Predictors of Discordance in the Assessment of Skeletal Muscle Mass between Computed Tomography and Bioimpedance Analysis
Abstract
:1. Introduction
2. Methods
2.1. Study Subjects
2.2. Data Collection
2.3. Fibrosis-4 Index Calculation
2.4. Measurements of Skeletal Muscle Area
2.5. Definition of Low SMM
2.6. Statistical Analysis
3. Results
3.1. Patients
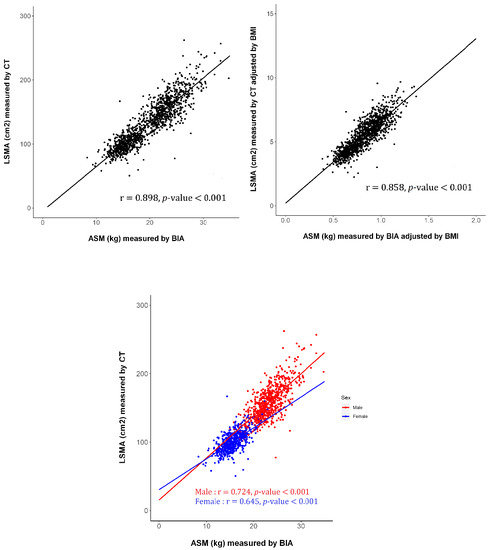
3.2. Association between SMM Assessed Using CT and BIA
3.3. Comparison between Subjects with and without Low SMM Assessed by CT
3.4. Comparison between Subjects with and without Low SMM Assessed by BIA
3.5. Prevalence and Predictors of Discordance in Defining Low SMM Assessed by CT and BIA
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Roubenoff, R. Origins and clinical relevance of sarcopenia. Can. J. Appl. Physiol. 2001, 26, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the european working group on sarcopenia in older people. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I. The epidemiology of sarcopenia. Clin. Geriatr. Med. 2011, 27, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Pahor, M.; Lauretani, F.; Zamboni, V.; Bandinelli, S.; Bernabei, R.; Guralnik, J.M.; Ferrucci, L. Skeletal muscle and mortality results from the inchianti study. J. Gerontol. A. Biol. Sci. Med. Sci. 2009, 64, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. A. Biol. Sci. Med. Sci. 2006, 61, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the asian working group for sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The fnih sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A. Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Vellas, B.; Fielding, R.A.; Bens, C.; Bernabei, R.; Cawthon, P.M.; Cederholm, T.; Cruz-Jentoft, A.J.; Del Signore, S.; Donahue, S.; Morley, J.; et al. Implications of ICD-10 for sarcopenia clinical practice and clinical trials: Report by the international conference on frailty and sarcopenia research task force. J. Frailty Aging 2018, 7, 2–9. [Google Scholar] [PubMed]
- Proctor, D.N.; O’Brien, P.C.; Atkinson, E.J.; Nair, K.S. Comparison of techniques to estimate total body skeletal muscle mass in people of different age groups. Am. J. Physiol. 1999, 277, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Wang, Z.M.; Heymsfield, S.B. Skeletal muscle mass and aging: Regional and whole-body measurement methods. Can. J. Appl. Physiol. 2001, 26, 102–122. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.Y.; Huang, T.Y.; Wu, Y.T. Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in taiwan. J. Am. Geriatr. Soc. 2008, 56, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Chumlea, W.C.; Guo, S.S. Bioelectrical impedance and body composition: Present status and future directions. Nutr. Rev. 1994, 52, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Mackenzie, T.A.; Barre, L.K.; Lopez-Jimenez, F.; Bartels, S.J. Sarcopenia, sarcopenic obesity and mortality in older adults: Results from the national health and nutrition examination survey III. Eur. J. Clin. Nutr. 2014, 68, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Genton, L.; Hans, D.; Pichard, C. Validation of a bioelectrical impedance analysis equation to predict appendicular skeletal muscle mass (ASMM). Clin. Nutr. 2003, 22, 537–543. [Google Scholar] [CrossRef]
- Sergi, G.; De Rui, M.; Veronese, N.; Bolzetta, F.; Berton, L.; Carraro, S.; Bano, G.; Coin, A.; Manzato, E.; Perissinotto, E. Assessing appendicular skeletal muscle mass with bioelectrical impedance analysis in free-living caucasian older adults. Clin. Nutr. 2015, 34, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Scafoglieri, A.; Clarys, J.P.; Bauer, J.M.; Verlaan, S.; Van Malderen, L.; Vantieghem, S.; Cederholm, T.; Sieber, C.C.; Mets, T.; Bautmans, I.; et al. Predicting appendicular lean and fat mass with bioelectrical impedance analysis in older adults with physical function decline - the provide study. Clin. Nutr. 2017, 36, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Karelis, A.D.; Chamberland, G.; Aubertin-Leheudre, M.; Duval, C.; Ecological mobility in Aging and Parkinson (EMAP) Group. Validation of a portable bioelectrical impedance analyzer for the assessment of body composition. Appl. Physiol. Nutr. Metab. 2013, 38, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.H.; de Craen, A.J.; Slagboom, P.E.; Gunn, D.A.; Stokkel, M.P.; Westendorp, R.G.; Maier, A.B. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin. Nutr. 2011, 30, 610–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Kim, C.H.; Kim, D.W.; Park, M.; Park, H.S.; Min, S.S.; Han, S.H.; Yee, J.Y.; Chung, S.; Kim, C. External cross-validation of bioelectrical impedance analysis for the assessment of body composition in korean adults. Nutr. Res. Pract. 2011, 5, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.L.; Holmes, J.C.; Desautels, R.L.; Edmonds, L.B.; Nuudi, L. Ability of new octapolar bioimpedance spectroscopy analyzers to predict 4-component-model percentage body fat in hispanic, black, and white adults. Am. J. Clin. Nutr. 2008, 87, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Malavolti, M.; Mussi, C.; Poli, M.; Fantuzzi, A.L.; Salvioli, G.; Battistini, N.; Bedogni, G. Cross-calibration of eight-polar bioelectrical impedance analysis versus dual-energy x-ray absorptiometry for the assessment of total and appendicular body composition in healthy subjects aged 21–82 years. Ann. Hum. Biol. 2003, 30, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.C. Segmental bioelectrical impedance analysis: An update. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, M.; Yasuno, M.; Ishiguro, M.; Yamauchi, S.; Kikuchi, A.; Tokura, M.; Ishikawa, T.; Nakatani, E.; Uetake, H. Morphologic change of the psoas muscle as a surrogate marker of sarcopenia and predictor of complications after colorectal cancer surgery. Int. J. Colorectal Dis. 2017, 32, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. Fib-4: An inexpensive and accurate marker of fibrosis in hcv infection. Comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Buckinx, F.; Reginster, J.Y.; Dardenne, N.; Croisiser, J.L.; Kaux, J.F.; Beaudart, C.; Slomian, J.; Bruyere, O. Concordance between muscle mass assessed by bioelectrical impedance analysis and by dual energy x-ray absorptiometry: A cross-sectional study. BMC Musculoskelet Disord. 2015, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Forslund, A.H.; Johansson, A.G.; Sjodin, A.; Bryding, G.; Ljunghall, S.; Hambraeus, L. Evaluation of modified multicompartment models to calculate body composition in healthy males. Am. J. Clin. Nutr. 1996, 63, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Han, D.S.; Chang, K.V.; Li, C.M.; Lin, Y.H.; Kao, T.W.; Tsai, K.S.; Wang, T.G.; Yang, W.S. Skeletal muscle mass adjusted by height correlated better with muscular functions than that adjusted by body weight in defining sarcopenia. Sci. Rep. 2016, 6, 19457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montano-Loza, A.J.; Meza-Junco, J.; Prado, C.M.; Lieffers, J.R.; Baracos, V.E.; Bain, V.G.; Sawyer, M.B. Muscle wasting is associated with mortality in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2012, 10, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S.; Merli, M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J. Hepatol. 2016, 65, 1232–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in new mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord 2017, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International clinical practice guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health. Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Park, M.S.; Yang, S.J.; Yoo, H.J.; Kang, H.J.; Song, W.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: The Korean Sarcopenic Obesity Study (KSOS). Diabetes Care 2010, 33, 1497–1499. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Yang, S.J.; Yoo, H.J.; Lim, K.I.; Kang, H.J.; Song, W.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: The Korean sarcopenic obesity study. Int. J. Obes. 2009, 33, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.M.; Lynn, H.S.; Woo, J.W.; Kwok, T.C.; Melton, L.J., 3rd. Prevalence of and risk factors for sarcopenia in elderly Chinese men and women. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kim, J.H.; Yoon, J.W.; Kang, S.M.; Choi, S.H.; Park, Y.J.; Kim, K.W.; Lim, J.Y.; Park, K.S.; Jang, H.C. Sarcopenic obesity: Prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 2010, 33, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Stauber, P.M.; McHugh, D.; Koehler, K.M.; Garry, P.J. Cross-sectional age differences in body composition in persons 60+ years of age. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, M307–M316. [Google Scholar] [CrossRef] [PubMed]
- Lloret Linares, C.; Ciangura, C.; Bouillot, J.L.; Coupaye, M.; Decleves, X.; Poitou, C.; Basdevant, A.; Oppert, J.M. Validity of leg-to-leg bioelectrical impedance analysis to estimate body fat in obesity. Obes. Surg. 2011, 21, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Pomeroy, E.; Macintosh, A.; Wells, J.C.K.; Cole, T.J.; Stock, J.T. Relationship between body mass, lean mass, fat mass, and limb bone cross-sectional geometry: Implications for estimating body mass and physique from the skeleton. Am. J. Phys. Anthropol. 2018, 166, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Bosy-Westphal, A.; Muller, M.J. Identification of skeletal muscle mass depletion across age and BMI groups in health and disease—There is need for a unified definition. Int. J. Obes. 2015, 39, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Jung, K.S.; Kim, S.U.; Yoon, H.J.; Yun, Y.J.; Lee, B.W.; Kang, E.S.; Han, K.H.; Lee, H.C.; Cha, B.S. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008-2011). J. Hepatol. 2015, 63, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, S.U.; Song, K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Lee, B.W.; Kang, E.S.; Cha, B.S.; Han, K.H. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008–2011). Hepatology 2016, 63, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Ahn, S.; Kim, Y.J.; Ji, M.J.; Kim, K.M.; Choi, S.H.; Jang, H.C.; Lim, S. Comparison between dual-energy X-ray absorptiometry and bioelectrical impedance analyses for accuracy in measuring whole body muscle mass and appendicular skeletal muscle mass. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, S.; Beckwee, D.; Vella-Azzopardi, R.; Beyer, I.; Knoop, V.; Jansen, B.; Delaere, A.; Antoine, A.; Bautmans, I.; Scafoglieri, A.; et al. Evaluation of appendicular lean mass using bio impedance in persons aged 80+: A new equation based on the BUTTERFLY-study. Clin. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- National Health Statistics. Available online: http://www.index.go.kr/potal/main/EachDtlPageDetail.do?idx_cd=1438 (accessed on 10 December 2018).



| Variables | All |
|---|---|
| Demographic parameters | |
| Age, years | 57 (18–92) |
| <40 | 59 (5.0) |
| 40–49 | 182 (15.6) |
| 50–59 | 445 (38.2) |
| 60–69 | 296 (25.4) |
| >70 | 181 (15.5) |
| Female gender | 521 (43.7) |
| Body mass index, kg/m2 | 24.0 (15.4–43.9) |
| Hypertension | 488 (41.0) |
| Diabetes mellitus | 314 (26.4) |
| Viral hepatitis | 57 (4.9) |
| Laboratory parameters | |
| Fasting glucose, mg/dL | 96 (58–340) |
| Aspartate aminotransferase, IU/L | 21 (8–140) |
| Alanine aminotransferase, IU/L | 19 (3–196) |
| Serum albumin, mg/dL | 4.3 (3.4–5.3) |
| Total bilirubin, mg/dL | 0.7 (0.2–4.0) |
| Gamma glutamyl-transpeptidase, IU/L | 23 (6–539) |
| Serum creatinine, mg/dL | 0.8 (0.4–7.3) |
| Platelet count, 109/L | 231 (89–846) |
| Prothrombin time, INR | 0.9 (0.7–2.3) |
| Total cholesterol, mg/dL | 187 (83–392) |
| Triglycerides, mg/dL | 103 (31–815) |
| High-density lipoprotein cholesterol, mg/dL | 48 (23–115) |
| Low-density lipoprotein cholesterol, mg/dL | 109 (27–299) |
| HbA1c, % | 5.8 (4.4–13.4) |
| Fibrosis-4 index | 1.17 (0.20–5.47) |
| Muscle mass parameters | |
| By computed tomography | |
| Whole body fat-free mass, kg | 45.3 (21.2–84.7) |
| Lumbar skeletal muscle index, cm2/m2 | 46.9 (20.0–85.6) |
| By bioimpedance analysis | |
| ASM, kg | 20.1 (8.3–34.7) |
| ASM index, kg/m2 | 7.1 (3.2–28.9) |
| ASM/body mass index | 0.82 (0.43–1.23) |
| Variables | without Low SMM | with Low SMM | p-Value |
|---|---|---|---|
| (n = 988, 84.9%) | (n = 176, 15.1%) | ||
| Demographic parameters | |||
| Age, years | 57 (19–92) | 63 (18–92) | 0.001 |
| Female gender | 435 (44.1) | 86 (48.8) | 0.017 |
| Body mass index, kg/m2 | 24.2 (16.5–43.8) | 22.4 (15.4–28.9) | 0.584 |
| Hypertension | 411 (41.5) | 77 (43.7) | 0.436 |
| Diabetes mellitus | 268 (27.1) | 46 (26.1) | 0.780 |
| Viral hepatitis | 45(4.5) | 12 (6.8) | 0.152 |
| Laboratory parameters | |||
| Fasting glucose, mg/dL | 96 (58–340) | 96 (65–325) | 0.820 |
| Aspartate aminotransferase, IU/L | 21 (8–140) | 20 (11–69) | 0.964 |
| Alanine aminotransferase, IU/L | 19 (3–196) | 18 (4–58) | 0.756 |
| Serum albumin, mg/dL | 4.3 (3.4–5.3) | 4.2 (3.5–4.8) | 0.025 |
| Total bilirubin, mg/dL | 0.7 (0.2–4.0) | 0.7 (0.2–2.8) | 0.441 |
| Gamma glutamyl-transpeptidase, IU/L | 23 (7–398) | 22 (6–539) | 0.407 |
| Serum creatinine, mg/dL | 0.81 (0.38–7.3) | 0.74 (0.41–2.74) | 0.828 |
| Platelet count, 109/L | 232 (89–846) | 229 (122–438) | 0.654 |
| Prothrombin time, INR | 0.93 (0.78–2.28) | 0.94 (0.73–2.15) | 0.574 |
| Total cholesterol, mg/dL | 188 (83–392) | 177 (98–302) | 0.007 |
| Triglycerides, mg/dL | 105 (31–684) | 84.5 (43–815) | 0.825 |
| High-density lipoprotein cholesterol, mg/dL | 48 (24–100) | 50 (23–115) | 0.027 |
| Low-density lipoprotein cholesterol, mg/dL | 111 (27–299) | 100 (43–213) | 0.038 |
| HbA1c, % | 5.8 (4.4–13.4) | 5.8 (4.7–12.4) | 0.436 |
| Fibrosis-4 index | 1.15 (0.20–5.47) | 1.31 (0.37–3.39) | 0.825 |
| Muscle mass parameters | |||
| By computed tomography | |||
| Whole body fat-free mass, kg | 46.4 (23.9–84.7) | 40.8 (21.2–79.3) | 0.530 |
| Lumbar skeletal muscle index, cm2/m2 | 48.7 (34.2–85.6) | 39.9 (20.0–46.0) | <0.001 |
| By bioimpedance analysis | |||
| ASM, kg | 20.3 (10.3–34.7) | 18.1 (8.3–27.6) | 0.973 |
| ASM index, kg/m2 | 7.30 (4.62–10.58) | 6.39 (3.24–8.64) | <0.001 |
| ASM/body mass index | 0.81 (0.45–1.23) | 0.82 (0.43–1.17) | 0.044 |
| Variables | without Low SMM | with Low SMM | p-Value |
|---|---|---|---|
| (n = 972, 83.6%) | (n = 191, 16.4%) | ||
| Demographic parameters | |||
| Age, years | 57 (19–92) | 60 (18–92) | <0.001 |
| Female gender | 392 (40.0) | 129 (67.5) | <0.001 |
| Body mass index, kg/m2 | 24.2 (17.1–43.8) | 21.8 (15.4–27.8) | 0.005 |
| Hypertension | 411 (42.2) | 77 (40.3) | 0.474 |
| Diabetes mellitus | 273 (28.0) | 41 (21.4) | 0.339 |
| Viral hepatitis | 48 (4.9) | 9 (4.7) | 0.757 |
| Laboratory parameters | |||
| Fasting glucose, mg/dL | 97 (58–340) | 94 (65–265) | 0.701 |
| Aspartate aminotransferase, IU/L | 21 (8–140) | 20 (11–69) | 0.604 |
| Alanine aminotransferase, IU/L | 20 (3–196) | 17 (5–66) | 0.683 |
| Serum albumin, mg/dL | 4.3 (3.4–5.2) | 4.2 (3.5–5.3) | 0.135 |
| Total bilirubin, mg/dL | 0.7 (0.2–4.0) | 0.7 (0.3–2.5) | 0.740 |
| Gamma glutamyl-transpeptidase, IU/L | 23 (6–398) | 19 (7–539) | <0.001 |
| Serum creatinine, mg/dL | 0.82 (0.38–7.01) | 0.69 (0.39–7.3) | 0.016 |
| Platelet count, 109/L | 230 (89–846) | 241 (122–458) | 0.256 |
| Prothrombin time, INR | 0.93 (0.73–2.28) | 0.94 (0.78–2.15) | 0.027 |
| Total cholesterol, mg/dL | 185 (83–392) | 194 (98–300) | 0.918 |
| Triglycerides, mg/dL | 106 (31–815) | 88 (36–435) | 0.915 |
| High-density lipoprotein cholesterol, mg/dL | 47 (23–98) | 53 (29–115) | 0.082 |
| Low-density lipoprotein cholesterol, mg/dL | 108 (27–299) | 112 (43–213) | 0.968 |
| HbA1c, % | 5.8 (4.7–13.4) | 5.8 (4.4–10.5) | 0.386 |
| Fibrosis-4 index | 1.16 (0.32–5.47) | 1.29 (0.20–4.82) | <0.001 |
| Muscle mass parameters | |||
| By computed tomography | |||
| Whole body fat-free mass, kg | 47.7 (21.2~84.7) | 34.4 (23.5~54.2) | 0.131 |
| Lumbar skeletal muscle index, cm2/m2 | 48.5 (20.0–85.6) | 38.0 (27.0–58.1) | <0.001 |
| By bioimpedance analysis | |||
| ASM, kg | 21.3 (12.8–34.7) | 14.0 (8.3–21.6) | <0.001 |
| ASM index, kg/m2 | 7.45 (5.70–10.58) | 5.56 (3.24–6.99) | <0.001 |
| ASMI/body mass index | 0.83 (0.45–1.23) | 0.76 (0.43–1.08) | 0.568 |
| Muscle Mass Assessed by CT | Muscle Mass Assessed by BIA | |
|---|---|---|
| * BIA Cutoff | ||
| without Low SMM | with Low SMM | |
| (n = 972, 83.6%) | (n = 191, 16.4%) | |
| ** CT cutoff | ||
| Without low SMM (n = 987, 84.9%) | 841 (72.3) | 146 (12.6) |
| With low SMM (n = 176, 15.1%) | 131 (11.3) | 45 (3.9) |
| Variables | Discordance between CT and BIA-Based Low SMM | ||
|---|---|---|---|
| Univariate | Multivariate | ||
| p-Value | p-Value | OR (95% CI) | |
| Demographic parameters | |||
| Age, years | <0.001 | <0.001 | 1.050 (1.035–1.069) |
| Female gender | <0.001 | 0.044 | 1.480 (1.012–2.303) |
| Body mass index, kg/m2 | <0.001 | <0.001 | 0.725 (0.668–0.790) |
| Hypertension | 0.420 | - | - |
| Diabetes mellitus | 0.219 | - | - |
| Viral hepatitis | 0.875 | - | - |
| Laboratory parameters | |||
| Fasting glucose, mg/dL | 0.728 | - | - |
| Aspartate aminotransferase, IU/L | 0.734 | - | - |
| Alanine aminotransferase, IU/L | 0.092 | 0.021 | 0.977 (0.955–0.996) |
| Serum albumin, mg/dL | 0.020 | 0.097 | 0.581 (0.264–1.117) |
| Total bilirubin, mg/dL | 0.691 | - | - |
| Gamma glutamyl-transpeptidase, IU/L | 0.002 | <0.001 | 1.009 (1.004–1.014) |
| Serum creatinine, mg/dL | 0.803 | - | - |
| Platelet count, 109/L | 0.759 | - | - |
| Prothrombin time, INR | 0.084 | 0.153 | 3.580 (0.629–19.312) |
| Total cholesterol, mg/dL | 0.277 | - | - |
| Triglycerides, mg/dL | 0.096 | 0.128 | 1.003 (0.999–1.005) |
| High-density lipoprotein cholesterol, mg/dL | 0.096 | 0.280 | 1.011 (0.993–1.023) |
| Low-density lipoprotein cholesterol, mg/dL | 0.172 | - | - |
| HbA1c, % | 0.696 | - | - |
| Fibrosis-4 index | 0.615 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, M.H.; Lim, T.S.; Jeon, M.Y.; Lee, H.W.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.-H.; Kim, S.U. Predictors of Discordance in the Assessment of Skeletal Muscle Mass between Computed Tomography and Bioimpedance Analysis. J. Clin. Med. 2019, 8, 322. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm8030322
Jo MH, Lim TS, Jeon MY, Lee HW, Kim BK, Park JY, Kim DY, Ahn SH, Han K-H, Kim SU. Predictors of Discordance in the Assessment of Skeletal Muscle Mass between Computed Tomography and Bioimpedance Analysis. Journal of Clinical Medicine. 2019; 8(3):322. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm8030322
Chicago/Turabian StyleJo, Min Ho, Tae Seop Lim, Mi Young Jeon, Hye Won Lee, Beom Kyung Kim, Jun Yong Park, Do Young Kim, Sang Hoon Ahn, Kwang-Hyub Han, and Seung Up Kim. 2019. "Predictors of Discordance in the Assessment of Skeletal Muscle Mass between Computed Tomography and Bioimpedance Analysis" Journal of Clinical Medicine 8, no. 3: 322. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm8030322