Route and Regulation of Zinc, Cadmium, and Iron Transport in Rice Plants (Oryza sativa L.) during Vegetative Growth and Grain Filling: Metal Transporters, Metal Speciation, Grain Cd Reduction and Zn and Fe Biofortification
Abstract
:1. Introduction
| Metal | Demands/Toxicity for Plants | Deficiency Diseases/Excess Toxicity for Humans |
|---|---|---|
| Zn | Demand: Cofactor of over 300 enzymes including DNA- and RNA-polymerases; Excess toxicity: Unregulated binding of Zn to S-, N- and O-containing molecules | Deficiency: Stunting, diarrhea, pneumonia; Excess toxicity: Interference of Fe and Cu uptake in the intestines |
| Cd | Toxicity: Binding to protein SH-residues, exchanges with divalent cations such as Zn2+ and Ca2+, and excessive production of reactive oxygen species | Excess toxicity: Itai-itai disease (spinal and leg bone pain) caused by Cd accumulation in the liver and kidneys resulting in tubular renal dysfunction, osteoporosis, cancer, and cardiovascular diseases |
| Fe | Demand: Proteins involved in redox and electron transport. Leaf pigment formation; Toxicity: Free Fe can generate toxic levels of oxygen and hydroxyl free radicals through the Fenton reaction | Deficiency: Anemia, impaired mental development; Iron overload: Excessive accumulation of Fe in the liver, heart, and pancreas. Hemosiderosis; Hemochromatosis |

2. Uptake at Root-Surface Membranes and Radial Transport to the Xylem

| Site | Zn | Cd | Fe |
|---|---|---|---|
| Acquisition at root cell membranes | OsIRT1 1; OsZIP4,5 2 | OsNRAMP5 3; OsIRT1/OsIRT2 4 | OsYSL15 5; OsIRT1/OsIRT2 6 |
| Vacuolar import through the tonoplast in root cells | (ZIF1) 7 | OsHMA3 8 | |
| Xylem loading at root pericycle cells | OsHMA2 9 | OsHMA2 5,9 | |
| Xylem-to-phloem transport at nodes | OsHMA2 10 | OsHMA2 10 | OsYSL16 11 |
| Phloem loading after mobilization in leaves | ZIP and YSL families | OYSL15 12 | |
| Phloem unloading at reproductive organs | OsYSL18 13 |
3. Xylem and Phloem Transport

| Metal | Chemical Forms in Xylem Sap (pH 6) | Chemical Forms in Phloem Sap (pH 8) |
|---|---|---|
| Zn | Free ions and partially bound 1 | Bound dominantly to NA 2 |
| Cd | Primarily in free ions 3 | Bound largely to specific proteins and slightly to thiol-compounds 4 |
| Fe | Bound largely to citrate (around 65%) and slightly to DMA (around 5%)and some in free ions 5 | Bound to DMA, citrate, and proteins 2 |
| Cu | Bound dominantly to DMA 6 | Bound to NA, histidine and proteins 6 |


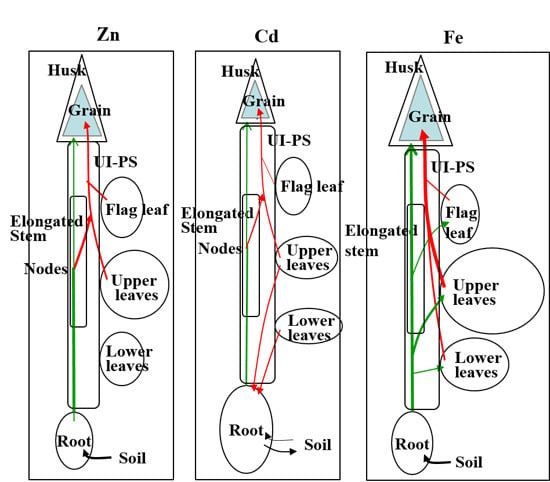
4. Accumulation of Zn, Cd, and Fe in Rice Grains



5. Molecular Technologies to Increase Grain Fe and Zn and to Reduce Grain Cd
| Field (Soil Cd mg·kg−1) | Grain Zn (mg·kg−1) | Grain Cd (mg·kg−1) | Grain Fe (mg·kg−1) | |||
|---|---|---|---|---|---|---|
| Koshi | Kan1 | Koshi | Kan1 | Koshi | Kan1 | |
| A (1.35) | 48.8 | 36.9 | 0.57 | ND | 21.5 | 16.4 |
| B (1.21) | 34.4 | 31.4 | 1.86 | 0.02 | 14.2 | 15.8 |
| C (0.35) | 50.4 | 37.5 | 0.97 | 0.03 | 15.1 | 14.3 |
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nouet, C.; Motte, P.; Hanikenne, M. Chloroplastic and mitochondrial metal homeostasis. Trends Plant Sci. 2011, 16, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.M.; Guerinot, M.L. Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nat. Chem. Biol. 2009, 5, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, M.G.; Clemens, S.; Williams, L.E.; Krämer, U.; Borg, S.; Schjørring, J.K.; Sanders, D. Zinc biofortification of cereals: Problems and solutions. Trends Plant Sci. 2008, 13, 464–473. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Physiological limits to zinc biofortification of edible crops. Front. Plant Sci. 2011, 2, 80. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Aarts, M.G.M.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2012, 18, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Hurrell, R.F. Improving iron, zinc, vitamin A nutrition through plant biotechnology. Curr. Opin. Biotechnol. 2002, 13, 142–145. [Google Scholar] [CrossRef]
- Dijkhuizen, M.A.; Wieringa, F.T.; West, C.E. Zinc plus β-carotene supplementation of pregnant women is superior to β-carotene supplementation alone in improving vitamin A status in both mothers and infants. Am. J. Clin. Nutr. 2004, 80, 1299–1307. [Google Scholar] [PubMed]
- Fukushima, M.; Ishizaki, A.; Sakamoto, M.; Kobayashi, E. Cadmium concentration in rice eaten by famers in the Jinzu River Basin. Jpn. J. Hyg. 1973, 28, 406–415. [Google Scholar] [CrossRef]
- Khoshgoftarmanesh, A.H.; Schulin, R.; Chaney, R.L.; Daneshbakhsh, B.; Afyuni, M. Micronutrient-efficient genotypes for crop yield and nutritional quality in sustainable agriculture. A review. Agron. Sustain. Dev. 2010, 30, 83–107. [Google Scholar] [CrossRef]
- Simmons, R.W.; Pongsakul, P.; Chaney, R.L.; Saiyasitpanich, D.; Klinphoklap, S.; Nobuntou, W. The relative exclusion of zinc and iron from rice grain in relation to rice grain cadmium as compared to soybean: Implications for human health. Plant Soil 2003, 257, 163–170. [Google Scholar] [CrossRef]
- Wissuwa, M.; Ismail, A.M.; Graham, R.D. Rice grain zinc concentrations as affected by genotype, native soil-zinc availability, and zinc fertilization. Plant Soil 2008, 306, 37–48. [Google Scholar] [CrossRef]
- Ito, H.; Iimura, K. Absorption of zinc and cadmium by rice plants and their influence on its growth. II. Effect of cadmium. Jpn. J. Soil Sci. Plant Nutr. 1976, 47, 44–48. [Google Scholar]
- Ito, H.; Iimura, K. Absorption of zinc and cadmium by rice plants and their influence on its growth. I. Effect of zinc. Jpn. J. Soil Sci. Plant Nutr. 1976, 47, 39–43. [Google Scholar]
- Jiang, W.; Struik, P.C.; van Keulen, H.; Zhao, M.; Jin, L.N.; Stomph, T.J. Does increased zinc uptake enhance grain zinc mass concentration in rice? Ann. Appl. Biol. 2008, 153, 135–147. [Google Scholar] [CrossRef]
- Shiratori, K.; Suzuki, T.; Miyoshi, H. Chemical forms of soil zinc in the salt-rich reclaimed fields, where zinc-deficiency symptoms were observed in rice plants. Jpn. J. Soil Sci. Plant Nutr. 1972, 43, 291–296. [Google Scholar]
- Khaokaew, S.; Chaney, R.L.; Landrot, G.; Ginder-Vogel, M.; Sparks, D.L. Speciation and release kinetics of cadmium in an alkaline paddy soil under various flooding periods and draining conditions. Environ. Sci. Technol. 2011, 45, 4249–4255. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Yamaguchi, N. Chemical speciation of cadmium and sulfur K-edge XANES spectroscopy in flooded paddy soils amended with zerovalent iron. Soil Sci. Soc. Am. J. 2013, 77, 1189–1198. [Google Scholar] [CrossRef]
- Lee, S.; An, G. Over-expression of OsIRT1 leads to iron and zinc accumulations in rice. Plant Cell Environ. 2009, 32, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Ishimaru, Y.; Igura, M.; Kuramata, M.; Abe, T.; Senoura, T.; Hase, Y.; Arao, T.; Nishizawa, N.K.; Nakanishi, H. Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc. Natl. Acad. Sci. USA 2012, 109, 19166–19171. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Yamaji, N.; Yokosho, K.; Ma, J.F. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Ogawa, I.; Ishimaru, Y.; Mori, S.; Nishizawa, N.K. Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Sci. Plant Nutr. 2006, 52, 464–469. [Google Scholar] [CrossRef]
- Inoue, H.; Kobayashi, T.; Nozoye, T.; Takahashi, M.; Kakei, Y.; Suzuki, K.; Nakazono, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Rice OsYSL15 is an iron-regulated iron(III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J. Biol. Chem. 2009, 284, 3470–3479. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, Y.; Suzuki, M.; Tsukamoto, T.; Suzuki, K.; Nakazono, M.; Kobayashi, T.; Wada, Y.; Watanabe, S.; Matsuhashi, S.; Takahashi, M.; et al. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J. 2006, 45, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Haydon, M.J.; Kawachi, M.; Wirtz, M.; Hillmer, S.; Hell, R.; Krämer, U. Vacuolar nicotianamine has critical and distinct roles under iron deficiency and for zinc sequestration in Arabidopsis. Plant Cell 2012, 24, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Ueno, D.; Yamaji, N.; Kono, I.; Huang, C.F.; Ando, T.; Yano, M.; Ma, J.F. Gene limiting cadmium accumulation in rice. Proc. Natl. Acad. Sci. USA 2010, 107, 16500–16505. [Google Scholar] [CrossRef] [PubMed]
- Nocito, F.F.; Lancilli, C.; Dendena, B.; Lucchini, G.; Sacchi, G.A. Cadmium retention in rice roots is influenced by cadmium availability, chelation and translocation. Plant Cell Environ. 2011, 34, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Ogo, Y.; Senoura, T.; Nishizawa, N.K.; Nakanishi, H. The OsHMA2 transporter is involved in root–to-shoot translocation of Zn and Cd in rice. Plant Cell Environ. 2012, 35, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Xia, J.; Mitani-Ueno, N.; Yokosho, K.; Ma, J.F. Preferential delivery of zinc to developing tissues in rice is mediated by P-type heavy metal ATPase OsHMA2. Plant Physiol. 2013, 162, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Kakei, Y.; Ishimaru, Y.; Kobayashi, T.; Yamakawa, T.; Nakanshi, H.; Nishizawa, N.K. OsYSL16 plays a role in the allocation of iron. Plant Mol. Biol. 2012, 79, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chiecko, J.C.; Kim, S.A.; Walker, E.L.; Lee, Y.; Guerinot, M.L.; An, G. Disruption of OsYSL15 leads to inefficiency in rice plants. Plant Physiol. 2009, 150, 786–800. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Kobayashi, T.; Takahashi, M.; Nagasaka, S.; Usuda, K.; Kakei, Y.; Ishimaru, Y.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. OsYSL18 is a rice iron(III)-deoxymugineic acid transporter specifically expressed in reproductive organs and phloem of lamina joints. Plant Mol. Biol. 2009, 70, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Widodo; Broadley, M.R.; Rose, T.; Frei, M.; Pariasca-Tanaka, J.; Yoshihashi, T.; Thomson, M.; Hammond, J.P.; Aprile, A.; Close, T.J.; et al. Response to zinc deficiency of two rice lines with contrasting tolerance is determined by root growth maintenance and organic acid exudation rate, and not by zinc-transport activity. New Phytol. 2010, 186, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Nomoto, K.; Takemoto, S. Physiological aspect of mugineic acid, a possible phytosiderophore of graminaceous plants. J. Plant Nutr. 1984, 7, 469–477. [Google Scholar] [CrossRef]
- Nozoye, T.; Nagasaka, S.; Kobayashi, T.; Takahashi, M.; Sato, Y.; Uozumi, N.; Nakanishi, H.; Nishizawa, N.K. Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. J. Biol. Chem. 2011, 286, 5446–5454. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.; von Wiren, N.; Hayen, H. Investigation of ascorbate-mediated iron release from ferric phytosiderophores in the presence of nicotianamine. Biometals 2008, 21, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Ariga, T.; Hazama, K.; Yanagisawa, S.; Yoneyama, T. Chemical forms of iron in xylem sap from graminaceous and non-graminaceous plants. Soil Sci. Plant Nutr. 2014, 60, 460–469. [Google Scholar] [CrossRef]
- Park, J.; Song, W.-Y.; Ko, D.; Eom, Y.; Hansen, T.H.; Schiller, M.; Lee, T.G.; Martinoia, E.; Lee, Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012, 69, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Suzui, N.; Ito-Tanabata, S.; Ishii, S.; Igura, M.; Abe, T.; Kuramata, M.; Kawachi, N.; Fujimaki, S. Real-time imaging and analysis of differences in cadmium dynamics in rice cultivars (Oryza sativa) using positron-emitting 107Cd trace. BMC Plant Biol. 2011, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Yokosho, K.; Yamaji, N.; Ueno, D.; Mitani, N.; Ma, J.F. OsFRDL1 is a citrate transporter required for efficient transformation of iron in rice. Plant Physiol. 2009, 149, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, S.; Fukumorita, T.; Chino, M. Collection of rice phloem sap from stylets of homopterous insects severed by YAG laser. Plant Cell Physiol. 1980, 21, 1319–1327. [Google Scholar]
- Nishiyama, R. Zinc Transport via Phloem in Rice Plants. Master thesis, Graduate School of Agricultural and Life Sciences, the University of Tokyo, Tokyo, Japan, 2006. [Google Scholar]
- Kato, M. The University of Tokyo: Tokyo, 2006; Unpublished work.
- Nishiyama, R.; Kato, M.; Nagata, S.; Yanagisawa, S.; Yoneyama, T. Identification of Zn-nicotianamine and Fe-2′-deoxymugineic acid in the phloem sap from rice plants (Oryza sativa L.). Plant Cell Physiol. 2012, 53, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Ishikawa, S.; Inagaki, K.; Chiba, K.; Hayashi, H.; Yanagisawa, S.; Yoneyama, T. 2010: Possible chemical forms of cadmium and varietal differences in cadmium concentrations in the phloem sap of rice plants (Oryza sativa L.). Soil Sci. Plant Nutr. 2010, 56, 839–847. [Google Scholar] [CrossRef]
- Álvarez-Fernández, A.; Díaz-Benito, P.; Abadía, A.; Lopez-Millán, A.-F.; Abadía, J. 2014: Metal species involved in long distance metal transport in plants. Front. Plant Sci. 2014, 5, 105. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Nagata, S.; Yanagisawa, S.; Yoneyama, T. Copper in xylem and phloem from rice (Oryza sativa): The effect of moderate copper concentrations in the growth medium on the accumulation of five essential metals and a speciation analysis of copper-containing compounds. Funct. Plant Biol. 2013, 40, 89–100. [Google Scholar] [CrossRef]
- Obata, H.; Kitagishi, K. Longitudinal distribution pattern of zinc and manganese in leaf with special reference to aging. Jpn. J. Soil Sci. Plant Nutr. 1980, 51, 285–291. [Google Scholar]
- Kitagishi, K.; Obata, H.; Kondo, T. Effect of zinc deficiency on 80S ribosome content of meristematic tissues of rice plant. Soil Sci. Plant Nutr. 1987, 33, 423–429. [Google Scholar] [CrossRef]
- Obata, H.; Kitagishi, K. Accumulation and retranslocation of zinc in a leaf of rice plant with reference to aging. Jpn. J. Soil Sci. Plant Nutr. 1982, 53, 235–240. [Google Scholar]
- Kitagishi, K.; Obata, H. Retrieval of zinc from transpiration stream in the nodes of rice plants as affected by the concentration of zinc in culture solution. Rep. Environ. Sci. Mie Univ. 1982, 7, 91–97. [Google Scholar]
- Yamaguchi, N.; Ishikawa, S.; Abe, T.; Baba, K.; Arao, T.; Terada, Y. Role of the node in controlling traffic of cadmium, zinc, and manganese in rice. J. Exp. Bot. 2012, 63, 2729–2737. [Google Scholar] [CrossRef] [PubMed]
- Kitagishi, K.; Obata, H. Distribution of 115mCd in rice plants absorbed by roots at vegetative stage. Rep. Environ. Sci. Mie Univ. 1979, 4, 59–65. [Google Scholar]
- Kobayashi, N.I.; Tanoi, K.; Hirose, A.; Nakanishi, T.M. Characterization of rapid intervascular transport of cadmium in rice stem by radioisotope imaging. J. Exp. Bot. 2013, 64, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, T.; Nakanishi, H.; Uchida, H.; Watanabe, S.; Matsuhashi, S.; Mori, S.; Nishizawa, N.K. 2009: 52Fe translocation in barley as monitored by a positron emitting tracer imaging system (PETIS): Evidence for the direct translocation of Fe from roots to young leaves via phloem. Plant Cell Physiol. 2009, 50, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, T.; Gosho, T.; Kato, M.; Goto, S.; Hayashi, H. Xylem and phloem transport of Cd, Zn and Fe into the grains of rice plants (Oryza sativa L.) grown in continuously flooded Cd-contaminated soil. Soil Sci. Plant Nutr. 2010, 56, 445–453. [Google Scholar] [CrossRef]
- Zee, S.-Y. Transfer cells and vascular tissue distribution in the vegetative nodes of rice. Aust. J. Bot. 1972, 20, 41–48. [Google Scholar]
- Kawahara, H.; Chonan, N.; Matsuda, T. Studies on morphogenesis in rice plants. 7. The morphology of vascular bundles in the vegetative nodes of the culm. Proc. Crop Sci. Soc. Jpn. 1974, 43, 389–401. [Google Scholar] [CrossRef]
- Chonan, N.; Kawahara, H.; Matsuda, T. Ultrastructure of elliptical and diffuse bundles in the vegetative nodes of rice. Jpn. J. Crop Sci. 1984, 54, 393–402. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Lu, L.-L.; Yang, X.-E.; Feng, Y.; Wei, Y.-Y.; Hao, H.-L.; Stoffella, P.J.; He, Z.-L. Uptake, translocation, and remobilization of zinc absorbed at different growth stages by rice genotypes of different Zn densities. J. Agric. Food Chem. 2010, 58, 6767–6773. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, R.; Tanoi, K.; Yanagisawa, S.; Yoneyama, T. 2013: Quantification of zinc transport to the grain in rice plants (Oryza sativa L.) at early grain-filling by a combination of mathematical modeling and 65Zn tracing. Soil Sci. Plant Nutr. 2013, 59, 750–755. [Google Scholar] [CrossRef]
- Tanaka, K.; Fujimaki, S.; Fujiwara, T.; Yoneyama, T.; Hayashi, H. Quantitative estimation of the contribution of the phloem in cadmium transport to grains in rice plants (Oryza sativa L.). Soil Sci. Plant Nutr. 2007, 53, 72–77. [Google Scholar] [CrossRef]
- Rodda, M.S.; Li, G.; Reid, R.J. The timing of grain Cd accumulation in rice plants: The relative importance of remobilization within the plant and root Cd uptake post-flowering. Plant Soil 2011, 347, 105–114. [Google Scholar] [CrossRef]
- Fujimaki, S.; Suzui, N.; Ishioka, N.S.; Kawachi, N.; Ito, S.; Chino, M.; Nakamura, S. Tracing cadmium from culture to spikelet: Noninvasive imaging and quantitative characterization of absorption, transport, and accumulation of cadmium in an intact rice plant. Plant Physiol. 2010, 152, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Zee, S.-Y. Vascular tissue and transfer cell distribution in the rice spikelet. Aust. J. Biol. Sci. 1972, 25, 411–414. [Google Scholar]
- Kawahara, H.; Matsuda, T.; Chonan, N. Studies on morphogenesis in rice plants. 9. On the structure of vascular bundles and phloem transport in the spikelet. Proc. Crop Sci. Soc. Jpn. 1977, 46, 82–90. [Google Scholar] [CrossRef]
- Herren, T.; Feller, U. Transfer of zinc from xylem to phloem in the peduncle of wheat. J. Plant Nutr. 1994, 17, 1587–1598. [Google Scholar] [CrossRef]
- Jiang, W.; Struik, P.C.; Lingna, J.; van Keulen, H.; Ming, Z.; Stomph, T.J. Uptake and distribution of root-applied or foliar-applied 65Zn after flowering in aerobic rice. Ann. Appl. Biol. 2007, 150, 383–391. [Google Scholar] [CrossRef]
- Sperotto, P.A.; Ricachenevsky, F.K.; Waldow, V.A.; Fett, J.P. Iron biofortification in rice: It’s a long way to the top. Plant Sci. 2012, 190, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Moritsugu, M. A study on combination of glutelin with cadmium in rice grain. Ber. Obara Inst. Landwirtsch. Biol. 1964, 12, 251–260. [Google Scholar]
- Schuler, M.; Rellán-Álvarez, R.; Fink-Straube, C.; Abadía, J.; Bauer, P. Nicotianamine functions in the phloem-based transport of iron to sink organs in pollen development and pollen tube growth in Arabidopsis. Plant Cell 2012, 24, 2380–2400. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Wu, L.; Yang, C.; Lv, Q. Effects of iron and zinc foliar applications on rice plants and their grain accumulation and grain quality. J. Sci. Food Agric. 2012, 93, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, Y.; Kim, S.; Tsukamoto, T.; Oki, H.; Kobayashi, T.; Watanabe, S.; Matsuhashi, S.; Takahashi, M.; Nakanishi, H.; Mori, S.; et al. Mutational reconstructed ferric chelate reductase confers enhanced tolerance in rice to iron deficiency in calcareous soil. Proc. Natl. Acad. Sci. USA 2007, 104, 7373–7378. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Usuda, K.; Kobayashi, T.; Ishimaru, Y.; Kakei, Y.; Takahashi, M.; Higuchi, K.; Nakanishi, H.; Mori, S.; Nishizawa, K.N. Overexpression of the barley nicotianamine synthase gene HvHAS1 increases iron and zinc concentrations in rice grains. Rice 2009, 2, 155–166. [Google Scholar] [CrossRef]
- Lee, S.; Jeon, U.S.; Lee, S.J.; Kim, Y.-K.; Persson, D.P.; Husted, S.; Schjørring, J.K.; Kakei, Y.; Masuda, H.; Nishizawa, N.K.; et al. Iron fortification of rice seeds through activation of the nicotianamine synthase gene. Proc. Nat. Acad. Sci. USA 2009, 106, 22014–22019. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Cheng, Z.; Ai, C.; Jiang, X.; Bei, X.; Zheng, Y.; Glahn, R.P.; Welch, R.M.; Miller, D.D.; Lei, X.G.; et al. Nicotianamine, a novel enhancer of rice iron bioavailability to humans. PLoS ONE 2010, 5, e10190. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Chaney, R.L. Bioavailability as an issue in risk assessment and management of food cadmium: A review. Sci. Total Environ. 2008, 398, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius (2014) CODEX STAN 193–1995, General Standard for Contaminants and Toxins in Food and Feed. 1995. Available online: http://www.codexalimentarius.org/download/standards/17/CXS_193e2015.pdf (accessed on 31 March 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoneyama, T.; Ishikawa, S.; Fujimaki, S. Route and Regulation of Zinc, Cadmium, and Iron Transport in Rice Plants (Oryza sativa L.) during Vegetative Growth and Grain Filling: Metal Transporters, Metal Speciation, Grain Cd Reduction and Zn and Fe Biofortification. Int. J. Mol. Sci. 2015, 16, 19111-19129. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160819111
Yoneyama T, Ishikawa S, Fujimaki S. Route and Regulation of Zinc, Cadmium, and Iron Transport in Rice Plants (Oryza sativa L.) during Vegetative Growth and Grain Filling: Metal Transporters, Metal Speciation, Grain Cd Reduction and Zn and Fe Biofortification. International Journal of Molecular Sciences. 2015; 16(8):19111-19129. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160819111
Chicago/Turabian StyleYoneyama, Tadakatsu, Satoru Ishikawa, and Shu Fujimaki. 2015. "Route and Regulation of Zinc, Cadmium, and Iron Transport in Rice Plants (Oryza sativa L.) during Vegetative Growth and Grain Filling: Metal Transporters, Metal Speciation, Grain Cd Reduction and Zn and Fe Biofortification" International Journal of Molecular Sciences 16, no. 8: 19111-19129. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms160819111