1. Introduction
Schizophrenia (SCZ) is a severe neurodevelopmental disorder affecting 1% of the general population worldwide [
1]. It represents a major concern of public health with very high rates of resistance to treatment. In fact, despite the introduction of atypical antipsychotics (especially clozapine) and despite all efforts implemented to individualize treatment, studies have shown that almost up to 30% of patients do no respond to treatment and still have a relapse during the first years of maintenance treatment.
Treatment-resistant schizophrenia is primarily defined by the severity of symptoms (positive) and the response to antipsychotics. A variety of representative scales, such as the positive and negative syndrome scale (PANSS), the brief psychiatric rating scale (BPRS), the scale for the assessment of negative symptoms (SANS) and the clinical global impression (CGI) clinical impression, were designed primarily to measure symptoms [
1,
2]. Using these different scales, studies have shown that treatment-resistant patients develop persistent positive psychotic symptoms, more pronounced negative symptoms and more severe cognitive impairment compared to treatment-responsive patients. Correctly identifying patients with resistance and understating the factors affecting the response to treatment could help health-care professionals better manage the patients’ disease thus reducing both health and economic burden to the patients, their families and the society [
3,
4]. In fact, studies have shown that individuals with treatment-resistant schizophrenia expressed the highest impairment in community functioning, low levels of achievements in functional milestones of everyday living, with high rates of unemployment, lack in psychological adjustment and sentimental relationship, leading to poorer quality of life and longer hospitalization rates/stays compared to non-resistant patients [
4,
5,
6,
7,
8,
9]. Heterogeneity of response to treatment is at the core of this challenge and different factors have been accounted for this variability including environmental and genetic factors [
10]. Clinical and social factors include the age of onset of the disease, the duration of illness, the severity of the psychotic symptoms, the compliance to the pharmacological treatment and to non-pharmacological interventions [
4,
10,
11,
12].
Several candidate genes have been explored in pharmacogenomics studies of response to treatment in schizophrenia including genetic variants of the dopamine receptors and pathways/signaling genes. Hence, it is clearly stated nowadays that patients with schizophrenia have an altered dopaminergic function and several authors have shown that, in treatment-resistant patients, distinct dopamine changes could be further identified: lower density of dopaminergic synapses in the caudate nucleus, lower dopamine synthesis capacity in the striatum [
13], and a decrease in the dopamine transporter protein expression, compared to patients who respond to antipsychotics. Moreover, numerous epidemiological and clinical studies suggested the role of inflammation in schizophrenia: authors speculated that pro-inflammatory cytokines may influence dopaminergic and glutaminergic pathways and cognition processes that are particularly altered in treatment-resistant patients [
14,
15,
16,
17,
18,
19].
Catechol-
O-methyltransferase (COMT) is an enzyme that metabolizes catecholamines and is a key modulator of cortical dopaminergic degradation [
20]. A common functional variant in the
COMT gene have been studied in particular: the c.472G > A polymorphism (rs4680; p.Val158Met) causes a valine (Val) to methionine (Met) substitution at codon 158 in the membrane-bound isoform enzyme, leading to a three- to four-fold reduced activity of the enzyme [
21,
22], lower protein expression [
21] and higher dopamine activity [
23] for the Met variant compared to the Val variant. In a study examining the expression levels of
COMT mRNA in post mortem cerebellum samples derived from psychiatric patients, including those with schizophrenia, the authors failed at identifying differences in
COMT expression or methylation in any psychiatric disorder. However, a strong sexual dimorphism in its expression was identified and a reduced expression with some
COMT SNPs such as rs737865 and rs165599 but not rs4680 [
24]. A recent study evaluated gene expression of 13 genes including
COMT in a context of resistance to treatment [
25]: no differences could be noted between patients with or without treatment-resistance schizophrenia in whole blood gene expression. The relationship between
COMT polymorphisms and response to antipsychotics have been extensively addressed in the literature, however, only a few studies addressed the
COMT rs4680 in relation to resistance to treatment [
6,
26,
27]. These studies yielded inconsistent results with relatively small sample sizes.
Another relevant gene that has been identified as an important modulator of response to treatment is the
DRD2 gene encoding the dopamine receptor 2, which is the most important target for antipsychotics. In acute schizophrenia patients, the mRNA expression levels of
DRD2 in peripheral blood samples were shown to be significantly lower than those in the healthy controls [
28], but higher in chronic schizophrenia patients receiving long-term clozapine treatment [
29].
Several studies have explored the association of single nucleotide polymorphisms (SNPs) in the genes encoding the dopamine receptors with the therapeutic effects of antipsychotics [
7,
30,
31]. One particular study investigated the association between SNPs in
DRD2 (rs1801028 and rs179932) and resistance to treatment but no significant difference was noted [
6]. We, therefore, thought to evaluate another SNP, the rs6277 that has never been explored in resistance to treatment.
Moreover, methylenete trahydrofolate reductase (MTHFR) is a pivotal enzyme that controls the intracellular methylation reactions, a key regulator to the production of neurotransmitters such as dopamine. It plays an essential role as well in the homocysteine level and neuroinflammation that could contribute to cognition impairment [
32,
33]. A single nucleotide polymorphism in
MTHFR (SNP; c.677C > T; p.Ala222Val; rs1801133) reduces MTHFR activity and may influence dopamine signaling by exacerbating underlying cortical dopamine deficiency in schizophrenia patients [
34]. The 677T variant has been associated with increased schizophrenia risk [
35,
36], more pronounced negative symptoms [
37], and more severe executive dysfunction in these patients [
37,
38]. In addition, it has been extensively studied when exploring the side effects of antipsychotics in schizophrenic patients, in particular, the metabolic syndrome [
39]. However, none of the studies have evaluated its association with resistance to treatment.
Finally, variants in the
OPRM1 gene encoding the μ-opioid receptor are of interest in the study of resistance to treatment as opioid receptors have been reported to regulate mesolimbic dopaminergic neuronal activities. Thus, the activation of μ-opioid receptors enhances extracellular dopamine concentration in the nucleus accumbens, which is known to be one of the main structures controlling physiological responses, behavior, and diseases including schizophrenia [
40]. No previous studies have evaluated the role of the
OPRM1 c.118A > G SNP (rs1799971) in resistance to treatment in schizophrenic patients.
Since the definition for treatment-resistance has varied across different studies [
10,
41,
42] and there is inconclusive or insufficient evidence of the association of all these SNPs in dopamine pathways genes and resistance to treatment and furthermore, none of the studies evaluated all these polymorphisms along with clinical factors, we conducted this case-control study to assess clinical and genetic factors affecting response to treatment in a sample of patients with schizophrenia (treatment-resistant patients versus treatment responders). We also aimed to examine if these factors are different when we consider two different resistance classifications (PANSS and BPRS).
4. Discussion
In schizophrenia, responses to antipsychotic treatment are complex and understanding the clinical and genetic affecting the variability in response is still a major challenge in psychiatry today. We conducted this study to evaluate clinical and genetic factors associated with resistance to treatment among a sample of Lebanese patients with schizophrenia.
In our study, we used both BPRS and PANSS scales and compared the factors that were significantly associated with the resistance definition stated by each of these scales. Surprisingly, we found some discrepancies between the two definitions. This inconsistency has been addressed by the “Treatment response and resistance in psychosis (TRRIP)” working group [
10] who agreed that there is a considerable variation in current approaches used to define resistance to treatment, which can contribute to failures to replicate findings. This factor alone could lead to inconsistent clinical management and treatment delay.
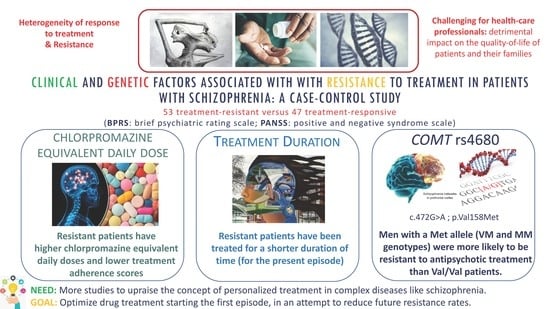
Our study showed that non-responder patients with schizophrenia had specific clinical features/patterns: they were more often men, had family history of schizophrenia and consumed more psychoactive substances than non-resistant patients, even if these factors did not remain significant in the multivariable analysis. These results are consistent with previously published studies [
4]. Hence, some authors argued that the age of onset of the disease varies by sex and determines the response to treatment, with men developing the disease earlier being more resistant to treatment [
61]. Moreover, some studies have identified a history of family psychosis as a predictor of treatment-resistant schizophrenia [
62,
63,
64]. Finally, patients with resistance had higher rates of smoking, alcohol and substance abuse [
3,
4].
Regarding treatment characteristics, our results showed that resistant patients have been treated for a shorter duration of time (for the present episode), had lower treatment adherence scores and higher chlorpromazine-equivalent daily doses. This could be explained by the fact that patients who started their treatment later, have not had the time to stabilize it yet (by adjusting the therapy: finding adequate doses, substituting molecules, using clozapine or adjuvant treatments, etc.) to overcome resistance. Therefore, these patients could suffer from what is called a higher “duration of untreated psychosis” (DUP) because their current treatment duration is relatively recent. This high DUP is associated with a poor response to antipsychotic treatment according to the studies of Perkins et al. [
11,
65]. Regarding the problem of adherence to treatment, it is well recognized to be the single largest source of unrecognized errors in studies of treatment resistance because poorly adherent patients could present false-positive “pseudo-resistance” [
10,
11]. However, this is not applicable to our patients since they were all were treated with a minimum duration of 12 weeks and a daily chlorpromazine equivalent daily dose higher than 600 mg.
Our study was the first to evaluate the allelic and genotypic frequencies of the
DRD2 SNP in the Lebanese population. Allelic frequencies observed were similar to those described in the Japanese and Chinese populations but not the Caucasian population. This is not surprising because even if the current majority of the Lebanese population is considered to be Arabs, many ethnic communities have undergone mixing in the course of history [
66]. For the other genes, allelic frequencies were similar to those previously reported in the Lebanese population.
Among all studied genetic factors, the
COMT p.Val158Met was the only one found to be associated with resistance to treatment, specifically in men. Patients with a Met allele (VM and MM genotypes) were more likely to be resistant to antipsychotic treatment. These results are consistent with the conclusions highlighted by three previous studies including treatment-resistant versus non-resistant patients, which demonstrated a higher frequency of the Met/Met genotype in patients with TRS [
26,
27,
67]. Sagud et al. [
27] identified a link between the Met/Met genotype and TRS in a group of 55 resistant female patients versus 331 non-resistant ones. Moreover, Inada et al. [
26] showed that patients with a Met/Met genotype had higher odds of being in the TRS group and had significantly received higher chlorpromazine equivalent doses compared to other genotypes. However, the authors did stratify their analysis according to gender. Finally, Escamilla et al. [
67] identified that treatment responders presented a higher frequency of the Val allele in comparison with patients in an ultra-resistance group (sample of 218 Mexican patients). Other studies did not find any association between this SNP of
COMT and TRS [
6,
25].
An explanation that can be put forward is that patients with Met/Met genotype for
COMT have a higher dopamine stimulation in the prefrontal cortex due to their fourfold lower functional enzyme activity [
13,
21,
22]. The brain tries to decrease the release of dopamine in the striatum, in order to protect the brain from excessive dopaminergic stimulation which could lead to severity of symptoms treatment-resistance [
13,
27]. The identified gender difference could be explained as well by the hypothesis that estrogens may affect the activity and functionality of COMT by influencing its gene expression [
58,
59].
For the other studied genetic factors, our study remains the first one to explore the role of
OPRM1 and
MTHFR variants with the resistance to treatment. Regarding
OPRM1, a study stipulated that the studied polymorphism can influence the myelination of axons especially in cortical neurons, which may play a role in the pathogenesis of schizophrenia [
68]. Another hypothesis demonstrated the role of opioid receptors, in particular, the μ-opioid receptor, in mesolimbic dopaminergic neuronal activities, known to be disrupted in patients with schizophrenia [
40]. MTHFR, as well, is widely recognized to be an important factor for the COMT metabolism of catecholamines, including cortical dopamine [
34]. Thus, the studied SNP, by reducing this cortical dopamine, could affect not only the symptoms of patients [
35,
36,
37,
38] but also their response to treatment. We failed to identify such associations in our study. Further larger studies may be required to better explore these gene variants.
Finally, very few studies have evaluated the gene-gene interaction and its impact on resistance to treatment in patients with schizophrenia [
69]. Rajagopal et al. [
69] have explored the interaction between
COMT rs4680 and
DRD4 120-bp duplication and demonstrated statistically significant epistasis between these polymorphisms and clinical response to clozapine. To the best of our knowledge, our study is the first to explore the interaction of these four genes affecting the dopamine transduction in the brain and their possible impact with the response to the treatment. Even though our study yielded a negative result, there is a need to replicate those findings in a larger independent sample; future studies exploring the functional effects of genes and polymorphisms would allow a better understanding of the mechanism(s) underlying their interaction.
Limitations and Strengths
Some limitations could be raised in our study. Due to the characteristics of the included population, the data lacks some highly important information regarding the pathology and its progress: date of the first episode, date of diagnosis, age of onset of treatment of the first schizophrenic episode, and most importantly the duration of treatment resistance, etc. Moreover, we acknowledge that the sample size was relatively small for genetic analyses and not gender-matched, which could explain some of the negative results due to a low statistical power; nevertheless, it remains big compared to the Lebanese population. Further multi-centered studies, including a larger sample of schizophrenic patients matched for gender, are required to confirm and generalize our results. Finally, the rating scales we used did not allow an evaluation of the cognitive symptom domain that could be altered in treatment-resistant patients [
10]. However, our study was the first to compare resistance to treatment using two different validated scales (PANSS and BPRS) and we correlated the results of each of these scales to a maximum number of socio-demographic, clinical and genetic factors. Furthermore, our study is the first to evaluate different polymorphisms in different genes that could potentially affect cortical dopamine pathways and explore the gene-gene interaction.