 2013, 56, 10–21. [Google Scholar] [CrossRef]
- Wittamer, V.; Franssen, J.D.; Vulcano, M.; Mirjolet, J.F.; le Poul, E.; Migeotte, I.; Brèzillon, S.; Tyldesley, R.; Blanpain, C.; Detheux, M.; et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 2003, 198, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007, 282, 28175–28141. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.Y.; Lee, D.C.; Chu, S.H.; Jeon, J.Y.; Lee, M.K.; Im, J.A.; Lee, J.W. Chemerin levels are positively correlated with abdominal visceral fat accumulation. Clin. Endocrinol. (Oxf.) 2012, 77, 47–50. [Google Scholar] [CrossRef]
- Chu, S.H.; Lee, M.K.; Ahn, K.Y.; Im, J.A.; Park, M.S.; Lee, D.C.; Jeon, J.Y.; Lee, J.W. Chemerin and adiponectin contribute reciprocally to metabolic syndrome. PLoS One 2012, 7, e34710. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Takahashi, Y.; Takahashi, K.; Zolotaryov, F.N.; Hong, K.S.; Kitazawa, R.; Lida, K.; Okimura, Y.; Kaji, H.; Kitazawa, S.; et al. Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3T3-L1 adipocytes. FEBS Lett. 2008, 582, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. Towards an integrative approach to understanding the role of chemerin in human health and disease. Obes. Rev. 2013, 14, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, H.; Kameshima, S.; Usui, T.; Okada, M.; Hara, Y. A novel adipocytokine, chemerin exerts anti-inflammatory roles in human vascular endothelial cells. Biochem. Biophys. Res. Commun. 2012, 423, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenkov, A. FGFs and metabolism. Curr. Opin. Pharmacol. 2009, 9, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Nakatake, Y.; Konishi, M.; Itoh, N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Biophys. Acta 2000, 1492, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Hondares, E.; Gallego-Escuredo, J.M.; Flachs, P.; Frontini, A.; Cereijo, R.; Goday, A.; Perugini, J.; Kopecky, P.; Giralt, M.; Cinti, S.; et al. Fibroblast growth factor-21 is expressed in neonatal and pheochromocytoma-induced adult human brown adipose tissue. Metabolism 2014, 63, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Berglund, E.D.; Li, C.Y.; Bina, H.A.; Lynes, S.E.; Michael, M.D.; Shanafelt, A.B.; Kharitonenkov, A.; Wasserman, D.H. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology 2009, 150, 4084–4093. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenkov, A.; Wroblewski, V.J.; Koester, A.; Chen, Y.F.; Clutinger, C.K.; Tigno, X.T.; Hansen, B.C.; Shanafelt, A.B.; Etgen, G.J. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 2007, 148, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Coskun, T.; Bina, H.A.; Schneider, M.A.; Dunbar, J.D.; Hu, C.C.; Chen, Y.; Moller, D.E.; Kharitonenkow, A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008, 149, 6018–6027. [Google Scholar] [CrossRef]
- Fisher, F.M.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012, 26, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, P.; Selgas, R.; Romero, S.; Diez, J.J. Biological role, clinical significance, and therapeutic possibilities of the recently discovered metabolic hormone fibroblastic growth factor 21. Eur. J. Endocrinol. 2012, 167, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Dushay, J.; Chui, P.C.; Gopalakrishnan, G.S.; Varela-Rey, M.; Crawley, M.; Fisher, F.M.; Badman, M.K.; Martinez-Chantar, M.L.; Maratos-Flier, E. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 2010, 139, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Giannini, C.; Feldstein, A.E.; Santoro, N.; Kim, G.; Kursawe, R.; Pierpont, B.; Caprio, S. Circulating levels of FGF-21 in obese youth: Associations with liver fat content and markers of liver damage. J. Clin. Endocrinol. Metable 2013, 98, 2993–3000. [Google Scholar] [CrossRef]
- Feldstein, A.E.; Alkhouri, N.; de Vito, R.; Alisi, A.; Lopez, R.; Nobili, V. Serum cytokeratin-18 fragment levels are useful biomarkers for nonalcoholic steatohepatitis in children. Am. J. Gastroenterol. 2013, 108, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Mai, K.; Andres, J.; Biedasek, K.; Weicht, J.; Bobbert, T.; Sabath, M.; Meinus, S.; Reinecke, F.; Mohlig, M.; Weickert, M.O.; et al. Free fatty acids link metabolism and regulation of the insulin-sensitizing fibroblast growth factor-21. Diabetes 2009, 58, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, L.; Johnsen, A.H.; Sengelov, H.; Borregaard, N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 1993, 268, 10425–10432. [Google Scholar] [PubMed]
- Auguet, T.; Quintero, Y.; Terra, X.; Martinez, S.; Lucas, A.; Pellitero, S.; Aguilar, C.; Hernandez, M.; del Castillo, D.; Richart, C. Upregulation of lipocalin 2 in adipose tissues of severely obese women: Positive relationship with proinflammatory cytokines. Obesity 2011, 19, 2295–2300. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lam, K.S.; Kraegen, E.W.; Sweeney, G.; Zhang, J.; Tso, A.W.; Chow, W.S.; Wat, N.M.; Xu, J.Y.; Hoo, R.L.; et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin. Chem. 2007, 53, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hamnvik, O.P.; Petrou, M.; Gong, H.; Chamberland, J.P.; Christophi, C.A.; Kales, S.N.; Christiani, D.C.; Mantzoros, C.S. Circulating lipocalin 2 is associated with body fat distribution at baseline but is not an independent predictor of insulin resistance: The prospective cyprus metabolism study. Eur. J. Endocrinol. 2011, 165, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S.; Uehori, J.; Matsumoto, M.; Suzuki, Y.; Matsuhisa, A.; Toyoshima, K.; Seya, T. Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall. J. Biol. Chem. 2001, 276, 23456–23463. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Z.; Lee, M.J.; Hu, H.; Pray, J.; Wu, H.B.; Hansen, B.C.; Shuldiner, A.R.; Fried, S.K.; McLenithan, J.C.; Gong, D.W. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: Possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metable 2006, 290, E1253–E1261. [Google Scholar] [CrossRef]
- Jaikanth, C.; Gurumurthy, P.; Cherian, K.M.; Indhumathi, T. Emergence of omentin as a pleiotropic adipocytokine. Exp. Clin. Endocrinol. Diabetes 2013, 121, 377–383. [Google Scholar] [CrossRef] [PubMed]
- De Souza Batista, C.M.; Yang, R.Z.; Lee, M.J.; Glynn, N.M.; Yu, D.Z.; Pray, J.; Ndubuizu, K.; Patil, S.; Schwartz, A. Omentin plasma levels and gene expression are decreased in obesity. Diabetes 2007, 56, 1655–1661. [Google Scholar]
- Prats-Puig, A.; Bassols, J.; Bargallo, E.; Mas-Parareda, M.; Ribot, R.; Soriano-Rodriguez, P.; Berebgui, A.; Diaz, M.; de Zegher, F.; Ibanez, L.; et al. Toward an early marker of metabolic dysfunction: Omentin-1 in prepubertal children. Obesity 2011, 19, 1905–1907. [Google Scholar] [CrossRef] [PubMed]
- Kloting, N.; Kovacs, P.; Kern, M.; Heiker, J.T.; Fasshauer, M.; Schon, M.R.; Stumvoll, M.; Beck-Sickinger, A.G.; Bluher, M. Central vaspin administration acutely reduces food intake and has sustained blood glucose-lowering effects. Diabetologia 2011, 54, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Wada, J.; Eguchi, J.; Zhang, H.; Baba, M.; Seida, A.; Hashimoto, I.; Okada, T.; Yasuhara, A.; Nakatsuka, A.; et al. Visceral adipose tissue-derived serine protease inhibitor: A unique insulin-sensitizing adipocytokine in obesity. Proc. Natl. Acad. Sci. USA 2005, 102, 10610–10615. [Google Scholar] [CrossRef] [PubMed]
- Bluher, M. Vaspin in obesity and diabetes: Pathophysiological and clinical significance. Endocrine 2012, 41, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Youn, B.S.; Kloting, N.; Kratzsch, J.; Lee, N.; Park, J.W.; Song, E.S.; Ruschke, K.; Oberbach, A.; Fasshauer, M.; Stumvoll, M.; et al. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes 2008, 57, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Kloting, N.; Berndt, J.; Kralisch, S.; Kovacs, P.; Fasshauer, M.; Schon, M.R.; Stumvoll, M.; Blüher, M. Vaspin gene expression in human adipose tissue: Association with obesity and type 2 diabetes. Biochem. Biophys. Res. Commun. 2006, 339, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Jekal, Y.; Im, J.A.; Kim, E.; Lee, S.H.; Park, J.H.; Chu, S.H.; Chung, K.M.; Lee, H.C.; Oh, E.G.; et al. Reduced serum vaspin concentrations in obese children following short-term intensive lifestyle modification. Clin. Chim. Acta 2010, 411, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Spiroglou, S.G.; Kostopoulos, C.G.; Varakis, J.N.; Papadaki, H.H. Adipokines in periaortic and epicardial adipose tissue: Differential expression and relation to atherosclerosis. J. Atheroscler. Thromb. 2010, 17, 115–130. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barraco, G.M.; Luciano, R.; Semeraro, M.; Prieto-Hontoria, P.L.; Manco, M. Recently Discovered Adipokines and Cardio-Metabolic Comorbidities in Childhood Obesity. Int. J. Mol. Sci. 2014, 15, 19760-19776. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms151119760
Barraco GM, Luciano R, Semeraro M, Prieto-Hontoria PL, Manco M. Recently Discovered Adipokines and Cardio-Metabolic Comorbidities in Childhood Obesity. International Journal of Molecular Sciences. 2014; 15(11):19760-19776. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms151119760
Chicago/Turabian StyleBarraco, Gloria Maria, Rosa Luciano, Michela Semeraro, Pedro L. Prieto-Hontoria, and Melania Manco. 2014. "Recently Discovered Adipokines and Cardio-Metabolic Comorbidities in Childhood Obesity" International Journal of Molecular Sciences 15, no. 11: 19760-19776. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms151119760