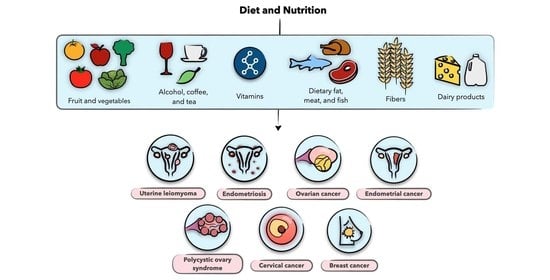
Diet and Nutrition in Gynecological Disorders: A Focus on Clinical Studies
Abstract
:1. Introduction
2. Search Criteria
3. An Overview of Dietary Constituents
3.1. Sources of Dietary Elements
3.2. Role in Health and Disease
3.3. Role of Diet in Oxidative Stress and Inflammation
4. Diet and Nutrition in Uterine Leiomyoma
4.1. Uterine Leiomyoma
4.2. Vegetables and Fruit
4.3. Vitamins
4.4. Dietary Fat, Meat, and Fish
4.5. Alcohol and Coffee
4.6. Epigallocatechin-3-Gallate (EGCG)
4.7. Miscellaneous
5. Diet and Nutrition in Endometriosis
5.1. Endometriosis
5.2. Vegetables and Fruit
5.3. Vitamins
5.4. Dietary Fat, Meat, and Fish
5.5. Whole Grain and Fiber
5.6. Plant-Derived Compounds
5.6.1. Resveratrol
5.6.2. Epigallocatechin-3-Gallate (EGCG)
5.6.3. Curcumin
6. Diet and Nutrition in Polycystic Ovary Syndrome
6.1. Polycystic Ovary Syndrome (PCOS)
6.2. Weight Loss Diets
6.3. Vitamin D
6.4. Coenzyme Q10 and Vitamin E
6.5. Inositol
6.6. Alternative Therapies
7. Diet and Nutrition in Gynecological Malignancies
7.1. Gynecological Malignancies
7.2. Diet and Nutrition in Cervical Cancer
7.3. Diet and Nutrition in Ovarian Cancer
7.4. Diet and Nutrition in Endometrial Cancer
7.5. Diet and Nutrition in Breast Cancer
8. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardozo, E.R.; Clark, A.D.; Banks, N.K.; Henne, M.B.; Stegmann, B.J.; Segars, J.H. The estimated annual cost of uterine leiomyomata in the United States. Am. J. Obstet. Gynecol. 2012, 206, 211.e1–211.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoens, S.; Dunselman, G.; Dirksen, C.; Hummelshoj, L.; Bokor, A.; Brandes, I.; Brodszky, V.; Canis, M.; Colombo, G.L.; DeLeire, T. The burden of endometriosis: Costs and quality of life of women with endometriosis and treated in referral centres. Hum. Reprod. 2012, 27, 1292–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azziz, R.; Marin, C.; Hoq, L.; Badamgarav, E.; Song, P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J. Clin. Endocrinol. Metab. 2005, 90, 4650–4658. [Google Scholar] [CrossRef] [Green Version]
- Pavone, D.; Clemenza, S.; Sorbi, F.; Fambrini, M.; Petraglia, F. Epidemiology and risk factors of uterine fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 46, 3–11. [Google Scholar] [CrossRef]
- Laughlin, S.K.; Schroeder, J.C.; Baird, D.D. New directions in the epidemiology of uterine fibroids. Semin. Reprod. Med. 2010, 28, 204–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinelli, A.; Vinciguerra, M.; Malvasi, A.; Andjić, M.; Babović, I.; Sparić, R. Uterine Fibroids and Diet. Int. J. Environ. Res. Public Health 2021, 18, 1066. [Google Scholar] [CrossRef]
- Parazzini, F.; Viganò, P.; Candiani, M.; Fedele, L. Diet and endometriosis risk: A literature review. Reprod. Biomed. Online 2013, 26, 323–336. [Google Scholar] [CrossRef] [Green Version]
- Harris, H.R.; Eke, A.C.; Chavarro, J.E.; Missmer, S.A. Fruit and vegetable consumption and risk of endometriosis. Hum. Reprod. 2018, 33, 715–727. [Google Scholar] [CrossRef]
- Norman, R.J.; Davies, M.J.; Lord, J.; Moran, L.J. The role of lifestyle modification in polycystic ovary syndrome. Trends Endocrinol. Metab. 2002, 13, 251–257. [Google Scholar] [CrossRef]
- Sirmans, S.M.; Pate, K.A. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin. Epidemiol. 2013, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bandera, E.V.; Kushi, L.H.; Moore, D.F.; Gifkins, D.M.; McCullough, M.L. Fruits and vegetables and endometrial cancer risk: A systematic literature review and meta-analysis. Nutr. Cancer 2007, 58, 6–21. [Google Scholar] [CrossRef] [PubMed]
- González, C.A.; Travier, N.; Luján-Barroso, L.; Castellsagué, X.; Bosch, F.X.; Roura, E.; Bueno-de-Mesquita, H.B.; Palli, D.; Boeing, H.; Pala, V.; et al. Dietary factors and in situ and invasive cervical cancer risk in the European prospective investigation into cancer and nutrition study. Int. J. Cancer 2011, 129, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Crane, T.E.; Khulpateea, B.R.; Alberts, D.S.; Basen-Engquist, K.; Thomson, C.A. Dietary intake and ovarian cancer risk: A systematic review. Cancer Epidemiol. Biomark. Prev. 2014, 23, 255–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castelló, A.; Pollán, M.; Buijsse, B.; Ruiz, A.; Casas, A.M.; Baena-Cañada, J.M.; Lope, V.; Antolín, S.; Ramos, M.; Muñoz, M.; et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: Case-control EpiGEICAM study. Br. J. Cancer 2014, 111, 1454–1462. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P. Nutrients and oxidative stress: Friend or foe? Oxid. Med. Cell Longev. 2018, 2018, 9719584. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A review of the role of green tea (Camellia sinensis) in antiphotoaging, stress resistance, neuroprotection, and autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef] [Green Version]
- Shenkin, A. Micronutrients in health and disease. Postgrad. Med. J. 2006, 82, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Ryan-Harshman, M.; Aldoori, W. Health benefits of selected vitamins. Can. Fam. Physician 2005, 51, 965–968. [Google Scholar]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [Green Version]
- Oteng, A.-B.; Kersten, S. Mechanisms of action of trans fatty acids. Adv. Nutr. 2020, 11, 697–708. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramteke, P.; Deb, A.; Shepal, V.; Bhat, M.K. Hyperglycemia associated metabolic and molecular alterations in cancer risk, progression, treatment, and mortality. Cancers 2019, 11, 1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AlAshqar, A.; Patzkowsky, K.; Afrin, S.; Wild, R.; Taylor, H.S.; Borahay, M.A. Cardiometabolic risk factors and benign gynecologic disorders. Obstet. Gynecol. 2019, 74, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P.; Bath, S.C.; Westaway, J.; Williams, P.; Mao, J.; Vanderlelie, J.J.; Perkins, A.V.; Redman, C.W.G. Selenium status in UK pregnant women and its relationship with hypertensive conditions of pregnancy. Br. J. Nutr. 2015, 113, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattmiller, S.A.; Carlson, B.A.; Sordillo, L.M. Regulation of inflammation by selenium and selenoproteins: Impact on eicosanoid biosynthesis. J. Nutr. Sci. 2013, 2, e28. [Google Scholar] [CrossRef] [Green Version]
- Lewandowska, M.G.; Sajdak, S.; Marciniak, W.; Lubiński, J. First trimester serum copper or zinc levels, and risk of pregnancy-induced hypertension. Nutrients 2019, 11, 2479. [Google Scholar] [CrossRef] [Green Version]
- Baird, D.D.; Dunson, D.B.; Hill, M.C.; Cousins, D.; Schectman, J.M. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am. J. Obstet. Gynecol. 2003, 188, 100–107. [Google Scholar] [CrossRef]
- Borahay, M.A.; Vincent, K.; Motamedi, M.; Sbrana, E.; Kilic, G.S.; Al-Hendy, A.; Boehning, D. Novel Effects of Simvastatin on Uterine Fibroids: In vitro and Patient-Derived Xenograft Mouse Model Study. Am. J. Obstet. Gynecol. 2015, 213, e191–e196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritton, K.; Borahay, M.A. New and emerging therapies for uterine fibroids. Semin. Reprod. Med. 2017, 35, 549–559. [Google Scholar] [CrossRef]
- Wise, L.A.; Radin, R.G.; Palmer, J.R.; Kumanyika, S.K.; Boggs, D.A.; Rosenberg, L. Intake of fruit, vegetables, and carotenoids in relation to risk of uterine leiomyomata. Am. J. Clin. Nutr. 2011, 94, 1620–1631. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Y.; Lu, Q.; Ren, M. Vegetarian diet and reduced uterine fibroids risk: A case–control study in Nanjing, China. J. Obstet. Gynaecol. Res. 2016, 42, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Chiaffarino, F.; Parazzini, F.; La Vecchia, C.; Chatenoud, L.; Di Cintio, E.; Marsico, S. Diet and uterine myomas. Obstet. Gynecol. 1999, 94, 395–398. [Google Scholar] [PubMed]
- He, Y.; Zeng, Q.; Dong, S.; Qin, L.; Li, G.; Wang, P. Associations between uterine fibroids and lifestyles including diet, physical activity and stress: A case-control study in China. Asia Pac. J. Clin. Nutr. 2013, 22, 109–117. [Google Scholar] [PubMed]
- Armstrong, B.K.; Brown, J.B.; Clarke, H.T.; Crooke, D.K.; Hähnel, R.; Masarei, J.R.; Ratajczak, T. Diet and reproductive hormones: A study of vegetarian and nonvegetarian postmenopausal women. J. Natl. Cancer Inst. 1981, 67, 761–767. [Google Scholar] [PubMed]
- Islam, M.S.; Akhtar, M.M.; Ciavattini, A.; Giannubilo, S.R.; Protic, O.; Janjusevic, M.; Procopio, A.D.; Segars, J.H.; Castellucci, M.; Ciarmela, P. Use of dietary phytochemicals to target inflammation, fibrosis, proliferation, and angiogenesis in uterine tissues: Promising options for prevention and treatment of uterine fibroids? Mol. Nutr. Food Res. 2014, 58, 1667–1684. [Google Scholar] [CrossRef] [Green Version]
- Terry, K.L.; Missmer, S.A.; Hankinson, S.E.; Willett, W.C.; De Vivo, I. Lycopene and other carotenoid intake in relation to risk of uterine leiomyomata. Am. J. Obstet. Gynecol. 2008, 198, 37. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.L.; Huber, L.R.B.; Thompson, M.E.; Racine, E.F. Serum micronutrient concentrations and risk of uterine fibroids. J. Women’s Health 2011, 20, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Mitro, S.D.; Zota, A.R. Vitamin D and uterine leiomyoma among a sample of US women: Findings from NHANES, 2001–2006. Reprod. Toxicol. 2015, 57, 81–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, D.D.; Hill, M.C.; Schectman, J.M.; Hollis, B.W. Vitamin D and risk of uterine fibroids. Epidemiology 2013, 24, 447. [Google Scholar] [CrossRef] [Green Version]
- Sabry, M.; Halder, S.K.; Allah, A.S.A.; Roshdy, E.; Rajaratnam, V.; Al-Hendy, A. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: A cross-sectional observational study. Int. J. Womens Health 2013, 5, 93–100. [Google Scholar]
- Paffoni, A.; Somigliana, E.; Vigano, P.; Benaglia, L.; Cardellicchio, L.; Pagliardini, L.; Papaleo, E.; Candiani, M.; Fedele, L. Vitamin D status in women with uterine leiomyomas. J. Clin. Endocrinol. Metab. 2013, 98, E1374–E1378. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Barik, A.; Imam, N. Vitamin D3 level in women with uterine fibroid: An observational study in eastern Indian population. J. Obstet. Gynaecol. India 2019, 69, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Ciavattini, A.; Carpini, G.D.; Serri, M.; Vignini, A.; Sabbatinelli, J.; Tozzi, A.; Aggiusti, A.; Clemente, N. Hypovitaminosis D and “small burden” uterine fibroids: Opportunity for a vitamin D supplementation. Medicine 2016, 95, e5698. [Google Scholar] [CrossRef] [PubMed]
- Oskovi Kaplan, Z.A.; Taşçi, Y.; Topçu, H.O.; Erkaya, S. 25-Hydroxy vitamin D levels in premenopausal Turkish women with uterine leiomyoma. Gynecol. Endocrinol. 2018, 34, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, B.; Sheng, B.; Wang, J.; Zhu, X. The associations between serum vitamin D, calcium and uterine fibroids in Chinese women: A case-controlled study. J. Int. Med. Res. 2020, 48, 1–11. [Google Scholar]
- Srivastava, P.; Gupta, H.P.; Singhi, S.; Khanduri, S.; Rathore, B. Evaluation of 25-hydroxy vitamin D3 levels in patients with a fibroid uterus. J. Obstet. Gynaecol. 2020, 40, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Włodarczyk, M.; Słabuszewska-Jóźwiak, A.; Nowicka, G.; Jakiel, G. Influence of vitamin D and transforming growth factor β3 serum concentrations, obesity, and family history on the risk for uterine fibroids. Fertil. Steril. 2016, 106, 1787–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasky, T.M.; Bethea, T.N.; Wesselink, A.K.; Wegienka, G.R.; Baird, D.D.; Wise, L.A. Dietary fat intake and risk of uterine leiomyomata: A prospective ultrasound study. Am. J. Epidemiol. 2020, 189, 1538–1546. [Google Scholar] [CrossRef]
- Nagata, C.; Nakamura, K.; Oba, S.; Hayashi, M.; Takeda, N.; Yasuda, K. Association of intakes of fat, dietary fibre, soya isoflavones and alcohol with uterine fibroids in Japanese women. Br. J. Nutr. 2009, 101, 1427–1431. [Google Scholar] [CrossRef] [Green Version]
- Borahay, M.A.; Fang, X.; Baillargeon, J.G.; Kilic, G.S.; Boehning, D.F.; Kuo, Y.F. Statin use and uterine fibroid risk in hyperlipidemia patients: A nested case-control study. Am. J. Obstet. Gynecol. 2016, 215, 751–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afrin, S.; Islam, M.S.; Patzkowsky, K.; Malik, M.; Catherino, W.H.; Segars, J.H.; Borahay, M.A. Simvastatin ameliorates altered mechanotransduction in uterine leiomyoma cells. Am. J. Obstet. Gynecol. 2020, 223, e731–e733. [Google Scholar] [CrossRef]
- Zeybek, B.; Costantine, M.; Kilic, G.S.; Borahay, M.A. Therapeutic Roles of Statins in Gynecology and Obstetrics: The Current Evidence. Reprod. Sci. 2018, 26, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.M.; Spiegelman, D.; Barbieri, R.L.; Goldman, M.B.; Manson, J.E.; Colditz, G.A.; Willett, W.C.; Hunter, D.J. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet. Gynecol. 1997, 90, 967–973. [Google Scholar] [CrossRef]
- Wise, L.A.; Palmer, J.R.; Harlow, B.L.; Spiegelman, D.; Stewart, E.A.; Adams-Campbell, L.L.; Rosenberg, L. Risk of uterine leiomyomata in relation to tobacco, alcohol and caffeine consumption in the Black Women’s Health Study. Hum. Reprod. 2004, 19, 1746–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Templeman, C.; Marshall, S.F.; Clarke, C.A.; Henderson, K.D.; Largent, J.; Neuhausen, S.; Reynolds, P.; Ursin, G.; Bernstein, L. Risk factors for surgically removed fibroids in a large cohort of teachers. Fertil. Steril. 2009, 92, 1436–1446. [Google Scholar] [CrossRef] [Green Version]
- Roshdy, E.; Rajaratnam, V.; Maitra, S.; Sabry, M.; Allah, A.S.A.; Al-Hendy, A. Treatment of symptomatic uterine fibroids with green tea extract: A pilot randomized controlled clinical study. Int. J. Womens Health 2013, 5, 477. [Google Scholar] [PubMed] [Green Version]
- Gao, M.; Wang, H. Frequent milk and soybean consumption are high risks for uterine leiomyoma: A prospective cohort study. Medicine 2018, 97, e12009. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Radin, R.G.; Palmer, J.R.; Kumanyika, S.K.; Rosenberg, L. A prospective study of dairy intake and risk of uterine leiomyomata. Am. J. Epidemiol. 2010, 171, 221–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- AlAshqar, A.; Reschke, L.; Kirschen, G.W.; Borahay, M.A. Role of Inflammation in Benign Gynecologic Disorders: From Pathogenesis to Novel Therapies. Biol. Reprod. 2021. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; Chiaffarino, F.; Surace, M.; Chatenoud, L.; Cipriani, S.; Chiantera, V.; Benzi, G.; Fedele, L. Selected food intake and risk of endometriosis. Hum. Reprod. 2004, 19, 1755–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trabert, B.; Peters, U.; De Roos, A.J.; Scholes, D.; Holt, V.L. Diet and risk of endometriosis in a population-based case–control study. Br. J. Nutr. 2011, 105, 459–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mier-Cabrera, J.; Genera-García, M.; De la Jara-Díaz, J.; Perichart-Perera, O.; Vadillo-Ortega, F.; Hernández-Guerrero, C. Effect of vitamins C and E supplementation on peripheral oxidative stress markers and pregnancy rate in women with endometriosis. Int. J. Gynaecol. Obstet. 2008, 100, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Mier-Cabrera, J.; Aburto-Soto, T.; Burrola-Méndez, S.; Jiménez-Zamudio, L.; Tolentino, M.C.; Casanueva, E.; Hernández-Guerrero, C. Women with endometriosis improved their peripheral antioxidant markers after the application of a high antioxidant diet. Reprod. Biol. Endocrinol. 2009, 7, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santanam, N.; Kavtaradze, N.; Murphy, A.; Dominguez, C.; Parthasarathy, S. Antioxidant supplementation reduces endometriosis-related pelvic pain in humans. Transl. Res. 2013, 161, 189–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyashita, M.; Koga, K.; Izumi, G.; Sue, F.; Makabe, T.; Taguchi, A.; Nagai, M.; Urata, Y.; Takamura, M.; Harada, M. Effects of 1, 25-dihydroxy vitamin D3 on endometriosis. J. Clin. Endocrinol. Metab. 2016, 101, 2371–2379. [Google Scholar] [CrossRef] [Green Version]
- Mehdizadehkashi, A.; Rokhgireh, S.; Tahermanesh, K.; Eslahi, N.; Minaeian, S.; Samimi, M. The effect of vitamin D supplementation on clinical symptoms and metabolic profiles in patients with endometriosis. Gynecol. Endocrinol. 2021. [Google Scholar] [CrossRef]
- Nodler, J.L.; DiVasta, A.D.; Vitonis, A.F.; Karevicius, S.; Malsch, M.; Sarda, V.; Fadayomi, A.; Harris, H.R.; Missmer, S.A. Supplementation with vitamin D or ω-3 fatty acids in adolescent girls and young women with endometriosis (SAGE): A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2020, 112, 229–236. [Google Scholar] [CrossRef]
- Almassinokiani, F.; Khodaverdi, S.; Solaymani-Dodaran, M.; Akbari, P.; Pazouki, A. Effects of vitamin D on endometriosis-related pain: A double-blind clinical trial. Med. Sci. Monit. 2016, 22, 4960. [Google Scholar] [CrossRef] [Green Version]
- Pazhohan, A.; Danaei-Mehrabad, S.; Mohamad-Rezaeii, Z.; Amidi, F.; Khodarahmian, M.; Shabani Nashtaei, M.; Sobhani, A.; Farajzadeh, M.A. The modulating effects of vitamin D on the activity of β-catenin in the endometrium of women with endometriosis: A randomized exploratory trial. Gynecol. Endocrinol. 2021, 37, 278–282. [Google Scholar] [CrossRef]
- Missmer, S.A.; Chavarro, J.E.; Malspeis, S.; Bertone-Johnson, E.R.; Hornstein, M.D.; Spiegelman, D.; Barbieri, R.L.; Willett, W.C.; Hankinson, S.E. A prospective study of dietary fat consumption and endometriosis risk. Hum. Reprod. 2010, 25, 1528–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heilier, J.F.; Donnez, J.; Nackers, F.; Rousseau, R.; Verougstraete, V.; Rosenkranz, K.; Donnez, O.; Grandjean, F.; Lison, D.; Tonglet, R. Environmental and host-associated risk factors in endometriosis and deep endometriotic nodules: A matched case-control study. Environ. Res. 2007, 103, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.-M.; Skakkebaek, N.E. Exposure to exogenous estrogens in food: Possible impact on human development and health. Eur. J. Endocrinol. 1999, 140, 477–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savaris, A.L.; do Amaral, V.F. Nutrient intake, anthropometric data and correlations with the systemic antioxidant capacity of women with pelvic endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 158, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef] [PubMed]
- Dull, A.-M.; Moga, M.A.; Dimienescu, O.G.; Sechel, G.; Burtea, V.; Anastasiu, C.V. Therapeutic approaches of resveratrol on endometriosis via anti-inflammatory and anti-angiogenic pathways. Molecules 2019, 24, 667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maia, H., Jr.; Haddad, C.; Pinheiro, N.; Casoy, J. Advantages of the association of resveratrol with oral contraceptives for management of endometriosis-related pain. Int. J. Womens Health 2012, 4, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Mendes da Silva, D.; Gross, L.A.; Neto, E.d.P.G.; Lessey, B.A.; Savaris, R.F. The use of resveratrol as an adjuvant treatment of pain in endometriosis: A randomized clinical trial. J. Endocr. Soc. 2017, 1, 359–369. [Google Scholar] [CrossRef]
- Ricci, A.G.; Olivares, C.N.; Bilotas, M.A.; Baston, J.I.; Singla, J.J.; Meresman, G.F.; Barañao, R.I. Natural therapies assessment for the treatment of endometriosis. Hum. Reprod. 2013, 28, 178–188. [Google Scholar] [CrossRef] [Green Version]
- Di Chen, S.B.W.; Yang, H.; Yuan, J.; Chan, T.H.; Dou, Q.P. EGCG, green tea polyphenols and their synthetic analogs and prodrugs for human cancer prevention and treatment. Adv. Clin. Chem. 2011, 53, 155–177. [Google Scholar]
- Bisht, K.; Wagner, K.-H.; Bulmer, A.C. Curcumin, resveratrol and flavonoids as anti-inflammatory, cyto-and DNA-protective dietary compounds. Toxicology 2010, 278, 88–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.-H.; Lee, E.N.; Park, J.K.; Lee, J.-R.; Kim, J.-H.; Choi, H.-J.; Kim, B.-S.; Lee, H.-W.; Lee, K.-S.; Yoon, S. Curcumin attenuates TNF-α-induced expression of intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and proinflammatory cytokines in human endometriotic stromal cells. Phytother. Res. 2012, 26, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wei, Y.-X.; Zhou, Q.; Zhang, Y.; Guo, X.-P.; Zhang, J. Inhibitory effect of curcumin in human endometriosis endometrial cells via downregulation of vascular endothelial growth factor. Mol. Med. Rep. 2017, 16, 5611–5617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jana, S.; Paul, S.; Swarnakar, S. Curcumin as anti-endometriotic agent: Implication of MMP-3 and intrinsic apoptotic pathway. Biochem. Pharmacol. 2012, 83, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Swarnakar, S.; Paul, S. Curcumin arrests endometriosis by downregulation of matrix metalloproteinase-9 activity. Indian J. Biochem. Biophys. 2009, 46, 59–65. [Google Scholar] [PubMed]
- Lizneva, D.S.L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, R.K. Polycystic Ovary Syndrome. Nurs. Clin. N. Am. 2018, 53, 407–420. [Google Scholar] [CrossRef]
- Nardo, L.G.; Patchava, S.; Laing, I. Polycystic ovary syndrome: Pathophysiology, molecular aspects and clinical implications. Panminerva Med. 2008, 50, 267–278. [Google Scholar]
- Carmina, E.; Lobo, R.A. Use of fasting blood to assess the prevalence of insulin resistance in women with polycystic ovary syndrome. Fertil. Steril. 2004, 82, 661–665. [Google Scholar] [CrossRef]
- Crosignani, P.G.; Colombo, M.; Vegetti, W.; Somigliana, E.; Gessati, A.; Ragni, G. Overweight and obese anovulatory patients with polycystic ovaries: Parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum. Reprod. 2003, 18, 1928–1932. [Google Scholar] [CrossRef] [Green Version]
- Nybacka, A.; Carlstrom, K.; Stahle, A.; Nyren, S.; Hellstrom, P.M.; Hirschberg, A.L. Randomized comparison of the influence of dietary management and/or physical exercise on ovarian function and metabolic parameters in overweight women with polycystic ovary syndrome. Fertil. Steril. 2011, 96, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Einarsson, S.; Bergh, C.; Friberg, B.; Pinborg, A.; Klajnbard, A.; Karlstrom, P.O.; Kluge, L.; Larsson, I.; Loft, A.; Mikkelsen-Englund, A.L.; et al. Weight reduction intervention for obese infertile women prior to IVF: A randomized controlled trial. Hum. Reprod. 2017, 32, 1621–1630. [Google Scholar] [CrossRef] [Green Version]
- Marzouk, T.M.; Sayed Ahmed, W.A. Effect of Dietary Weight Loss on Menstrual Regularity in Obese Young Adult Women with Polycystic Ovary Syndrome. J. Pediatr. Adolesc. Gynecol. 2015, 28, 457–461. [Google Scholar] [CrossRef]
- Thomson, R.L.; Buckley, J.D.; Lim, S.S.; Noakes, M.; Clifton, P.M.; Norman, R.J.; Brinkworth, G.D. Lifestyle management improves quality of life and depression in overweight and obese women with polycystic ovary syndrome. Fertil. Steril. 2010, 94, 1812–1816. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; McBreairty, L.E.; Chizen, D.R.; Pierson, R.A.; Chilibeck, P.D.; Zello, G.A. A comparison of a pulse-based diet and the therapeutic lifestyle changes diet in combination with exercise and health counselling on the cardio-metabolic risk profile in women with polycystic ovary syndrome: A randomized controlled trial. Nutrients 2018, 10, 1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asemi, Z.; Esmaillzadeh, A. DASH diet, insulin resistance, and serum hs-CRP in polycystic ovary syndrome: A randomized controlled clinical trial. Horm. Metab. Res. 2015, 47, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Azadi-Yazdi, M.; Karimi-Zarchi, M.; Salehi-Abargouei, A.; Fallahzadeh, H.; Nadjarzadeh, A. Effects of dietary approach to stop hypertension diet on androgens, antioxidant status and body composition in overweight and obese women with polycystic ovary syndrome: A randomised controlled trial. J. Hum. Nutr. Diet. 2017, 30, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. Effects of caloric intake timing on insulin resistance and hyperandrogenism in lean women with polycystic ovary syndrome. Clin. Sci. 2013, 125, 423–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papakonstantinou, E.; Kechribari, I.; Mitrou, P.; Trakakis, E.; Vassiliadi, D.; Georgousopoulou, E.; Zampelas, A.; Kontogianni, M.D.; Dimitriadis, G. Effect of meal frequency on glucose and insulin levels in women with polycystic ovary syndrome: A randomised trial. Eur. J. Clin. Nutr. 2016, 70, 588–594. [Google Scholar] [CrossRef]
- Stamets, K.; Taylor, D.S.; Kunselman, A.; Demers, L.M.; Pelkman, C.L.; Legro, R.S. A randomized trial of the effects of two types of short-term hypocaloric diets on weight loss in women with polycystic ovary syndrome. Fertil. Steril. 2004, 81, 630–637. [Google Scholar] [CrossRef]
- Sorensen, L.B.; Soe, M.; Halkier, K.H.; Stigsby, B.; Astrup, A. Effects of increased dietary protein-to-carbohydrate ratios in women with polycystic ovary syndrome. Am. J. Clin. Nutr. 2012, 95, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Kasim-Karakas, S.E.; Almario, R.U.; Cunningham, W. Effects of protein versus simple sugar intake on weight loss in polycystic ovary syndrome (according to the National Institutes of Health criteria). Fertil. Steril. 2009, 92, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Toscani, M.K.; Mario, F.M.; Radavelli-Bagatini, S.; Wiltgen, D.; Matos, M.C.; Spritzer, P.M. Effect of high-protein or normal-protein diet on weight loss, body composition, hormone, and metabolic profile in southern Brazilian women with polycystic ovary syndrome: A randomized study. Gynecol. Endocrinol. 2011, 27, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.; Krssak, M.; Bernroider, E.; Anderwald, C.; Brehm, A.; Meyerspeer, M.; Nowotny, P.; Roth, E.; Waldhausl, W.; Roden, M. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes 2002, 51, 599–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linn, T.; Santosa, B.; Gronemeyer, D.; Aygen, S.; Scholz, N.; Busch, M.; Bretzel, R.G. Effect of long-term dietary protein intake on glucose metabolism in humans. Diabetologia 2000, 43, 1257–1265. [Google Scholar] [CrossRef] [Green Version]
- Galletly, C.; Moran, L.; Noakes, M.; Clifton, P.; Tomlinson, L.; Norman, R. Psychological benefits of a high-protein, low-carbohydrate diet in obese women with polycystic ovary syndrome—A pilot study. Appetite 2007, 49, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Gower, B.A.; Chandler-Laney, P.C.; Ovalle, F.; Goree, L.L.; Azziz, R.; Desmond, R.A.; Granger, W.M.; Goss, A.M.; Bates, G.W. Favourable metabolic effects of a eucaloric lower-carbohydrate diet in women with PCOS. Clin. Endocrinol. 2013, 79, 550–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, S.; Reeves, S.; Sharp, K.; Jeanes, Y.M. An isocaloric low glycemic index diet improves insulin sensitivity in women with polycystic ovary syndrome. J. Acad. Nutr. Diet. 2013, 113, 1523–1531. [Google Scholar] [CrossRef]
- Liu, S.; Willett, W.C.; Stampfer, M.J.; Hu, F.B.; Franz, M.; Sampson, L.; Hennekens, C.H.; Manson, J.E. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am. J. Clin. Nutr. 2000, 71, 1455–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKeown, N.M.; Meigs, J.B.; Liu, S.; Saltzman, E.; Wilson, P.W.; Jacques, P.F. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care 2004, 27, 538–546. [Google Scholar] [CrossRef] [Green Version]
- Perelman, D.; Coghlan, N.; Lamendola, C.; Carter, S.; Abbasi, F.; McLaughlin, T. Substituting poly- and mono-unsaturated fat for dietary carbohydrate reduces hyperinsulinemia in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2017, 33, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Kasim-Karakas, S.E.; Almario, R.U.; Gregory, L.; Wong, R.; Todd, H.; Lasley, B.L. Metabolic and endocrine effects of a polyunsaturated fatty acid-rich diet in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 615–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phelan, N.; O’Connor, A.; Kyaw Tun, T.; Correia, N.; Boran, G.; Roche, H.M.; Gibney, J. Hormonal and metabolic effects of polyunsaturated fatty acids in young women with polycystic ovary syndrome: Results from a cross-sectional analysis and a randomized, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2011, 93, 652–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, J.M.; Gallagher, M.; Gooding, H.; Feldman, H.A.; Gordon, C.M.; Ludwig, D.S.; Ebbeling, C.B. A randomized pilot study of dietary treatments for polycystic ovary syndrome in adolescents. Pediatr. Obes. 2016, 11, 210–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner-McGrievy, G.M.; Davidson, C.R.; Wingard, E.E.; Billings, D.L. Low glycemic index vegan or low-calorie weight loss diets for women with polycystic ovary syndrome: A randomized controlled feasibility study. Nutr. Res. 2014, 34, 552–558. [Google Scholar] [CrossRef]
- Ardabili, H.R.; Gargari, B.P.; Farzadi, L. Vitamin D supplementation has no effect on insulin resistance assessment in women with polycystic ovary syndrome and vitamin D deficiency. Nutr. Res. 2012, 32, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Foroozanfard, F.; Rahmani, E.; Talebi, M.; Bahmani, F.; Asemi, Z. Effect of two different doses of vitamin D supplementation on metabolic profiles of insulin-resistant patients with polycystic ovary syndrome. Nutrients 2017, 9, 1280. [Google Scholar] [CrossRef] [Green Version]
- Pal, L.; Berry, A.; Coraluzzi, L.; Kustan, E.; Danton, C.; Shaw, J.; Taylor, H. Therapeutic implications of vitamin D and calcium in overweight women with polycystic ovary syndrome. Gynecol. Endocrinol. 2012, 28, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Jafari-Sfidvajani, S.; Ahangari, R.; Hozoori, M.; Mozaffari-Khosravi, H.; Fallahzadeh, H.; Nadjarzadeh, A. The effect of vitamin D supplementation in combination with low-calorie diet on anthropometric indices and androgen hormones in women with polycystic ovary syndrome: A double-blind, randomized, placebo-controlled trial. J. Endocrinol. Investig. 2018, 41, 597–607. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Ashraf, A. Role of vitamin d in insulin secretion and insulin sensitivity for glucose homeostasis. Int. J. Endocrinol. 2010, 2010, 351385. [Google Scholar] [CrossRef] [Green Version]
- Izadi, A.; Shirazi, S.; Taghizadeh, S.; Gargari, B.P. Independent and Additive Effects of Coenzyme Q10 and Vitamin E on Cardiometabolic Outcomes and Visceral Adiposity in Women With Polycystic Ovary Syndrome. Arch. Med. Res. 2019, 50, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Izadi, A.; Ebrahimi, S.; Shirazi, S.; Taghizadeh, S.; Parizad, M.; Farzadi, L.; Gargari, B.P. Hormonal and metabolic effects of coenzyme Q10 and/or vitamin E in patients with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 319–327. [Google Scholar] [CrossRef]
- Shokrpour, M.; Asemi, Z. The effects of magnesium and vitamin E co-supplementation on hormonal status and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. Biol. Trace Elem. Res. 2019, 191, 54–60. [Google Scholar] [CrossRef]
- Genazzani, A.D. Inositol as putative integrative treatment for PCOS. Reprod. Biomed. Online 2016, 33, 770–780. [Google Scholar] [CrossRef] [Green Version]
- Genazzani, A.D.; Prati, A.; Santagni, S.; Ricchieri, F.; Chierchia, E.; Rattighieri, E.; Campedelli, A.; Simoncini, T.; Artini, P.G. Differential insulin response to myo-inositol administration in obese polycystic ovary syndrome patients. Gynecol. Endocrinol. 2012, 28, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.D.; Santagni, S.; Rattighieri, E.; Chierchia, E.; Despini, G.; Marini, G.; Prati, A.; Simoncini, T. Modulatory role of D-chiro-inositol (DCI) on LH and insulin secretion in obese PCOS patients. Gynecol. Endocrinol. 2014, 30, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, A.; Lagana, A.S.; Barbaro, L. Comparison between effects of myo-inositol and D-chiro-inositol on ovarian function and metabolic factors in women with PCOS. Gynecol. Endocrinol. 2014, 30, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Coskun, A.; Arikan, T.; Kilinc, M.; Arikan, D.C.; Ekerbicer, H.C. Plasma selenium levels in Turkish women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Jamilian, M.; Kashan, Z.F.; Heidar, Z.; Mohseni, M.; Ghandi, Y.; Bagherian, T.; Asemi, Z. Selenium supplementation and the effects on reproductive outcomes, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome. Horm. Metab. Res. 2016, 48, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Karamali, M.; Kashanian, M.; Alaeinasab, S.; Asemi, Z. The effect of dietary soy intake on weight loss, glycaemic control, lipid profiles and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: A randomised clinical trial. J. Hum. Nutr. Diet. 2018, 31, 533–543. [Google Scholar] [CrossRef]
- USCS. United States Cancer Statistics: Data Visualizations. Available online: https://gis.cdc.gov/Cancer/USCS/DataViz.html. (accessed on 23 March 2021).
- Shaw, E.; Farris, M.; McNeil, J.; Friedenreich, C. Obesity and Endometrial Cancer. Recent Results Cancer Res. 2016, 208, 107–136. [Google Scholar] [PubMed]
- Garcia-Closas, R.; Castellsague, X.; Bosch, X.; Gonzalez, C. The role of diet and nutrition in cervical carcinogenesis: A review of recent evidence. Int. J. Cancer 2005, 117, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Hu, T.; Hang, C.-Y.; Yang, R.; Li, X.; Chen, Z.-L.; Mei, Y.-D.; Zhang, Q.-H.; Huang, K.-C.; Xiang, Q.-Y. Case-control study of diet in patients with cervical cancer or precancerosis in Wufeng, a high incidence region in China. Asian Pac. J. Cancer Prev. 2012, 13, 5299–5302. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H. Green tea: Health benefits as cancer preventive for humans. Chem. Rec. 2005, 5, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, M.; Saha, A.; Fujiki, H. New cancer treatment strategy using combination of green tea catechins and anticancer drugs. Cancer Sci. 2011, 102, 317–323. [Google Scholar] [CrossRef]
- Sedjo, R.L.; Roe, D.J.; Abrahamsen, M.; Harris, R.B.; Craft, N.; Baldwin, S.; Giuliano, A.R. Vitamin A, carotenoids, and risk of persistent oncogenic human papillomavirus infection. Cancer Epidemiol. Biomark. Prev. 2002, 11, 876–884. [Google Scholar]
- Ghosh, C.; Baker, J.A.; Moysich, K.B.; Rivera, R.; Brasure, J.R.; McCann, S.E. Dietary intakes of selected nutrients and food groups and risk of cervical cancer. Nutr. Cancer 2008, 60, 331–341. [Google Scholar] [CrossRef]
- VanEenwyk, J.; Davis, F.G.; Colman, N. Folate, vitamin C, and cervical intraepithelial neoplasia. Cancer Epidemiol. Biomark. Prev. 1992, 1, 119–124. [Google Scholar]
- Slattery, M.L.; Abbott, T.M.; Overall, J.C., Jr.; Robison, L.M.; French, T.K.; Jolles, C.; Gardner, J.W.; West, D.W. Dietary vitamins A, C, and E and selenium as risk factors for cervical cancer. Epidemiology 1990, 1, 8–15. [Google Scholar] [CrossRef]
- Butterworth, C.E.; Hatch, K.D.; Gore, H.; Mueller, H.; Krumdieck, C.L. Improvement in cervical dysplasia associated with folic acid therapy in users of oral contraceptives. Am. J. Clin. Nutr. 1982, 35, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Butterworth, C.E.; Hatch, K.D.; Macaluso, M.; Cole, P.; Sauberlich, H.E.; Soong, S.-J.; Borst, M.; Baker, V.V. Folate deficiency and cervical dysplasia. JAMA 1992, 267, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Hao, M.; Wang, Y.; Feng, N.; Wang, Z.; Wang, W.; Wang, J.; Ding, L. Association between folate status and cervical intraepithelial neoplasia. Eur. J. Clin. Nutr. 2016, 70, 837–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrero, R.; Potischman, N.; Brinton, L.A.; Reeves, W.C.; Brenes, M.M.; Tenorio, F.; de Britton, R.C.; Gaitan, E. A case-control study of nutrient status and invasive cervical cancer: I. Dietary indicators. Am. J. Epidemiol. 1991, 134, 1335–1346. [Google Scholar] [CrossRef] [PubMed]
- AICR, W. World Cancer Research Fund/American Institute for Cancer Research: Diet, Nutrition, Physical Activity and Ovarian Cancer. Continuous Update Project Expert Report. Available online: https://www.wcrf.org/dietandcancer/ovarian-cancer (accessed on 12 March 2021).
- Zhang, M.; Yang, Z.Y.; Binns, C.W.; Lee, A.H. Diet and ovarian cancer risk: A case-control study in China. Br. J. Cancer 2002, 86, 712–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekström, A.M.; Serafini, M.; Nyrén, O.; Hansson, L.-E.; Ye, W.; Wolk, A. Dietary antioxidant intake and the risk of cardia cancer and noncardia cancer of the intestinal and diffuse types: A population-based case-control study in Sweden. Int. J. Cancer 2000, 87, 133–140. [Google Scholar] [CrossRef]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit, and cancer prevention: A review. J. Acad. Nutr. Diet. 1996, 96, 1027–1039. [Google Scholar]
- Ji, B.-T.; Chow, W.-H.; Yang, G.; McLaughlin, J.K.; Zheng, W.; Shu, X.-O.; Jin, F.; Gao, R.-N.; Gao, Y.-T.; Fraumeni, F.J., Jr. Dietary habits and stomach cancer in Shanghai, China. Int. J. Cancer 1998, 76, 659–664. [Google Scholar] [CrossRef]
- O’Neill, I.K.; Chen, J.; Bartsch, H. International Agency for Research on Cancer. Relevance to Human Cancer of N-Nitroso Compounds, Tobacco, and Mycotoxins; International Agency for Research on Cancer; IARC Publications: Lyon, France, 1991; p. 614. [Google Scholar]
- Blank, M.M.; Wentzensen, N.; Murphy, M.A.; Hollenbeck, A.; Park, Y. Dietary fat intake and risk of ovarian cancer in the NIH-AARP Diet and Health Study. Br. J. Cancer 2012, 106, 596–602. [Google Scholar] [CrossRef] [Green Version]
- Larsson, S.C.; Bergkvist, L.; Wolk, A. Milk and lactose intakes and ovarian cancer risk in the Swedish Mammography Cohort. Am. J. Clin. Nutr. 2004, 80, 1353–1357. [Google Scholar] [CrossRef] [Green Version]
- Birt, D.F. The influence of dietary fat on carcinogenesis: Lessons from experimental models. Nutr. Rev. 1990, 48, 1–5. [Google Scholar] [CrossRef]
- Ness, R.B.; Cottreau, C. Possible role of ovarian epithelial inflammation in ovarian cancer. J. Natl. Cancer Inst. 1999, 91, 1459–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, D.P. Diet, hormones, and cancer. Annu. Rev. Public Health 1993, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Prentice, R.L.; Thomson, C.A.; Caan, B.; Hubbell, F.A.; Anderson, G.L.; Beresford, S.A.A.; Pettinger, M.; Lane, D.S.; Lessin, L.; Yasmeen, S. Low-fat dietary pattern and cancer incidence in the Women’s Health Initiative Dietary Modification Randomized Controlled Trial. J. Natl. Cancer Inst. 2007, 99, 1534–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairfield, K.M.; Hunter, D.J.; Colditz, G.A.; Fuchs, C.S.; Cramer, D.W.; Speizer, F.E.; Willett, W.C.; Hankinson, S.E. A prospective study of dietary lactose and ovarian cancer. Int. J. Cancer 2004, 110, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Rota, M.; Pasquali, E.; Scotti, L.; Pelucchi, C.; Tramacere, I.; Islami, F.; Negri, E.; Boffetta, P.; Bellocco, R.; Corrao, G. Alcohol drinking and epithelial ovarian cancer risk. A systematic review and meta-analysis. Gynecol. Oncol. 2012, 125, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Foong, K.W.; Bolton, H. Obesity and ovarian cancer risk: A systematic review. Post Reprod. Health 2017, 23, 183–198. [Google Scholar] [CrossRef]
- Wang, T.; Rohan, T.E.; Gunter, M.J.; Xue, X.; Wactawski-Wende, J.; Rajpathak, S.N.; Cushman, M.; Strickler, H.D.; Kaplan, R.C.; Wassertheil-Smoller, S. A prospective study of inflammation markers and endometrial cancer risk in postmenopausal hormone nonusers. Cancer Epidemiol. Biomark. Prev. 2011, 20, 971–977. [Google Scholar] [CrossRef] [Green Version]
- Dossus, L.; Rinaldi, S.; Becker, S.; Lukanova, A.; Tjonneland, A.; Olsen, A.; Stegger, J.; Overvad, K.; Chabbert-Buffet, N.; Jimenez-Corona, A. Obesity, inflammatory markers, and endometrial cancer risk: A prospective case-control study. Endocr. Relat. Cancer 2010, 17, 1007–1019. [Google Scholar] [CrossRef]
- Ricceri, F.; Giraudo, M.T.; Fasanelli, F.; Milanese, D.; Sciannameo, V.; Fiorini, L.; Sacerdote, C. Diet and endometrial cancer: A focus on the role of fruit and vegetable intake, Mediterranean diet and dietary inflammatory index in the endometrial cancer risk. BMC Cancer 2017, 17, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bravi, F.; Scotti, L.; Bosetti, C.; Zucchetto, A.; Talamini, R.; Montella, M.; Greggi, S.; Pelucchi, C.; Negri, E.; Franceschi, S. Food groups and endometrial cancer risk: A case-control study from Italy. Am. J. Obstet. Gynecol. 2009, 200, 293.e1–293.e7. [Google Scholar] [CrossRef]
- Filomeno, M.; Bosetti, C.; Bidoli, E.; Levi, F.; Serraino, D.; Montella, M.; La Vecchia, C.; Tavani, A. Mediterranean diet and risk of endometrial cancer: A pooled analysis of three Italian case-control studies. Br. J. Cancer 2015, 112, 1816–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckland, G.; Travier, N.; Cottet, V.; Gonzalez, C.A.; Luján-Barroso, L.; Agudo, A.; Trichopoulou, A.; Lagiou, P.; Trichopoulos, D.; Peeters, P.H. Adherence to the mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int. J. Cancer 2013, 132, 2918–2927. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kang, D.; Lee, S.A. Effect of dietary patterns on serum C-reactive protein level. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Sales-Campos, H.; Reis de Souza, P.; Crema Peghini, B.; Santana da Silva, J.; Ribeiro Cardoso, C. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar] [PubMed]
- Khodarahmi, M.; Azadbakht, L. The association between different kinds of fat intake and breast cancer risk in women. Int. J. Prev. Med. 2014, 5, 6–15. [Google Scholar] [PubMed]
- Schouten, L.J.; Goldbohm, R.A.; Van Den Brandt, P.A. Anthropometry, physical activity, and endometrial cancer risk: Results from the Netherlands Cohort Study. J. Natl. Cancer Inst. 2004, 96, 1635–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dougan, M.M.; Hankinson, S.E.; Vivo, I.D.; Tworoger, S.S.; Glynn, R.J.; Michels, K.B. Prospective study of body size throughout the life-course and the incidence of endometrial cancer among premenopausal and postmenopausal women. Int. J. Cancer 2015, 137, 625–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, E.R.; Mendelson, C.R. Effect of aging and obesity on aromatase activity of human adipose cells. Am. J. Clin. Nutr. 1987, 45, 290–295. [Google Scholar] [CrossRef]
- Simó, R.; Sáez-López, C.; Barbosa-Desongles, A.; Hernández, C.; Selva, D.M. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol. Metab. 2015, 26, 376–383. [Google Scholar] [CrossRef] [PubMed]
- McCampbell, A.S.; Broaddus, R.R.; Loose, D.S.; Davies, P.J.A. Overexpression of the insulin-like growth factor I receptor and activation of the AKT pathway in hyperplastic endometrium. Clin. Cancer Res. 2006, 12, 6373–6378. [Google Scholar] [CrossRef] [Green Version]
- Mu, N.; Zhu, Y.; Wang, Y.; Zhang, H.; Xue, F. Insulin resistance: A significant risk factor of endometrial cancer. Gynecol. Oncol. 2012, 125, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Onstad, M.A.; Schmandt, R.E.; Lu, K.H. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J. Clin. Oncol. 2016, 34, 4225–4230. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, M. The second world cancer research fund/american institute for cancer research expert report. Food, nutrition, physical activity, and the prevention of cancer: A global perspective. Proc. Nutr. Soc. 2008, 67, 253–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, R.; Orsini, N.; Mignone, L.; Saji, S.; Wolk, A. Alcohol intake and risk of breast cancer defined by estrogen and progesterone receptor status- a meta-analysis of epidemiological studies. Int. J. Cancer 2008, 122, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Gavaler, J.S.; Rosenblum, E. Exposure-dependent effects of ethanol on serum estradiol and uterus mass in sexually mature oophorectomized rats: A model for bilaterally ovariectomized-postmenopausal women. J. Stud. Alcohol 1987, 48, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Meng, Q.; Gao, B.; Grossman, J.; Yadegari, M.; Goldberg, I.D.; Rosen, E.M. Alcohol stimulates estrogen receptor signaling in human breast cancer cell lines. Cancer Res. 2000, 60, 5635–5639. [Google Scholar]
- Etique, N.; Grillier-Vuissoz, I.; Flament, S. Ethanol stimulates the secretion of matrix metalloproteinases 2 and 9 in MCF-7 human breast cancer cells. Oncol. Rep. 2006, 15, 603–608. [Google Scholar] [CrossRef] [Green Version]
- Freedman, L.S.; Kipnis, V.; Schatzkin, A.; Potischman, N. Methods of epidemiology: Evaluating the fat-breast cancer hypothesis-comparing dietary instruments and other developments. Cancer J. 2008, 14, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.-S.; Hu, X.-J.; Zhao, Y.-M.; Yang, J.; Li, D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: Meta-analysis of data from 21 independent prospective cohort studies. BMJ 2013, 346, f3706. [Google Scholar] [CrossRef] [Green Version]
- Chajès, V.; Torres-Mejía, G.; Biessy, C.; Ortega-Olvera, C.; Angeles-Llerenas, A.; Ferrari, P.; Lazcano-Ponce, E.; Romieu, I. ω-3 and ω-6 Polyunsaturated fatty acid intakes and the risk of breast cancer in Mexican women: Impact of obesity status. Cancer Epidemiol. Biomark. Prev. 2012, 21, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Chajès, V.; Thiébaut, A.C.M.; Rotival, M.; Gauthier, E.; Maillard, V.; Boutron-Ruault, M.-C.; Joulin, V.; Lenoir, G.M.; Clavel-Chapelon, F. Association between serum trans-monounsaturated fatty acids and breast cancer risk in the E3N-EPIC Study. Am. J. Epidemiol. 2008, 167, 1312–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chajes, V.; Joulin, V.; Clavel-Chapelon, F. The fatty acid desaturation index of blood lipids, as a biomarker of hepatic stearoyl-CoA desaturase expression, is a predictive factor of breast cancer risk. Curr. Opin. Lipidol. 2011, 22, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shikany, J.M.; Redden, D.T.; Neuhouser, M.L.; Chlebowski, R.T.; Rohan, T.E.; Simon, M.S.; Liu, S.; Lane, D.S.; Tinker, L. Dietary glycemic load, glycemic index, and carbohydrate and risk of breast cancer in the Women’s Health Initiative. Nutr. Cancer 2011, 63, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Barclay, A.W.; Petocz, P.; McMillan-Price, J.; Flood, V.M.; Prvan, T.; Mitchell, P.; Brand-Miller, J.C. Glycemic index, glycemic load, and chronic disease risk-a meta-analysis of observational studies. Am. J. Clin. Nutr. 2008, 87, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Wolever, T.M.; Collier, G.R.; Ocana, A.; Rao, A.V.; Buckley, G.; Lam, Y.; Mayer, A.; Thompson, L.U. Metabolic effects of a low-glycemic-index diet. Am. J. Clin. Nutr. 1987, 46, 968–975. [Google Scholar] [CrossRef]
- Li, F.; Zhu, Y.; Ding, L.; Zhang, Y. Effects of dietary glucose on serum estrogen levels and onset of puberty in gilts. Asian-Australas. J. Animal Sci. 2016, 29, 1309–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Rohan, T. Role of the insulin-like growth factor family in cancer development and progression. J. Natl. Cancer Inst. 2000, 92, 1472–1489. [Google Scholar] [CrossRef]
- Bauer, S.R.; Hankinson, S.E.; Bertone-Johnson, E.R.; Ding, E.L. Plasma vitamin D levels, menopause, and risk of breast cancer: Dose-response meta-analysis of prospective studies. Medicine 2013, 92, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühn, T.; Kaaks, R.; Becker, S.; Eomois, P.-P.; Clavel-Chapelon, F.; Kvaskoff, M.; Dossus, L.; Tjønneland, A.; Olsen, A.; Overvad, K. Plasma 25-hydroxyvitamin D and the risk of breast cancer in the European prospective investigation into cancer and nutrition: A nested case–control study. Int. J. Cancer 2013, 133, 1689–1700. [Google Scholar] [CrossRef] [Green Version]
- Engin, A. Obesity-associated breast cancer: Analysis of risk factors. Adv. Exp. Med. Biol. 2017, 960, 571–606. [Google Scholar]
- Chlebowski, R.T.; Aiello, E.; McTiernan, A. Weight loss in breast cancer patient management. J. Clin. Oncol. 2002, 20, 1128–1143. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afrin, S.; AlAshqar, A.; El Sabeh, M.; Miyashita-Ishiwata, M.; Reschke, L.; Brennan, J.T.; Fader, A.; Borahay, M.A. Diet and Nutrition in Gynecological Disorders: A Focus on Clinical Studies. Nutrients 2021, 13, 1747. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13061747
Afrin S, AlAshqar A, El Sabeh M, Miyashita-Ishiwata M, Reschke L, Brennan JT, Fader A, Borahay MA. Diet and Nutrition in Gynecological Disorders: A Focus on Clinical Studies. Nutrients. 2021; 13(6):1747. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13061747
Chicago/Turabian StyleAfrin, Sadia, Abdelrahman AlAshqar, Malak El Sabeh, Mariko Miyashita-Ishiwata, Lauren Reschke, Joshua T. Brennan, Amanda Fader, and Mostafa A. Borahay. 2021. "Diet and Nutrition in Gynecological Disorders: A Focus on Clinical Studies" Nutrients 13, no. 6: 1747. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13061747