Comprehensive and Quantitative Proteomic Analysis of Metamorphosis-Related Proteins in the Veined Rapa Whelk, Rapana venosa
Abstract
:1. Introduction
2. Results
2.1. General Characterization of Proteomic Data
2.2. Functional Analysis of DEPs with Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG)
2.3. Association Analysis of Transcriptome and Proteome Data
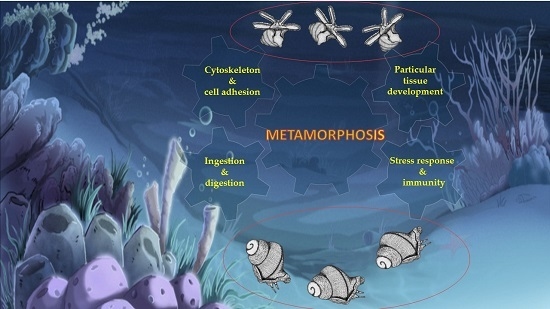
3. Discussion
3.1. Cytoskeleton and Cell Adhesion
3.2. Ingestion and Digestion
3.3. Stress Response and Immunity
3.4. Specific Tissue Development
4. Materials and Methods
4.1. Larvae Culture and Sample Collection
4.2. Protein Extraction, Digestion, and iTRAQ Labelling
4.3. Strong Cation Exchange (SCX) Fractionation and Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS) Analysis
4.4. Protein Identification and Quantification
4.5. Enrichment of GO and KEGG Pathways
4.6. Correlation Analysis of Transcriptomic and Proteomic Data
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 2DE | Two-Dimensional Electrophoresis |
| ACN | Acetonitrile |
| CHAPS | 3-[(3-cholamidopropyl)dimethylammonio]propanesulfonate |
| DEPs | Differentially Expressed Proteins |
| DTT | DL-Dithiothreitol |
| ECM | Extracellular Matrix |
| EDTA | Ethylene Diamine Tetraacetic Acid |
| GO | Gene Ontology |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic Acid |
| HPLC | High Performance Liquid Chromatography |
| iTRAQ | isobaric Tags for Relative and Absolute Quantitation |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LC–MS/MS | Fractionation and Liquid Chromatography–Tandem Mass Spectrometry |
| PMSF | Phenylmethanesulfonyl Fluoride |
| SCX | Strong Cation Exchange |
| SDS-PAGE | Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis |
References
- Yuan, C.-Y. Primary exploration on aquaculture of Rapana venosa. Fish. Sci. 1992, 11, 16–18. (In Chinese) [Google Scholar]
- Mann, R.; Harding, J.M. Salinity tolerance of larval Rapana venosa: Implications for dispersal and establishment of an invading predatory gastropod on the North American Atlantic coast. Biol. Bull. 2003, 204, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.; Harding, J.M.; Westcott, E. Occurrence of imposex and seasonal patterns of gametogenesis in the invading veined rapa whelk Rapana venosa from Chesapeake Bay, USA. Mar. Ecol. Prog. Ser. 2006, 310, 129–138. [Google Scholar] [CrossRef]
- Giberto, D.A.; Bremec, C.S.; Schejter, L.; Schiariti, A.; Mianzan, H.; Acha, E.M. The invasive Rapa Whelk Rapana venosa (Valenciennes 1846): status and potential ecological impacts in the Río de la Plata estuary, Argentina-Uruguay. J. Shellfish Res. 2006, 25, 919–924. [Google Scholar]
- Leppäkoski, E.; Gollasch, S.; Olenin, S. Invasive Aquatic Species of Europe. Distribution, Impacts and Management; Springer Science & Business Media: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Çulha, M.; Bat, L.; Doğan, A.; Dağlı, E. Ecology and distribution of the veined rapa whelk Rapana venosa (Valenciennes, 1846) in Sinop peninsula (Southern Central Black Sea), Turkey. J. Anim. Vet. Adv. 2009, 8, 51–58. [Google Scholar]
- Pan, Y.; Qiu, T.; Zhang, T.; Wang, P.; Ban, S. Morphological studies on the early development of Rapana venosa. J. Fish. China 2013, 37, 1503–1512. (In Chinese) [Google Scholar] [CrossRef]
- Yang, Z.; Yu, H.; Yu, R.; Li, Q. Induced metamorphosis in larvae of the veined rapa whelk Rapana venosa using chemical cues. Mar. Biol. Res. 2015, 11, 1–8. [Google Scholar] [CrossRef]
- Song, H.; Yu, Z.L.; Sun, L.N.; Gao, Y.; Zhang, T.; Wang, H.Y. De novo transcriptome sequencing and analysis of Rapana venosa from six different developmental stages using Hi-seq 2500. Comp. Biochem. Physiol. D-Genom. Proteom. 2016, 17, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Glisovic, T.; Bachorik, J.L.; Yong, J.; Dreyfuss, G. RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 2008, 582, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Deribe, Y.L.; Pawson, T.; Dikic, I. Post-translational modifications in signal integration. Nat. Struct. Mol. Biol. 2010, 17, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Vengatesen, T.; Tim, W.; Pei-Yuan, Q. 2D gel-based proteome and phosphoproteome analysis during larval metamorphosis in two major marine biofouling invertebrates. J. Proteome Res. 2009, 8, 2708–2719. [Google Scholar]
- Mok, F.S.; Thiyagarajan, V.; Qian, P.Y. Proteomic analysis during larval development and metamorphosis of the spionid polychaete Pseudopolydora vexillosa. Proteome Sci. 2009, 7, 178–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huoming, Z.; Him, W.Y.; Hao, W.; Zhangfan, C.; Arellano, S.M.; Timothy, R.; Pei-Yuan, Q. Quantitative proteomics identify molecular targets that are crucial in larval settlement and metamorphosis of Bugula neritina. J. Proteome Res. 2011, 10, 349–360. [Google Scholar]
- Thiyagarajan, V.; Qian, P. Proteomic analysis of larvae during development, attachment, and metamorphosis in the fouling barnacle, Balanus amphitrite. Proteomics 2008, 8, 3164–3172. [Google Scholar] [CrossRef] [PubMed]
- Zieske, L.R. A perspective on the use of iTRAQ reagent technology for protein complex and profiling studies. In Proceedings of the IEEE International Conference on Electronics, Circuits & Systems, Nice, France, 10–13 December 2006; pp. 1501–1508.
- Wu, W.W.; Guanghui, W.; Seung Joon, B.; Rong-Fong, S. Comparative study of three proteomic quantitative methods, DIGE, cICAT, and iTRAQ, using 2D gel- or LC-MALDI TOF/TOF. J. Proteome Res. 2006, 5, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Karp, N.A.; Huber, W.; Sadowski, P.G.; Charles, P.D.; Hester, S.V.; Lilley, K.S. Addressing accuracy and precision issues in iTRAQ quantitation. Mol. Cell. Proteom. 2010, 9, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.W.; Cai, D.; Verhey, K.J. Tubulin modifications and their cellular functions. Curr. Opin. Cell Biol. 2008, 20, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Erck, C.; Peris, L.; Andrieux, A.; Meissirel, C.; Gruber, A.D.; Vernet, M.; Schweitzer, A.; Saoudi, Y.; Pointu, H.; Bosc, C. A vital role of tubulin-tyrosine-ligase for neuronal organization. Proc. Natl. Acad. Sci. USA 2005, 102, 7853–7858. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, K.; Heier, R.L.; Taruishi, M.; Takagi, H.; Mukai, M.; Shimma, S.; Taira, S.; Hatanaka, K.; Morone, N.; Yao, I. Loss of α-tubulin polyglutamylation in ROSA22 mice is associated with abnormal targeting of KIF1A and modulated synaptic function. Proc. Natl. Acad. Sci. USA 2007, 104, 3213–3218. [Google Scholar] [CrossRef] [PubMed]
- Blake, J.A.; Woodwick, K.H. Reproduction and larval development of Pseudopolydora paucibranchiata (Okuda) and Pseudopolydora kempi (Southern) (Polychaeta: Spionidae). Biol. Bull. 1975, 149, 109–127. [Google Scholar] [CrossRef]
- Jacobson, M.D.; Weil, M.; Raff, M.C. Programmed cell death in animal development. Cell 1997, 88, 347–354. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Xiao, K.; Arellano, S.M.; Thiyagarajan, V.; Qian, P.-Y. 2D gel-based multiplexed proteomic analysis during larval development and metamorphosis of the biofouling polychaete tubeworm Hydroides elegans. J. Proteome Res. 2010, 9, 4851–4860. [Google Scholar] [CrossRef] [PubMed]
- Timpl, R.; Brown, J.C. Supramolecular assembly of basement membranes. Bioessays 1996, 18, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-B.; Fu, L.; Hasebe, T.; Ishizuya-Oka, A. Regulation of extracellular matrix remodeling and cell fate determination by matrix metalloproteinase stromelysin-3 during thyroid hormone-dependent post-embryonic development. Pharmacol. Ther. 2007, 116, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Nakajima, K.; Yaoita, Y. Expression of matrix metalloproteinase genes in regressing or remodeling organs during amphibian metamorphosis. Dev. Growth Differ. 2007, 49, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Royer, V.; Hourdry, A.; Fraichard, S.; Bouhin, H. Characterization of a putative extracellular matrix protein from the beetle Tenebrio molitor: Hormonal regulation during metamorphosis. Dev. Genes Evol. 2004, 214, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Huan, P.; Wang, H.; Liu, B. A label-free proteomic analysis on competent larvae and juveniles of the pacific oyster Crassostrea gigas. PLoS ONE 2015, 10, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Altincicek, B.; Vilcinskas, A. Identification of a lepidopteran matrix metalloproteinase with dual roles in metamorphosis and innate immunity. Dev. Comp. Immunol. 2008, 32, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Huang, B.; Ke, C.; Xu, Y.; Wang, D. Activities of several digestive enzymes of Babylonia areolata (Gastropoda: Buccinidae) during early development. J. Trop. Oceanogr. 2006, 26, 55–59. [Google Scholar]
- Jensen, R.G. Detection and determination of lipase (acylglycerol hydrolase) activity from various sources. Lipids 1983, 18, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Olivera, B.M.; Cruz, L.J.; Gray, W.R.; Rivier, J.E.F. Conotoxins. J. Biol. Chem. 1991, 266, 22067–22137. [Google Scholar] [PubMed]
- Milne, T.J.; Abbenante, G.; Tyndall, J.D.; Halliday, J.; Lewis, R.J. Isolation and characterization of a cone snail protease with homology to CRISP proteins of the pathogenesis-related protein superfamily. J. Biol. Chem. 2003, 278, 31105–31110. [Google Scholar] [CrossRef] [PubMed]
- Heyland, A.; Moroz, L.L. Signaling mechanisms underlying metamorphic transitions in animals. Integr. Comp. Biol. 2006, 46, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Sato, E.F.; Nishikawa, M.; Hiramoto, K.; Kashiwagi, A.; Utsumi, K. Free radical theory of apoptosis and metamorphosis. Redox Rep. 2004, 9, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Manzo, W.; Gardner, E.; Menon, J. Reactive oxygen species and anti-oxidant defenses in tail of tadpoles, Xenopus laevis. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2013, 158, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Strobel, A.; Hu, M.Y.A.; Gutowska, M.A.; Lieb, B.; Lucassen, M.; Melzner, F.; Pörtner, H.O.; Mark, F.C. Influence of temperature, hypercapnia, and development on the relative expression of different hemocyanin isoforms in the common cuttlefish sepia officinalis. J. Exp. Zool. Part A 2012, 317, 511–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coates, C.J.; Nairn, J. Diverse immune functions of hemocyanins. Dev. Comp. Immunol. 2014, 45, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Olga, A.; Lilia, Y.; Rada, S.; Stefan, S.; Pavlina, D.; Draga, T. Changes in the gene expression profile of the bladder cancer cell lines after treatment with Helix lucorum and Rapana venosa hemocyanin. J. Balk. Union Oncol. 2015, 20, 180–187. [Google Scholar]
- Dolashka, P.; Moshtanska, V.; Borisova, V.; Dolashki, A.; Stevanovic, S.; Dimanov, T.; Voelter, W. Antimicrobial proline-rich peptides from the hemolymph of marine snail Rapana venosa. Peptides 2011, 32, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Velkova, L.; Todorov, D.; Dimitrov, I.; Shishkov, S.; Beeumen, J.V.; Dolashkaangelova, P. Rapana Venosa hemocyanin with antiviral activity. Biotechnol. Biotechnol. Equip. 2014, 23, 606–610. [Google Scholar] [CrossRef]
- Pavlina, D.; Ludmyla, V.; Stoyan, S.; Kalina, K.; Aleksander, D.; Ivan, D.; Boris, A.; Bart, D.; Wolfgang, V.; Jozef, V.B. Glycan structures and antiviral effect of the structural subunit RvH2 of Rapana hemocyanin. Carbohydr. Res. 2010, 345, 2361–2367. [Google Scholar]
- Wong, S.G.; Dessen, A. Structure of a bacterial α2-macroglobulin reveals mimicry of eukaryotic innate immunity. Nat. Commun. 2014, 5, 4917–4917. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef] [PubMed]
- Degnan, B.M.; Groppe, J.C.; Morse, D.E. Chymotrypsin mRNA expression in digestive gland amoebocytes: Cell specification occurs prior to metamorphosis and gut morphogenesis in the gastropod, Haliotis rufescens. Dev. Genes Evol. 1995, 205, 97–101. [Google Scholar] [CrossRef]
- Degnan, B.; Degnan, S.M.; Morse, D.E. Muscle-specific regulation of tropomyosin gene expression and myofibrillogenesis differs among muscle systems examined at metamorphosis of the gastropod Haliotis rufescens. Dev. Genes Evol. 1997, 206, 464–471. [Google Scholar] [CrossRef]
- Xu, D.; Sun, L.; Liu, S.; Zhang, L.; Yang, H. Understanding the heat shock response in the sea cucumber Apostichopus japonicus, using iTRAQ-based proteomics. Int. J. Mol. Sci. 2016, 17, 150. [Google Scholar] [CrossRef] [PubMed]
- Gene Ontology Consortium. Available online: http://www.geneontology.org/ (accessed on 1 March 2015).
- KEGG: Kyoto Encyclopedia of Genes and Genomes. Available online: http://www.genome.jp/kegg/ (accessed on 1 March 2015).



| Item | Value |
|---|---|
| Total Spectra | 224,473 |
| Spectra | 53,723 |
| Unique Spectra | 46,485 |
| Peptide | 21,626 |
| Unique Peptide | 20,175 |
| Protein | 5312 |
| Upregulated protein | 470 |
| Downregulated protein | 668 |
| Accession | FC | p-Value | Annotation | Organism Species | Description |
|---|---|---|---|---|---|
| Cytoskeleton and Cell Adhesion | |||||
| c111395_g1 | 0.49 | 8.22 × 10−3 | Paramyosin | Mytilus galloprovincialis | cytoskeleton component |
| c119060_g1 | 1.01 | 6.45 × 10−5 | Paramyosin | Mytilus galloprovincialis | cytoskeleton component |
| c67246_g1 | 0.80 | 1.29 × 10−3 | Paramyosin | Mytilus galloprovincialis | cytoskeleton component |
| c128871_g1 | 0.30 | 3.16 × 10−2 | Tropomyosin-2 | Biomphalaria glabrata | cytoskeleton component |
| c128871_g1 | 0.30 | 3.16 × 10−2 | Tropomyosin-2 | Biomphalaria glabrata | cytoskeleton component |
| c64757_g1 | 1.42 | 1.03 × 10−4 | Tubulin α chain | Plasmodium falciparum | cytoskeleton component |
| c144449_g1 | −0.63 | 1.02 × 10−2 | Tubulin α-1 chain | Paracentrotus lividus | cytoskeleton component |
| c19674_g1 | −0.46 | 1.08 × 10−2 | Tubulin α-2 chain | Gossypium hirsutum | cytoskeleton component |
| c65878_g1 | 0.60 | 5.21 × 10−3 | Tubulin α-8 chain (Fragment) | Gallus gallus | cytoskeleton component |
| c129550_g1 | −0.52 | 2.78 × 10−2 | Tubulin β chain (Fragment) | Haliotis discus | cytoskeleton component |
| c52663_g1 | −0.59 | 3.64 × 10−3 | Tubulin β-2 chain | Drosophila melanogaster | cytoskeleton component |
| c91498_g1 | −0.52 | 1.39 × 10−3 | Tubulin β-4B chain | Mesocricetus auratus | cytoskeleton component |
| c154903_g1 | −0.46 | 2.80 × 10−4 | Collagen α-1(XV) chain | Homo sapiens | extracellular matrix |
| c136294_g1 | −1.19 | 5.14 × 10−3 | Collagen α-1(XXI) chain | Xenopus laevis | extracellular matrix |
| c156326_g1 | −1.23 | 1.42 × 10−4 | Collagen α-1(XXII) chain | Homo sapiens | extracellular matrix |
| c155801_g1 | −0.56 | 1.18 × 10−3 | Collagen α-4(VI) chain | Crassostrea gigas | extracellular matrix |
| c156014_g6 | −0.85 | 1.72 × 10−2 | Collagen α-5(VI) chain | Crassostrea gigas | extracellular matrix |
| c154603_g1 | −0.91 | 2.20 × 10−4 | Collagen α-6(VI) chain | Homo sapiens | extracellular matrix |
| c156014_g2 | −1.06 | 9.95 × 10−6 | Collagen α-6(VI) chain | Homo sapiens | extracellular matrix |
| c169434_g1 | 0.81 | 1.38 × 10−2 | Extracellular matrix protein 3 | Lytechinus variegatus | extracellular matrix |
| c215931_g1 | 0.87 | 2.65 × 10−2 | FRAS1-related extracellular matrix protein 2 | Homo sapiens | extracellular matrix |
| c157006_g5 | −0.61 | 2.06 × 10−2 | Laminin subunit alpha-2 | Mus musculus | extracellular matrix |
| c155563_g1 | −0.87 | 2.04 × 10−4 | Laminin-like protein epi-1 | Crassostrea gigas | extracellular matrix |
| c154307_g2 | −0.79 | 4.65 × 10−2 | Matrix metalloproteinase-19 | Homo sapiens | extracellular matrix |
| c147589_g2 | −0.89 | 1.45 × 10−2 | Cadherin-89D | Drosophila melanogaster | involved in adhesion |
| c149462_g1 | 0.42 | 9.10 × 10−3 | Kinectin | Mus musculus | involved in adhesion |
| c104353_g1 | −0.55 | 2.02 × 10−2 | Lactadherin | Rattus norvegicus | involved in adhesion |
| c156870_g1 | 0.64 | 8.39 × 10−4 | Macrophage mannose receptor 1 | Homo sapiens | involved in adhesion |
| c156842_g1 | −0.36 | 1.64 × 10−3 | Neural cell adhesion molecule 1 | Bos taurus | involved in adhesion |
| c151606_g1 | −0.40 | 6.31 × 10−3 | Neural cell adhesion molecule 1 | Rattus norvegicus | involved in adhesion |
| c136200_g1 | −0.31 | 2.49 × 10−2 | Neuroglian | Drosophila melanogaster | involved in adhesion |
| c154303_g4 | 0.68 | 2.35 × 10−2 | Non-neuronal cytoplasmic intermediate filament protein | Helix aspersa | involved in adhesion |
| c135777_g1 | −1.18 | 5.99 × 10−5 | Periostin | Mus musculus | involved in adhesion |
| c157397_g1 | −1.38 | 1.25 × 10−5 | Protocadherin Fat 4 | Homo sapiens | involved in adhesion |
| c142570_g1 | −1.11 | 2.18 × 10−4 | Protocadherin-like wing polarity protein stan | Drosophila melanogaster | involved in adhesion |
| Ingestion and Digestion | |||||
| c128401_g2 | −0.78 | 2.31 × 10−2 | Beta-galactosidase-1-like protein 2 | Homo sapiens | involved in carbohydrates hydrolysis |
| c135558_g1 | −1.78 | 1.09 × 10−3 | Endo-1,4-β-xylanase Z | Clostridium thermocellum | involved in carbohydrates hydrolysis |
| c96519_g1 | −1.58 | 5.17 × 10−3 | Endoglucanase | Mytilus edulis | involved in carbohydrates hydrolysis |
| c137870_g1 | −1.17 | 1.63 × 10−3 | Endoglucanase E-4 | Thermobifida fusca | involved in carbohydrates hydrolysis |
| c154739_g1 | −0.98 | 7.49 × 10−4 | Endoglucanase E-4 | Thermobifida fusca | involved in carbohydrates hydrolysis |
| c150903_g1 | −1.78 | 1.09 × 10−3 | Exoglucanase XynX | Clostridium thermocellum | involved in carbohydrates hydrolysis |
| c145604_g1 | 1.18 | 6.57 × 10−5 | Inactive pancreatic lipase-related protein 1 | Rattus norvegicus | involved in fat hydrolysis |
| c71768_g2 | 2.20 | 5.20 × 10−7 | Pancreatic triacylglycerol lipase | Myocastor coypus | involved in fat hydrolysis |
| c141966_g1 | 1.21 | 2.76 × 10−3 | Chymotrypsin-like elastase family member 3B | Mus musculus | involved in proteins hydrolysis |
| c140662_g1 | 0.74 | 3.47 × 10−3 | Chymotrypsin-like serine proteinase | Haliotis rufescens | involved in proteins hydrolysis |
| c141241_g2 | 0.33 | 2.94 × 10−2 | Glutamate carboxypeptidase 2 | Rattus norvegicus | involved in proteins hydrolysis |
| c150838_g1 | 1.44 | 4.46 × 10−5 | Prolyl endopeptidase | Mus musculus | involved in proteins hydrolysis |
| c153823_g1 | 0.43 | 3.91 × 10−3 | Trypsin | Sus scrofa | involved in proteins hydrolysis |
| c149315_g1 | 1.96 | 4.51 × 10−4 | Zinc carboxypeptidase A 1 | Anopheles gambiae | involved in proteins hydrolysis |
| c150282_g1 | 2.22 | 2.11 × 10−4 | Zinc metalloproteinase nas-13 | Caenorhabditis elegans | involved in proteins hydrolysis |
| c146629_g1 | 1.74 | 3.63 × 10−4 | Zinc metalloproteinase nas-14 | Caenorhabditis elegans | involved in proteins hydrolysis |
| c149138_g1 | −0.45 | 3.15 × 10−3 | Zinc metalloproteinase nas-30 | Caenorhabditis elegans | involved in proteins hydrolysis |
| c128907_g1 | 1.87 | 1.72 × 10−3 | Zinc metalloproteinase nas-38 | Caenorhabditis elegans | involved in proteins hydrolysis |
| c153700_g1 | 1.79 | 1.27 × 10−4 | Zinc metalloproteinase nas-6 | Caenorhabditis elegans | involved in proteins hydrolysis |
| c156669_g2 | 2.30 | 3.77 × 10−5 | Zinc metalloproteinase nas-8 | Caenorhabditis elegans | involved in proteins hydrolysis |
| c131553_g1 | 0.83 | 4.63 × 10−3 | Conotoxin Cl14.12 | Conus californicus | involved in secretory venom for predation |
| c147316_g1 | 1.33 | 9.47 × 10−4 | Cysteine-rich venom protein | Conus textile | involved in secretory venom for predation |
| c143655_g1 | 2.27 | 2.96 × 10−4 | Cysteine-rich venom protein Mr30 | Conus marmoreus | involved in secretory venom for predation |
| Stress Response and Immunity | |||||
| c122242_g1 | 1.59 | 1.84 × 10−4 | Myeloperoxidase | Mus musculus | anti-oxidant protein |
| c88819_g1 | 1.68 | 1.13 × 10−3 | Peroxidase-like protein 3 (Fragment) | Lottia gigantea | anti-oxidant protein |
| c156674_g2 | 0.49 | 1.21 × 10−2 | Peroxidasin homolog | Mus musculus | anti-oxidant protein |
| c140657_g1 | −0.38 | 1.54 × 10−2 | Peroxiredoxin-2 | Rattus norvegicus | anti-oxidant protein |
| c142245_g1 | −0.37 | 1.23 × 10−2 | Peroxiredoxin-6 | Gallus gallus | anti-oxidant protein |
| c156482_g1 | 0.30 | 1.01 × 10−2 | Probable deferrochelatase/peroxidase YfeX | Escherichia coli | anti-oxidant protein |
| c130129_g1 | −0.69 | 3.65 × 10−3 | Thioredoxin-T | Drosophila melanogaster | anti-oxidant protein |
| c152296_g4 | −0.85 | 1.93 × 10−3 | Angiotensin-converting enzyme (Fragment) | Gallus gallus | immune-related protein |
| c154571_g1 | −2.22 | 1.81 × 10−3 | Uncharacterized protein C1orf194 homolog | Danio rerio | immune-related protein |
| c120194_g1 | 2.15 | 5.35 × 10−5 | Hemocyanin A-type, units Ode to Odg (Fragment) | Enteroctopus dofleini | oxygen supply, immune-related protein |
| c147531_g1 | 2.31 | 2.43 × 10−5 | Hemocyanin A-type, units Ode to Odg (Fragment) | Enteroctopus dofleini | oxygen supply, immune-related protein |
| c153812_g1 | 2.41 | 2.00 × 10−4 | Hemocyanin G-type, units Oda to Odg | Enteroctopus dofleini | oxygen supply, immune-related protein |
| c146636_g1 | 2.42 | 1.95 × 10−4 | Hemocyanin G-type, units Oda to Odg | Enteroctopus dofleini | oxygen supply, immune-related protein |
| c156294_g1 | 2.43 | 7.60 × 10−5 | Hemocyanin G-type, units Oda to Odg | Enteroctopus dofleini | oxygen supply, immune-related protein |
| c153794_g2 | 1.02 | 1.50 × 10−3 | Alpha-2-macroglobulin | Pongo abelii | proteolysis, immune-related protein |
| c155750_g1 | 0.45 | 5.33 × 10−5 | 60 kDa heat shock protein, mitochondrial | Cricetulus griseus | response to stress |
| c155284_g2 | 0.42 | 7.02 × 10−3 | Heat shock protein 75 kDa, mitochondrial | Mus musculus | response to stress |
| Particular Tissue Development | |||||
| c157271_g1 | −0.73 | 2.29 × 10−2 | Dynein heavy chain 10, axonemal | Strongylocentrotus purpuratus | cilia-specific protein |
| c156807_g2 | −0.76 | 1.09 × 10−2 | Dynein heavy chain 12, axonemal | Xenopus laevis | cilia-specific protein |
| c123013_g1 | −0.56 | 3.64 × 10−2 | Dynein heavy chain 5, axonemal | Bos taurus | cilia-specific protein |
| c155384_g3 | −0.64 | 2.82 × 10−3 | Dynein heavy chain 6, axonemal | Rattus norvegicus | cilia-specific protein |
| c154803_g2 | −0.76 | 4.90 × 10−3 | Dynein heavy chain 7, axonemal | Homo sapiens | cilia-specific protein |
| c157287_g2 | −0.79 | 2.31 × 10−4 | Dynein heavy chain 8, axonema | Mus musculus | cilia-specific protein |
| c154991_g1 | −1.02 | 5.78 × 10−4 | Dynein intermediate chain 2, ciliary | Heliocidaris crassispina | cilia-specific protein |
| c122667_g1 | −0.87 | 1.98 × 10−3 | Dynein light chain 1, axonemal | Homo sapiens | cilia-specific protein |
| c156053_g3 | 0.89 | 2.24 × 10−3 | Myosin essential light chain, striated adductor muscle | Homo sapiens | cilia-specific protein |
| c85433_g2 | 0.89 | 8.64 × 10−4 | Myosin heavy chain, striated muscle | Homo sapiens | cilia-specific protein |
| c151606_g1 | −0.40 | 6.31 × 10−3 | Neural cell adhesion molecule 1 | Homo sapiens | cilia-specific protein |
| c136200_g1 | −0.31 | 2.49 × 10−2 | Neuroglian | Homo sapiens | cilia-specific protein |
| c150230_g2 | −1.42 | 7.67 × 10−6 | Tektin-1 | Homo sapiens | cilia-specific protein |
| c153806_g1 | −1.58 | 2.39 × 10−4 | Tektin-2 | Homo sapiens | cilia-specific protein |
| c155866_g1 | −1.30 | 2.54 × 10−4 | Tektin-3 | Rattus norvegicus | cilia-specific protein |
| c28062_g1 | −0.89 | 1.80 × 10−4 | Tektin-4 | Tripneustes gratilla | cilia-specific protein |
| c153806_g3 | −1.19 | 1.10 × 10−4 | Tektin-B1 | Heliocidaris crassispina | cilia-specific protein |
| c131813_g1 | −1.08 | 1.05 × 10−2 | Dynein beta chain, ciliary | Argopecten irradians | muscle-specific protein |
| c95355_g1 | −0.93 | 4.43 × 10−3 | Dynein beta chain, ciliary | Argopecten irradians | muscle-specific protein |
| c157057_g1 | −0.68 | 1.06 × 10−2 | Dynein heavy chain 1, axonemal | Drosophila melanogaster | neuron-specific protein |
| c155993_g1 | −0.64 | 4.95 × 10−3 | Dynein heavy chain 10, axonemal | Rattus norvegicus | neuron-specific protein |
| # | Pathway | Differential Proteins with Pathway Annotation (347) | All Proteins with Pathway Annotation (2056) | p-Value | q-Value | Pathway ID |
|---|---|---|---|---|---|---|
| 1 | Phototransduction | 7 (2.02%) | 11 (0.54%) | 0.000655 | 0.039412 | ko04744 |
| 2 | Caprolactam degradation | 7 (2.02%) | 11 (0.54%) | 0.000655 | 0.039412 | ko00930 |
| 3 | Pentose and glucuronate Interconversions | 12 (3.46%) | 27 (1.31%) | 0.000685 | 0.039412 | ko00040 |
| 4 | Olfactory transduction | 9 (2.59%) | 18 (0.88%) | 0.001175 | 0.039412 | ko04740 |
| 5 | Glycerolipid metabolism | 11 (3.17%) | 25 (1.22%) | 0.001275 | 0.039412 | ko00561 |
| 6 | Galactose metabolism | 11 (3.17%) | 25 (1.22%) | 0.001275 | 0.039412 | ko00052 |
| 7 | Salivary secretion | 17 (4.9%) | 48 (2.33%) | 0.001326 | 0.039412 | ko04970 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Wang, H.-Y.; Zhang, T. Comprehensive and Quantitative Proteomic Analysis of Metamorphosis-Related Proteins in the Veined Rapa Whelk, Rapana venosa. Int. J. Mol. Sci. 2016, 17, 924. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17060924
Song H, Wang H-Y, Zhang T. Comprehensive and Quantitative Proteomic Analysis of Metamorphosis-Related Proteins in the Veined Rapa Whelk, Rapana venosa. International Journal of Molecular Sciences. 2016; 17(6):924. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17060924
Chicago/Turabian StyleSong, Hao, Hai-Yan Wang, and Tao Zhang. 2016. "Comprehensive and Quantitative Proteomic Analysis of Metamorphosis-Related Proteins in the Veined Rapa Whelk, Rapana venosa" International Journal of Molecular Sciences 17, no. 6: 924. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17060924