Low-Molecular-Weight Fe(III) Complexes for MRI Contrast Agents
Abstract
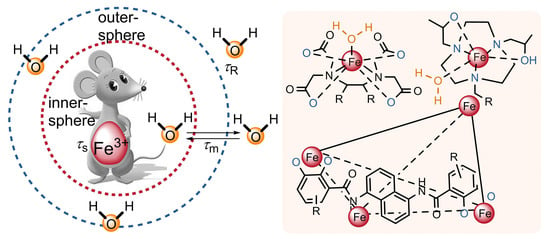
:1. Introduction
2. Mononuclear Fe(III) Complexes
2.1. Mononuclear Fe(III) Complexes with Open Ligands
| Complex | Log KML | r1(mM−1s−1) in Buffer | r2(mM−1s−1) in Buffer | q |
|---|---|---|---|---|
| Fe(III)–HBED [31] | 39.7 | 0.49 a | 0.52 a | 0 |
| Fe(III)–HBEDP [33] | - | 0.9 a | 1.0 a | 0 |
| Fe(III)–SHBED [33] | - | 1.3 a | 1.6 a | |
| Fe(III)–HBEDP- (CH2OH)2 [33] | - | 0.9 a | 1.2 a | 0 |
| Fe(III)–HBEDP- (CH2OH)3 [33] | - | 1.5 a | 1.7 a | 0 |
| Gd(III)–DTPA [25] | 22.5 | 3.4 b | 3.9 b | 1 |
| Fe(III)–DTPA [25] | 27.3 | 0.6 b | 0.6 b | 0 |
| Fe(III)–tCDTA [25] | 27.5 | 2.0 b | 2.2 b | 1 |
| Fe(III)–tCDTA [35] | 27.5 | 1.56 c | 1.72 c | 1 |
| Fe(III)–(en-tCDTA) [35] | - | 0.78 c | 0.81 c | 0 |
| Fe(III)–(trans-tCDTA) [35] | - | 1.92 c | 2.16 c | 1 |
| Fe(III)–(DFX)2 [39] | 38.6 | 2.3 d | 3.1 d | 0 |
| Fe(III)–PyC3A [48] | 23.2 e | 1.8 f | - | 1 |
2.2. Mononuclear Fe(III) Complexes with Macrocyclic Ligands
3. Multinuclear Fe(III) Complexes
| Complex | r1 (mM−1s−1)a in Buffer | r2 (mM−1s−1)a in Buffer | r1 (mM−1s−1) in HSA | r2 (mM−1s−1) in HSA |
|---|---|---|---|---|
| Fe(III)–TOLOP [66] | 1.83 | 5.81 | 2.71 | 4.32 |
| Fe(III)2-META [66] | 4.06 | 13.70 | 6.56 | 8.85 |
| Fe(III)2-PARA [66] | 5.26 | 13.44 | 6.71 | 11.90 |
| Fe(III)2-DIP [66] | 4.36 | 10.93 | 5.82 | 7.56 |
| MOC-Fe(III)2 [68] | 1.07 b | 1.28 b | - | - |
| MOC-Fe(III)4 [68] | 1.43 b | 1.63 b | - | - |
| MOC-Fe(III)4 [70] | 8.7 c | - | 21 c | - |
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lauffer, R.B. Paramagnetic metal complexes as water proton relaxation agents for NMR imaging: Theory and design. Chem. Rev. 1987, 87, 901–927. [Google Scholar] [CrossRef]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI contrast agents: Current challenges and new frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Lohrke, J.; Frenzel, T.; Endrikat, J.; Alves, F.C.; Grist, T.M.; Law, M.; Lee, J.M.; Leiner, T.; Li, K.-C.; Nikolaou, K.; et al. 25 years of contrast-enhanced MRI: Developments, current challenges and future perspectives. Adv. Ther. 2016, 33, 1–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Runge, V.M. Critical questions regarding gadolinium deposition in the brain and body after injections of the gadolinium-based contrast agents, safety, and clinical recommendations in consideration of the ema’s pharmacovigilance and risk assessment committee recommendation for suspension of the marketing authorizations for 4 linear agents. Invest. Radiol. 2017, 52, 317–323. [Google Scholar]
- Li, H.; Meade, T.J. Molecular magnetic resonance imaging with gd(III)-based contrast agents: Challenges and key advances. J. Am. Chem. Soc. 2019, 141, 17025–17041. [Google Scholar] [CrossRef]
- Boros, E.; Gale, E.M.; Caravan, P. Mr imaging probes: Design and applications. Dalton Trans. 2015, 44, 4804–4818. [Google Scholar] [CrossRef]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef]
- Liu, J.; Xiong, Z.; Zhang, J.; Peng, C.; Klajnert-Maculewicz, B.; Shen, M.; Shi, X. Zwitterionic gadolinium(III)-complexed dendrimer-entrapped gold nanoparticles for enhanced computed tomography/magnetic resonance imaging of lung cancer metastasis. ACS Appl. Mater. Interfaces 2019, 11, 15212–15221. [Google Scholar] [CrossRef]
- Yang, Z.; Lin, H.; Huang, J.; Li, A.; Sun, C.; Richmond, J.; Gao, J. A gadolinium-complex-based theranostic prodrug for in vivo tumour-targeted magnetic resonance imaging and therapy. Chem. Commun. 2019, 55, 4546–4549. [Google Scholar] [CrossRef]
- Marckmann, P.; Skov, L.; Rossen, K.; Dupont, A.; Damholt, M.B.; Heaf, J.G.; Thomsen, H.S. Nephrogenic systemic fibrosis: Suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J. Am. Soc. Nephrol. 2006, 17, 2359–2362. [Google Scholar] [CrossRef] [Green Version]
- Grobner, T. Gadolinium—A specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol. Dial. Transpl. 2006, 21, 1104–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanal, E.; Tweedle, M.F. Residual or retained gadolinium: Practical implications for radiologists and our patients. Radiology 2015, 275, 630–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, M.L.; Chettle, D.R.; Gräfe, J.L.; Noseworthy, M.D.; McNeill, F.E. Observed deposition of gadolinium in bone using a new noninvasive in vivo biomedical device: Results of a small pilot feasibility study. Radiology 2018, 287, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Fukusato, T.; Matsuda, M.; Toyoda, K.; Oba, H.; Kotoku, J.I.; Haruyama, T.; Kitajima, K.; Furui, S. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: Evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015, 276, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, J.; Semelka, R.C.; Ramalho, M.; Nunes, R.H.; AlObaidy, M.; Castillo, M. Gadolinium-based contrast agent accumulation and toxicity: An update. Am. J. Neuroradiol. 2016, 37, 1192–1198. [Google Scholar] [CrossRef] [Green Version]
- Kras, E.A.; Snyder, E.M.; Sokolow, G.E.; Morrow, J.R. Distinct coordination chemistry of Fe(III)-based MRI probes. Acc. Chem. Res. 2022, 55, 1435–1444. [Google Scholar] [CrossRef]
- Kuźnik, N.; Wyskocka, M. Iron (III) contrast agent candidates for MRI: A survey of the structure–effect relationship in the last 15 years of studies. Eur. J. Inorg. Chem. 2016, 2016, 445–458. [Google Scholar] [CrossRef]
- Wesolowski, J.R.; Kaiser, A. Alternatives to GBCA: Are we there yet? Top. Magn. Reson. Imaging 2016, 25, 171–175. [Google Scholar] [CrossRef]
- Gutowsky, H.S.; Woessner, D.E. Nuclear magnetic spin-lattice relaxation in liquids. Phys. Rev. 1956, 104, 843–844. [Google Scholar] [CrossRef]
- Strandberg, E.; Westlund, P.-O. 1H NMRD profile and ESR lineshape calculation for an isotropic electron spin system with S = 7/2. A generalized modified solomon–bloembergen–morgan theory for nonextreme-narrowing conditions. J. Magn. Reson. A 1996, 122, 179–191. [Google Scholar] [CrossRef]
- Bloembergen, N. Proton relaxation times in paramagnetic solutions. J. Chem. Phys. 1957, 27, 572–573. [Google Scholar] [CrossRef]
- Baranyai, Z.; Carniato, F.; Nucera, A.; Horváth, D.; Tei, L.; Platas-Iglesias, C.; Botta, M. Defining the conditions for the development of the emerging class of FeIII-based MRI contrast agents. Chem. Sci. 2021, 12, 11138–11145. [Google Scholar] [CrossRef] [PubMed]
- Pujales-Paradela, R.; Regueiro-Figueroa, M.; Esteban-Gómez, D.; Platas-Iglesias, C. Chapter 5 Transition Metal-Based T1 Contrast Agents. In Contrast Agents for MRI: Experimental Methods; The Royal Society of Chemistry: London, UK, 2018; pp. 448–478. [Google Scholar]
- An, L.; Cai, Y.; Tian, Q.; Lin, J.; Yang, S. Ultrasensitive iron-based magnetic resonance contrast agent constructed with natural polyphenol tannic acid for tumor theranostics. Sci. China Mater. 2020, 64, 498–509. [Google Scholar] [CrossRef]
- Boehm-Sturm, P.; Haeckel, A.; Hauptmann, R.; Mueller, S.; Kuhl, C.K.; Schellenberger, E.A. Low-molecular-weight iron chelates may be an alternative to gadolinium-based contrast agents for T1-weighted contrast-enhanced MR imaging. Radiology 2018, 286, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Anderson, G.J.; Frazer, D.M. Current understanding of iron homeostasis. Am. J. Clin. Nutr. 2017, 106, 1559S–1566S. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Asik, D.; Snyder, E.M.; Dilillo, A.E.; Cullen, P.J.; Morrow, J.R. Binding and release of FeIII complexes from glucan particles for the delivery of T1 MRI contrast agents. ChemMedChem 2020, 15, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, M.; Bauer, H.; Mintorovitch, J.; Requardt, M.; Weinmann, H.-J. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest. Radiol. 2005, 40, 715–724. [Google Scholar] [CrossRef] [Green Version]
- Hoener, B.-A.; Engelstad, B.L.; Ramos, E.C.; Macapinlac, H.A.; Price, D.C.; Johnson, T.R.; White, D.L. Comparison of Fe-HBED and Fe-EHPG as hepatobiliary MR contrast agents. J. Magn. Reson. Imaging 1991, 1, 357–362. [Google Scholar] [CrossRef]
- Lauffer, R.B.; Vincent, A.C.; Padmanabhan, S.; Villringer, A.; Saini, S.; Elmaleh, D.R.; Brady, T.J. Hepatobiliary MR contrast agents: 5-substituted iron-ehpg derivatives. Magn. Reson. Med. 1987, 4, 582–590. [Google Scholar] [CrossRef]
- Eplattenier, F.L.; Murase, I.; Martell, A.E. New multidentate ligands. Vi. Chelating tendencies of n,n′-di(2-hydroxybenzyl)ethylenediamine-n,n′-diacetic acid. J. Am. Chem. Soc. 1967, 89, 837–843. [Google Scholar] [CrossRef]
- Larsen, S.K.; Jenkins, B.G.; Memon, N.G.; Lauffer, R.B. Structure-affinity relationships in the binding of unsubstituted iron phenolate complexes to human serum albumin. Molecular structure of iron(III) n, n’-bis(2-hydroxybenzyl)ethylenediamine-n,n’-diacetate. Inorg. Chem. 1990, 29, 1147–1152. [Google Scholar] [CrossRef]
- Bales, B.C.; Grimmond, B.; Johnson, B.F.; Luttrell, M.T.; Meyer, D.E.; Polyanskaya, T.; Rishel, M.J.; Roberts, J. Fe-HBED analogs: A promising class of iron-chelate contrast agents for magnetic resonance imaging. Contrast Media Mol. Imaging 2019, 2019, 8356931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fornasiero, D.; Bellen, J.C.; Baker, R.J.; Chatterton, B.E. Paramagnetic complexes of manganese(II), iron(III), and gadolinium(III) as contrast agents for magnetic resonance imaging: The influence of stability constants on the biodistribution of radioactive aminopolycarboxylate complexes. Invest. Radiol. 1987, 22, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Haeckel, A.; Hauptmann, R.; Ray, I.P.; Limberg, C.; Kulak, N.; Hamm, B.; Schellenberger, E. Iron(III)-TCDTA derivatives as MRI contrast agents: Increased T1 relaxivities at higher magnetic field strength and ph sensing. Magn. Reson. Med. 2021, 85, 3370–3382. [Google Scholar] [CrossRef] [PubMed]
- Thews, O.; Riemann, A. Tumor pH and metastasis: A malignant process beyond hypoxia. Cancer Metast. Rev. 2019, 38, 113–129. [Google Scholar] [CrossRef]
- Szomolanyi, P.; Rohrer, M.; Frenzel, T.; Noebauer-Huhmann, I.M.; Jost, G.; Endrikat, J.; Trattnig, S.; Pietsch, H. Comparison of the relaxivities of macrocyclic gadolinium-based contrast agents in human plasma at 1.5, 3, and 7 T, and blood at 3 T. Invest. Radiol. 2019, 54, 559–564. [Google Scholar] [CrossRef]
- Shirley, M.; Plosker, G.L. Deferasirox: A review of its use for chronic iron overload in patients with non-transfusion-dependent thalassaemia. Drugs 2014, 74, 1017–1027. [Google Scholar] [CrossRef]
- Palagi, L.; Di Gregorio, E.; Costanzo, D.; Stefania, R.; Cavallotti, C.; Capozza, M.; Aime, S.; Gianolio, E. Fe(deferasirox)(2): An iron(III)-based magnetic resonance imaging T1 contrast agent endowed with remarkable molecular and functional characteristics. J. Am. Chem. Soc. 2021, 143, 14178–14188. [Google Scholar] [CrossRef]
- Heinz, U.; Hegetschweiler, K.; Acklin, P.; Faller, B.; Lattmann, R.; Schnebli, H.P. 4-[3,5-bis(2-hydroxyphenyl)-1,2,4-triazol-1-yl]- benzoic acid: A novel efficient and selective iron(III) complexing agent. Angew. Chem. Int. Ed. 1999, 38, 2568–2570. [Google Scholar] [CrossRef]
- Bruin, G.J.M.; Faller, T.; Wiegand, H.; Schweitzer, A.; Nick, H.; Schneider, J.; Boernsen, K.-O.; Waldmeier, F. Pharmacokinetics, distribution, metabolism, and excretion of deferasirox and its iron complex in rats. Drug Metab. Dispos. 2008, 36, 2523–2538. [Google Scholar] [CrossRef]
- Gale, E.M.; Zhu, J.; Caravan, P. Direct measurement of the Mn(II) hydration state in metal complexes and metalloproteins through 17O NMR line widths. J. Am. Chem. Soc. 2013, 135, 18600–18608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maheshwaran, D.; Nagendraraj, T.; Sekar Balaji, T.; Kumaresan, G.; Senthil Kumaran, S.; Mayilmurugan, R. Smart dual T1 MRI-optical imaging agent based on a rhodamine appended Fe(III)-catecholate complex. Dalton Trans. 2020, 49, 14680–14689. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.-L.; Kramberger, I.; Davis, J.J. Environmentally responsive MRI contrast agents. Chem. Commun. 2013, 49, 9704–9721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelovski, G. What we can really do with bioresponsive MRI contrast agents. Angew. Chem. Int. Ed. 2016, 55, 7038–7046. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, D.V.; Bernstein, A.S.; Pagel, M.D. A review of responsive MRI contrast agents: 2005–2014. Contrast Media Mol. Imaging 2015, 10, 245–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, S.M.; Tomé, V.; Calvete, M.J.F.; Castro, M.M.C.A.; Tóth, É.; Geraldes, C.F.G.C. Metal-based redox-responsive MRI contrast agents. Coord. Chem. Rev. 2019, 390, 1–31. [Google Scholar] [CrossRef]
- Wang, H.; Jordan, V.C.; Ramsay, I.A.; Sojoodi, M.; Fuchs, B.C.; Tanabe, K.K.; Caravan, P.; Gale, E.M. Molecular magnetic resonance imaging using a redox-active iron complex. J. Am. Chem. Soc. 2019, 141, 5916–5925. [Google Scholar] [CrossRef]
- Gale, E.M.; Jones, C.M.; Ramsay, I.; Farrar, C.T.; Caravan, P. A janus chelator enables biochemically responsive MRI contrast with exceptional dynamic range. J. Am. Chem. Soc. 2016, 138, 15861–15864. [Google Scholar] [CrossRef] [Green Version]
- Poole, L.B. The basics of thiols and cysteines in redox biology and chemistry. Free Radi. Biol. Med. 2015, 80, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Wood, P.M. The potential diagram for oxygen at pH 7. Biochem. J. 1988, 253, 287–289. [Google Scholar] [CrossRef]
- Delgado, R.; Sun, Y.; Motekaitis, R.J.; Martell, A.E. Stabilities of divalent and trivalent metal ion complexes of macrocyclic triazatriacetic acids. Inorg. Chem. 1993, 32, 3320–3326. [Google Scholar] [CrossRef]
- Martell, A.E.; Motekaitis, R.J.; Welch, M.J. N,n′,n″-tris(3-hydroxy-6-methyl-2-pyridylmethyl)-1,4,7-triazacyclonana, a new effective ligand for iron (III). J. Chem. Soc., Chem. Commun. 1990, 24, 1748–1749. [Google Scholar] [CrossRef]
- Snyder, E.M.; Asik, D.; Abozeid, S.M.; Burgio, A.; Bateman, G.; Turowski, S.G.; Spernyak, J.A.; Morrow, J.R. A class of FeIII macrocyclic complexes with alcohol donor groups as effective T1 MRI contrast agents. Angew. Chem. Int. Ed. 2020, 59, 2414–2419. [Google Scholar] [CrossRef]
- Asik, D.; Smolinski, R.; Abozeid, S.M.; Mitchell, T.B.; Turowski, S.G.; Spernyak, J.A.; Morrow, J.R. Modulating the properties of Fe(III) macrocyclic MRI contrast agents by appending sulfonate or hydroxyl groups. Molecules 2020, 25, 2291. [Google Scholar] [CrossRef] [PubMed]
- Kras, E.A.; Abozeid, S.M.; Eduardo, W.; Spernyak, J.A.; Morrow, J.R. Comparison of phosphonate, hydroxypropyl and carboxylate pendants in Fe(III) macrocyclic complexes as MRI contrast agents. J. Inorg. Biochem. 2021, 225, 111594. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Asik, D.; Spernyak, J.A.; Cullen, P.J.; Morrow, J.R. MRI and fluorescence studies of saccharomyces cerevisiae loaded with a bimodal Fe(III) T1contrast agent. J. Inorg. Biochem. 2019, 201, 110832. [Google Scholar] [CrossRef]
- Salaam, J.; Rivat, M.; Fogeron, T.; Hasserodt, J. Molecular sensors operating by a spin-state change in solution: Application to magnetic resonance imaging. Anal. Sens. 2020, 1, 11–29. [Google Scholar] [CrossRef]
- Sahoo, D.; Quesne, M.G.; de Visser, S.P.; Rath, S.P. Hydrogen-bonding interactions trigger a spin-flip in iron(III) porphyrin complexes. Angew. Chem. Int. Ed. 2015, 54, 4796–4800. [Google Scholar] [CrossRef] [Green Version]
- Venkataramani, S.; Jana, U.; Dommaschk, M.; Sönnichsen, F.D.; Tuczek, F.; Herges, R. Magnetic bistability of molecules in homogeneous solution at room temperature. Science 2011, 331, 445–448. [Google Scholar] [CrossRef]
- Dommaschk, M.; Peters, M.; Gutzeit, F.; Schütt, C.; Näther, C.; Sönnichsen, F.D.; Tiwari, S.; Riedel, C.; Boretius, S.; Herges, R. Photoswitchable magnetic resonance imaging contrast by improved light-driven coordination-induced spin state switch. J. Am. Chem. Soc. 2015, 137, 7552–7555. [Google Scholar] [CrossRef]
- Shankar, S.; Peters, M.; Steinborn, K.; Krahwinkel, B.; Sönnichsen, F.D.; Grote, D.; Sander, W.; Lohmiller, T.; Rüdiger, O.; Herges, R. Light-controlled switching of the spin state of iron(III). Nat. Commun. 2018, 9, 4750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasserodt, J.; Kolanowski, J.L.; Touti, F. Magnetogenesis in water induced by a chemical analyte. Angew. Chem. Int. Ed. 2014, 53, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Touti, F.; Maurin, P.; Hasserodt, J. Magnetogenesis under physiological conditions with probes that report on (bio-)chemical stimuli. Angew. Chem. Int. Ed. 2013, 52, 4654–4658. [Google Scholar] [CrossRef] [PubMed]
- Gondrand, C.; Touti, F.; Godart, E.; Berezhanskyy, Y.; Jeanneau, E.; Maurin, P.; Hasserodt, J. Spring-loaded iron(II) complexes as magnetogenic probes reporting on a chemical analyte in water. Eur. J. Inorg. Chem. 2015, 2015, 1376–1382. [Google Scholar] [CrossRef]
- Asik, D.; Abozeid, S.M.; Turowski, S.G.; Spernyak, J.A.; Morrow, J.R. Dinuclear Fe(III) hydroxypropyl-appended macrocyclic complexes as MRI probes. Inorg. Chem. 2021, 60, 8651–8664. [Google Scholar] [CrossRef]
- Aime, S.; Botta, M.; Fasano, M.; Terreno, E. Prototropic and water-exchange processes in aqueous solutions of Gd(III) chelates. Acc. Chem. Res. 1999, 32, 941–949. [Google Scholar] [CrossRef]
- Wang, R.; An, L.; He, J.; Li, M.; Jiao, J.; Yang, S. A class of water-soluble Fe(III) coordination complexes as T1-weighted MRI contrast agents. J. Mater. Chem. B 2021, 9, 1787–1791. [Google Scholar] [CrossRef]
- Jiao, J.; He, J.; Li, M.; Yang, J.; Yang, H.; Wang, X.; Yang, S. A porphyrin-based metallacage for enhanced photodynamic therapy. Nanoscale 2022, 14, 6373–6383. [Google Scholar] [CrossRef]
- Sokolow, G.E.; Crawley, M.R.; Morphet, D.R.; Asik, D.; Spernyak, J.A.; McGray, A.J.R.; Cook, T.R.; Morrow, J.R. Metal-organic polyhedron with four Fe(III) centers producing enhanced T1 magnetic resonance imaging contrast in tumors. Inorg. Chem. 2022, 61, 2603–2611. [Google Scholar] [CrossRef]
- Percastegui, E.G.; Ronson, T.K.; Nitschke, J.R. Design and applications of water-soluble coordination cages. Chem. Rev. 2020, 120, 13480–13544. [Google Scholar] [CrossRef]
- Kersting, B.; Meyer, M.; Powers, R.E.; Raymond, K.N. Dinuclear catecholate helicates: Their inversion mechanism. J. Am. Chem. Soc. 1996, 118, 7221–7222. [Google Scholar] [CrossRef]
- Weisser, J.T.; Nilges, M.J.; Sever, M.J.; Wilker, J.J. Epr investigation and spectral simulations of iron−catecholate complexes and iron−peptide models of marine adhesive cross-links. Inorg. Chem. 2006, 45, 7736–7747. [Google Scholar] [CrossRef] [PubMed]
- Kosman, D.J. The teleos of metallo-reduction and metallo-oxidation in eukaryotic iron and copper trafficking. Metallomics 2018, 10, 370–377. [Google Scholar] [CrossRef]
- Khan, R.K.; Gadiraju, S.P.; Kumar, M.; Hatmaker, G.A.; Fisher, B.J.; Natarajan, R.; Reiner, J.E.; Collinson, M.M. Redox potential measurements in red blood cell packets using nanoporous gold electrodes. ACS Sens. 2018, 3, 1601–1608. [Google Scholar] [CrossRef]
- Koppenol, W.H. The haber-weiss cycle–70 years later. Redox Rep. 2001, 6, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H.; Hider, R.H. Iron and redox cycling. Do’s and don’ts. Free Radic. Biol. Med. 2019, 133, 3–10. [Google Scholar] [CrossRef]
- Dorazio, S.J.; Morrow, J.R. The development of iron(II) complexes as paracest MRI contrast agents. Eur. J. Inorg. Chem. 2012, 2012, 2006–2014. [Google Scholar] [CrossRef]
- Horrocks, W.D.; Sudnick, D.R. Lanthanide ion luminescence probes of the structure of biological macromolecules. Acc. Chem. Res. 1981, 14, 384–392. [Google Scholar] [CrossRef]
- Theil, E.C.; Goss, D.J. Living with iron (and oxygen): Questions and answers about iron homeostasis. Chem. Rev. 2009, 109, 4568–4579. [Google Scholar] [CrossRef] [Green Version]











| Complex | Log K1 a | r1 (mM−1s−1)b in Buffer | r2 (mM−1s−1)b in Buffer | r1 (mM−1s−1)b in HSA | r2 (mM−1s−1)b in HSA |
|---|---|---|---|---|---|
| Fe(III)–TOB [54] | 7.05 | 2.2 | 4.5 | 2.5 | 4.2 |
| Fe(III)–TOBA [54] | 6.75 | 0.81 | 1.5 | 1.1 | 1.6 |
| Fe(III)–TzB [54] | 6.91 | 1.7 | 5.0 | 2.2 | 3.6 |
| Fe(III)–TzBC [54] | 7.44 | 0.42 | 1.5 | 0.96 | 2.0 |
| Gd(III)–DTPA [54] | 22.5 c | 3.1 | 3.9 | 3.2 | 4.0 |
| Fe(III)–TOSO [55] | 7.55 d | 2.0 e | 6.1 e | 2.5 e | 5.5 e |
| Fe(III)–NOHP [55] | 11.78 d | 10.97 e | 1.8 e | 1.2 e | 2.3 e |
| Fe(III)–NOTP [56] | 29.6 c | 0.72 e | 1.26 e | 1.05 e | 1.55 e |
| Fe(III)–TOCs [57] | - | 1.10 | 1.6 | - | - |
| Fe(III)–TOCO151 [57] | - | 1.06 | 2.04 | - | - |
| Fe(III)–DTPA [57] | - | 0.51 | 1.20 | - | - |
| Fe(III)–EDTA [57] | - | 1.37 | 2.28 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; An, L.; Yang, S. Low-Molecular-Weight Fe(III) Complexes for MRI Contrast Agents. Molecules 2022, 27, 4573. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27144573
Chen S, An L, Yang S. Low-Molecular-Weight Fe(III) Complexes for MRI Contrast Agents. Molecules. 2022; 27(14):4573. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27144573
Chicago/Turabian StyleChen, Shangjun, Lu An, and Shiping Yang. 2022. "Low-Molecular-Weight Fe(III) Complexes for MRI Contrast Agents" Molecules 27, no. 14: 4573. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27144573