Recognizing Depression from the Microbiota–Gut–Brain Axis
Abstract
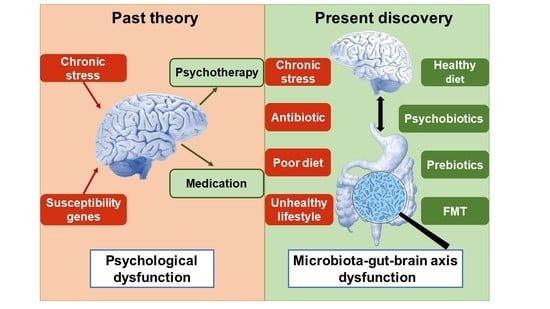
:1. Introduction
2. The Pathophysiology of Major Depressive Disorder
2.1. The Brain Dysfunction
2.2. The HPA Axis Dysfunction
2.3. Immune System Abnormalities
2.4. Gut Brain Dysfunction
3. The Latest Research Progress of Depression: The Microbiota Hypothesis
3.1. Depressed Patients Have Different Gut Microbiota from Healthy Persons
3.2. Depressive Symptoms Can Be Transmitted Following Fecal Microbiota Transplantation
3.3. Gut Microbiota Disturbances Increase the Susceptibilities of Depression
3.4. Gut Microbiota Restoration Alleviates Depression
3.5. The Mechanisms of Traditional Antidepressant Therapies are Probably Related with Microbiota
3.6. New Therapies Integrating Gut Microbiota Regulation Present Promising Effects
4. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
References
- Aan het Rot, M.; Mathew, S.J.; Charney, D.S. Neurobiological Mechanisms in Major Depressive Disorder. Can. Med. Assoc. J. 2009, 180, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, D.M.; Wohleb, E.S.; Duman, R.S. Emerging Treatment Mechanisms for Depression: Focus on Glutamate and Synaptic Plasticity. Drug Discov. Today 2016, 21, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H. Medical Research: If Depression Were Cancer. Nature 2014, 515, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.; Lau, W.K.; Sim, J.; Sum, M.Y.; Baldessarini, R.J. Prevention of Relapse and Recurrence in Adults with Major Depressive Disorder: Systematic Review and Meta-Analyses of Controlled Trials. Int. J. Neuropsychopharmacol. 2015, 19. [Google Scholar] [CrossRef] [PubMed]
- Miret, M.; Ayuso-Mateos, J.L.; Sanchez-Moreno, J.; Vieta, E. Depressive Disorders and Suicide: Epidemiology, Risk Factors, and Burden. Neurosci. Biobehav. Rev. 2013, 37, 2372–2374. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Torres, C.D. Mental Health: A World of Depression. Nature 2014, 515, 181. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.J.; Charlson, F.J.; Norman, R.E.; Patten, S.B.; Freedman, G.; Murray, C.J.; Vos, T.; Whiteford, H.A. Burden of Depressive Disorders by Country, Sex, Age, and Year: Findings from the Global Burden of Disease Study 2010. PLoS Med. 2013, 10, E1001547. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 DALYs and HALE Collaborators. Global, Regional, and National Disability-Adjusted Life-Years (Dalys) for 333 Diseases and Injuries and Healthy Life Expectancy (Hale) for 195 Countries and Territories, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1260–1344. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Wang, M.; Zhang, Y.; Li, L. From Serotonin to Neuroplasticity: Evolvement of Theories for Major Depressive Disorder. Front. Cell. Neurosci. 2017, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, D.; Liu, H.; Han, M.H. Neuronal Correlates of Depression. Cell. Mol. Life Sci. 2015, 72, 4825–4848. [Google Scholar] [CrossRef] [PubMed]
- Belmaker, R.H.; Agam, G. Major Depression Disorder. N. Engl. J. Med. 2008, 358, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Corfield, E.C.; Yang, Y.; Martin, N.G.; Nyholt, D.R. A Continuum of Genetic Liability for Minor and Major Depression. Transl. Psychiatry 2017, 7, E1131. [Google Scholar] [CrossRef] [PubMed]
- Bukh, J.D.; Bock, C.; Vinberg, M.; Werge, T.; Gether, U.; Vedel Kessing, L. Interaction between Genetic Polymorphisms and Stressful Life Events in First Episode Depression. J. Affect. Disord. 2009, 119, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, P.; Sudo, N.; Dinan, T.; Taylor, V.H.; Bienenstock, J. Mood and Gut Feelings. Brain Behav. Immun. 2010, 24, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Evrensel, A.; Ceylan, M.E. The Gut-Brain Axis: The Missing Link in Depression. Clin. Psychopharmacol. Neurosci. 2015, 13, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Clarke, G.; Berk, M.; Jacka, F.N. The Gut Microbiome and Diet in Psychiatry: Focus on Depression. Curr. Opin. Psychiatry 2015, 28, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; Blacher, E.; Elinav, E.; Pettersson, S. Our Gut Microbiome: The Evolving Inner Self. Cell 2017, 171, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wang, T.; Hu, X.; Li, W.; Jin, F.; Wang, L. Microorganism and Behavior and Psychiatric Disorders. Adv. Psychol. Sci. 2012, 20, 75–97. (In Chinese) [Google Scholar] [CrossRef]
- Leonard, B.E. The HPA and Immune Axes in Stress: The Involvement of the Serotonergic System. Eur. Psychiatry 2005, 20, 302–306. [Google Scholar] [CrossRef]
- Mahar, I.; Bambico, F.R.; Mechawar, N.; Nobrega, J.N. Stress, Serotonin, and Hippocampal Neurogenesis in Relation to Depression and Antidepressant Effects. Neurosci. Biobehav. Rev. 2014, 38, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.W.; Lowry, C.A. The Hygiene Hypothesis and Psychiatric Disorders. Trends Immunol. 2008, 29, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Lima-Ojeda, J.M.; Rupprecht, R.; Baghai, T.C. “I Am I and My Bacterial Circumstances”: Linking Gut Microbiome, Neurodevelopment, and Depression. Front. Psychiatry 2017, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Bercik, P. The Relationship Between Intestinal Microbiota and the Central Nervous System in Normal Gastrointestinal Function and Disease. Gastroenterology 2009, 136, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- O’mahony, S.M.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Irritable Bowel Syndrome and Stress-Related Psychiatric Co-Morbidities: Focus on Early Life Stress. Handb. Exp. Pharmacol. 2017, 239, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsen, I. Brain-Gut Axis as an Example of the Bio-Psycho-Social Model. Gut 2000, 47, 5–7. [Google Scholar] [CrossRef]
- Hamon, M.; Blier, P. Monoamine Neurocircuitry in Depression and Strategies for New Treatments. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Lener, M.S.; Niciu, M.J.; Ballard, E.D.; Park, M.; Park, L.T.; Nugent, A.C.; Zarate, C.A., Jr. Glutamate and Gamma-Aminobutyric Acid Systems in the Pathophysiology of Major Depression and Antidepressant Response to Ketamine. Biol. Psychiatry 2017, 81, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Rm, H. History and Evolution of the Monoamine Hypothesis of Depression. J. Clin. Psychiatry 2000, 61 (Suppl. 6), 4–6. [Google Scholar]
- Pytka, K.; Dziubina, A.; Mlyniec, K.; Dziedziczak, A.; Zmudzka, E.; Furgala, A.; Olczyk, A.; Sapa, J.; Filipek, B. The Role of Glutamatergic, Gaba-Ergic, and Cholinergic Receptors in Depression and Antidepressant-Like Effect. Pharmacol. Rep. 2016, 68, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Murrough, J.W.; Abdallah, C.G.; Mathew, S.J. Targeting Glutamate Signalling in Depression: Progress and Prospects. Nat. Rev. Drug Discov. 2017, 16, 472–486. [Google Scholar] [CrossRef] [PubMed]
- Serafini, G. Neuroplasticity and Major Depression, the Role of Modern Antidepressant Drugs. World J. Psychiatry 2012, 2, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Sahay, A.; Hen, R. Adult Hippocampal Neurogenesis in Depression. Nat. Neurosci. 2007, 10, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Kraus, C.; Castren, E.; Kasper, S.; Lanzenberger, R. Serotonin and Neuroplasticity—Links between Molecular, Functional and Structural Pathophysiology in Depression. Neurosci. Biobehav. Rev. 2017, 77, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.D.; Correia, J.S.; Patricio, P.; Mateus-Pinheiro, A.; Machado-Santos, A.R.; Loureiro-Campos, E.; Morais, M.; Bessa, J.M.; Sousa, N.; Pinto, L. Adult Hippocampal Neuroplasticity Triggers Susceptibility to Recurrent Depression. Transl. Psychiatry 2017, 7, E1058. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Lane, H.Y.; Lin, C.H. New Treatment Strategies of Depression: Based on Mechanisms Related to Neuroplasticity. Neural Plast. 2017, 2017, 4605971. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M. Precision Psychiatry: A Neural Circuit Taxonomy for Depression and Anxiety. Lancet Psychiatry 2016, 3, 472–480. [Google Scholar] [CrossRef]
- Barden, N. Implication of the Hypothalamic-Pituitary-Adrenal Axis in the Physiopathology of Depression. J. Psychiatry Neurosci. 2004, 29, 185–193. [Google Scholar] [PubMed]
- Juruena, M.F.; Cleare, A.J.; Pariante, C.M. The Hypothalamic Pituitary Adrenal Axis, Glucocorticoid Receptor Function and Relevance to Depression. Rev. Bras. Psiquiatr. 2004, 26, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Kunugi, H.; Hori, H.; Adachi, N.; Numakawa, T. Interface between Hypothalamic-Pituitary-Adrenal Axis and Brain-Derived Neurotrophic Factor in Depression. Psychiatry Clin. Neurosci. 2010, 64, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Leonard, B.E.; Myint, A.M.; Kubera, M.; Verkerk, R. The New ‘5-Ht’ Hypothesis of Depression: Cell-Mediated Immune Activation Induces Indoleamine 2,3-Dioxygenase, Which Leads to Lower Plasma Tryptophan and an Increased Synthesis of Detrimental Tryptophan Catabolites (Trycats), Both of Which Contribute to the Onset of Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 702–721. [Google Scholar] [CrossRef] [PubMed]
- Nasca, C.; Bigio, B.; Zelli, D.; Nicoletti, F.; Mcewen, B.S. Mind the Gap: Glucocorticoids Modulate Hippocampal Glutamate Tone Underlying Individual Differences in Stress Susceptibility. Mol. Psychiatry 2015, 20, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Singhal, G.; Baune, B.T. Microglia: An Interface between the Loss of Neuroplasticity and Depression. Front. Cell. Neurosci. 2017, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Schiepers, O.J.; Wichers, M.C.; Maes, M. Cytokines and Major Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, D.; Dhabhar, F.S.; James, S.J.; Hough, C.M.; Jain, F.A.; Bersani, F.S.; Reus, V.I.; Verhoeven, J.E.; Epel, E.S.; Mahan, L.; et al. Oxidative Stress, Inflammation and Treatment Response in Major Depression. Psychoneuroendocrinology 2017, 76, 197–205. [Google Scholar] [CrossRef] [PubMed]
- O’brien, S.M.; Scott, L.V.; Dinan, T.G. Cytokines: Abnormalities in Major Depression and Implications for Pharmacological Treatment. Hum. Psychopharmacol. 2004, 19, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Wichers, M.; Maes, M. The Psychoneuroimmuno-Pathophysiology of Cytokine-Induced Depression in Humans. Int. J. Neuropsychopharmacol. 2002, 5, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.E. Inflammation and Depression: A Causal or Coincidental Link to the Pathophysiology? Acta Neuropsychiatr. 2018, 30, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Haroon, E.; Miller, A.H. Inflammation Effects on Brain Glutamate in Depression: Mechanistic Considerations and Treatment Implications. Curr. Top. Behav. Neurosci. 2017, 31, 173–198. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.E.; Hinz, R.; Conen, S.; Gregory, C.J.; Matthews, J.C.; Anton-Rodriguez, J.M.; Gerhard, A.; Talbot, P.S. Elevated Translocator Protein in Anterior Cingulate in Major Depression and a Role for Inflammation in Suicidal Thinking: A Positron Emission Tomography Study. Biol. Psychiatry 2018, 83, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Franklin, T.C.; Wohleb, E.S.; Zhang, Y.; Fogaca, M.; Hare, B.; Duman, R.S. Persistent Increase in Microglial Rage Contributes to Chronic Stress-Induced Priming of Depressive-Like Behavior. Biol. Psychiatry 2018, 83, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Gershon, M.D. The Bowel and Beyond: The Enteric Nervous System in Neurological Disorders. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 517–528. [Google Scholar] [CrossRef] [PubMed]
- O’hara, A.M.; Shanahan, F. The Gut Flora as a Forgotten Organ. Embo Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M. The Microbial Organ in the Gut as a Driver of Homeostasis And Disease. Med. Hypotheses 2010, 74, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Avetisyan, M.; Schill, E.M.; Heuckeroth, R.O. Building a Second Brain in the Bowel. J. Clin. Investig. 2015, 125, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.; Callewaert, C.; Marotz, C.; Hyde, E.R.; Debelius, J.W.; Mcdonald, D.; Sogin, M.L. The Microbiome and Human Biology. Annu. Rev. Genomics Hum. Genet. 2017, 18, 65–86. [Google Scholar] [CrossRef] [PubMed]
- Logan, A.C.; Katzman, M. Major Depressive Disorder: Probiotics May Be an Adjuvant Therapy. Med. Hypotheses 2005, 64, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered Fecal Microbiota Composition in Patients with Major Depressive Disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linlokken, A.; Wilson, R.; Rudi, K. Correlation between the Human Fecal Microbiota and Depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- O’mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, Tryptophan Metabolism and the Brain-Gut-Microbiome Axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef] [PubMed]
- O’mahony, S.M.; Hyland, N.P.; Dinan, T.G.; Cryan, J.F. Maternal Separation as a Model of Brain-Gut Axis Dysfunction. Psychopharmacology 2011, 214, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.V.; Clarke, G.; Dinan, T.G. The Brain-Gut Axis: A Target for Treating Stress-Related Disorders. Mod. Trends Pharmacopsychiatry 2013, 28, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, K.A.; Foster, J.A. Effects of Gut Microbiota on the Brain: Implications for Psychiatry. J. Psychiatry Neurosci. 2009, 34, 230–231. [Google Scholar] [PubMed]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/Brain Axis and the Microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-Gut-Microbiota Axis: Challenges for Translation in Psychiatry. Ann. Epidemiol. 2016, 26, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and Mental Health: A Review. Brain Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Murphy, A.B.; Cryan, J.F.; Ross, P.R.; Dinan, T.G.; Stanton, C. Microbiome in Brain Function and Mental Health. Trends Food Sci. Technol. 2016, 57, 289–301. [Google Scholar] [CrossRef]
- Yarandi, S.S.; Peterson, D.A.; Treisman, G.J.; Moran, T.H.; Pasricha, P.J. Modulatory Effects of Gut Microbiota on the Central Nervous System: How Gut Could Play a Role in Neuropsychiatric Health and Diseases. J. Neurogastroenterol. Motil. 2016, 22, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Fond, G.; Boukouaci, W.; Chevalier, G.; Regnault, A.; Eberl, G.; Hamdani, N.; Dickerson, F.; Macgregor, A.; Boyer, L.; Dargel, A.; et al. The “Psychomicrobiotic”: Targeting Microbiota in Major Psychiatric Disorders: A Systematic Review. Pathol.-Biol. 2015, 63, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.A. Brain, Meet Gut. Nature 2015, 526, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut Microbes and the Brain: Paradigm Shift in Neuroscience. J. Neurosci. 2014, 34, 15490–15496. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Lyte, M.; Meyer, E.; Cryan, J.F. Gut Microbiota and Brain Function: An Evolving Field in Neuroscience. Int. J. Neuropsychopharmacol. 2016, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N. Microbiome, HPA Axis and Production of Endocrine Hormones in the Gut. Adv. Exp. Med. Biol. 2014, 817, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Gareau, M.G.; Jury, J.; Macqueen, G.; Sherman, P.M.; Perdue, M.H. Probiotic Treatment of Rat Pups Normalises Corticosterone Release and Ameliorates Colonic Dysfunction Induced by Maternal Separation. Gut 2007, 56, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Eutamene, H.; Bueno, L. Role of Probiotics in Correcting Abnormalities of Colonic Flora Induced by Stress. Gut 2007, 56, 1495–1497. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Kubo, C. Postnatal Microbial Colonization Programs the Hypothalamic-Pituitary-Adrenal System for Stress Response in Mice. J. Psychosom. Res. 2005, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Littman, D.R. The Microbiota in Adaptive Immune Homeostasis and Disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Levy, M.; Suez, J.; Elinav, E. The Interplay between the Innate Immune System and the Microbiota. Curr. Opin. Immunol. 2014, 26, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, H.; Yim, Y.S.; Ha, S.; Atarashi, K.; Tan, T.G.; Longman, R.S.; Honda, K.; Littman, D.R.; Choi, G.B.; et al. Maternal Gut Bacteria Promote Neurodevelopmental Abnormalities in Mouse Offspring. Nature 2017, 549, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Bien-Ly, N.; Watts, R.J. The Blood-Brain Barrier’s Gut Check. Sci. Transl. Med. 2014, 6, 263fs46. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central Gaba Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Ogbonnaya, E.S.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F.; O’leary, O.F. Adult Hippocampal Neurogenesis Is Regulated by the Microbiome. Biol. Psychiatry 2015, 78, E7–E9. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Ruiz, A.; Mosley, M.; George, A.J.; Mussaji, L.F.; Fullerton, E.F.; Ruszkowski, E.M.; Jacobs, A.J.; Gewirtz, A.T.; Chassaing, B.; Forger, N.G. The Microbiota Influences Cell Death and Microglial Colonization in the Perinatal Mouse Brain. Brain Behav. Immun. 2018, 67, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Hrabe De Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host Microbiota Constantly Control Maturation and Function of Microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Hoban, A.E.; Stilling, R.M.; Ryan, F.J.; Shanahan, F.; Dinan, T.G.; Claesson, M.J.; Clarke, G.; Cryan, J.F. Regulation of Prefrontal Cortex Myelination by the Microbiota. Transl. Psychiatry 2016, 6, E774. [Google Scholar] [CrossRef] [PubMed]
- Borre, Y.E.; O’keeffe, G.W.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota and Neurodevelopmental Windows: Implications for Brain Disorders. Trends Mol. Med. 2014, 20, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Bjorkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal Gut Microbiota Modulates Brain Development and Behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Borre, Y.; O’brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the Blues: Depression-Associated Gut Microbiota Induces Neurobehavioural Changes in the Rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Luna, R.A.; Foster, J.A. Gut Brain Axis: Diet Microbiota Interactions and Implications for Modulation of Anxiety and Depression. Curr. Opin. Biotechnol. 2015, 32, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, R.; Stone, T.W. The Gut-Brain Axis, BDNF, NMDA and CNS Disorders. Neurochem. Res. 2016, 41, 2819–2835. [Google Scholar] [CrossRef] [PubMed]
- Farmer, A.D.; Randall, H.A.; Aziz, Q. It’s a Gut Feeling: How the Gut Microbiota Affects the State of Mind. J. Physiol. 2014, 592, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine Pathway Metabolism and the Microbiota-Gut-Brain Axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Wang, X.; Wang, Z.; Zhang, J.; Jiang, R.; Wang, X.; Wang, K.; Liu, Z.; Xia, Z.; et al. Similar Fecal Microbiota Signatures in Patients with Diarrhea-Predominant Irritable Bowel Syndrome and Patients with Depression. Clin. Gastroenterol. Hepatol. 2016, 14, 1602–1611.E5. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible Association of Bifidobacterium and Lactobacillus in the Gut Microbiota of Patients with Major Depressive Disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut Microbiome Remodeling Induces Depressive-Like Behaviors through a Pathway Mediated by the Host’s Metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Ding, B.; Feng, C.; Yin, S.; Zhang, T.; Qi, X.; Lv, H.; Guo, X.; Dong, K.; Zhu, Y.; et al. Prevotella and Klebsiella Proportions in Fecal Microbial Communities Are Potential Characteristic Parameters for Patients with Major Depressive Disorder. J. Affect. Disord. 2017, 207, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Park, A.J.; Collins, J.; Blennerhassett, P.A.; Ghia, J.E.; Verdu, E.F.; Bercik, P.; Collins, S.M. Altered Colonic Function and Microbiota Profile in a Mouse Model of Chronic Depression. Neurogastroenterol. Motil. 2013, 25, 733-e575. [Google Scholar] [CrossRef] [PubMed]
- O’mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.M.; Quigley, E.M.; Cryan, J.F.; Dinan, T.G. Early Life Stress Alters Behavior, Immunity, and Microbiota in Rats: Implications for Irritable Bowel Syndrome and Psychiatric Illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Bharwani, A.; Mian, M.F.; Surette, M.G.; Bienenstock, J.; Forsythe, P. Oral Treatment with Lactobacillus Rhamnosus Attenuates Behavioural Deficits and Immune Changes in Chronic Social Stress. BMC Med. 2017, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Jia, H.; Zhou, C.; Yang, Y.; Zhao, Y.; Yang, M.; Zou, Z. Variations in Gut Microbiota and Fecal Metabolic Phenotype Associated with Depression by 16s rRNA Gene Sequencing and Lc/Ms-Based Metabolomics. J. Pharm. Biomed. Anal. 2017, 138, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wang, T.; Hu, X.; Luo, J.; Li, W.; Wu, X.; Duan, Y.; Jin, F. Administration of Lactobacillus Helveticus Ns8 Improves Behavioral, Cognitive, and Biochemical Aberrations Caused by Chronic Restraint Stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human Gut Microbiome Viewed across Age and Geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, T.; Liang, S.; Li, W.; Wu, X.; Jin, F. Antibiotic-Induced Imbalances in Gut Microbiota Aggravates Cholesterol Accumulation and Liver Injuries in Rats Fed a High-Cholesterol Diet. Appl. Microbiol. Biotechnol. 2015, 99, 9111–9122. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, E.E.; Farzi, A.; Mayerhofer, R.; Reichmann, F.; Jacan, A.; Wagner, B.; Zinser, E.; Bordag, N.; Magnes, C.; Frohlich, E.; et al. Cognitive Impairment by Antibiotic-Induced Gut Dysbiosis: Analysis of Gut Microbiota-Brain Communication. Brain Behav. Immun. 2016, 56, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Collins, S.M. The Effects of Inflammation, Infection and Antibiotics on the Microbiota-Gut-Brain Axis. Adv. Exp. Med. Biol. 2014, 817, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hu, X.; Liang, S.; Li, W.; Wu, X.; Wang, L.; Jin, F. Lactobacillus Fermentum Ns9 Restores the Antibiotic Induced Physiological and Psychological Abnormalities in Rats. Benef. Microbes 2015, 6, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Lurie, I.; Yang, Y.X.; Haynes, K.; Mamtani, R.; Boursi, B. Antibiotic Exposure and the Risk for Depression, Anxiety, or Psychosis: A Nested Case-Control Study. J. Clin. Psychiatry 2015, 76, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Kohler, O.; Petersen, L.; Mors, O.; Mortensen, P.B.; Yolken, R.H.; Gasse, C.; Benros, M.E. Infections and Exposure to Anti-Infective Agents and the Risk of Severe Mental Disorders: A Nationwide Study. Acta Psychiatr. Scand. 2017, 135, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Slykerman, R.F.; Thompson, J.; Waldie, K.E.; Murphy, R.; Wall, C.; Mitchell, E.A. Antibiotics in the First Year of Life and Subsequent Neurocognitive Outcomes. Acta Paediatr. 2017, 106, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Turco, F.; Iannotta, M.; De Gregorio, D.; Palumbo, I.; Sarnelli, G.; Furiano, A.; Napolitano, F.; Boccella, S.; Luongo, L.; et al. Antibiotic-Induced Microbiota Perturbation Causes Gut Endocannabinoidome Changes, Hippocampal Neuroglial Reorganization and Depression in Mice. Brain Behav. Immun. 2018, 67, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Holdeman, L.V.; Good, I.J.; Moore, W.E. Human Fecal Flora Variation in Bacterial Composition within Individuals and a Possible Effect of Emotional Stress. Appl. Environ. Microbiol. 1976, 31, 359–375. [Google Scholar] [PubMed]
- Galley, J.D.; Nelson, M.C.; Yu, Z.T.; Dowd, S.E.; Walter, J.; Kumar, P.S.; Lyte, M.; Bailey, M.T. Exposure to a Social Stressor Disrupts the Community Structure of the Colonic Mucosa-Associated Microbiota. BMC Microbiol. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.T.; Coe, C.L. Maternal Separation Disrupts the Integrity of the Intestinal Microflora in Infant Rhesus Monkeys. Dev. Psychobiol. 1999, 35, 146–155. [Google Scholar] [CrossRef]
- Gur, T.L.; Worly, B.L.; Bailey, M.T. Stress and the Commensal Microbiota: Importance in Parturition and Infant Neurodevelopment. Front. Psychiatry 2015, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Regulation of the Stress Response by the Gut Microbiota: Implications for Psychoneuroendocrinology. Psychoneuroendocrinology 2012, 37, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Collins, S.M.; Bercik, P.; Verdu, E.F. The Microbiota-Gut-Brain Axis in Gastrointestinal Disorders: Stressed Bugs, Stressed Brain or Both? J. Physiol. 2014, 592, 2989–2997. [Google Scholar] [CrossRef] [PubMed]
- Marin, I.A.; Goertz, J.E.; Ren, T.; Rich, S.S.; Onengut-Gumuscu, S.; Farber, E.; Wu, M.; Overall, C.C.; Kipnis, J.; Gaultier, A. Microbiota Alteration Is Associated with the Development of Stress-Induced Despair Behavior. Sci. Rep. 2017, 7, 43859. [Google Scholar] [CrossRef] [PubMed]
- Frei, R.; Lauener, R.P.; Crameri, R.; O’mahony, L. Microbiota and Dietary Interactions—An Update to the Hygiene Hypothesis? Allergy 2012, 67, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-Induced Extinctions in the Gut Microbiota Compound over Generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, K.V.; Sherwin, E.; Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding the Microbiota-Gut-Brain Axis: Diet, Microbiome, and Neuropsychiatry. Transl. Res. 2017, 179, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Slyepchenko, A.; Maes, M.; Jacka, F.N.; Köhler, C.A.; Barichello, T.; Mcintyre, R.S.; Berk, M.; Grande, I.; Foster, J.A.; Vieta, E.; et al. Gut Microbiota, Bacterial Translocation, and Interactions with Diet: Pathophysiological Links between Major Depressive Disorder and Non-Communicable Medical Comorbidities. Psychother. Psychosom. 2017, 86, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.; Corfe, B. The Role of Diet and Nutrition on Mental Health and Wellbeing. Proc. Nutr. Soc. 2017, 76, 425–426. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.E.; Hsu, T.M.; Kanoski, S.E. Gut to Brain Dysbiosis: Mechanisms Linking Western Diet Consumption, The Microbiome, and Cognitive Impairment. Front. Behav. Neurosci. 2017, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Bereswill, S.; Pyndt Jørgensen, B.; Hansen, J.T.; Krych, L.; Larsen, C.; Klein, A.B.; Nielsen, D.S.; Josefsen, K.; Hansen, A.K.; Sørensen, D.B. A Possible Link between Food and Mood: Dietary Impact on Gut Microbiota and Behavior in Balb/C Mice. PLoS ONE 2014, 9, E103398. [Google Scholar] [CrossRef]
- Oriach, C.S.; Robertson, R.C.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Food for Thought: The Role of Nutrition in the Microbiota-Gut–Brain Axis. Clin. Nutr. Exp. 2016, 6, 25–38. [Google Scholar] [CrossRef]
- Ng, K.M.; Ferreyra, J.A.; Higginbottom, S.K.; Lynch, J.B.; Kashyap, P.C.; Gopinath, S.; Naidu, N.; Choudhury, B.; Weimer, B.C.; Monack, D.M.; et al. Microbiota-Liberated Host Sugars Facilitate Post-Antibiotic Expansion of Enteric Pathogens. Nature 2013, 502, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Roca-Saavedra, P.; Mendez-Vilabrille, V.; Miranda, J.M.; Nebot, C.; Cardelle-Cobas, A.; Franco, C.M.; Cepeda, A. Food Additives, Contaminants and Other Minor Components: Effects on Human Gut Microbiota—A Review. J. Physiol. Biochem. 2017, 71, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-Gut-Microbe Communication in Health and Disease. Front. Physiol. 2011, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, F. Linking Lifestyle with Microbiota and Risk of Chronic Inflammatory Disorders. In The Hygiene Hypothesis and Darwinian Medicine; Rook, G.A.W., Ed.; Birkhäuser: Basel, Switzerland, 2009; pp. 93–102. [Google Scholar]
- Liu, X.; Cao, S.; Zhang, X. Modulation of Gut Microbiota-Brain Axis by Probiotics, Prebiotics, and Diet. J. Agric. Food Chem. 2015, 63, 7885–7895. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Ianiro, G.; Bibbo, S.; Gasbarrini, A. Gut Microbiota Modulation: Probiotics, Antibiotics or Fecal Microbiota Transplantation? Intern. Emerg. Med. 2014, 9, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Marques, T.M.; Cryan, J.F.; Shanahan, F.; Fitzgerald, G.F.; Ross, R.P.; Dinan, T.G.; Stanton, C. Gut Microbiota Modulation and Implications for Host Health: Dietary Strategies to Influence the Gut–Brain Axis. Innov. Food Sci. Emerg. Technol. 2014, 22, 239–247. [Google Scholar] [CrossRef]
- FAO; WHO. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and Metabolic Response to Probiotic Administration in Patients with Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.J.K.; Milev, R. The Effects of Probiotics on Depressive Symptoms in Humans: A Systematic Review. Ann. Gen. Psychiatry 2017, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Pirbaglou, M.; Katz, J.; De Souza, R.J.; Stearns, J.C.; Motamed, M.; Ritvo, P. Probiotic Supplementation Can Positively Affect Anxiety and Depressive Symptoms: A Systematic Review of Randomized Controlled Trials. Nutr. Res. 2016, 36, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, A.; Elfving, B.; Hokland, M.; Wegener, G.; Lund, S. Probiotic Treatment Reduces Depressive-Like Behaviour in Rats Independently of Diet. Psychoneuroendocrinology 2017, 79, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the Probiotic Bifidobacterium Infantis in the Maternal Separation Model of Depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (Isapp) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Cowen, P.J.; Harmer, C.J.; Tzortzis, G.; Errington, S.; Burnet, P.W.J. Prebiotic Intake Reduces the Waking Cortisol Response and Alters Emotional Bias in Healthy Volunteers. Psychopharmacology 2015, 232, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.C.; Seira Oriach, C.; Murphy, K.; Moloney, G.M.; Cryan, J.F.; Dinan, T.G.; Paul Ross, R.; Stanton, C. Omega-3 Polyunsaturated Fatty Acids Critically Regulate Behaviour and Gut Microbiota Development in Adolescence and Adulthood. Brain Behav. Immun. 2017, 59, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Mika, A.; Day, H.E.; Martinez, A.; Rumian, N.L.; Greenwood, B.N.; Chichlowski, M.; Berg, B.M.; Fleshner, M. Early Life Diets with Prebiotics and Bioactive Milk Fractions Attenuate the Impact of Stress on Learned Helplessness Behaviours and Alter Gene Expression within Neural Circuits Important for Stress Resistance. Eur. J. Neurosci. 2017, 45, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.; Dias, G.P.; Thuret, S. Effects of Diet on Brain Plasticity in Animal and Human Studies: Mind the Gap. Neural Plast. 2014, 2014, 563160. [Google Scholar] [CrossRef] [PubMed]
- Heiman, M.L.; Greenway, F.L. A Healthy Gastrointestinal Microbiome Is Dependent on Dietary Diversity. Mol. Metab. 2016, 5, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Evrensel, A.; Ceylan, M.E. Fecal Microbiota Transplantation and Its Usage in Neuropsychiatric Disorders. Clin. Psychopharmacol. Neurosci. 2016, 14, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Kali, A. Psychobiotics: An Emerging Probiotic in Psychiatric Practice. Biomed. J. 2016, 39, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Macedo, D.; Filho, A.; Soares De Sousa, C.N.; Quevedo, J.; Barichello, T.; Junior, H.V.N.; Freitas De Lucena, D. Antidepressants, Antimicrobials or Both? Gut Microbiota Dysbiosis in Depression and Possible Implications of the Antimicrobial Effects of Antidepressant Drugs for Antidepressant Effectiveness. J. Affect. Disord. 2017, 208, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.F.; Ferreira Rocha, N.B.; Paes, F.; Arias-Carrión, O.; Machado, S.; de Sá Filho, A.S. Neural Mechanisms of Exercise—Effects on Gut Miccrobiota and Depression. CNS Neurol. Disord. Drug Targets 2015, 14, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, M.; Herring, M.P.; Owen, N.; Dunstan, D.; Ekblom, O.; Helgadottir, B.; Nakitanda, O.A.; Forsell, Y. Exercise, Physical Activity, And Sedentary Behavior in the Treatment of Depression: Broadening the Scientific Perspectives and Clinical Opportunities. Front. Psychiatry 2016, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Schnorr, S.L.; Bachner, H.A. Integrative Therapies in Anxiety Treatment with Special Emphasis on the Gut Microbiome. Yale J. Biol. Med. 2016, 89, 397–422. [Google Scholar] [PubMed]
- Bambling, M.; Edwards, S.C.; Hall, S.; Vitetta, L. A Combination of Probiotics and Magnesium Orotate Attenuate Depression in a Small Ssri Resistant Cohort: An Intestinal Anti-Inflammatory Response Is Suggested. Inflammopharmacology 2017, 25, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Bain, L.; Stroud, C. Enabling Discovery, Development, And Translation Of Treatments For Cognitive Dysfunction In Depression: Workshop Summary; National Academies Press: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
- Garay, R.P.; Zarate, C.A., Jr.; Charpeaud, T.; Citrome, L.; Correll, C.U.; Hameg, A.; Llorca, P.M. Investigational Drugs in Recent Clinical Trials for Treatment-Resistant Depression. Expert Rev. Neurother. 2017, 17, 593–609. [Google Scholar] [CrossRef] [PubMed]
- Hyman, S. Mental Health: Depression Needs Large Human-Genetics Studies. Nature 2014, 515, 189–191. [Google Scholar] [CrossRef] [PubMed]
- The Economist Intelligence Unit. Provision for Supporting People with Mental Illness: A Comparison of 15 Asia Pacific Countries; The Economist Intelligence Unit: London, UK, 2016. [Google Scholar]
- Dinan, T.G.; Stilling, R.M.; Stanton, C.; Cryan, J.F. Collective Unconscious: How Gut Microbes Shape Human Behavior. J. Psychiatr. Res. 2015, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Parashar, A.; Udayabanu, M. Gut Microbiota Regulates Key Modulators of Social Behavior. Eur. Neuropsychopharmacol. 2016, 26, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Yang, Y.; Zhu, W. Gut Microbiota: The Brain Peacekeeper. Front. Microbiol. 2016, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Bested, A.C.; Logan, A.C.; Selhub, E.M. Intestinal Microbiota, Probiotics and Mental Health: From Metchnikoff to Modern Advances: Part III—Convergence toward Clinical Trials. Gut Pathog. 2013, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bested, A.C.; Logan, A.C.; Selhub, E.M. Intestinal Microbiota, Probiotics and Mental Health: From Metchnikoff to Modern Advances: Part I—Autointoxication Revisited. Gut Pathog. 2013, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bested, A.C.; Logan, A.C.; Selhub, E.M. Intestinal Microbiota, Probiotics and Mental Health: From Metchnikoff to Modern Advances: Part II—Contemporary Contextual Research. Gut Pathog. 2013, 5, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, S.M.; Surette, M.; Bercik, P. The Interplay between the Intestinal Microbiota and the Brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Collins, S.M.; Verdu, E.F. Microbes and the Gut-Brain Axis. Neurogastroenterol. Motil. 2012, 24, 405–413. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.; Wu, X.; Hu, X.; Wang, T.; Jin, F. Recognizing Depression from the Microbiota–Gut–Brain Axis. Int. J. Mol. Sci. 2018, 19, 1592. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19061592
Liang S, Wu X, Hu X, Wang T, Jin F. Recognizing Depression from the Microbiota–Gut–Brain Axis. International Journal of Molecular Sciences. 2018; 19(6):1592. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19061592
Chicago/Turabian StyleLiang, Shan, Xiaoli Wu, Xu Hu, Tao Wang, and Feng Jin. 2018. "Recognizing Depression from the Microbiota–Gut–Brain Axis" International Journal of Molecular Sciences 19, no. 6: 1592. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19061592