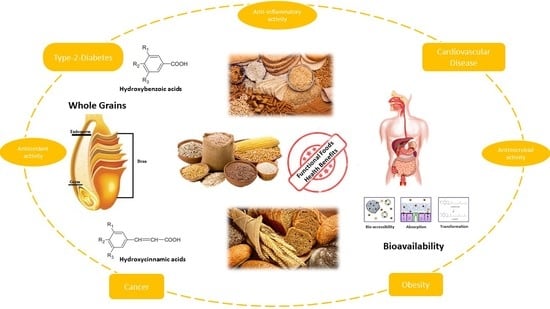
Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability
Abstract
:1. Introduction
2. Whole Grains and Their Main Fractions
Bioactive Compounds
3. Phenolic Acids
3.1. Biological Activities of Phenolic Acids in the Human Body
3.1.1. Antioxidant Effect
3.1.2. Anti-Inflammatory Effect
3.1.3. Antimicrobial Effect
4. Health Outcomes Associated with Consumption of Whole Grains and Bran Fractions
4.1. Type-2 Diabetes (T2D)
4.2. Obesity
4.3. Cardiovascular Disease (CVD)
4.4. Cancer
5. Whole Grains and Bran Health Claims
6. Whole Grains and Bran as Functional Foods
7. Bioavailability
7.1. Bioaccessibility and Intestinal Absorption
7.2. Food Processing Influences the Bioaccessibility and Bioavailability
7.3. Strategies to Increase Phenolic Content Bioavailability and Bioaccessibility in Whole Grains and Whole Grain Based-Products
7.3.1. Bioprocessing Treatments
7.3.2. Mechanical Processing
7.3.3. Encapsulation
8. Future Perspectives and Outlooks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agriculture, Forestry and Fishery Statistics—2016 Edition. Available online: https://ec.europa.eu/eurostat/web/products-statistical-books/-/KS-FK-16-001 (accessed on 10 September 2018).
- Watson, R.R.; Preedy, V.R.; Zibadi, S. Wheat and Rice in Disease Prevention and Health: Benefits, Risks and Mechanisms of Whole Grains in health Promotion; Academic Press: Cambridge, MA, USA, 2014; ISBN 978-0-12-404604-7. [Google Scholar]
- Björck, I.; Östman, E.; Kristensen, M.; Mateo Anson, N.; Price, R.K.; Haenen, G.R.M.M.; Havenaar, R.; Bach Knudsen, K.E.; Frid, A.; Mykkänen, H.; et al. Cereal grains for nutrition and health benefits: Overview of results from in vitro, animal and human studies in the HEALTHGRAIN project. Trends Food Sci. Technol. 2012, 25, 87–100. [Google Scholar] [CrossRef]
- He, M.; van Dam, R.M.; Rimm, E.; Hu, F.B.; Qi, L. Whole-grain, cereal fiber, bran, and germ intake and the risks of all-cause and cardiovascular disease-specific mortality among women with type 2 diabetes mellitus. Circulation 2010, 121, 2162–2168. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A.; Rock, E.; Rémésy, C. Is the in vitro antioxidant potential of whole-grain cereals and cereal products well reflected in vivo? J. Cereal Sci. 2008, 48, 258–276. [Google Scholar] [CrossRef]
- Gong, L.; Cao, W.; Chi, H.; Wang, J.; Zhang, H.; Liu, J.; Sun, B. Whole cereal grains and potential health effects: Involvement of the gut microbiota. Food Res. Int. 2018, 103, 84–102. [Google Scholar] [CrossRef] [PubMed]
- Prevention and Control of Noncommunicable Diseases in the European Region: A Progress Report. Available online: http://www.euro.who.int/en/health-topics/noncommunicable-diseases/ncd-background-information/prevention-and-control-of-noncommunicable-diseases-in-the-european-region-a-progress-report (accessed on 10 September 2018).
- Cho, S.S.; Qi, L.; Fahey, G.C.; Klurfeld, D.M. Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. Am. J. Clin. Nutr. 2013, 98, 594–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Lillioja, S.; Neal, A.L.; Tapsell, L.; Jacobs, D.R. Whole Grains, Type 2 Diabetes, Coronary Heart Disease, and Hypertension: Links to the Aleurone preferred over Indigestible Fiber. BioFactors Oxf. Engl. 2013, 39, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Murtaugh, M.A.; Jacobs, D.R.; Jacob, B.; Steffen, L.M.; Marquart, L. Epidemiological support for the protection of whole grains against diabetes. Proc. Nutr. Soc. 2003, 62, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Munter, J.S.L.; Hu, F.B.; Spiegelman, D.; Franz, M.; van Dam, R.M. Whole Grain, Bran, and Germ Intake and Risk of Type 2 Diabetes: A Prospective Cohort Study and Systematic Review. PLoS Med. 2007, 4, e261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montonen, J.; Knekt, P.; Järvinen, R.; Aromaa, A.; Reunanen, A. Whole-grain and fiber intake and the incidence of type 2 diabetes. Am. J. Clin. Nutr. 2003, 77, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Giacco, R.; Costabile, G.; Della Pepa, G.; Anniballi, G.; Griffo, E.; Mangione, A.; Cipriano, P.; Viscovo, D.; Clemente, G.; Landberg, R.; et al. A whole-grain cereal-based diet lowers postprandial plasma insulin and triglyceride levels in individuals with metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. NMCD 2014, 24, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Flint, A.J.; Qi, Q.; van Dam, R.M.; Sampson, L.A.; Rimm, E.B.; Holmes, M.D.; Willett, W.C.; Hu, F.B.; Sun, Q. Association between dietary whole grain intake and risk of mortality: Two large prospective studies in US men and women. JAMA Intern. Med. 2015, 175, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.T.; Kuznesof, S.; Richardson, D.P.; Seal, C.J. Behavioural, attitudinal and dietary responses to the consumption of wholegrain foods. Proc. Nutr. Soc. 2003, 62, 455–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frølich, W.; Åman, P.; Tetens, I. Whole grain foods and health—A Scandinavian perspective. Food Nutr. Res. 2013, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laddomada, B.; Caretto, S.; Mita, G. Wheat Bran Phenolic Acids: Bioavailability and Stability in Whole Wheat-Based Foods. Molecules 2015, 20, 15666–15685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouns, F.; Hemery, Y.; Price, R.; Anson, N.M. Wheat aleurone: Separation, composition, health aspects, and potential food use. Crit. Rev. Food Sci. Nutr. 2012, 52, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. Available online: https://www.hindawi.com/journals/bmri/2015/905215/abs/ (accessed on 29 August 2018).
- Sevgi, K.; Tepe, B.; Sarikurkcu, C. Antioxidant and DNA damage protection potentials of selected phenolic acids. Food Chem. Toxicol. 2015, 77, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Andrés-Lacueva, C. Polyphenols and health: current state and progress. J. Agric. Food Chem. 2012, 60, 8773–8775. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Liu, C.; Luo, S.; Chen, J.; Gong, E. The Profile and Bioaccessibility of Phenolic Compounds in Cereals Influenced by Improved Extrusion Cooking Treatment. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Parada, J.; Aguilera, J.M. Food Microstructure Affects the Bioavailability of Several Nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Stampfer, M.J.; Hu, F.B.; Giovannucci, E.; Rimm, E.; Manson, J.E.; Hennekens, C.H.; Willett, W.C. Whole-grain consumption and risk of coronary heart disease: Results from the Nurses’ Health Study. Am. J. Clin. Nutr. 1999, 70, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.D.; Liu, S.; Van Horn, L.; Tinker, L.F.; Shikany, J.M.; Eaton, C.B.; Margolis, K.L. The association of whole grain consumption with incident type 2 diabetes: The Women’s Health Initiative Observational Study. Ann. Epidemiol. 2013, 23, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Diet and Cancer. Available online: https://www.wcrf.org/dietandcancer (accessed on 10 October 2018).
- Adom, K.K.; Sorrells, M.E.; Liu, R.H. Phytochemicals and Antioxidant Activity of Milled Fractions of Different Wheat Varieties. J. Agric. Food Chem. 2005, 53, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Mitrea, L.; Trif, M.; Catoi, A.-F.; Vodnar, D.-C. Utilization of biodiesel derived-glycerol for 1,3-PD and citric acid production. Microb. Cell Factories 2017, 16, 190. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Cătoi, A.-F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing by-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Anson, N.M.; Hemery, Y.M.; Bast, A.; Haenen, G.R.M.M. Optimizing the bioactive potential of wheat bran by processing. Food Funct. 2012, 3, 362. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit. Rev. Food Sci. Nutr. 2017, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Foschia, M.; Peressini, D.; Sensidoni, A.; Brennan, M.A.; Brennan, C.S. How combinations of dietary fibres can affect physicochemical characteristics of pasta. LWT Food Sci. Technol. 2015, 61, 41–46. [Google Scholar] [CrossRef]
- Sanz Penella, J.M.; Collar, C.; Haros, M. Effect of wheat bran and enzyme addition on dough functional performance and phytic acid levels in bread. J. Cereal Sci. 2008, 48, 715–721. [Google Scholar] [CrossRef]
- Van der Kamp, J.W.; Poutanen, K.; Seal, C.J.; Richardson, D.P. The HEALTHGRAIN definition of ‘whole grain’. Food Nutr. Res. 2014, 58, 22100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavin, J.; Tucker, M.; Harriman, C.; Jonnalagadda, S.S. Whole Grains: Definition, Dietary Recommendations, and Health Benefits. Cereal Foods World 2013, 58, 191–198. [Google Scholar] [CrossRef]
- Go for the Whole Grain Poster. Available online: https://nutritioneducationstore.com/products/go-for-the-whole-grain-poster (accessed on 25 September 2018).
- Ozcan, T.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L.; Delikanli, B. Phenolics in Human Health. Int. J. Chem. Eng. Appl. 2014, 5, 393–396. [Google Scholar] [CrossRef]
- NEVO. Available online: https://nevo-online.rivm.nl/ProductenDetailsGetabt.aspx?tabid=10 (accessed on 6 September 2018).
- Slavin, J.L.; Jacobs, D.; Marquart, L. Grain Processing and Nutrition. Crit. Rev. Food Sci. Nutr. 2000, 40, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Gani, A.; Sm, W.; Fa, M.; Hameed, G. Whole-Grain Cereal Bioactive Compounds and Their Health Benefits: A Review. J. Food Process. Technol. 2012, 3. [Google Scholar] [CrossRef]
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Sibakov, N.; Poutanen, K.; Micard, V. How does wheat grain, bran and aleurone structure impact their nutritional and technological properties? Trends Food Sci. Technol. 2015, 41, 118–134. [Google Scholar] [CrossRef]
- Kim, K.; Tsao, R.; Yang, R.; Cui, S. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Li, L.; Shewry, P.R.; Ward, J.L. Phenolic acids in wheat varieties in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2008, 56, 9732–9739. [Google Scholar] [CrossRef] [PubMed]
- Dicko, M.H.; Gruppen, H.; Barro, C.; Traore, A.S.; van Berkel, W.J.H.; Voragen, A.G.J. Impact of phenolic compounds and related enzymes in sorghum varieties for resistance and susceptibility to biotic and abiotic stresses. J. Chem. Ecol. 2005, 31, 2671–2688. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawat, N.; Laddomada, B.; Gill, B.S. Genomics of Cereal-Based Functional Foods. In Cereal Genomics II; Gupta, P.K., Varshney, R.K., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 247–274. ISBN 978-94-007-6401-9. [Google Scholar]
- Fernandez-Orozco, R.; Li, L.; Harflett, C.; Shewry, P.R.; Ward, J.L. Effects of Environment and Genotype on Phenolic Acids in Wheat in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2010, 58, 9341–9352. [Google Scholar] [CrossRef] [PubMed]
- Laddomada, B.; Durante, M.; Mangini, G.; D’Amico, L.; Lenucci, M.S.; Simeone, R.; Piarulli, L.; Mita, G.; Blanco, A. Genetic variation for phenolic acids concentration and composition in a tetraploid wheat (Triticum turgidum L.) collection. Genet. Resour. Crop Evol. 2017, 64, 587–597. [Google Scholar] [CrossRef]
- Andersson, A.A.M.; Dimberg, L.; Åman, P.; Landberg, R. Recent findings on certain bioactive components in whole grain wheat and rye. J. Cereal Sci. 2014, 59, 294–311. [Google Scholar] [CrossRef]
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Technol. 2008, 19, 451–463. [Google Scholar] [CrossRef]
- Pang, Y.; Ahmed, S.; Xu, Y.; Beta, T.; Zhu, Z.; Shao, Y.; Bao, J. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem. 2018, 240, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidi, F.; Yeo, J.; Shahidi, F.; Yeo, J. Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Verma, B.; Hucl, P.; Chibbar, R.N. Phenolic Content and Antioxidant Properties of Bran in 51 Wheat Cultivars. Cereal Chem. 2008, 85, 544–549. [Google Scholar] [CrossRef]
- Juurlink, B.H.; Azouz, H.J.; Aldalati, A.M.; AlTinawi, B.M.; Ganguly, P. Hydroxybenzoic acid isomers and the cardiovascular system. Nutr. J. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.M.; Bennett, R.N.; Mellon, F.A.; Kroon, P.A.; Garcia-Conesa, M.-T. Absorption of Hydroxycinnamates in Humans after High-Bran Cereal Consumption. J. Agric. Food Chem. 2003, 51, 6050–6055. [Google Scholar] [CrossRef] [PubMed]
- Adom, K.K.; Liu, R.H. Antioxidant Activity of Grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Whole grain phytochemicals and health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Andreasen, M.F.; Kroon, P.A.; Williamson, G.; Garcia-Conesa, M.-T. Intestinal release and uptake of phenolic antioxidant diferulic acids. Free Radic. Biol. Med. 2001, 31, 304–314. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Dietary fiber as a carrier of dietary antioxidants: An essential physiological function. J. Agric. Food Chem. 2011, 59, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Price, R.K.; Wallace, J.M.W.; Hamill, L.L.; Keaveney, E.M.; Strain, J.J.; Parker, M.J.; Welch, R.W. Evaluation of the effect of wheat aleurone-rich foods on markers of antioxidant status, inflammation and endothelial function in apparently healthy men and women. Br. J. Nutr. 2012, 108, 1644–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, R.K.; Welch, R.W.; Lee-Manion, A.M.; Bradbury, I.; Strain, J.J. Total Phenolics and Antioxidant Potential in Plasma and Urine of Humans after Consumption of Wheat Bran. Cereal Chem. 2008, 85, 152–157. [Google Scholar] [CrossRef]
- Serpen, A.; Capuano, E.; Fogliano, V.; Gökmen, V. A New Procedure to Measure the Antioxidant Activity of Insoluble Food Components. J. Agric. Food Chem. 2007, 55, 7676–7681. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, S.; Kurokawa, T.; Nakata, C.; Saito, Y.; Oikawa, S.; Kobayashi, M.; Hirano, T.; Iseki, K. In vitro and in vivo antioxidant properties of ferulic acid: A comparative study with other natural oxidation inhibitors. Food Chem. 2009, 114, 466–471. [Google Scholar] [CrossRef]
- Rukkumani, R.; Aruna, K.; Varma, P.S.; Menon, V.P. Influence of ferulic acid on circulatory prooxidant-antioxidant status during alcohol and PUFA induced toxicity. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2004, 55, 551–561. [Google Scholar]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables, and Grains—A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–918. [Google Scholar] [CrossRef] [PubMed]
- Laddomada, B.; Durante, M.; Minervini, F.; Garbetta, A.; Cardinali, A.; D’Antuono, I.; Caretto, S.; Blanco, A.; Mita, G. Phytochemical composition and anti-inflammatory activity of extracts from the whole-meal flour of Italian durum wheat cultivars. Int. J. Mol. Sci. 2015, 16, 3512–3527. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Mennella, I.; Ferracane, R.; Rivellese, A.A.; Giacco, R.; Ercolini, D.; Gibbons, S.M.; La Storia, A.; Gilbert, J.A.; Jonnalagadda, S.; et al. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: Role of polyphenols bound to cereal dietary fiber. Am. J. Clin. Nutr. 2015, 101, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Mateo Anson, N.; Aura, A.-M.; Selinheimo, E.; Mattila, I.; Poutanen, K.; van den Berg, R.; Havenaar, R.; Bast, A.; Haenen, G.R.M.M. Bioprocessing of wheat bran in whole wheat bread increases the bioavailability of phenolic acids in men and exerts antiinflammatory effects ex vivo. J. Nutr. 2011, 141, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Structure–function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Ripari, V.; Bai, Y.; Gänzle, M.G. Metabolism of phenolic acids in whole wheat and rye malt sourdoughs. Food Microbiol. 2019, 77, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Saavedra, M.J.; Simões, M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Taguri, T.; Tanaka, T.; Kouno, I. Antibacterial Spectrum of Plant Polyphenols and Extracts Depending upon Hydroxyphenyl Structure. Biol. Pharm. Bull. 2006, 29, 2226–2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elegir, G.; Kindl, A.; Sadocco, P.; Orlandi, M. Development of antimicrobial cellulose packaging through laccase-mediated grafting of phenolic compounds. Enzym. Microb. Technol. 2008, 43, 84–92. [Google Scholar] [CrossRef]
- Antimicrobial Properties of Grape Seed Extracts and Their Effectiveness after Incorporation into Pea Starch Films—Corrales—2009—International Journal of Food Science and Technology—Wiley Online Library. Available online: https://0-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/full/10.1111/j.1365-2621.2008.01790.x (accessed on 20 September 2018).
- Jonnalagadda, S.S.; Harnack, L.; Hai Liu, R.; McKeown, N.; Seal, C.; Liu, S.; Fahey, G.C. Putting the Whole Grain Puzzle Together: Health Benefits Associated with Whole Grains—Summary of American Society for Nutrition 2010 Satellite Symposium. J. Nutr. 2011, 141, 1011S–1022S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seal, C.J.; Brownlee, I.A. Whole-grain foods and chronic disease: Evidence from epidemiological and intervention studies. Proc. Nutr. Soc. 2015, 74, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhao, Q.; Guo, W.; Bao, W.; Wang, X. Association of whole grain intake with all-cause, cardiovascular, and cancer mortality: A systematic review and dose-response meta-analysis from prospective cohort studies. Eur. J. Clin. Nutr. 2018, 72, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Benisi-Kohansal, S.; Saneei, P.; Salehi-Marzijarani, M.; Larijani, B.; Esmaillzadeh, A. Whole-Grain Intake and Mortality from All Causes, Cardiovascular Disease, and Cancer: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2016, 7, 1052–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Punder, K.; Pruimboom, L. The Dietary Intake of Wheat and other Cereal Grains and Their Role in Inflammation. Nutrients 2013, 5, 771–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catoi, A.F.; Parvu, A.; Muresan, A.; Galea, R.F.; Catoi, C. Evaluation of nitric oxide synthesis in morbid obesity. Free Radic. Res. 2006, 40, S140. [Google Scholar]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef] [PubMed]
- Diabetes. Available online: http://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 19 September 2018).
- Belobrajdic, D.P.; Bird, A.R. The potential role of phytochemicals in wholegrain cereals for the prevention of type-2 diabetes. Nutr. J. 2013, 12, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- InterAct Consortium. Dietary fibre and incidence of type 2 diabetes in eight European countries: The EPIC-InterAct Study and a meta-analysis of prospective studies. Diabetologia 2015, 58, 1394–1408. [Google Scholar] [CrossRef] [PubMed]
- Hauner, H.; Bechthold, A.; Boeing, H.; Brönstrup, A.; Buyken, A.; Leschik-Bonnet, E.; Linseisen, J.; Schulze, M.; Strohm, D.; Wolfram, G.; et al. Evidence-based guideline of the German Nutrition Society: Carbohydrate intake and prevention of nutrition-related diseases. Ann. Nutr. Metab. 2012, 60 (Suppl. 1), 1–58. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.-M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Della Pepa, G.; Vetrani, C.; Vitale, M.; Riccardi, G.; Della Pepa, G.; Vetrani, C.; Vitale, M.; Riccardi, G. Wholegrain Intake and Risk of Type 2 Diabetes: Evidence from Epidemiological and Intervention Studies. Nutrients 2018, 10, 1288. [Google Scholar] [CrossRef] [PubMed]
- Kyrø, C.; Tjønneland, A.; Overvad, K.; Olsen, A.; Landberg, R. Higher Whole-Grain Intake Is Associated with Lower Risk of Type 2 Diabetes among Middle-Aged Men and Women: The Danish Diet, Cancer, and Health Cohort. J. Nutr. 2018, 148, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.K.; Kullman, E.L.; Scelsi, A.R.; Haus, J.M.; Filion, J.; Pagadala, M.R.; Godin, J.-P.; Kochhar, S.; Ross, A.B.; Kirwan, J.P. A whole-grain diet reduces peripheral insulin resistance and improves glucose kinetics in obese adults: A randomized-controlled trial. Metabolism 2018, 82, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Heaton, K.W.; Marcus, S.N.; Emmett, P.M.; Bolton, C.H. Particle size of wheat, maize, and oat test meals: Effects on plasma glucose and insulin responses and on the rate of starch digestion in vitro. Am. J. Clin. Nutr. 1988, 47, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Whincup, P.H.; Donin, A.S. Cereal fibre and type 2 diabetes: Time now for randomised controlled trials? Diabetologia 2015, 58, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Obesity and Overweight. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 19 September 2018).
- Kikuchi, Y.; Nozaki, S.; Makita, M.; Yokozuka, S.; Fukudome, S.; Yanagisawa, T.; Aoe, S. Effects of Whole Grain Wheat Bread on Visceral Fat Obesity in Japanese Subjects: A Randomized Double-Blind Study. Plant Foods Hum. Nutr. 2018, 73, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Choumenkovitch, S.F.; McKeown, N.M.; Tovar, A.; Hyatt, R.R.; Kraak, V.I.; Hastings, A.V.; Herzog, J.B.; Economos, C.D. Whole grain consumption is inversely associated with BMI Z-score in rural school-aged children. Public Health Nutr. 2013, 16, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Bahadoran, Z.; Golzarand, M.; Shiva, N.; Azizi, F. Association between dietary phytochemical index and 3-year changes in weight, waist circumference and body adiposity index in adults: Tehran Lipid and Glucose study. Nutr. Metab. 2012, 9, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Willett, W.C.; Manson, J.E.; Hu, F.B.; Rosner, B.; Colditz, G. Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Am. J. Clin. Nutr. 2003, 78, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Pol, K.; Christensen, R.; Bartels, E.M.; Raben, A.; Tetens, I.; Kristensen, M. Whole grain and body weight changes in apparently healthy adults: A systematic review and meta-analysis of randomized controlled studies. Am. J. Clin. Nutr. 2013, 98, 872–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, M.; Toubro, S.; Jensen, M.G.; Ross, A.B.; Riboldi, G.; Petronio, M.; Bügel, S.; Tetens, I.; Astrup, A. Whole Grain Compared with Refined Wheat Decreases the Percentage of Body Fat Following a 12-Week, Energy-Restricted Dietary Intervention in Postmenopausal Women. J. Nutr. 2012, 142, 710–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harland, J.I.; Garton, L.E. Whole-grain intake as a marker of healthy body weight and adiposity. Public Health Nutr. 2008, 11, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.; Pelletier, X.; Ross, A.; Thielecke, F.; Kristensen, M.; Pelletier, X.; Ross, A.B.; Thielecke, F. A High Rate of Non-Compliance Confounds the Study of Whole Grains and Weight Maintenance in a Randomised Intervention Trial—The Case for Greater Use of Dietary Biomarkers in Nutrition Intervention Studies. Nutrients 2017, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cardiovascular Diseases (CVDs). Available online: http://www.who.int/cardiovascular_diseases/en/ (accessed on 19 September 2018).
- Zong, G.; Gao, A.; Hu, F.B.; Sun, Q. Whole Grain Intake and Mortality from All Causes, Cardiovascular Disease, and Cancer: A Meta-Analysis of Prospective Cohort Studies. Circulation 2016, 133, 2370–2380. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Barera, A.; Buscemi, S.; Monastero, R.; Caruso, C.; Caldarella, R.; Ciaccio, M.; Vasto, S. β-glucans: Ex vivo inflammatory and oxidative stress results after pasta intake. Immun. Ageing 2016, 13, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tighe, P.; Duthie, G.; Vaughan, N.; Brittenden, J.; Simpson, W.G.; Duthie, S.; Mutch, W.; Wahle, K.; Horgan, G.; Thies, F. Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: A randomized controlled trial. Am. J. Clin. Nutr. 2010, 92, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Hollænder, P.L.B.; Ross, A.B.; Kristensen, M. Whole-grain and blood lipid changes in apparently healthy adults: A systematic review and meta-analysis of randomized controlled studies. Am. J. Clin. Nutr. 2015, 102, 556–572. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, Y.; Zhang, L.; Su, H.; Zheng, X. Blackberry subjected to in vitro gastrointestinal digestion affords protection against Ethyl Carbamate-induced cytotoxicity. Food Chem. 2016, 212, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.S.; Fleenor, B.S. Whole grain consumption is negatively correlated with obesity-associated aortic stiffness: A hypothesis. Nutrition 2018, 45, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Bernard, W.S.; Christopher, P.W. World Cancer Report 2014; World Health Organization: Geneva, Switzerland, 2014; ISBN 978-92-832-0429-9. [Google Scholar]
- Masisi, K.; Beta, T.; Moghadasian, M.H. Antioxidant properties of diverse cereal grains: A review on in vitro and in vivo studies. Food Chem. 2016, 196, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, R.H. Potential Mechanisms of Action of Dietary Phytochemicals for Cancer Prevention by Targeting Cellular Signaling Transduction Pathways. J. Agric. Food Chem. 2018, 66, 3260–3276. [Google Scholar] [CrossRef] [PubMed]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Novel chelators for cancer treatment: Where are we now? Antioxid. Redox Signal. 2013, 18, 973–1006. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R.; Marquart, L.; Slavin, J.; Kushi, L.H. Whole-grain intake and cancer: An expanded review and meta-analysis. Nutr. Cancer 1998, 30, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Chan, D.S.M.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2011, 343, d6617. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Giovannucci, E.; Bergkvist, L.; Wolk, A. Whole grain consumption and risk of colorectal cancer: A population-based cohort of 60000 women. Br. J. Cancer 2005, 92, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Kyrø, C.; Skeie, G.; Loft, S.; Landberg, R.; Christensen, J.; Lund, E.; Nilsson, L.M.; Palmqvist, R.; Tjønneland, A.; Olsen, A. Intake of whole grains from different cereal and food sources and incidence of colorectal cancer in the Scandinavian HELGA cohort. Cancer Causes Control 2013, 24, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Makarem, N.; Nicholson, J.M.; Bandera, E.V.; McKeown, N.M.; Parekh, N. Consumption of whole grains and cereal fiber in relation to cancer risk: A systematic review of longitudinal studies. Nutr. Rev. 2016, 74, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Zheng, H.; Bi, J.; Wang, X.; Jiang, T.; Gao, X.; Tian, F.; Xu, M.; Wu, C.; Zhang, L.; et al. Whole Grain Intake Reduces Pancreatic Cancer Risk. Medicine 2016, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreher, M.L. Dietary Patterns, Whole Plant Foods, Nutrients and Phytochemicals in Colorectal Cancer Prevention and Management. In Dietary Patterns and Whole Plant Foods in Aging and Disease; Dreher, M.L., Ed.; Nutrition and Health; Springer International Publishing: Cham, Switzerland, 2018; pp. 521–555. ISBN 978-3-319-59180-3. [Google Scholar]
- Antitumor Activity of Wheats with High Orthophenolic Content: Nutrition and Cancer: Vol 47, No 2. Available online: https://0-www-tandfonline-com.brum.beds.ac.uk/doi/abs/10.1207/s15327914nc4702_12 (accessed on 20 September 2018).
- Scientific Opinion on the Substantiation of Health Claims Related to Wheat Bran Fibre and Increase in Faecal Bulk (ID 3066), Reduction in Intestinal Transit Time (ID 828, 839, 3067, 4699) and Contribution to the Maintenance or Achievement of a Normal Body Weight (ID 829) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006—2010—EFSA Journal—Wiley Online Library. Available online: https://0-efsa-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/10.2903/j.efsa.2010.1817 (accessed on 6 September 2018).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to whole grain (ID 831, 832, 833, 1126, 1268, 1269, 1270, 1271, 1431) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1766. [Google Scholar] [CrossRef] [Green Version]
- Caleja, C.; Ribeiro, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Phenolic Compounds as Nutraceuticals or Functional Food Ingredients. Curr. Pharm. Des. 2017, 23. [Google Scholar] [CrossRef] [PubMed]
- World Agriculture: Towards 2015/2030—An FAO Perspective. Available online: http://www.fao.org/docrep/005/Y4252E/y4252e05.htm (accessed on 6 September 2018).
- Glei, M.; Hofmann, T.; Küster, K.; Hollmann, J.; Lindhauer, M.G.; Pool-Zobel, B.L. Both Wheat (Triticum aestivum) Bran Arabinoxylans and Gut Flora-Mediated Fermentation Products Protect Human Colon Cells from Genotoxic Activities of 4-Hydroxynonenal and Hydrogen Peroxide. J. Agric. Food Chem. 2006, 54, 2088–2095. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Possemiers, S.; Druart, C.; de Wiele, T.V.; Backer, F.D.; Cani, P.D.; Larondelle, Y.; Delzenne, N.M. Prebiotic Effects of Wheat Arabinoxylan Related to the Increase in Bifidobacteria, Roseburia and Bacteroides/Prevotella in Diet-Induced Obese Mice. PLoS ONE 2011, 6, e20944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uda, Y.; Price, K.R.; Williamson, G.; Rhodes, M.J.C. Induction of the anticarcinogenic marker enzyme, quinone reductase, in murine hepatoma cells in vitro by flavonoids. Cancer Lett. 1997, 120, 213–216. [Google Scholar] [CrossRef]
- Price, K.R.; Rhodes, M.J.C. Analysis of the Major Flavonol Glycosides Present in Four Varieties of Onion (Allium cepa) and Changes in Composition Resulting from Autolysis. J. Sci. Food Agric. 1997, 74, 331–339. [Google Scholar] [CrossRef]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The Effects of Plant Flavonoids on Mammalian Cells: Implications for Inflammation, Heart Disease, and Cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Shankar, A.H.; Prasad, A.S. Zinc and immune function: The biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998, 68, 447S–463S. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000; ISBN-10: 0-309-06949-1. [Google Scholar]
- Iron Deficiency and Reduced Work Capacity: A Critical Review of the Research to Determine a Causal Relationship. Oxford Academic. Available online: https://0-academic-oup-com.brum.beds.ac.uk/jn/article/131/2/676S/4686866 (accessed on 6 September 2018).
- Schneeman, B.O. Fiber, Inulin and Oligofructose: Similarities and Differences. J. Nutr. 1999, 129, 1424S–1427S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keegstra, K.; Walton, J. β-Glucans—Brewer’s Bane, Dietician’s Delight. Science 2006, 311, 1872–1873. [Google Scholar] [CrossRef] [PubMed]
- Menga, V.; Fares, C.; Troccoli, A.; Cattivelli, L.; Baiano, A. Effects of genotype, location and baking on the phenolic content and some antioxidant properties of cereal species. Int. J. Food Sci. Technol. 2010, 45, 7–16. [Google Scholar] [CrossRef]
- Mao, J.; Burt, A.J.; Ramputh, A.-I.; Simmonds, J.; Cass, L.; Hubbard, K.; Miller, S.; Altosaar, I.; Arnason, J.T. Diverted secondary metabolism and improved resistance to European corn borer (Ostrinia nubilalis) in maize (Zea mays L.) transformed with wheat oxalate oxidase. J. Agric. Food Chem. 2007, 55, 2582–2589. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.I.; Ferreira, I.C.F.R.; Barreiro, M.F. Microencapsulation of bioactives for food applications. Food Funct. 2015, 6, 1035–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, J.C.R.; Campos, M.R.S. New Polymers for Encapsulation of Nutraceutical Compounds; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 978-1-119-22879-0. [Google Scholar]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy: Bioavailability of bioactive food compounds. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Zou, L.; Zhang, R.; Salvia-Trujillo, L.; Kumosani, T.; Xiao, H. Enhancing Nutraceutical Performance Using Excipient Foods: Designing Food Structures and Compositions to Increase Bioavailability. Compr. Rev. Food Sci. Food Saf. 2015, 14, 824–847. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.-J. Dietary Polyphenols, Gut Microbiota, and Intestinal Epithelial Health. In Nutritional and Therapeutic Interventions for Diabetes and Metabolic Syndrome; Elsevier: Amsterdam, The Netherlands, 2018; pp. 295–314. ISBN 978-0-12-812019-4. [Google Scholar]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.; Lean, M.E.J.; Roberts, S.A.; Crozier, A. Bioavailability of dietary (poly)phenols: A study with ileostomists to discriminate between absorption in small and large intestine. Food Funct. 2013, 4, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Erk, T.; Hauser, J.; Williamson, G.; Renouf, M.; Steiling, H.; Dionisi, F.; Richling, E. Structure- and dose-absorption relationships of coffee polyphenols. BioFactors Oxf. Engl. 2014, 40, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, U.; Szewczyk, K.; Hrabec, E.; Janecka, A.; Gorlach, S. Overview of Metabolism and Bioavailability Enhancement of Polyphenols. J. Agric. Food Chem. 2013, 61, 12183–12199. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marze, S. Bioavailability of Nutrients and Micronutrients: Advances in Modeling and in Vitro Approaches. Annu. Rev. Food Sci. Technol. 2017, 8, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Ifie, I.; Marshall, L.J. Food processing and its impact on phenolic constituents in food. Cogent Food Agric. 2018, 4, 1–11. [Google Scholar] [CrossRef]
- Nagah, A.M.; Seal, C.J. In vitro procedure to predict apparent antioxidant release from wholegrain foods measured using three different analytical methods. J. Sci. Food Agric. 2005, 85, 1177–1185. [Google Scholar] [CrossRef]
- Wang, T.; He, F.; Chen, G. Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: A concise review. J. Funct. Foods 2014, 7, 101–111. [Google Scholar] [CrossRef]
- Hithamani, G.; Srinivasan, K. Bioaccessibility of polyphenols from wheat (Triticum aestivum), sorghum (Sorghum bicolor), green gram (Vigna radiata), and chickpea (Cicer arietinum) as influenced by domestic food processing. J. Agric. Food Chem. 2014, 62, 11170–11179. [Google Scholar] [CrossRef] [PubMed]
- Hithamani, G.; Srinivasan, K. Effect of domestic processing on the polyphenol content and bioaccessibility in finger millet (Eleusine coracana) and pearl millet (Pennisetum glaucum). Food Chem. 2014, 164, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Hübner, F.; Arendt, E.K. Germination of cereal grains as a way to improve the nutritional value: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.-S.M.; Rabalski, I. Effect of baking on free and bound phenolic acids in wholegrain bakery products. J. Cereal Sci. 2013, 57, 312–318. [Google Scholar] [CrossRef]
- Li, W.; Pickard, M.D.; Beta, T. Effect of thermal processing on antioxidant properties of purple wheat bran. Food Chem. 2007, 104, 1080–1086. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Călinoiu, L.F.; Dulf, F.V.; Ştefănescu, B.E.; Crişan, G.; Socaciu, C. Identification of the bioactive compounds and antioxidant, antimutagenic and antimicrobial activities of thermally processed agro-industrial waste. Food Chem. 2017, 231, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Calinoiu, L.-F.; Mitrea, L.; Precup, G.; Bindea, M.; Rusu, B.; Dulf, F.-V.; Stefanescu, B.-E.; Vodnar, D.-C. Characterization of Grape and Apple Peel Wastes’ Bioactive Compounds and Their Increased Bioavailability After Exposure to Thermal Process. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca-Food Sci. Technol. 2017, 74, 80–89. [Google Scholar] [CrossRef]
- Bryngelsson, S.; Dimberg, L.H.; Kamal-Eldin, A. Effects of Commercial Processing on Levels of Antioxidants in Oats (Avena sativa L.). J. Agric. Food Chem. 2002, 50, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, H.; Kozlowska, H.; Lewczuk, B. Bioactive compounds in the cereal grains before and after hydrothermal processing. Innov. Food Sci. Emerg. Technol. 2001, 2, 159–169. [Google Scholar] [CrossRef]
- Lopez, S.; Julieta, N.; Loarca-Piña, G.; Campos-Vega, R.; Gaytán Martínez, M.; Morales Sánchez, E.; Esquerra-Brauer, J.M.; Gonzalez-Aguilar, G.A.; Robles Sánchez, M. The Extrusion Process as an Alternative for Improving the Biological Potential of Sorghum Bran: Phenolic Compounds and Antiradical and Anti-Inflammatory Capacity. Available online: https://www.hindawi.com/journals/ecam/2016/8387975/ (accessed on 24 September 2018).
- Altan, A.; McCarthy, K.L.; Maskan, M. Effect of extrusion process on antioxidant activity, total phenolics and β-glucan content of extrudates developed from barley-fruit and vegetable by-products. Int. J. Food Sci. Technol. 2009, 44, 1263–1271. [Google Scholar] [CrossRef]
- Chávez, D.W.H.; Ascheri, J.L.R.; Carvalho, C.W.P.; Godoy, R.L.O.; Pacheco, S. Sorghum and roasted coffee blends as a novel extruded product: Bioactive compounds and antioxidant capacity. J. Funct. Foods 2017, 29, 93–103. [Google Scholar] [CrossRef]
- De Cardoso, L.M.; Pinheiro, S.S.; de Carvalho, C.W.P.; Queiroz, V.A.V.; de Menezes, C.B.; Moreira, A.V.B.; de Barros, F.A.R.; Awika, J.M.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Phenolic compounds profile in sorghum processed by extrusion cooking and dry heat in a conventional oven. J. Cereal Sci. 2015, 65, 220–226. [Google Scholar] [CrossRef]
- Sultan, N.; Wani, I.A.; Masoodi, F.A. Moisture mediated effects of γ-irradiation on physicochemical, functional, and antioxidant properties of pigmented brown rice (Oryza sativa L.) flour. J. Cereal Sci. 2018, 79, 399–407. [Google Scholar] [CrossRef]
- Fares, C.; Platani, C.; Baiano, A.; Menga, V. Effect of processing and cooking on phenolic acid profile and antioxidant capacity of durum wheat pasta enriched with debranning fractions of wheat. Food Chem. 2010, 119, 1023–1029. [Google Scholar] [CrossRef]
- Gómez, M.; Jiménez, S.; Ruiz, E.; Oliete, B. Effect of extruded wheat bran on dough rheology and bread quality. LWT Food Sci. Technol. 2011, 44, 2231–2237. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Rocculi, P.; Dalla Rosa, M. Strategies to improve food functionality: Structure–property relationships on high pressures homogenization, vacuum impregnation and drying technologies. Trends Food Sci. Technol. 2015, 46, 1–12. [Google Scholar] [CrossRef]
- Delcour, J.A.; Rouau, X.; Courtin, C.M.; Poutanen, K.; Ranieri, R. Technologies for enhanced exploitation of the health-promoting potential of cereals. Trends Food Sci. Technol. 2012, 25, 78–86. [Google Scholar] [CrossRef]
- Pasqualone, A.; Delvecchio, L.N.; Gambacorta, G.; Laddomada, B.; Urso, V.; Mazzaglia, A.; Ruisi, P.; Di, G.M. Effect of Supplementation with Wheat Bran Aqueous Extracts Obtained by Ultrasound-Assisted Technologies on the Sensory Properties and the Antioxidant Activity of Dry Pasta. Nat. Prod. Commun. 2015, 10, 1739–1742. [Google Scholar] [PubMed]
- Salmenkallio-Marttila, M.; Katina, K.; Autio, K. Effects of Bran Fermentation on Quality and Microstructure of High-Fiber Wheat Bread. Cereal Chem. 2001, 78, 429–435. [Google Scholar] [CrossRef]
- Lebesi, D.M.; Tzia, C. Effect of the Addition of Different Dietary Fiber and Edible Cereal Bran Sources on the Baking and Sensory Characteristics of Cupcakes. Food Bioprocess Technol. 2011, 4, 710–722. [Google Scholar] [CrossRef]
- Blandino, M.; Sovrani, V.; Marinaccio, F.; Reyneri, A.; Rolle, L.; Giacosa, S.; Locatelli, M.; Bordiga, M.; Travaglia, F.; Coïsson, J.D.; et al. Nutritional and technological quality of bread enriched with an intermediated pearled wheat fraction. Food Chem. 2013, 141, 2549–2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemery, Y.; Rouau, X.; Lullien-Pellerin, V.; Barron, C.; Abecassis, J. Dry processes to develop wheat fractions and products with enhanced nutritional quality. J. Cereal Sci. 2007, 46, 327–347. [Google Scholar] [CrossRef]
- Gazzola, A.; Marinaccio, F.; Sovrani, V.; Blandino, M. Effect of adding wheat and barley pearled fractions on the rheological properties of doughs for bakery products. Tec. Mol. 2014, 65, 266–274. [Google Scholar]
- Vodnar, D.C.; Venus, J.; Schneider, R.; Socaciu, C. Lactic Acid Production by Lactobacillus paracasei 168 in Discontinuous Fermentation Using Lucerne Green juice as Nutrient Substitute. Chem. Eng. Technol. 2010, 33, 468–474. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, V.; Banerjee, R.; Sharma, S.; Kuila, A. Perspectives of cell-wall degrading enzymes in cereal polishing. Food Biosci. 2016, 15, 81–86. [Google Scholar] [CrossRef]
- Bhanja Dey, T.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar] [CrossRef]
- Coda, R.; Cagno, R.D.; Gobbetti, M.; Rizzello, C.G. Sourdough lactic acid bacteria: exploration of non-wheat cereal-based fermentation. Food Microbiol. 2014, 37, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Zhai, F.-H.; Wang, Q.; Han, J.-R. Nutritional components and antioxidant properties of seven kinds of cereals fermented by the basidiomycete Agaricus blazei. J. Cereal Sci. 2015, 65, 202–208. [Google Scholar] [CrossRef]
- Konopka, I.; Tańska, M.; Faron, A.; Czaplicki, S. Release of free ferulic acid and changes in antioxidant properties during the wheat and rye bread making process. Food Sci. Biotechnol. 2014, 23, 831–840. [Google Scholar] [CrossRef]
- Singh, J.P.; Kaur, A.; Shevkani, K.; Singh, N. Influence of jambolan (Syzygium cumini) and xanthan gum incorporation on the physicochemical, antioxidant and sensory properties of gluten-free eggless rice muffins. Int. J. Food Sci. Technol. 2015, 50, 1190–1197. [Google Scholar] [CrossRef]
- Amaya Villalva, M.F.; González-Aguilar, G.; Sández, O.R.; Astiazarán García, H.; Ledesma Osuna, A.I.; López-Ahumada, G.A.; Robles-Sánchez, R.M. Bioprocessing of wheat (Triticum aestivum cv. Kronstad) bran from Northwest Mexico: Effects on ferulic acid bioaccessibility in breads. CyTA J. Food 2018, 16, 570–579. [Google Scholar] [CrossRef]
- Nordlund, E.; Katina, K.; Aura, A.-M.; Poutanen, K. Changes in bran structure by bioprocessing with enzymes and yeast modifies the in vitro digestibility and fermentability of bran protein and dietary fibre complex. J. Cereal Sci. 2013, 58, 200–208. [Google Scholar] [CrossRef]
- Nordlund, E.; Aura, A.-M.; Mattila, I.; Kössö, T.; Rouau, X.; Poutanen, K. Formation of Phenolic Microbial Metabolites and Short-Chain Fatty Acids from Rye, Wheat, and Oat Bran and Their Fractions in the Metabolical in Vitro Colon Model. J. Agric. Food Chem. 2012, 60, 8134–8145. [Google Scholar] [CrossRef] [PubMed]
- Rosa, N.N.; Barron, C.; Gaiani, C.; Dufour, C.; Micard, V. Ultra-fine grinding increases the antioxidant capacity of wheat bran. J. Cereal Sci. 2013, 57, 84–90. [Google Scholar] [CrossRef]
- Lappi, J.; Aura, A.-M.; Katina, K.; Nordlund, E.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Comparison of postprandial phenolic acid excretions and glucose responses after ingestion of breads with bioprocessed or native rye bran. Food Funct. 2013, 4, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Katina, K.; Laitila, A.; Juvonen, R.; Liukkonen, K.-H.; Kariluoto, S.; Piironen, V.; Landberg, R.; Åman, P.; Poutanen, K. Bran fermentation as a means to enhance technological properties and bioactivity of rye. Food Microbiol. 2007, 24, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, R.; Liu, C.; Zheng, X.; Liu, B. Enhancing antioxidant activity and antiproliferation of wheat bran through steam flash explosion. J. Food Sci. Technol. 2016, 53, 3028–3034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, E.; Wu, Z.; Long, J.; Wang, F.; Pan, X.; Xu, X.; Jin, Z.; Jiao, A. Effect of Thermostable α-Amylase Addition on the Physicochemical Properties, Free/Bound Phenolics and Antioxidant Capacities of Extruded Hulled and Whole Rice. Food Bioprocess Technol. 2015, 8, 1958–1973. [Google Scholar] [CrossRef]
- Shewry, P.R.; Charmet, G.; Branlard, G.; Lafiandra, D.; Gergely, S.; Salgó, A.; Saulnier, L.; Bedo, Z.; Mills, E.N.C.; Ward, J.L. Developing new types of wheat with enhanced health benefits. Trends Food Sci. Technol. 2012, 25, 70–77. [Google Scholar] [CrossRef]
- Hung, P.V.; Hatcher, D.W.; Barker, W. Phenolic acid composition of sprouted wheats by ultra-performance liquid chromatography (UPLC) and their antioxidant activities. Food Chem. 2011, 126, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Van Hung, P.; Maeda, T.; Morita, N. Improvement of nutritional composition and antioxidant capacity of high-amylose wheat during germination. J. Food Sci. Technol. 2015, 52, 6756–6762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Kwak, H.; Kim, S.; Kim, M.J.; Kwak, H.S.; Kim, S.S. Effects of Germination on Protein, γ-Aminobutyric Acid, Phenolic Acids, and Antioxidant Capacity in Wheat. Molecules 2018, 23, 2244. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Saxena, D.C.; Riar, C.S. Analysing the effect of germination on phenolics, dietary fibres, minerals and γ-amino butyric acid contents of barnyard millet (Echinochloa frumentaceae). Food Biosci. 2016, 13, 60–68. [Google Scholar] [CrossRef]
- Garzón, A.G.; Torres, R.L.; Drago, S.R. Effects of malting conditions on enzyme activities, chemical, and bioactive compounds of sorghum starchy products as raw material for brewery. Starch Stärke 2016, 68, 1048–1054. [Google Scholar] [CrossRef]
- Protonotariou, S.; Mandala, I.; Rosell, C.M. Jet Milling Effect on Functionality, Quality and in Vitro Digestibility of Whole Wheat Flour and Bread. Food Bioprocess Technol. 2015, 8, 1319–1329. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Coda, R.; Mazzacane, F.; Minervini, D.; Gobbetti, M. Micronized by-products from debranned durum wheat and sourdough fermentation enhanced the nutritional, textural and sensory features of bread. Food Res. Int. 2012, 46, 304–313. [Google Scholar] [CrossRef]
- Hemery, Y.M.; Anson, N.M.; Havenaar, R.; Haenen, G.R.M.M.; Noort, M.W.J.; Rouau, X. Dry-fractionation of wheat bran increases the bioaccessibility of phenolic acids in breads made from processed bran fractions. Food Res. Int. 2010, 43, 1429–1438. [Google Scholar] [CrossRef]
- Hemery, Y.; Rouau, X.; Dragan, C.; Bilici, M.; Beleca, R.; Dascalescu, L. Electrostatic properties of wheat bran and its constitutive layers: Influence of particle size, composition, and moisture content. J. Food Eng. 2009, 93, 114–124. [Google Scholar] [CrossRef]
- Yao, M.; McClements, D.J.; Xiao, H. Improving oral bioavailability of nutraceuticals by engineered nanoparticle-based delivery systems. Curr. Opin. Food Sci. 2015, 2, 14–19. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Socaciu, C. Selenium enriched green tea increase stability of Lactobacillus casei and Lactobacillus plantarum in chitosan coated alginate microcapsules during exposure to simulated gastrointestinal and refrigerated conditions. LWT Food Sci. Technol. 2014, 57, 406–411. [Google Scholar] [CrossRef]
- Calinoiu, L.-F.; Vodnar, D.-C.; Precup, G. The Probiotic Bacteria Viability under Different Conditions. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca-Food Sci. Technol. 2016, 73, 55–60. [Google Scholar] [CrossRef]
- Araiza-Calahorra, A.; Akhtar, M.; Sarkar, A. Recent advances in emulsion-based delivery approaches for curcumin: From encapsulation to bioaccessibility. Trends Food Sci. Technol. 2018, 71, 155–169. [Google Scholar] [CrossRef]
- Akhavan, S.; Assadpour, E.; Katouzian, I.; Jafari, S.M. Lipid nano scale cargos for the protection and delivery of food bioactive ingredients and nutraceuticals. Trends Food Sci. Technol. 2018, 74, 132–146. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. Nutr. 2018, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Moral, S.; Teixeira, M.C.; Fernandes, A.R.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A.; Souto, E.B. Lipid nanocarriers for the loading of polyphenols—A comprehensive review. Adv. Colloid Interface Sci. 2018, 260, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Faridi Esfanjani, A.; Assadpour, E.; Jafari, S.M. Improving the bioavailability of phenolic compounds by loading them within lipid-based nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]
- Bondi, M.L.; Montana, G.; Craparo, E.F.; Picone, P.; Capuano, G.; Carlo, M.D.; Giammona, G. Ferulic Acid-Loaded Lipid Nanostructures as Drug Delivery Systems for Alzheimer’s Disease: Preparation, Characterization and Cytotoxicity Studies. Available online: https://0-www-ingentaconnect-com.brum.beds.ac.uk/content/ben/cnano/2009/00000005/00000001/art00003 (accessed on 25 September 2018).
- Medina-Remón, A.; Kirwan, R.; Lamuela-Raventós, R.M.; Estruch, R. Dietary patterns and the risk of obesity, type 2 diabetes mellitus, cardiovascular diseases, asthma, and neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2018, 58, 262–296. [Google Scholar] [CrossRef] [PubMed]
- Granata, G.; Consoli, G.M.L.; Lo Nigro, R.; Geraci, C. Hydroxycinnamic acids loaded in lipid-core nanocapsules. Food Chem. 2018, 245, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Panwar, R.; Raghuwanshi, N.; Srivastava, A.K.; Sharma, A.K.; Pruthi, V. In-vivo sustained release of nanoencapsulated ferulic acid and its impact in induced diabetes. Mater. Sci. Eng. C 2018, 92, 381–392. [Google Scholar] [CrossRef] [PubMed]



| Whole Wheat Flour | White Wheat Flour, 75% Extraction * | Whole Rye Flour | Rye Flour, 60% Extraction * | Whole Barley Grain | Pearl Barley | |
|---|---|---|---|---|---|---|
| Carbohydrates, g (% of energy) | 62 (75.6) | 71 (80.6) | 59.2 (71.4) | 73 (85) | 60.8 (72.8) | 67 (79) |
| Protein, g (% of energy) | 10 (12.2) | 12.6 (14.3) | 10 (13) | 8 (9.3) | 10.6 (12.7) | 9 (10.6) |
| Fat, g (% of energy) | 2 (5.5) | 1.1 (2.8) | 2 (5.8) | 1 (2.6) | 2.1 (5.7) | 2 (5.3) |
| Dietary fiber, g | 11 | 4 | 15 | 5 | 14.8 | 8.6 |
| Vitamin B1, mg | 0.4 | 0.07 | 0.4 | 0.15 | 0.31 | 0.03 |
| Vitamin B2, mg | 0.15 | 0.04 | 0.2 | 0.07 | 0.10 | 0.03 |
| Vitamin B3, mg | 5.7 | 1 | 1.7 | 1 | 5.2 | 3 |
| Vitamin B6, mg | 0.35 | 0.12 | 0.22 | 0.23 | 0.56 | 0.25 |
| Vitamin B9, µg | 37 | 22 | 78 | 28 | 50 | 20 |
| Iron, mg | 4 | 0.8 | 4 | 1.5 | 6.0 | 2 |
| Zinc, mg | 2.9 | 0.64 | 3 | 1.3 | 3.3 | 2 |
| Magnesium, mg | 124 | 20 | 92 | 51 | 91 | 44 |
| Sodium, mg | 5 | 2 | 5 | 10 | 4 | 5 |
| B-glucan, g | 0.7 | 0.08 | 1.9 | n.d | 4.4 | 4.0 |
Cinnamic Acid Derivatives | Substitutions | ||
|---|---|---|---|
| R1 | R2 | R3 | |
| Cinnamic acid | H | H | H |
| p-Coumaric acid | H | OH | H |
| Caffeic acid | OH | OH | H |
| Ferulic acid | CH3O | OH | H |
| Sinapic acid | CH3O | OH | CH3O |
Benzoic Acid Derivatives | Substitutions | ||
|---|---|---|---|
| R1 | R2 | R3 | |
| Benzoic acid | H | H | H |
| p-Hydroxybenzoic acid | H | OH | H |
| Protocatechuic acid | H | OH | OH |
| Vanillic acid | CH3O | OH | H |
| Syringic acid | CH3O | OH | CH3O |
| Gallic acid | OH | OH | OH |
| g/100 g | Wheat | Oat | Barley | Ref | |||
|---|---|---|---|---|---|---|---|
| Whole | Bran | Whole | Bran | Whole | Bran | ||
| TDF | 11.6–17.0 | 36.5–52.4 | 11.5–37.7 | 18.1–25.2 | 14.6–27.1 | - | [19,44] |
| IDF | 10.2–14.7 | 35.0–48.4 | 8.6–33.9 | 14.5–20.2 | 12.0–22.1 | - | [19,44] |
| SDF | 1.4–2.3 | 1.5–4.0 | 2.9–3.8 | 3.6–5.0 | 2.6–5.0 | - | [19,44] |
| FA | 4.5–1270 | 942–5 400 | 359 | - | 168–723 | 2002–2017 | [19,44] |
| PCA | 0.2–37.2 | 100–457 | - | - | 4–374 | 2565–3367 | [19,44] |
| SA | 1.3–63 | 300 | 55 | - | - | - | [19,44] |
| TPC | 350–1505 | 2800–5643 | 1223 | 1950 | 1022–1193 | - | [19,44] |
| Wheat Fractions | FA | DHD | DHT | SA | p-CA | Total | Ref |
|---|---|---|---|---|---|---|---|
| Bran | 5.26 | 1.01 | 0.24 | 0.25 | 0.09 | 6.85 | [30,45] |
| Endosperm | 0.10 | 0.03 | 0.00 | 0.01 | 0.00 | 0.14 | [30,45] |
| Aleurone | 8.17 | 1.07 | 0.11 | 0.44 | 0.21 | 10.00 | [30,45] |
| Intermediate layer | 5.92 | 0.91 | 0.07 | 0.08 | 0.07 | 7.05 | [30,45] |
| Pericarp | 8.18 | 5.12 | 1.21 | 0.01 | 0.04 | 14.56 | [30,45] |
| Scutellum | 3.48 | 0.37 | 0.03 | 0.01 | 0.01 | 3.90 | [30,45] |
| Cereals Bran | Major Antioxidants |
|---|---|
| Wheat | Ferulic, vanillic, caffeic, coumaric and syringic acid |
| Barley | Protocatechuic acid, p-hydroxybenzoic acid, salicylic acid, vanillic acid, syringic acid, ferulic acid, coumaric acid, sinapic acid |
| Oat | p-hydroxybenzoic acid, vanillic acid, |
| Rye | Protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, syringic acid, ferulic acid, p-coumaric acid, caffeic acid, sinapic acid |
| Source of Intake | Type 2 Diabetes (T2D) | Obesity | Cardiovascular Diseases (CVD) |
|---|---|---|---|
| Mixtures of whole grains and bran | Moderate | Moderate-Limited | Moderate |
| Cereal fiber | Moderate | Moderate-Limited | Moderate |
| Whole grains | Limited | Limited-Inadequate | Limited |
| Grain Fraction | Bioactive Compound | Whole Grain | Functional Potential | Reference |
|---|---|---|---|---|
| Pericarp, Testa and Aleurone | Arabinoxylans | Wheat, barley, rice, rye | Increase fecal biomass, enhance gut health and lipid metabolism | [130,131] |
| Phenolic Acids and Flavonoids | All | Enhance redox potential; antioxidant and anticancer effects; hepatoprotective and antiaging properties | [132,133,134] | |
| Aleurone | Minerals | All | Mg enhances cardiac health, sustains muscle properties; Fe, Zn and Cu sustain proper blood circulation, growth, development and body functions; Ca enhances bone health | [135,136,137] |
| Inulin | Wheat, barley, rye | Prebiotic effect; enhances gut health and glycemic response | [138] | |
| Endosperm | B-glucans | Oat, barley, rye | Decrease glycemic index; prebiotic effect | [139] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Călinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10111615
Călinoiu LF, Vodnar DC. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients. 2018; 10(11):1615. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10111615
Chicago/Turabian StyleCălinoiu, Lavinia Florina, and Dan Cristian Vodnar. 2018. "Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability" Nutrients 10, no. 11: 1615. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10111615