Low-Molecular-Weight Metabolites from Diatoms: Structures, Biological Roles and Biosynthesis
Abstract
:1. Introduction
2. Lipids
2.1. Fatty Acids and Triacylglycerols


2.2. Polar Lipids


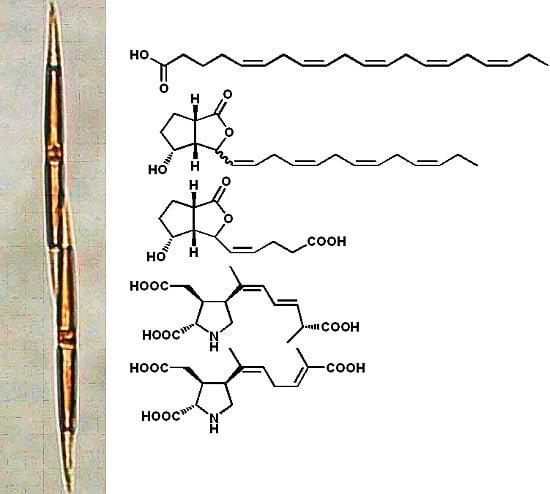
3. Oxylipins





4. Sterols


5. Isoprenoid and Other Hydrocarbons
5.1. Hydrocarbons, Derived from Fatty Acids

5.2. Isoprenoid Hydrocarbons and Related Compounds


6. Pigments


7. Halogenated Compounds

8. Toxins

9. Miscellaneous Natural Products
9.1. Attractants
9.2. Long-Chain Polyamines

10. Genomic and Post-Genomics Approaches to the Studies on Bioactive Low-Molecular Metabolites from Diatoms
11. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mann, D.G. Biodiversity, biogeography and conservation of diatoms. Hydrobiologia 1996, 336, 19–32. [Google Scholar] [CrossRef]
- Mann, D.G. The species concept in diatoms: Evidences for morphologically distinct, sympatric gamodemes in four epipelic species. Plant Syst. Evol. 1989, 164, 215–237. [Google Scholar] [CrossRef]
- Round, F.E.; Crawford, D.G.; Mann, D.G. The Diatoms: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990; pp. 3–123. [Google Scholar]
- Pivateau, F.; Gandemer, G.; Baud, J.-P. Changes in lipid and fatty acids compositions of European oysters fattened with Skeletonema costatum diatom for six weeks in ponds. Aquac. Int. 1999, 7, 341–355. [Google Scholar] [CrossRef]
- Ackman, R.G.; Jangaard, P.M.; Hoyle, R.J.; Brosherholf, H. Origin of marine fatty acids. I. Analysis of fatty acids produced by the diatom Skeletonema costatum. J. Fish. Res. Board Can. 1964, 21, 747–756. [Google Scholar] [CrossRef]
- Ackman, R.G.; Tocher, C.S.; McLahlan, J. Marine phytoplankter fatty acids. J. Fish. Res. Board Can. 1968, 25, 1603–1620. [Google Scholar] [CrossRef]
- Brokerhoff, H.; Yurkowsky, M.; Hoyle, R.J.; Ackman, R.G. Fatty acids distribution in lipids of marine phytoplankton. J. Fish. Res. Board Can. 1964, 21, 1379–1384. [Google Scholar] [CrossRef]
- Ackman, R.G. A history of fats and oils in Canada. Lipids 2003, 38, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, G.A.; Volkman, J.K.; Barret, S.M.; Leroi, J.-M.; Jeffrey, S.W. Essential polyunsaturated fatty acids from 14 species of diatom (Bacillariophyceae). Phytochemistry 1994, 35, 155–161. [Google Scholar] [CrossRef]
- Viso, A.C.; Marty, J.C. Fatty acids from 28 marine microalgae. Phytochemistry 1993, 34, 1521–1533. [Google Scholar] [CrossRef]
- Viron, C.; Saunois, A.; Andre, P.; Perly, B.; Lafosse, M. Isolation and identification of unsaturated fatty acid methyl esters from marine microalgae. Anal. Chim. Acta 2000, 409, 257–266. [Google Scholar] [CrossRef]
- Arao, T.; Kawaguchi, A.; Yamada, M. Positional distribution of fatty acids in lipids of the marine diatom Phaeodactylum tricornutum. Phytochemistry 1987, 35, 2573–2576. [Google Scholar] [CrossRef]
- Medina, A.R.; Grima, E.M.; Gimenez, A.G.; Gonzales, M.J.I. Downstream processing of algal polyunsaturated fatty acids. Biotechnol. Adv. 1998, 16, 517–580. [Google Scholar] [CrossRef]
- Vereshchagin, A.L.; Glyzina, O.Y.; Basharina, T.N.; Safonova, T.A.; Latyshev, N.A.; Liubochko, S.A.; Korneva, E.S.; Petrova, D.P.; Annenkov, W.; Danolovtseva, E.N.; et al. Culturing of a fresh-water diatom alga Synedra acus in a 100L bioreactor and analysis of the biomass composition. Biotekhnologia 2008, 4, 55–63. [Google Scholar]
- Shishlyannikov, S.M.; Klimenkov, I.V.; Bedoshvili, E.D.; Mikhailov, I.S.; Gorshkov, A.G. Effect of mixotrophic growth on the ultrastructure and fatty acid composition of the diatom Synedra acus from Lake Baikal. J. Biol. Res.–Thessalon. 2014, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Arao, T.; Yamada, M. Biosynthesis of polyunsaturated fatty acids in the marine diatom Phaeodactylum tricornutum. Phytochemistry 1993, 35, 1177–1181. [Google Scholar] [CrossRef]
- Mansour, M.P.; Frampton, D.M.F.; Nichols, P.D.; Volkman, J.K.; Blackburn, S.I. Lipid and fatty acid yield of nine stationary-phase microalgae, applications and unusual C24–C28 polyunsaturated fatty acids. J. Appl. Phycol. 2005, 17, 287–300. [Google Scholar] [CrossRef]
- Arao, T.; Yamada, M. Biosynthesis of polyunsaturated fatty acids in the marine diatom Phaeodactylum tricornotum. Phytochemistry 1994, 36, 629–635. [Google Scholar] [CrossRef]
- Bowler, C.; Allen, A.E.; Badger, J.H.; Grimwood, J.; Jabbari, K.; Kuo, A.; Maheswari, U.; Martens, C.; Maumas, F.; Otillar, R.P.; et al. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 2008, 456, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Fukuda, Y.; Yoshino, T.; Maeda, Y.; Muto, M.; Matsumoto, M.; Mayama, S.; Matsunaga, T. High throughput pyrosequencing of the chloroplast genome of a highly neutral-lipid-producing marine pennate diatom, Fistulifera sp., strain JPCC DA 0580. Photosynth. Res. 2011, 109, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Nojima, D.; Yoshino, T.; Maeda, Y.; Tanaka, M.; Nemoto, M.; Tanaka, T. Proteomics analysis of oil body-associated proteins in the oleaginous diatom. J. Proteome Res. 2013, 12, 5293–5301. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Maeda, Y.; Sunaga, Y.; Muto, M.; Matsumoto, M.; Yoshino, T.; Tanaka, T. Biosynthesis of polyunsaturated fatty acids in the oleaginous marine diatom Fistulifera sp. Strain JPCC DA 0580. Mar. Drugs 2013, 11, 5008–5023. [Google Scholar] [CrossRef] [PubMed]
- Berge, J.P.; Couygou, J.P.; Dubacq, J.P.; Durand, P. Reassessment of the lipid composition of the diatom, Skeletonema costatum. Phytochemistry 1995, 39, 1017–1021. [Google Scholar] [CrossRef]
- Hildebrand, M.; Davis, A.K.; Smith, S.R.; Trailer, J.C.; Abbriano, R. The place of diatoms in the biofuel industry. Biofuels 2012, 3, 221–240. [Google Scholar] [CrossRef]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High lipid induction in microalgae for biodiesal production. Energies 2012, 5, 1532–1533. [Google Scholar] [CrossRef]
- Thompson, G.A. Lipid and membrane function in green algae. Biochim. Biophys. Acta 1996, 1302, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.T.; Zendejas, E.J.; Lane, P.D.; Gaucher, S.; Simmons, B.A.; Lane, T.W. Triacylglycerols accumulation and profiling in the model diatoms Thalassiosira pseudonana, and Phaeodactylum tricornutum (Baccilarriophyceae) during starvation. J. Appl. Phycol. 2009, 21, 669–681. [Google Scholar] [CrossRef]
- Siron, R.; Giusti’, G.; Berland, B. Changes in the fatty acid composition of Phaeodactylum tricornutum and Dunaliella tertiolecta during growth and under phosphorus deficiency. Mar. Ecol. Prog. Ser. 1989, 55, 85–100. [Google Scholar] [CrossRef]
- Li, S.; Xu, J.L.; Chen, J.; Chen, J.J.; Zhou, C.X.; Yan, X.J. Characterization of the triacylglycerol profile in marine diatoms by ultra performance liquid chromatography coupled with electrospray ionization Q1-quadrupole time-of-flight mass spectrometry. J. Appl. Phycol. 2014, 26, 1389–1398. [Google Scholar] [CrossRef]
- Roughan, P.G.; Slack, C.R. Glycerolipid synthesis in leaves. Trends Biochem. Sci. 1984, 9, 383–386. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacyglycerols as feedstocks for biofuel production, perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Peng, K.T.; Huang, T.; Niu, Y.F.; Xie, W.H.; Yang, W.D.; Liu, J.S.; Li, H.Y. Glycerol and neutral lipid production in the oleaginous marine diatom promoted by overexpression of glycerol-3-phosphate dehydrogenase. Biotechnol. Biofuels 2014, 7, 110. [Google Scholar] [CrossRef]
- Liang, Y.; Maeda, Y.; Yoahino, T.; Matsumoto, M.; Tanaka, T. Profiling of polar lipids in marine oleagineous diatom Fistulifera sp. JPCC DA0580: Prediction of the potential mechanism for eicosapentaenic acid-incorporation into triacylglycerols. Mar. Drugs 2014, 12, 3218–3230. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.; Kates, M.; Volcani, B.E. Identification of the sulfolipids in the non-synthetic diatom Nitzschia alba. Biochem. Biophys. Acta 1978, 528, 89–106. [Google Scholar]
- Bissert, P.; Ito, S.; Tremblay, P.A.; Volcani, B.E.; Dessort, D.; Kates, M. Occurrence of phosphatidylsulfocholine, the sulfonium analog of phospatidylcholine in some diatoms and algae. Biochim. Bophys. Acta 1984, 796, 320–327. [Google Scholar] [CrossRef]
- Anderson, R.; Kates, M.; Volcani, B.E. Studies on the biosynthesis of sulfolipids in the diatoms. Biochem. Biophys. Acta 1979, 573, 576–561. [Google Scholar] [CrossRef]
- Kabara, J.J.; Swieczkowsky, D.M.; Conley, A.J.; Truant, J.P. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Jüttner, F. Liberation of 5,8,11,14,17-eicosapentaenic acid and other polyunsaturated fatty acids as a grazer defense reaction in epilithic diatom biofilms. J. Phycol. 2001, 37, 744–755. [Google Scholar] [CrossRef]
- Sieg, R.D.; Poulson-Ellestad, K.L.; Kubanek, J. Chemical ecology of the marine plankton. J. Nat. Prod. Rep. 2011, 28, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Poulson, K.L.; Sieg, R.D.; Kubanek, J. Chemical ecology of the marine plankton. Nat. Prod. Rep. 2009, 26, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Paul, V.L.; Ritson-Williams, R. Marine Chemical Ecology. Nat. Prod. Rep. 2008, 25, 662–695. [Google Scholar] [CrossRef] [PubMed]
- Miralto, A.; Barone, G.; Romano, G.; Poulet, S.A.; Ianora, A.; Russo, G.L.; Buttino, I.; Mazzarella, G.; Laabir, M.; Cabrini, M.; et al. The insidious effect of diatoms on copepod reproduction. Nature 1999, 402, 173–176. [Google Scholar] [CrossRef]
- Irigoien, X.; Harris, R.P.; Verheye, H.M.; Joly, P.; Runge, J.; Starr, M.; Pond, D.; Campbell, R.; Shreeve, R.; Ward, P.; et al. Copepod hatching success in marine ecosystems with high diatom concentrations. Nature 2002, 419, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Bartual, A.; Arandia-Gorostidi, N.; Cozar, A.; Morillo-Garcia, S.; Ortega, M.J.; Vidal, M.; Cabello, A.M.; Gonzales-Cordillo, J.I.; Echevarria, F. Polyunsaturated aldehydes from large phytoplankton of the Atlantic Ocean Surface (42° N to 33° S). Mar. Drugs 2014, 12, 682–699. [Google Scholar] [CrossRef] [PubMed]
- Vardi, A.; Formiggini, F.; Casotti, R.; de Martino, A.; Ribalet, F.; Miralto, A.; Bowler, C. A stress surveillance system based on calcium and nitric oxide in marine diatoms. PLoS Biol. 2006, 4, e60. [Google Scholar] [CrossRef] [PubMed]
- Lauritiano, C.; Borra, M.; Carotenuto, Y.; Biffali, E.; Mralto, A.; Procaccini, G.; Ianora, A. First molecular evidence of diatom effects in the copepod Calanus elgolandicus. J. Exp. Mar. Biol. Ecol. 2011, 404, 79–86. [Google Scholar] [CrossRef]
- Ianora, A.; Miralto, A.; Poulet, S.A.; Carotenuto, Y.; Buttino, I.; Romano, G.; Casotti, R.; Ponhert, G.; Wichard, T.; Colucci-D’Amato, L.; et al. Aldehyde suppression of copepod recruitment in blooms of a ubiquitous planktonic diatom. Nature 2004, 429, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Wichard, T.; Poulet, S.A.; Halsband-Lenk, C.; Albana, A.; Harris, R.; Liu, D.; Pohnert, G. Studies on the chemical defense potential of diatoms: Screening of fifty one species for alpha, beta, gamma, delta-unsaturated aldehydes. J. Chem. Ecol. 2005, 31, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Fontano, A.; D’Ippolito, G.; Cutignano, A.; Miralto, A.; Ianora, A.; Romano, G.; Cimino, G. Chemistry of oxylipin pathways in marine diatoms. Pure Appl. Chem. 2007, 79, 481–490. [Google Scholar] [CrossRef]
- D’Ippolito, G.; Romano, G.; Caruso, T.; Spinella, A.; Cimino, G.; Fontano, A. Production of octadienal in the marine diatom Skeletonema costatum. Org. Lett. 2003, 5, 885–887. [Google Scholar] [CrossRef] [PubMed]
- D’Ippolito, G.; Romano, G.; Iadicicco, O.; Miralto, A.; Ianara, A.; Cimino, G.; Fontana, A. New birth-control aldehydes from the marine diatom Skeletonema costatum: Characterization and biogenesis. Tetrahedron Lett. 2002, 43, 6133–6136. [Google Scholar] [CrossRef]
- D’Ippolito, G.; Citignano, A.; Briante, R.; Febbrario, R.; Cimino, G.; Fontana, A. New C16 fatty-acid based oxylipin pathway in the marine diatom Thalassiosira rotula. Org. Biomol. Chem. 2005, 3, 4065–4070. [Google Scholar] [CrossRef] [PubMed]
- Barriero, A.; Carotenuto, Y.; Lamari, N.; Esposito, F.; D’Ippolito, G.; Fontana, A.; Romano, G.; Ianora, A.; Miralto, A.; Guisande, C. Diatom induction of reproductive failure in copepod: The effect of PUAs versus non volatile oxylipins. J. Exp. Mar. Biol. Ecol. 2011, 401, 13–19. [Google Scholar] [CrossRef]
- D’Ippolito, G.; Lamari, N.; Montresor, M.; Romano, G.; Cutignano, A.; Gerecht, A.; Cimino, G.; Fontana, A. 15S-lipogenase metabolism in the marine diatom Pseudo-nitzschia dilicatissima. New Phytol. 2009, 183, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Nanjappa, D.; D’Ippolito, G.; Gallo, C.; Zingone, A.; Fontana, A. Oxylipin diversity in the diatom family Leptocylindraceae reveals DHA derivatives in marine diatoms. Mar. Drugs 2014, 12, 368–384. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Shimizu, Y. Bacillariolides I and II, a new type of cyclopentane eicosanoids from the diatom Nitzschia pungens. J. Chem. Soc. Chem. Commun. 1990, 5, 413–414. [Google Scholar] [CrossRef]
- Zheng, N.; Shimizu, Y. The isolation and structure of bacillariolide III, an extracellular metabolite of the diatom Pseudo-nitschia multiseries. J. Chem. Soc. Chem. Commun. 1997, 4, 399–400. [Google Scholar] [CrossRef]
- Wang, R.; Shimizu, Y.; Steiner, J.R.; Clardy, J.J. The absolute configuration of bacillariolides I and II, a new type of cyclopentane eicosanoids from a marine diatom. Chem. Soc. Chem. Commun. 1993, 4, 379–380. [Google Scholar] [CrossRef]
- Jüttner, F.; Müller, H. Excretion of octadiene and octatrienes by a freshwater diatom. Naturwissenschaften 1979, 66, 363–364. [Google Scholar] [CrossRef]
- Derenbach, J.B.; Pesando, D. Investigations into a small fraction of volatile hydrocarbons. III. Two diatom cultures produce ectocarpene, a pheromone of brown algae. Mar. Chem. 1986, 19, 337–341. [Google Scholar] [CrossRef]
- Wendel, T.; Jüttner, F. Lipoxygenase-mediated formation of hydrocarbons and unsaturated aldehydes in freshwater diatoms. Phytochemistry 1996, 41, 1445–1449. [Google Scholar] [CrossRef]
- Pohnert, G.; Boland, W.; Pohnert, G.; Boland, W. Biosynthesis of the algal pheromone hormosirene by the freshwater diatom Gomphonema parvulum (Bacillariophyceae). Tetrahedron 1996, 52, 10073–10082. [Google Scholar] [CrossRef]
- Hombeck, M.; Boland, W. Biosynthesis of the algal pheromone fucoserratene by the freshwater diatom Asterionella formosa (Bacillariophyceae). Tetrahedron 1998, 54, 11033–11042. [Google Scholar] [CrossRef]
- Volkman, J.K. A review of sterol marker of for marine and terrigenous organic matter. Org. Geochem. 1986, 9, 83–99. [Google Scholar] [CrossRef]
- Volkman, J.K.; Barrett, S.M.; Dunstan, G.A.; Jeffrey, S.W. Geochemical significance of the occurrence of dinosterol and other 4-methyl sterols in a marine diatom. Org. Geochem. 1993, 20, 7–15. [Google Scholar] [CrossRef]
- Rampen, S.W.; Abbas, B.A.; Schouten, S.; Damste, J.J.S. A comprehensive study of sterols in marine diatoms (Bacillariophyta): Implication of their use as tracers for diatom productivity. Limnol. Oceanogr. 2010, 55, 91–105. [Google Scholar] [CrossRef]
- Volkman, J.K.; Hallegraeff, G.M. Lipid in marine diatoms of the genus Thalassiosira: Predominance of 24-methylenecholesterol. Phytochemistry 1988, 27, 1389–1394. [Google Scholar] [CrossRef]
- Ponomarenko, L.P.; Stonik, I.V.; Aizdaicher, N.A.; Orlova, T.J.; Popovskaya, G.I.; Pomazkina, G.; Stonik, V.A. Sterols of marine microalgae Pyramimonas cf. cordata (Prasinophyta), Atthea ussirensis sp. nov. (Bacillariophyta) and a spring diatom bloom from Lake Baikal. Comp. Biochem. Physiol. 2004, 138, 65–70. [Google Scholar]
- Volkman, J.K.; Barrett, S.M.; Blackburn, S.I.; Mansour, M.P.; Sikes, E.L.; Gelin, F. Microalgal biomarkers: A review of recent research developments. Org. Geochem. 1998, 29, 1163–1179. [Google Scholar] [CrossRef]
- Kalinovsky, A.I.; Gorshkov, A.G.; Ponomarenko, L.P.; Stonik, V.A.; Dmitrenok, A.S.; Grachev, M.A. Preparation of 13 C-24-methylcholesta-5,24(28)-dien-3β-ol by cultivation of the Baikal diatom Synedra acus in NaH13CO3. Russ. Chem. Bull. 2010, 59, 236–240. [Google Scholar] [CrossRef]
- Ballantine, J.A.; Lavis, A.; Morris, R.J. Sterols of phytoplankton: Effects of illumination and growth stage. Phytochemistry 1979, 18, 1459–1466. [Google Scholar] [CrossRef]
- Masse, G.; Belt, S.T.; Rowland, S.J.; Rohmer, R. Isoprenoid biosynthesis in the diatoms Rhisosolenia setigera (Brightwell) and Haslea osrearia (Simonsen). Proc. Natl. Acad. Sci. USA 2004, 101, 4413–4418. [Google Scholar] [CrossRef] [PubMed]
- Blumer, M.; Mullin, M.M.; Guillard, R.R.L. A polyunsaturated hydrocarbon (3,6,9,12,15,18-heneicosahexaene) in the marine food web. Mar. Biol. 1970, 6, 226–235. [Google Scholar] [CrossRef]
- Lee, R.F.; Loeblich, A.R., III. Distribution of 21:6 hydrocarbon and its relationship to 22:6 fatty acid in algae. Phytochemistry 1971, 10, 593–602. [Google Scholar] [CrossRef]
- Volkman, J.K.; Barrett, S.M.; Dunstan, G.A. C25 and C30 highly branched alkenes in laboratory cultures of two marine diatoms. Org. Geochem. 1994, 21, 407–414. [Google Scholar] [CrossRef]
- Grossi, V.; Beker, B.; Geenevasen, J.A.J.; Schouten, S.; Raphel, D.; Fontaine, M.-F.; Damste, J.S.S. C25-highly branched isoprenoid alkenes from the benthic diatom Pleurosigma strigosum. Phytochemistry. 2004, 65, 3049–3055. [Google Scholar] [CrossRef] [PubMed]
- Damste, J.S.S.; Schouten, S.; Rijpsta, W.I.C.; Hopmans, E.C.; Peletier, H.; Gieskes, W.W.C.; Geenevasen, J.A.J. Novel polyunsaturated n-alkenes in the marine diatom Rhizosolenia setigera. Eur. J. Biochem. 2000, 267, 5727–5732. [Google Scholar] [CrossRef] [PubMed]
- Kardys, M. Uptake of hydrocabons by the marine diatom Cyclotella cryptica. Micrbial Ecol. 1980, 5, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Robson, J.N.; Rowland, S.J. Identification of novel widely distributed sedimentary acyclic sesterterpenoids. Nature 1986, 324, 561–563. [Google Scholar] [CrossRef]
- Robson, J.N.; Rowland, S. Synthesis of highly branched C30 sedimentary hydrocarbon. Tetrahedron Lett. 1988, 29, 3837–3840. [Google Scholar] [CrossRef]
- Belt, S.T.; Cooke, D.A.; Robert, J.M.; Rowland, S. Structural characterization of widespread polyunsaturated isoprenoid biomarkers: A C25 triene, tetraene, and pentaene from the diatom Haslea ostrearia Simonsen. Tetrahedron Lett. 1996, 37, 4755–4758. [Google Scholar] [CrossRef]
- Wraige, E.J.; Belt, S.T.; Lewis, C.A.; Cooke, D.A.; Robert, J.-M.; Masse, G.; Rowland, S.J. Variations in structures and distributions of C25 highly branched isoprenoids in cultures of the diatom, Haslea ostrearia (Simonsen). Org. Geochem. 1997, 27, 497–505. [Google Scholar] [CrossRef]
- Wraige, E.J.; Johns, L.; Belt, S.T.; Masse, G.; Robert, J.-M.; Rowland, S.J. Highly branched C-25 isoprenoids in axenic cultures of Haslea ostrearia. Phytochemistry 1999, 51, 69–73. [Google Scholar] [CrossRef]
- Sininqhe Damste, J.S.; Schouten, S.; Rijpsta, W.I.C.; Hopmans, E.C.; Peletier, H.; van der Maarel, M.J.E.C.; Gieskes, W.W.C.; Geenwasen, J.A.J. Structural identification of the C25 highly branched pentaene in the marine diatom, Rhizosolenia setigera. Org. Geochem. 1999, 30, 1581–1583. [Google Scholar] [CrossRef]
- Damste, J.S.S.; Rijpsta, W.I.C.; Schouten, S.; Peletier, H.; van der Maarel, M.J.E.C.; Gieskes, W.W.C. A C25 highly branched isoprenoid alkene and C25 and C27 n-polyenes in the marine diatom, Rhizosolenia setigera. Org. Geochem. 1999, 30, 95–100. [Google Scholar] [CrossRef]
- Rowland, S.J.; Allard, W.G.; Belt, S.T.; Masse, G.; Robert, J.-M.; Blackburn, S.; Frampton, D.; Revill, A.T.; Volkman, J.K. Factors influencing the distribution of polyunsaturated terpenoids in the diatom Rhizololenia setigera. Phytochemistry 2001, 58, 717–728. [Google Scholar] [CrossRef]
- Belt, S.T.; Allard, W.G.; Masse, G.; Robert, J.-M.; Rowland, S.R. Highly branched isoprenoids (HBIs): Identification of the most common and abundant sedimentary isomers. Geochim. Cosmochim. Acta 2000, 64, 3839–3851. [Google Scholar] [CrossRef]
- Belt, S.T.; Masse, G.; Allard, W.G.; Robert, J.-M.; Rowland, S.J. C25 highly branched isoprenoid alkenes in planktonic diatoms of the Pleurosigma genus. Org. Geochem. 2001, 32, 1271–1275. [Google Scholar] [CrossRef]
- Nichols, P.D.; Palmisano, A.C.; Smith, A.G.; White, D.C. Lipids from the Antharctic sea ice diatom Nitzschia cylindrus. Phytochemistry 1986, 25, 1649–1653. [Google Scholar] [CrossRef]
- Nichols, P.D.; Volkman, J.K.; Palmisano, A.C.; Smith, A.G.; White, D.C. Occurrence of an isoprenoid C25 diunsaturated alkene and high neutral lipid content in Antarctic sea-ice diatom communities. J. Phycol. 1988, 24, 90–96. [Google Scholar] [CrossRef]
- Nichols, D.S.; Nichols, P.D.; Sullivan, C.W. Fatty acid, sterols and hydrocabon composition of Antarctic sea ice diatom communities during the spring bloom in McMurdo sound. Antarct. Sci. 1993, 5, 271–278. [Google Scholar] [CrossRef]
- Belt, S.T.; Masse, G.; Poulin, M.; Michel, C.; LeBlank, B. A novel chemical fossil of palaeosea ice: IP25. Org. Geochem. 2007, 38, 16–27. [Google Scholar] [CrossRef]
- Brown, T.A.; Belt, S.T.; Tatarek, A.; Mundy, C.J. Source identification of the Arctic sea ice proxy IP25. Nat. Commun. 2014, 5, 4197. [Google Scholar] [CrossRef] [PubMed]
- Belt, S.T.; Allard, W.G.; Masse, G.; Robert, J.M.; Rowland, S.J. Structural characterization of C30 highly branched isoprenoid alkenes (rhizenes) in the marine diatom Rhizosolenia setigera. Tetrahedron Lett. 2001, 42, 5583–5585. [Google Scholar] [CrossRef]
- Belt, S.M.; Masse, G.; Allard, W.G.; Robert, J.M.; Rowland, S.J. Novel monocyclic sester- and triterpenoids from the marine diatom, Rhizosolenia setigera. Tetrahedron Lett. 2003, 44, 9103–9106. [Google Scholar] [CrossRef]
- Pennington, F.; Guillard, R.R.L.; Jensen, S. Carotenoid distribution patterns in Bacillariophyceae (diatoms). Biochem. Syst. Ecol. 1988, 16, 589–592. [Google Scholar] [CrossRef]
- Zapata, M.; Rodriguez, F.; Fraga, S.; Barra, L.; Ruggiero, M.V. Chlorophyll c pigment patterns in 18 species (51 strains) of the genus Pseudo-nitzschia (Bacillariophyceae). J. Phycol. 2011, 47, 1274–1280. [Google Scholar] [CrossRef]
- Lohr, M.; Wilhelm, C. Algae displaying the diadinoxanthin cycle also possess the violaxanthin cycle. Proc. Natl. Acad. Sci. 1999, 96, 8784–8789. [Google Scholar] [CrossRef] [PubMed]
- Willstätter, R.; Page, H.R. Chlorophyll. XXIV. The pigments of the brown algae. Justus Liebigs Ann. Chem. 1914, 404, 237–271. [Google Scholar] [CrossRef]
- Englert, G.; Bjornland, T.; Liannen-Jensen, S. 1D and 2D NMR study of some allenic carotenoids of the fucoxanthin series. Magn. Reson. Chem. 1990, 28, 519–528. [Google Scholar] [CrossRef]
- Wang, J.-H.; Wu, C.-F.; Yuan, J.-P.; Yuan, P.J. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP-1 in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Abidov, M.; Ramazanov, Z.; Seifulla, R.; Grachev, S. The effect of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes. Metab. 2010, 12, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.; Choi, H.J.; Lee, J.Y.; Jeong, H.S.; Kim, C.H.; Joo, M.; Choi, J.Y.; Han, S.W.; Kim, S.Y.; Choi, J.S.; et al. Marine algal fucoxanthin inhibits the metastatic potential of cancer cells. Biochem. Biophys. Res. Commun. 2013, 439, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Yung, Y.I.; Kwon, O.-N.; Cha, K.H.; Um, B.H.; Chung, D.; Pan, C.-H. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Lohr, M.; Wilhelm, C. Xanthophyll synthesis in diatoms: Quantification of putative intermediates and comparison of pigment conversation kinetics with rate constants derived from a model. Planta 2001, 212, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Coesel, S.; Obornik, M.; Varela, J.; Falciotore, A.; Bowler, C. Evolutionary origin of the carotenoid biosynthetic pathway in marine diatoms. PLoS ONE 2008, 3, e2896. [Google Scholar] [CrossRef] [PubMed]
- Dambek, M.; Eilers, U.; Breitenbach, J.; Steiger, S.; Büchel, C.; Sandmann, G. Biosynthesis of fucoxanthin and diadinoxanthin and function of initial pathways genes in Phaeodactylum tricornutum. J. Exp. Bot. 2012, 63, 5607–5612. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.M.; Webb, M.; Tokarczuk, R.; Wever, R.J. Bromoperoxidase and iodoperoxidase enzymes and production of halogenated methanes in marine diatom cultures. J. Geophys. Res. 2012, 101, 20899–20908. [Google Scholar] [CrossRef]
- Hill, V.L.; Manley, S.L. Release of reactive bromine and iodine from diatoms and its possible role in halogen transfer in polar and tropical oceans. Limnol. Oceanogr. 2009, 54, 812–822. [Google Scholar] [CrossRef]
- Syrpas, M.; Ruysbergh, E.; Blommaert, L.; Vanelslander, B.M.; Sabbe, K.; Vyverman, W.; de Kimpe, N.; Mangelinckx, S. Haloperoxidase mediated quorum quenching by Nitzschia cf. pellucida: Study of the metabolization of N-acyl homoserine lactones by a bentic diatom. Mar. Drugs 2014, 12, 352–367. [Google Scholar]
- Bart, V.; Carsten, P.; Grueneberg, J.; Prince, E.K.; Gillard, J.; Sabbe, K.; Pohnert, G.; Vyverman, W. Daily bursts of biogenic cyanogen bromide (BrCN) controls biofilm formation around a marine benthic diatom. Proc. Natl. Acad. Sci. USA 2012, 109, 2412–2417. [Google Scholar]
- Wichard, T.; Pohnert, G. Formation of halogenated medium chain hydrocarbons by a lipoxygenase/hydroperoxide halolyase-mediated transformation in planktonic microalgae. J. Am. Chem. Soc. 2006, 128, 7114–7115. [Google Scholar] [CrossRef] [PubMed]
- Quilliam, M.A.; Wright, J.L. The amnesic shellfish poisoning mystery. Anal. Chem. 1989, 61, 1053A–1106A. [Google Scholar] [CrossRef] [PubMed]
- Iverson, F.; Truelove, J.; Nera, E.; Tryphonas, L.; Campbell, J.; Lok, E. Domoic acid poisoning and mussel-associated intoxication: Preliminary investigations into the response of mice and rats to toxic mussel extract. Food Chem. Toxicol. 1989, 27, 377–384. [Google Scholar] [CrossRef]
- Trainer, V.L.; Bates, S.S.; Lundholm, N.; Thessen, A.E.; Cochlan, W.P.; Adams, N.G.; Trick, C.G. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef]
- Takemoto, T.; Daigo, K.; Kondo, Y.; Kondo, K. Studies on the constituents of Chondria armata. VIII. On the structure of domoic acid. Yakugaku Zasshi 1966, 86, 874–877. [Google Scholar] [PubMed]
- Ohfune, Y.; Tomita, M. Total synthesis of domic acid. A revision of original structure. J. Am. Chem. Soc. 1982, 104, 3511–3513. [Google Scholar] [CrossRef]
- Bates, S.S.; Trainer, V.L. The ecology of harmful diatoms. In Ecological Studies; Graneli, E., Turner, J.T., Eds.; Springer-Verlag: Berlin, Germany, 2006; Volume 189, pp. 81–93. [Google Scholar]
- Trainer, V.L.; Hickey, B.M.; Bates, S.S. Toxic diatoms. In Ocean and Human Health: Risks and Remedies from the Sea; Walsh, P.J., Smith, S.L., Fleming, L.E., Solo-Gabriele, H., Gerwick, W.H., Eds.; Elsevier: New York, NY, USA, 2008; pp. 219–237. [Google Scholar]
- Lelong, A.; Hegaret, H.; Soudant, P.; Bates, S.S. Pseudo-nitzschia (Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: Revisting previous paradigm. Phycologia 2012, 51, 168–216. [Google Scholar] [CrossRef]
- Kobayashi, K.; Takata, Y.; Kodama, M. Direct contact between Pseudo-nitzschia multiseries and bacteria is necessary for the diatom to produce a high level of domoic acid. Fish. Sci. 2009, 75, 771–776. [Google Scholar] [CrossRef]
- Pulido, O.M. Domoic acid toxicologic pathology: A review. Mar. Drugs 2008, 6, 180–219. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, K.A.; Robertson, A. Domoic acid and human exposure risks: A review. Toxicon 2010, 56, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Hess, P.; Gallacher, L.A.; Bates, S.; Quilliam, M.A. Determination and confirmation of amnesic shellfish poisoning toxin, domoic acid in shellfish from Scotland by liquid chromatography and mass spectrometry. J. AOAC Int. 2001, 84, 1657–1667. [Google Scholar] [PubMed]
- Kotaki, Y.; Koike, K.; Sato, S.; Ogata, T.; Fukuyo, Y.; Kodama, M. Confirmation of domoic acid production of Pseudo-nitzschia multiseries isolated from Ofunato Bay, Japan. Toxicon 1999, 37, 677–682. [Google Scholar] [CrossRef]
- Amzil, Z.; Fresnel, J.; le, G.D.; Billard, C. Domic acid accumulation in French shellfish in relation to toxic species of Pseudo-nitzschia multiseries and P. pseudodelicatissima. Toxicon 2001, 39, 1245–1251. [Google Scholar] [CrossRef]
- James, K.J.; Gillman, M.; Amandi, M.F.; Lopez-Rivera, A.; Puente, P.F.; Lehane, M.; Mitovic, S.; Furey, A. Amnesic shellfish poisoning toxins in bivalve molluscs In Ireland. Toxicon 2005, 46, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Scholin, C.A.; Gulland, F.; Doucette, G.J.; Benson, S.; Busman, M.; Chavez, E.P.; Cordaro, J.; DeLong, R.; de Vogelaere, A.; Harvey, J.; et al. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature 2000, 403, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Kodama, T.; Tanaka, T.; Yoshimuzu, H.; Takemoto, T.; Nomoto, K.; Fujita, T. Structures of isodomoic acids A, B, and C, novel insecticidal amino acids from the red alga Chondria armata. Chem. Pharm. Bull. 1986, 34, 4892–4895. [Google Scholar] [CrossRef]
- Wright, J.L.C.; Falk, M.; McInnes, A.G.; Walter, J.A. Identification of isodomoic acid D and 2 new geometrical isomers of domoic acid in toxic mussels. Can. J. Chem. 1990, 68, 22–25. [Google Scholar] [CrossRef]
- Clayden, J.; Read, B.; Hebdich, K.R. Chemistry of domoic acid, isodomoic acids, and their analogues. Tetrahedron 2005, 61, 5713–5724. [Google Scholar] [CrossRef]
- Pearson, M.L.; Dortch, Q.; Turner, R.E. Sedimental evidence of an increase in Pseudo-nitzschia (Bacillariophyceae) abundance in response to coastal etuterophication. Limnol. Oceanogr. 2002, 47, 551–558. [Google Scholar] [CrossRef]
- Mos, L. Domoic acid: A fascinating marine toxin. Environ. Toxicol. Pharmacol. 2001, 9, 79–85. [Google Scholar] [CrossRef]
- Stonik, I.V.; Orlova, T.Y.; Lindholm, N. Diversity of Pseudo-nitzschia from the western North Pacific. Diatom Res. 2011, 1, 121–134. [Google Scholar] [CrossRef]
- Silver, M.W.; Bargu, S.; Coale, S.L.; Benitz-Nelson, C.R.; Garcia, A.C.; Roberts, K.J.; Sekula-Wood, E.; Bruland, K.W.; Coale, K.H. Toxic diatoms and domoic acid in natural and iron-enriched waters of the oceanic Pacific. Proc. Natl. Acad. Sci. USA 2010, 107, 20762–20767. [Google Scholar] [CrossRef] [PubMed]
- Gillard, J.; Frenkel, J.; Devos, V.; Sabbe, K.; Paul, C.; Rempt, M.; Inze, D.; Pohnert, G.; Vuylsteke, M.; Vyverman, W. Metabolomics enables the structure elucidation of a diatom sex pheromone. Angew. Chem. Int. Ed. Engl. 2013, 52, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Bucci, M. Proline draws a diatom. Nat. Chem. Biol. 2013, 9, 66. [Google Scholar]
- Frenkel, J.; Wess, C.; Vyverman, W.; Pohnert, G. Chiral separation of a diketopiperazine pheromone from marine diatoms using supercriticial fluid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 951–952, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Kroger, N.; Deitzmann, R.; Sumpler, M. Polykationic peptides from diatom biosilica that direct silica nanosphere formation. Science 1999, 286, 1129–1132. [Google Scholar] [PubMed]
- Sumper, M. A phase separation model for the nanopatterning of diatom bioslica. Science 2002, 295, 2430–2433. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, N.; Kroger, N. Silica morphogenesis by alternative processing of silaffins in the diatom Thalassiosira pseudonana. J. Biol. Chem. 2004, 279, 42993–42999. [Google Scholar] [CrossRef] [PubMed]
- Kroger, N.; Lorenz, S.; Brunner, E.; Sumper, M. Self-assembly of highly phosporylated silaffins and their function in biosilica morphogenesis. Science 2002, 298, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, N.; Sumper, M.; Kroger, N. Biosilica formation in diatoms. Characterization of native sillafin-2 and its role in silica morphogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 12075–12080. [Google Scholar] [CrossRef] [PubMed]
- Sumper, M.; Kroger, N. Silica formation in diatoms: The function of long-chain polyamines and silaffines. J. Mater. Chem. 2004, 14, 2059–2065. [Google Scholar] [CrossRef]
- Kroger, N.; Deutzmann, R.; Bergsdorf, C.; Sumper, M. Species-specific polyamines from diatoms control silica morphology. Proc. Natl. Acad. Sci. USA 2000, 97, 14133–14138. [Google Scholar] [CrossRef] [PubMed]
- Vardi, A.; Thamatrakoln, K.; Bidle, K.D.; Falkowski, P.G. Diatom genomes come of age. Genome Biol. 2008, 9, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Ravin, N.V.; Galachyantz, Y.P.; Mardanov, A.V.; Beketsky, A.V.; Petrova, D.P.; Sherbakova, T.A.; Zakharova, Y.R.; Likhoshway, E.V.; Skryabin, K.G.; Grachev, M.A. Complete sequence of the mitochondrial genome of a diatom alga Synedra acus and comparative analysis of diatom mitochondrial genomes. Curr. Genet. 2010, 56, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Outdot-Le Secq, M.P.; Grimwood, J.; Shapiro, H.; Armburst, E.V.; Bowler, C.; Green, B.R. Chloroplast genomes of the diatoms Thalassiosira pseudonana and Phaeodactylum tricornutum: Comparison with other plastid genomes of the red lineage. Mol. Genet. Genomics 2007, 277, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Galachyants, Y.P.; Morozov, A.A.; Mardanov, A.V.; Beketsky, A.V.; Ravin, N.V.; Petrova, D.P.; Likhoshway, E.V. Complete chloroplast genome sequence of freshwater araphid pennate diatom alga Synedra acus from Baikal Lake. Int. J. Biol. 2012, 4, 27–35. [Google Scholar]
- Khozin-Goldberg, I.; Cohen, Z. Unraveling algal lipid metabolism: Recent advances in gene identification. Biochimie 2011, 93, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Tonon, T.; Qing, R.W.; Harvey, D.; Li, Y.; Larson, T.R.; Graham, I.A. Identification of a long-chain polyunsaturated fatty acid acyl-coenzyme A synthetase from the diatom Thalassiosira pseudonana. Plant Physiol. 2005, 138, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Lu, Y.; Zheng, J.-W.; Yang, W.-D.; Liu, J.-S. Biochemical and genetic engineering of diatoms for polyunsaturated fatty acid biosynthesis. Mar. Drugs 2014, 12, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.F.; Zhang, M.H.; Li, D.W.; Yang, W.D.; Liu, J.S.; Bai, W.B.; Li, H.Y. Improvement of neutral lipid and polyunsaturated fatty acid biosynthesis by overexpressing a type 2 diacylglycerol acyltransferase in marine diatom Phaeodactylum tricornutum. Mar. Drugs 2013, 11, 4558–4569. [Google Scholar] [CrossRef] [PubMed]
- Bellou, S.; Baeshen, M.N.; Elazzazy, A.M.; Aggeli, D.; Sayegh, F. Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol. Adv. 2014, 32, 1476–1493. [Google Scholar] [CrossRef] [PubMed]
- Zaslavskaya, L.A.; Lippmeier, J.C.; Shih, C.; Ehrhardt, D.; Grossman, A.R.; Apt, K.E. Trophic conversion of an obligate photoautotrophic organism through metabolic engineering. Science 2001, 292, 2073–2075. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.J. Molecular machines encoded by bacterial-derived multi-domain gene fusions that potentially synthesize, N-methylate and transfer long chain polyamines in diatoms. FEBS Lett. 2011, 585, 2627–2634. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, M. Carotenoid biosynthesis in diatoms. Photosynth. Res. 2010, 106, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Fabris, M.; Matthijs, M.; Carbonelle, S.; Moses, T.; Pollier, J.; Dasseville, R.; Baart, G.J.E.; Vyvermann, W.; Goossens, A. Tracking the sterol biosynthesis pathway of the diatom. New Phytol. 2014, 204, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Boissonneault, K.R.; Henningsen, B.M.; Bates, S.S.; Robertson, D.L.; Milton, S.; Pelletier, J.; Hogan, D.A.; Housman, D.E. Gene expression studies for the analysis of the marine diatom Pseudo-nitzschia multiseries. BMC Mol. Biol. 2013, 14, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armbrust, E.V.; Berges, J.A.; Bowler, C.; Green, B.R.; Martinez, D.; Putnam, N.H.; Zhou, S.; Allen, A.E.; Apt, K.E.; Bechner, M.; et al. The genome of the diatom Thalassiosira pseudonana: Ecology, evolution, and metabolism. Science 2004, 306, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.R.; Abbriano, R.M.; Hildebrand, M. Comparative analysis of diatom genomes reveals substantial differences in the organization of carbon partitioning pathways. Algal Res. 2012, 1, 2–16. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stonik, V.; Stonik, I. Low-Molecular-Weight Metabolites from Diatoms: Structures, Biological Roles and Biosynthesis. Mar. Drugs 2015, 13, 3672-3709. https://0-doi-org.brum.beds.ac.uk/10.3390/md13063672
Stonik V, Stonik I. Low-Molecular-Weight Metabolites from Diatoms: Structures, Biological Roles and Biosynthesis. Marine Drugs. 2015; 13(6):3672-3709. https://0-doi-org.brum.beds.ac.uk/10.3390/md13063672
Chicago/Turabian StyleStonik, Valentin, and Inna Stonik. 2015. "Low-Molecular-Weight Metabolites from Diatoms: Structures, Biological Roles and Biosynthesis" Marine Drugs 13, no. 6: 3672-3709. https://0-doi-org.brum.beds.ac.uk/10.3390/md13063672