Phytofabrication of Silver/Silver Chloride Nanoparticles Using Aqueous Leaf Extract of Oedera genistifolia: Characterization and Antibacterial Potential
Abstract
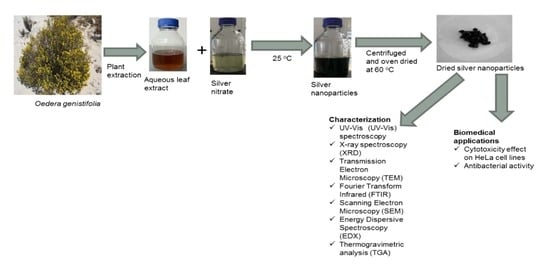
:1. Introduction
2. Results and Discussion
2.1. Screening of Phytochemical Compounds
2.2. UV-Vis Spectroscopy Analysis
2.3. FTIR Analysis
2.4. TEM, SEM and EDX Analyses
2.5. XRD Analysis
2.6. TGA Analysis
2.7. Cytotoxicity Assay Against HeLa Cells
2.8. Antibacterial Activity
3. Materials and Methods
3.1. Plant Collection and Extraction
3.2. Screening for Phytochemical Constituents
3.2.1. Test for Phenols
3.2.2. Test for Alkaloids
3.2.3. Test for Tannins
3.2.4. Test for Flavonoids
3.2.5. Test for Saponins
3.2.6. Test for Glycosides
3.2.7. Test for Terpenoids
3.2.8. Test for Protein
3.2.9. Test for Carbohydrates
3.2.10. Test for Anthraquinone
3.2.11. Test for Steroids
3.3. Biosynthesis of Ag/AgCl NPs
3.4. Characterization of Ag/AgCl NPs
3.5. Cytotoxicity Assay—Single Concentration Screening
3.6. Antibacterial Activity Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Marslin, G.; Siram, K.; Maqbool, Q.; Selvakesavan, R.K.; Kruszka, D.; Kachlicki, P.; Franklin, G. Secondary metabolites in the green synthesis of metallic nanoparticles. Materials 2018, 11, 940. [Google Scholar] [CrossRef]
- Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostructure Chem. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Nayak, D.; Pradhan, S.; Ashe, S.; Rauta, P.R.; Nayak, B. Biologically synthesised silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J. Colloid Interface Sci. 2015, 457, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Kalaiyarasan, T.; Bharti, V.K.; Chaurasia, O.P. One pot green preparation of Seabuckthorn silver nanoparticles (SBT@AgNPs) featuring high stability and long-evity, antibacterial, antioxidant potential: A nano disinfectant future perspective. RSC Adv. 2017, 7, 51130–51141. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan & its derivatives: A review in recent innovations. Int. J. Pharm. Sci. Res. 2015, 6, 14–30. [Google Scholar]
- Kharissova, O.V.; Dias, H.V.R.; Kharisov, B.I.; Perez, B.O.; Perez, V.M.J. The greener synthesis of nanoparticles. Trends Biotechnol. 2013, 31, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomedicine 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Mittal, J.; Batra, A.; Singh, A.; Sharma, M.M. Phytofabrication of nanoparticles through plant as nanofactories. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 043002. [Google Scholar] [CrossRef]
- Arya, G.R.; Kumari, M.; Sharma, N.; Chatterjee, S.; Gupta, N.; Kumar, A.; Nimesh, S. Evaluation of antibiofilm and catalytic activity of biogenic silver nanoparticles synthesized from Acacia nilotica leaf extract. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 045003. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Sdv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Dhuper, S.; Panda, D.; Nayak, P.L. Green synthesis and characterization of zero valent iron nanoparticles from the leaf extract of Mangifera indica. Nano. Trends J. Nanotech. App. 2012, 13, 16–22. [Google Scholar]
- Rahmaniyan, F.; Shamel, A.; Shafaghatlonbar, A. Evaluation of biologically synthesized silver nanoparticles by the bioreduction method. Synth. React. Inorg. M. 2015, 45, 1495–1500. [Google Scholar] [CrossRef]
- Veerasamy, R.; ZiXin, T.; Gunasagaran, S.; Wei, T.F.X.; Yang, E.F.C.; Kumar, N.J.; Dhanaraj, S.A. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J. Saudi Chem. Soc. 2011, 15, 113–120. [Google Scholar] [CrossRef]
- Yu, M.K.; Park, J.; Jon, S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheddi, E.S.; Farshori, N.N.; Al-Oqail, M.M.; Al-Massarani, S.M.; Saquib, Q.; Wahab, R.; Musarrat, J.; Al-Khedhairy, A.A.; Siddiqui, M.A. Anticancer potential of green synthesized silver nanoparticles using extract of Nepeta deflersiana against human cervical cancer cells (HeLa). Bioinorg. Chem. Appl. 2018, 12, 9390784. [Google Scholar] [CrossRef]
- Ashish, J.; Kumar, M.S.; Vanaja, N.; Abhinav, R.; Naisarg, M.; Sughosh, R.; Singh, K.M.J. Biosynthesis of nanoparticles from Ficus benjamina (fig tree) and comparing AgNP’s synthesized by cocktails of plant extracts. Int. Res. J. Biol. Sci. 2014, 3, 2278–3202. [Google Scholar]
- Mohammadinejad, R.; Karimi, S.; Iravani, S.; Varma, R.S. Plant-derived nanostructures: Types and applications. Green Chem. 2015, 18, 20–52. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.Y.; Zhou, H.; Hwang, S.; Koh, K.; Dong-Wook, H.; Lee, J.J. Green synthesis of phytochemical-stabilized Au nanoparticles under ambient conditions and their biocompatibility and antioxidative activity. Mater. Chem. 2011, 21, 13316–13326. [Google Scholar] [CrossRef]
- Ramesh, A.V.; Devi, D.R.; Battu, G.R.; Basavaiah, K. A facile plant mediated synthesis of silver nanoparticles using an aqueous leaf extract of Ficus hispida Linn. f. for catalytic, antioxidant and antibacterial applications. S. Afr. J. Chem. Eng. 2018, 26, 25–34. [Google Scholar] [CrossRef]
- Philip, D.; Unni, C.; Aromal, S.A.; Vidhu, V.K. Murraya Koenigii leaf-assisted rapid green synthesis of silver and gold nanoparticles. Spectrochimica Acta Part A 2011, 78, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Aslam, U.; Boerigter, C.; Morabito, M. Photochemical transformations on plasmonic metal nanoparticles. Nat. Mater. 2015, 14, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Settu, S.; Segaran, G.; Sundar, R.D.V.; Ravi, L. Phytochemical constituents of Dracaena mahatma leaves and their anti-bacterial, anti-oxidant and anti-inflammatory significance. Biotechnol. Res. Innov. 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Rastogi, L.; Arunachalam, J. Sunlight based irradiation strategy for rapid green synthesis of highly stable silver nanoparticles using aqueous garlic (Allium sativum) extract and their antibacterial potential. Mater. Chem. Phys. 2011, 129, 558–563. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Priya, K.; Nancy, F.T.; Noorlidah, A.; Ahmed, A.B.A. Biosynthesis, characterisation and anti-bacterial effect of plant-mediated silver nanoparticles using Artemisia nilagirica. Ind. Crop. Prod. 2013, 41, 235–240. [Google Scholar] [CrossRef]
- Ashokkumar, S.; Ravi, S.; Kathiravan, V.; Velmurugan, S. Synthesis, characterization and catalytic activity of silver nanopar-ticles using Tribulus terrestris leaf extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 121, 88–93. [Google Scholar] [CrossRef]
- Prakash, P.; Gnanaprakasama, P.; Emmanuel, R.; Arokiyaraj, S.; Saravanan, M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf. B Biointerfaces 2013, 108, 255–259. [Google Scholar] [CrossRef]
- Raja, S.; Ramesh, V.; Thivaharan, V. Green biosynthesis of silver nanoparticles using Calliandra haematocephala leaf extract, their antibacterial activity and hydrogen peroxide sensing capability. Arab. J. Chem. 2017, 10, 253–261. [Google Scholar] [CrossRef]
- Parthiban, E.; Nandhagopal, M.; Ramanibai, R.; Mathivanan, N. Green synthesis of silver-nanoparticles from Annona reticulata leaves aqueous extract and its mosquito larvicidal and anti-microbial activity on human pathogens. Biotechnol. Rep. 2018, 20, e00297. [Google Scholar] [CrossRef]
- Hembram, K.C.; Kumar, R.; Kandha, L.; Parhi, P.K.; Kundu, C.N.; Bindhani, B.K. Therapeutic prospective of plant-induced silver nanoparticles: Application as antimicrobial and anticancer agent. Artif. Cells Nanomed. Biotechnol. 2018, 46, S38–S51. [Google Scholar] [CrossRef] [PubMed]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; Pyne, S.; Ajay, M. Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 212–216. [Google Scholar] [CrossRef]
- Larayetan, R.; Ojemaye, M.O.; Okoh, O.O.; Okoh, A.I. Silver nanoparticles mediated by Callistemon citrinus extracts and their antimalaria, antitrypanosoma and antibacterial efficacy. J. Mol. Liq. 2019, 273, 615–625. [Google Scholar] [CrossRef]
- Tripathy, A.; Raichur, A.M.; Chandrasekaran, N.; Prathna, T.C.; Mukherjee, A. Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachta indica (Neem) leaves. J. Nanopart. Res. 2010, 12, 237–246. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Gold and silver nanoparticles: A class of chromophores with colors tunable in the range from 400 to 750 nm. Analyst 2003, 128, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Slocik, J.M.; Zabinski, J.S., Jr.; Phillips, D.M.; Naik, R.R. Colorimetric response of peptide-functionalized gold nanoparticles to metal ions. Small 2008, 4, 548–551. [Google Scholar] [CrossRef]
- Bagherzade, G.; Tavakoli, M.M.; Namaei, M.H. Green synthesis of silver nanoparticles using aqueous extract of saffron (Crocus sativus L.) wastages and its antibacterial activity against six bacteria. Asian Pac. J. Trop. Biomed. 2017, 7, 227–233. [Google Scholar] [CrossRef]
- Tavakoli, F.; Salavati-Niasari, M.; Mohandes, F. Green synthesis and characterization of graphene nanosheets. Mater. Res. Bull. 2015, 63, 51–57. [Google Scholar] [CrossRef]
- Kelkawi, A.H.; Kajani, A.A.; Bordbar, A.K. Green synthesis of silver nanoparticles using Mentha pulegium and investigation of their antibacterial, antifungal and anticancer activity. IET Nanobiotechnol. 2017, 11, 370–376. [Google Scholar] [CrossRef]
- Jinu, U.; Gomathi, M.; Saiqa, I.; Geetha, N.; Benelli, G.; Venkatachalam, P. Green engineered biomolecule-capped silver and copper nanohybrids using Prosopis cineraria leaf extract: Enhanced antibacterial activity against microbial pathogens of public health relevance and cytotoxicity on human breast cancer cells (MCF-7). Microb. Pathog. 2017, 105, 86–95. [Google Scholar] [CrossRef]
- Das, S.; Das, J.; Samadder, A.; Bhattacharyya, S.S.; Das, D.; Khuda-Bukhsh, A.R. Biosynthesized silver nanoparticles by ethanolic extracts of Phytolacca decandra, Gelsemium sempervirens, Hydrastis canadensis and Thuja occidentalis induce differential cytotoxicity through G2/M arrest in A375 cells. Colloids Surf. B Biointerfaces 2013, 101, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, B.; Subramanian, V.; Tumala, A.; Vellaichamy, E. Rapid synthesis of biocompatible silver nanoparticles using aqueous extract of Rosa damascena petals and evaluation of their anticancer activity. Asian Pac. J. Trop. Med. 2014, 7, S294–S300. [Google Scholar] [CrossRef] [Green Version]
- Edison, T.J.I.; Sethuraman, M.G. Instant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blue. Proc. Biochem. 2012, 47, 1351–1357. [Google Scholar] [CrossRef]
- Carmona, E.R.; Benito, N.; Plaza, T.; Recio-Sánchez, G. Green synthesis of silver nanoparticles by using leaf extracts from the endemic Buddleja globosa hope. Green Chem. Lett. Rev. 2017, 10, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Devi1, B.; Ahmaruzzaman, M. Bio-inspired sustainable and green synthesis of plasmonic Ag/AgCl nanoparticles for enhanced degradation of organic compound from aqueous phase. Environ. Sci. Pollut. Res. 2016, 23, 17702–17714. [Google Scholar] [CrossRef]
- Devi, T.B.; Ahmaruzzaman, M.; Begum, S. A rapid, facile and green synthesis of Ag@AgCl nanoparticles for the effective reduction of 2,4-dinitrophenyl hydrazine. New J. Chem. 2016, 40, 1497. [Google Scholar] [CrossRef]
- Baghkheiratia, E.K.; Bagherieh-Najjar, M.B.; Fadafanb, H.K.; Abdolzadeha, A. Synthesis and antibacterial activity of stable bio-conjugated nanoparticles mediated by walnut (Juglans regia) green husk extract. J. Exp. Nanosci. 2015, 11, 512–517. [Google Scholar] [CrossRef] [Green Version]
- Al Aboody, M.S. Silver/silver chloride (Ag/AgCl) nanoparticles synthesized from Azadirachta indica lalex and its antibiofilm activity against fluconazole resistant Candida tropicalis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2107–2113. [Google Scholar] [CrossRef] [Green Version]
- Feizia, S.; Taghipour, E.; Ghadam, P.; Mohammadi, P. Antifungal, antibacterial, antibiofilm and colorimetric sensing of toxic metals activities of eco friendly, economical synthesized Ag/AgCl nanoparticles using Malva sylvestris leaf extracts. Microb. Pathog. 2018, 125, 33–42. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Bhanage, B.M. Ag@AgCl nanomaterial synthesis using sugar cane juice and its application in degradation of azo dyes. ACS Sustain. Chem. Eng. 2014, 2, 1007–1013. [Google Scholar] [CrossRef]
- Sarkar, S.; Kotteeswaran, V. Green synthesis of silver nanoparticles from aqueous leaf extract of Pomegranate (Punica granatum) and their anticancer activity on human cervical cancer cells. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025014. [Google Scholar] [CrossRef]
- Vasanth, K.; Ilango, K.; MohanKumara, R.; Agrawal, A.; Dubey, G.P. Anticancer activity of Moringa oleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Colloid Surf. B. 2014, 117, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Moodley, J.S.; Krishna, S.B.N.; Pillay, K.; Sershen; Govender, P. Green synthesis of silver nanoparticles from Moringa oleifera leaf extracts and its antimicrobial potential. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 015011. [Google Scholar] [CrossRef] [Green Version]
- Krishnaraj, C.; Jagan, E.G.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.T.; Mohan, N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B. Biointerf. 2010, 76, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.V.; Borase, H.P.; Patil, C.D.; Salunke, B.K. Biosynthesis of silver nanoparticles using latex from few euphorbian plants and their antimicrobial potential. Appl. Biochem. Biotechnol. 2012, 167, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.N.S.; Agarwala, M. Phytochemical analysis of some medicinal plants. J. Phytol. 2011, 3, 10–14. [Google Scholar]
- Karthigaiselvi, K.; Rameshwari, K.S. Green synthesis of silver nanoparticles from aqueous extract of Stemodia viscosa and its evaluation of antimicrobial activity. Eur. J. Pharm. Med. Res. 2016, 3, 417–421. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |






| Phytochemicals | Degree |
|---|---|
| Phenolics | +++ |
| Alkaloids | + |
| Tannins | +++ |
| Flavonoids | +++ |
| Saponin | +++ |
| Glycosides | − |
| Terpenoids | + |
| Steroids | + |
| Proteins | + |
| Carbohydrates | + |
| Anthraquinone | − |
| Minimum Inhibitory Concentration (MIC) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacterial Strains | 32 mg/mL | 16 mg/mL | 8 mg/mL | 4 mg/mL | 2 mg/mL | 1 mg/mL | 0.5 mg/mL | 0.25 mg/mL | 0.125 mg/mL | |||||||||
| a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | |
| Enterobacter cloacae (ATCC 13047) | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | + |
| Listeria ivanovic (ATCC 19119) | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | − | + | + |
| Streptococcus uberis (ATCC 700407) | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | + |
| Staphylococcus aureus (ATCC 29213) | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | + |
| Mycobacterium smergatis (ATCC 19420) | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + |
| Vibrio spp. (PCR confirmed isolate) | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + |
| Minimum Bactericidal Concentration (MBC) | ||||||||||||||||||
| Enterobacter cloacae (ATCC 13047) | − | − | − | − | + | − | − | − | + | − | + | − | + | − | + | − | + | + |
| Listeria ivanovic (ATCC 19119) | − | − | − | − | − | − | + | − | + | − | + | − | + | + | + | + | + | + |
| Streptococcus uberis (ATCC 700407) | − | − | − | − | + | − | + | − | + | − | + | − | + | + | + | + | + | + |
| Staphylococcus aureus (ATCC 29213) | − | − | − | − | + | − | + | − | + | − | + | − | + | + | + | + | + | + |
| Mycobacterium smergatis (ATCC 19420) | − | − | − | − | − | − | − | − | − | − | + | − | + | + | + | + | + | + |
| Vibrio spp. (PCR confirmed isolate) | − | − | − | − | + | − | + | − | + | − | + | − | + | + | + | + | + | + |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okaiyeto, K.; Ojemaye, M.O.; Hoppe, H.; Mabinya, L.V.; Okoh, A.I. Phytofabrication of Silver/Silver Chloride Nanoparticles Using Aqueous Leaf Extract of Oedera genistifolia: Characterization and Antibacterial Potential. Molecules 2019, 24, 4382. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24234382
Okaiyeto K, Ojemaye MO, Hoppe H, Mabinya LV, Okoh AI. Phytofabrication of Silver/Silver Chloride Nanoparticles Using Aqueous Leaf Extract of Oedera genistifolia: Characterization and Antibacterial Potential. Molecules. 2019; 24(23):4382. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24234382
Chicago/Turabian StyleOkaiyeto, Kunle, Mike O. Ojemaye, Heinrich Hoppe, Leonard V. Mabinya, and Anthony I. Okoh. 2019. "Phytofabrication of Silver/Silver Chloride Nanoparticles Using Aqueous Leaf Extract of Oedera genistifolia: Characterization and Antibacterial Potential" Molecules 24, no. 23: 4382. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24234382