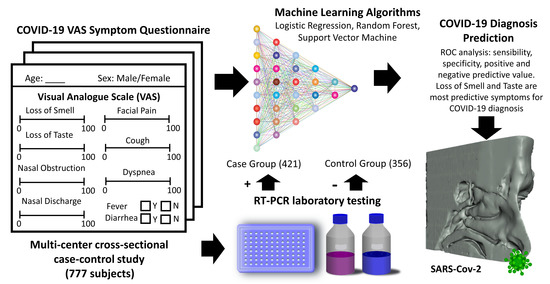
Loss of Smell and Taste Can Accurately Predict COVID-19 Infection: A Machine-Learning Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Participants
2.2. Study Variables
2.3. Data Analysis and Statistical Methods
2.4. Machine Leaning (ML) Approach to Predict a COVID-19 Diagnosis
3. Results
3.1. Demographic Characteristics of the Sample
3.2. Prevalence and Intensity of Symptoms between Cases and Controls
3.3. VAS Cut-off Points That Optimally Predicted COVID-19 Diagnosis
3.4. Multivariate Logistic Regression (LR) Model
3.5. ML Results: Comparison of Accuracy between Algorithms and Model Datasets
4. Discussion
4.1. Prevalence of Smell and Taste Disorders in COVID-19 Subjects
4.2. Predictive Value of Smell and Taste Disorders in the COVID-19 Disease
4.3. Prediction Models for COVID-19 Diagnosis Based on Smell and Taste Disorders
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.W.; Chee, J.; Subramaniam, S.; Ng, C.L. Frequency and Clinical Utility of Olfactory Dysfunction in COVID-19: A System-atic Review and Meta-analysis. Curr. Allergy Asthma Rep. 2020, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Mullol, J.; Alobid, I.; Mariño-Sánchez, F.; Izquierdo-Domínguez, A.; Marin, C.; Klimek, L.; Wang, D.-Y.; Liu, Z. The Loss of Smell and Taste in the COVID-19 Outbreak: A Tale of Many Countries. Curr. Allergy Asthma Rep. 2020, 20, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rocke, J.; Hopkins, C.; Philpott, C.M.; Kumar, N. Is loss of sense of smell a diagnostic marker in COVID-19: A systematic review and meta-analysis. Clin. Otolaryngol. 2020, 45, 914–922. [Google Scholar] [CrossRef]
- Izquierdo-Dominguez, A.; Rojas-Lechuga, M.J.; Mullol, J.; Alobid, I. Olfactory Dysfunction in the COVID-19 Outbreak. J. Investig. Allergol. Clin. Immunol. 2020, 30, 317–326. [Google Scholar] [CrossRef]
- Bagheri, S.H.; Asghari, A.; Farhadi, M.; Shamshiri, A.R.; Kabir, A.; Kamrava, S.K.; Jalessi, M.; Mohebbi, A.; Alizadeh, R.; Honarmand, A.A.; et al. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak in Iran. Med. J. Islam. Repub. Iran 2020, 34, 62. [Google Scholar]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683. [Google Scholar] [CrossRef] [Green Version]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Oto Rhino Laryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef]
- Menni, C.; Valdes, A.M.; Freidin, M.B.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Ganesh, S.; Varsavsky, T.; Cardoso, M.J.; Moustafa, J.S.E.-S.; et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 2020, 26, 1037–1040. [Google Scholar] [CrossRef]
- Roland, L.T.; Ii, J.G.G.; Loftus, P.A.; Cheung, S.W.; Chang, J.L. Smell and taste symptom-based predictive model for COVID-19 diagnosis. Int. Forum Allergy Rhinol. 2020, 10, 832–838. [Google Scholar] [CrossRef]
- von Bartheld, C.S.; Hagen, M.M.; Butowt, R. Prevalence of Chemosensory Dysfunction in COVID-19 Patients: A Systematic Re-view and Meta-analysis Reveals Significant Ethnic Differences. ACS Chem. Neurosci. 2020 11, 2944–2961.
- Beltrán-Corbellini, Á.; Chico-García, J.L.; Martínez-Poles, J.; Rodríguez-Jorge, F.; Natera-Villalba, E.; Gómez-Corral, J.; Gómez-López, A.; Monreal, E.; Parra-Díaz, P.; Cortés-Cuevas, J.L.; et al. Acute-onset smell and taste disorders in the context of COVID-19: A pilot multicentre polymerase chain reaction based case–control study. Eur. J. Neurol. 2020, 27, 1738–1741. [Google Scholar] [CrossRef]
- Villarreal, I.M.; Morato, M.; Martínez-RuizCoello, M.; Navarro, A.; Garcia-Chillerón, R.; Ruiz, Á.; De Almeida, I.V.; Mazón, L.; Plaza, G. Olfactory and taste disorders in healthcare workers with COVID-19 infection. Eur. Arch. Oto-Rhino-Laryngol. 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Clemency, B.M.; Varughese, R.; Do, D.K.S.; Ludwig, B.; Welch, J.V.; McCormack, R.F.; Ma, C.; Nan, N.; Rn, T.G.; Raab, T. Symptom Criteria for COVID-19 Testing of Heath Care Workers. Acad. Emerg. Med. 2020, 27, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Gerkin, R.C.; Ohla, K.; Veldhuizen, M.G.; Joseph, P.V.; Kelly, C.; Bakke, A.J.; Steele, K.; Farruggia, M.C.; Pellegrino, R.; Pepino, M.Y.; et al. Recent smell loss is the best predictor of COVID-19 among individuals with recent respiratory symptoms. Chem. Senses 2020. [Google Scholar] [CrossRef]
- Pierron, D.; Pereda-Loth, V.; Mantel, M.; Moranges, M.; Bignon, E.; Alva, O.; Kabous, J.; Heiske, M.; Pacalon, J.; David, R.; et al. Smell and taste changes are early indicators of the COVID-19 pandemic and political decision effectiveness. Nat. Commun. 2020, 11, 5152. [Google Scholar] [CrossRef]
- Ramakrishnan, V.R.; Arbet, J.; Mace, J.C.; Smith, S.S.; Soler, Z.M.; Smith, T.L. Predicting Olfactory Loss In Chronic Rhinosinusitis Using Machine Learning. medRxiv 2020. [Google Scholar]
- Yadaw, A.S.; Li, Y.-C.; Bose, S.; Iyengar, R.; Bunyavanich, S.; Pandey, G. Clinical features of COVID-19 mortality: Development and validation of a clinical prediction model. Lancet Digit. Health 2020, 2, e516–e525. [Google Scholar] [CrossRef]
- Tong, J.Y.; Wong, A.; Zhu, D.; Fastenberg, J.H.; Tham, T. The Prevalence of Olfactory and Gustatory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis. Otolaryngol. Head Neck Surg. 2020, 163, 3–11. [Google Scholar] [CrossRef]
- Yan, C.H.; Faraji, F.; Prajapati, D.; Boone, C.E.; DeConde, A.S. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int. Forum Allergy Rhinol. 2020, 10, 806–813. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo-Domínguez, A.; Rojas-Lechuga, M.J.; Chiesa-Estomba, C.; Calvo-Henríquez, C.; Ninchritz-Becerra, E.; Soriano-Reixach, M.; Poletti-Serafini, D.; Villarreal, I.M.; Maza-Solano, J.M.; Moreno-Luna, R.; et al. Smell and Taste Dysfunction in COVID-19 Is Associated With Younger Age in Ambulatory Settings: A Multicenter Cross-Sectional Study. J. Investig. Allergol. Clin. Immunol. 2020, 30, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Borsetto, D.; Hopkins, C.; Philips, V.; Obholzer, R.; Tirelli, G.; Polesel, J.; Calvanese, L.; Boscolo-Rizzo, P. Self-reported alteration of sense of smell or taste in patients with COVID-19: A systematic review and meta-analysis on 3563 patients. Rhinol. J. 2020, 58, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Larco, R.M.; Altez-Fernandez, C. Anosmia and dysgeusia in COVID-19: A systematic review. Wellcome Open Res. 2020, 5, 94. [Google Scholar] [CrossRef] [PubMed]
- Costa, K.V.T.d.; Carnaúba, A.T.L.; Rocha, K.W.; Andrade, K.C.L.d.; Ferreira, S.M.S.; Menezes, P.d.L. Olfactory and taste disorders in COVID-19: A systematic review. Braz. J. Otorhinolaryngol. 2020, 86, 781–792. [Google Scholar] [CrossRef]
- Mercante, G.; Ferreli, F.; De Virgilio, A.; Gaino, F.; Di Bari, M.; Colombo, G.; Russo, E.; Costantino, A.; Pirola, F.; Cugini, G.; et al. Prevalence of Taste and Smell Dysfunction in Coronavirus Disease 2019. JAMA Otolaryngol. Neck Surg. 2020, 146, 723. [Google Scholar] [CrossRef]
- Lovato, A.; De Filippis, C. Clinical Presentation of COVID-19: A Systematic Review Focusing on Upper Airway Symptoms. Ear, Nose Throat J. 2020, 99, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Kang, H.; Li, S.; Zhao, X. Understanding the neurotropic characteristics of SARS-CoV-2: From neurological manifestations of COVID-19 to potential neurotropic mechanisms. J. Neurol. 2020, 267, 2179–2184. [Google Scholar] [CrossRef]
- Dubé, M.; Coupanec, A.L.; Wong, A.H.M.; Rini, J.M.; Desforges, M.; Talbot, P.J. Axonal Transport Enables Neuron-to-Neuron Propa-gation of Human Coronavirus OC43. J. Virol. 2018, 92(17), 1–21. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, D.L.; Athmer, J.; Meyerholz, D.K.; Perlman, S. Murine Olfactory Bulb Interneurons Survive Infection with a Neuro-tropic Coronavirus. J. Virol. 2017, 91, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Shen, W.; Rowan, N.R.; Kulaga, H.; Hillel, A.; Ramanathan, M.; Lane, A.P. Elevated ACE-2 expression in the olfactory neuroepithelium: Implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur. Respir. J. 2020, 56, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, T.; Yang, N.; Han, D.; Mi, X.; Li, Y.; Liu, K.; Vuylsteke, A.; Xiang, H.; Guo, X. Neurological manifestations of patients with COVID-19: Potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front. Med. 2020, 14, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Tong, J.; Liu, M.; Shen, Y.; Guo, D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J. Med. Virol. 2020, 92, 589–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.; Duan, F.; Luo, C.; Liu, Q.; Qu, X.; Liang, L.; Wu, K. Characteristics of Ocular Findings of Patients With Coronavirus Disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020, 138, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Lozada-Nur, F.; Chainani-Wu, N.; Fortuna, G.; Sroussi, H. Dysgeusia in COVID-19: Possible Mechanisms and Implications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Kowall, B.; Nonnemacher, M.; Brune, B.; Brinkmann, M.; Dudda, M.; Böttcher, J.; Schmidt, B.; Standl, F.; Stolpe, S.; Dittmer, U.; et al. A model to identify individuals with a high probability of a SARS-CoV-2 infection. J. Infect. 2020. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pmc/articles/PMC7686708/ (accessed on 6 December 2020). [CrossRef] [PubMed]
- Wynants, L.; Van Calster, B.; Collins, G.S.; Riley, R.D.; Heinze, G.; Schuit, E.; Bonten, M.M.J.; Dahly, D.L.; Damen, J.; Debray, T.P.; et al. Prediction models for diagnosis and prognosis of covid-19: Systematic review and critical appraisal. BMJ 2020, 369, m1328. [Google Scholar] [CrossRef] [Green Version]
- Riley, R.D.; Ensor, J.; Snell, K.I.; Jr, F.E.H.; Martin, G.P.; Reitsma, J.B.; Moons, K.G.M.; Collins, G.S.; Van Smeden, M. Calculating the sample size required for developing a clinical prediction model. BMJ 2020, 368, m441. [Google Scholar] [CrossRef] [Green Version]
- Haller, D.M.; Sebo, P.; Tudrej, B.; Maisonneuve, H. Is a COVID-19 prediction model based on symptom tracking through an app applicable in primary care? Fam. Pract. 2020, 37. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pmc/articles/PMC7454492/ (accessed on 23 December 2020). [CrossRef] [PubMed]
- Barra, G.B.; Santa Rita, T.H.; Mesquita, P.G.; Jácomo, R.H.; Nery, L.F.A. Analytical Sensitivity and Specificity of Two RT-qPCR Protocols for SARS-CoV-2 Detection Performed in an Automated Workflow. Genes 2020, 11, 1183. [Google Scholar] [CrossRef]




| Symptom | Total (n = 777) | Cases (n = 421) | Controls (n = 356) | Odds Ratio | CI 95% | p-Value |
|---|---|---|---|---|---|---|
| Loss of smell | 312 (40%) | 257 (61%) | 55 (15%) | 8.58 | [6.06, 12.14] | <0.0001 |
| Loss of taste | 332 (43%) | 266 (63%) | 66 (19%) | 7.54 | [5.41, 10.52] | <0.0001 |
| Nasal obstruction | 262 (34%) | 153 (36%) | 109 (31%) | 1.29 | [0.95, 1.74] | 0.0946 |
| Nasal Discharge | 378 (49%) | 210 (50%) | 168 (47%) | 1.11 | [0.84, 1.47] | 0.4718 |
| Facial Pain | 161 (21%) | 114 (27%) | 47 (13%) | 2.44 | [1.67, 3.55] | 0.6173 |
| Cough | 471 (61%) | 307 (73%) | 164 (46%) | 3.15 | [2.34, 4.25] | <0.001 |
| Dyspnea | 260 (33%) | 182 (43%) | 78 (22%) | 2.71 | [1.98, 3.73] | <0.001 |
| Fever | 339 (44%) | 258 (61%) | 81 (23%) | 5.37 | [3.92, 7.37] | <0.001 |
| Diarrhea | 299 (39%) | 220 (53%) | 79 (22%) | 3.84 | [2.80, 5.26] | 0.0011 |
| OR | CI 95% | p-Value | VAS Cutoff Points | AUC | Sensitivity | Specificity | ||
|---|---|---|---|---|---|---|---|---|
| Loss of smell | 10.85 | 7.47 | 15.77 | <0.0001 | 21 | 0.76 | 0.60 | 0.88 |
| Loss of taste | 12.62 | 8.50 | 18.73 | <0.0001 | 44 | 0.76 | 0.59 | 0.90 |
| Nasal obstruction | 1.75 | 1.19 | 2.56 | 0.0046 | 52 | 0.53 | 0.21 | 0.87 |
| Nasal discharge | 1.46 | 1.06 | 2.02 | 0.0232 | 41 | 0.52 | 0.30 | 0.77 |
| Facial pain | 2.54 | 1.73 | 3.74 | <0.0001 | 15 | 0.57 | 0.26 | 0.88 |
| Cough | 3.19 | 2.36 | 4.31 | <0.0001 | 3 | 0.65 | 0.73 | 0.54 |
| Dyspnea | 3.06 | 2.16 | 4.33 | <0.0001 | 28 | 0.61 | 0.36 | 0.84 |
| OR | CI 95% | p-Value | ||
|---|---|---|---|---|
| Loss of smell | 2.42 | 1.30 | 4.50 | 0.0053 |
| Loss of taste | 6.21 | 3.21 | 12.04 | <0.001 |
| Dyspnea | 2.21 | 1.34 | 3.64 | 0.002 |
| Fever | 1.84 | 1.18 | 2.87 | 0.007 |
| Diarrhea | 2.02 | 1.29 | 3.16 | 0.002 |
| Sex | 3.11 | 1.97 | 4.90 | <0.001 |
| AUC | SE | SP | PPV | NPV | ||
|---|---|---|---|---|---|---|
Dataset 1 (11 predictors)
| LR | 0.759 | 0.792 | 0.722 | 0.772 | 0.747 |
| RF | 0.777 | 0.798 | 0.752 | 0.792 | 0.758 | |
| SVM | 0.771 | 0.811 | 0.724 | 0.777 | 0.764 | |
Dataset 2 (11 predictors)
| LR | 0.777 | 0.783 | 0.771 | 0.803 | 0.751 |
| RF | 0.798 | 0.818 | 0.775 | 0.812 | 0.782 | |
| SVM | 0.799 | 0.818 | 0.778 | 0.814 | 0.783 | |
Dataset 3 (11 predictors)
| LR | 0.773 | 0.787 | 0.757 | 0.794 | 0.751 |
| RF | 0.791 | 0.812 | 0.765 | 0.804 | 0.775 | |
| SVM | 0.794 | 0.826 | 0.755 | 0.800 | 0.786 | |
Dataset 4 (4 predictors)
| LR | 0.764 | 0.737 | 0.798 | 0.812 | 0.718 |
| RF | 0.755 | 0.768 | 0.741 | 0.779 | 0.731 | |
| SVM | 0.759 | 0.784 | 0.730 | 0.776 | 0.742 | |
Dataset 5 (2 predictors)
| LR | 0.768 | 0.812 | 0.715 | 0.772 | 0.762 |
| RF | 0.768 | 0.797 | 0.733 | 0.780 | 0.753 | |
| SVM | 0.765 | 0.799 | 0.724 | 0.775 | 0.753 |
| Menni et al. [9] | Roland et al. [10] | Clemency et al. [14] | Kowall et al. [35] | Gerkin et al. [15] | Our Study | |
|---|---|---|---|---|---|---|
| Sample | (UK)6452+/9186− (US)726+/2037− | 145+/157− | 225+/736− | 296+/1641− | 4148+/546− | 421+/356− |
| Demographic Data | (UK) Positive group: Mean Age: 41.25 71.88% female Negative group: Mean Age: 43.2 76.40 % female | Mean age: 39 Sex: 72% female | N/A | Mean age: 53.5 years Sex: 61.3% females in the negative group and 57.8% in the positive group | Positive group: Mean Age: 40.6 74% female Negative group: Mean Age: 43.2 78% female | Positive group: Mean Age: 47.3 61% female Negative group: Mean Age: 45.2 78% female |
| Data collection | App-based symptom tracker | Public survey posted on social media | Nurse call center for healthcare workers (HCW) | Self-administered questionnaire | Online survey | Self-administered questionnaire |
| Variable Types | Categorical | Categorical | Categorical | Categorical | Categorical, continuous VAS | Categorical, Continuous VAS |
| Classification Methods | ● Stepwise (forward and backward) ● Logistic Regression ● Akaike Information Criterion (AIC) Classifier threshold at 0.5 | ● Stepwise Logistic Regression ● (p = 0.05 for entry and 0.10 for removal with maximum iterations set at 20) Classifier threshold at 0.5 | Logistic regression with maximum positive likelihood ratio (PLR) criterion | Stepwise backward logistic regression (p = 0.10 for entry and for removal) | L1 regularized logistic regression (penalty α = 1) | ● Stepwise (forward and backward) ● Logistic Regression ● Bayesian Information Criterion (BIC) ● Random Forest (RF) ● Support Vector Machine (SVM) Classifier threshold at 0.5 |
| Predictors | Age, sex, loss of smell and taste, severe or significant persistent cough, severe fatigue, skipped meals | (1) Smell or taste change, fever, body ache, shortness of breath, sore throat (2) Smell or taste change, fever and/or myalgia | (1) Fever, shortness of breath, dry cough (2) Fever, loss of taste or smell (3) Fever, shortness of breath, dry cough, loss of taste or smell | Age, sex, age, return from abroad, close contact with a confirmed case, the presence of fever, cough, exhaustion, taste or smell disorder, current smoking, general health condition and number of comorbidities | (1) Loss of smell, time duration (2) Model with 70 features | Five model datasets (see Table 4) including different variables among: age, sex, loss of smell, loss of taste, nasal obstruction, nasal discharge, facial pain, cough, dyspnea, fever and diarrhea |
| Validation method | ● Holdout 80:20% ● training/test ● 10-fold cross-validation in the UK sample ● US validation sample | Holdout 75:25% training/test | N/A | Holdout 60:40% training-test | 100-fold cross-validation with 80:20% training-test | ● Holdout 75:25% training-test ● 50-fold cross-validation with ● 75:25% training-test |
| Accuracy Parameters | AUC = 0.76 SE = 0.66 SP = 0.83 PPV = 0.58 NPV = 0.87 | (1) AUC = 0.82 SE = 0.56 (2) AUC = 0.75 SE = 0.70 SP = 0.73 | (1) AUC = 0.63 SE = 0.93 SP = 0.09 (2) AUC = 0.75 SE = 0.89 SP = 0.48 (3) AUC = 0.77 SE = 0.98 SP = 0.08 | AUC = 0.821 | AUC = 0.72 SE = 0.85 SP = 0.75 | AUC = 0.80 SE = 0.82 SP = 0.78 PPV = 0.81 NPV = 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Callejon-Leblic, M.A.; Moreno-Luna, R.; Del Cuvillo, A.; Reyes-Tejero, I.M.; Garcia-Villaran, M.A.; Santos-Peña, M.; Maza-Solano, J.M.; Martín-Jimenez, D.I.; Palacios-Garcia, J.M.; Fernandez-Velez, C.; et al. Loss of Smell and Taste Can Accurately Predict COVID-19 Infection: A Machine-Learning Approach. J. Clin. Med. 2021, 10, 570. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm10040570
Callejon-Leblic MA, Moreno-Luna R, Del Cuvillo A, Reyes-Tejero IM, Garcia-Villaran MA, Santos-Peña M, Maza-Solano JM, Martín-Jimenez DI, Palacios-Garcia JM, Fernandez-Velez C, et al. Loss of Smell and Taste Can Accurately Predict COVID-19 Infection: A Machine-Learning Approach. Journal of Clinical Medicine. 2021; 10(4):570. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm10040570
Chicago/Turabian StyleCallejon-Leblic, María A, Ramon Moreno-Luna, Alfonso Del Cuvillo, Isabel M Reyes-Tejero, Miguel A Garcia-Villaran, Marta Santos-Peña, Juan M Maza-Solano, Daniel I Martín-Jimenez, Jose M Palacios-Garcia, Carlos Fernandez-Velez, and et al. 2021. "Loss of Smell and Taste Can Accurately Predict COVID-19 Infection: A Machine-Learning Approach" Journal of Clinical Medicine 10, no. 4: 570. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm10040570