More than a Toxin: Protein Inventory of Clostridium tetani Toxoid Vaccines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Vaccines Used in This Study
2.2. Tryptic Digest and C18 Clean up
2.3. Mass Spectrometry
2.4. Label-Free Quantitative Protein Analysis
2.5. Proteome Prediction of C. tetani
2.6. Prediction of Putative Antigens
2.7. Data Availability Statement
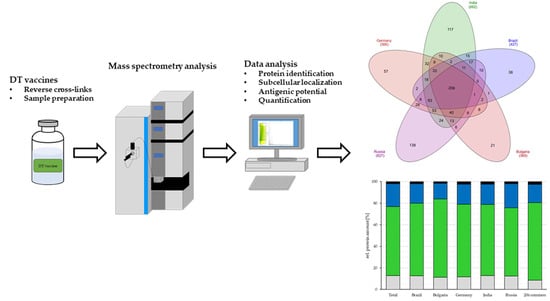
3. Results
3.1. Mass Spectrometric Analysis of Tetanus Vaccines
3.1.1. Identification of Proteins
3.1.2. Protein Localization
3.1.3. Bioinformatic Analysis of the 54 Common Unique Proteins in All Analyzed Vaccines
3.1.4. Quantification of Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schiavo, G.; Poulain, B.; Rossetto, O.; Benfenati, F.; Tauc, L.; Montecucco, C. Tetanus toxin is a zinc protein and its inhibition of neurotransmitter release and protease activity depend on zinc. EMBO J. 1992, 11, 3577–3583. [Google Scholar] [CrossRef] [PubMed]
- WHO position paper Diphtheria vaccine: WHO position paper. Wkly epidemiol. Rec. 2017, 31, 417–436.
- Cohen, J.E.; Wang, R.; Shen, R.F.; Wu, W.W.; Keller, J.E. Comparative pathogenomics of Clostridium tetani. PLoS ONE 2017, 12, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Roper, M.H.; Wassilak, S.G.F.; Tiwari, T.S.P.; Orenstein, W.A. Tetanus Toxoid. In Plotkin’s Vaccines, 7th ed.; Pltokin, S.A., Orenstein, W.A., Offit, P.A., Edwards, K.M., Eds.; Elsevier: Philadelphia, PA, USA, 2017; pp. 1052–1079. [Google Scholar] [CrossRef]
- Tjalsma, H.; Lambooy, L.; Hermans, P.W.; Swinkels, D.W. Shedding & shaving: Disclosure of proteomic expressions on a bacterial face. Proteomics 2008, 8, 1415–1428. [Google Scholar] [CrossRef]
- Hansmeier, N.; Chao, T.C.; Daschkey, S.; Müsken, M.; Kalinowski, J.; Pühler, A.; Tauch, A. A comprehensive proteome map of the lipid-requiring nosocomial pathogen Corynebacterium jeikeium K411. Proteomics 2007, 7, 1076–1096. [Google Scholar] [CrossRef]
- Hansmeier, N.; Chao, T.C.; Kalinowski, J.; Pühler, A.; Tauch, A. Mapping and comprehensive analysis of the extracellular and cell surface proteome of the human pathogen Corynebacterium diphtheriae. Proteomics 2006, 6, 2465–2476. [Google Scholar] [CrossRef]
- Bittel, M.; Gastiger, S.; Amin, B.; Hofmann, J.; Burkovski, A. Surface and extracellular proteome of the emerging pathogen Corynebacterium ulcerans. Proteomes 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Enany, S.; Yoshida, Y.; Magdeldin, S.; Zhang, Y.; Bo, X.; Yamamoto, T. Extensive proteomic profiling of the secretome of European community acquired methicillin resistant Staphylococcus aureus clone. Peptides 2012, 37, 128–137. [Google Scholar] [CrossRef]
- Möller, J.; Kraner, M.; Sonnewald, U.; Sangal, V.; Tittlbach, H.; Winkler, J.; Winkler, T.H.; Melnikov, V.; Lang, R.; Sing, A.; et al. Proteomics of diphtheria toxoid vaccines reveals multiple proteins that are immunogenic and may contribute to protection of humans against Corynebacterium diphtheriae. Vaccine. in revision.
- Wisniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Meth. 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Herzberg, C.; Weidinger, L.A.F.; Dörrbecker, B.; Hübner, S.; Stülke, J.; Commichau, F.M. SPINE: A method for the rapid detection and analysis of protein-protein interactions in vivo. Proteomics 2007, 7, 4032–4035. [Google Scholar] [CrossRef]
- Lyng, J. Quantitative estimation of diphtheria and tetanus toxoids. 4. Toxoids as international reference materials defining Lf-units for diphtheria and tetanus toxoids. Biologicals 1990, 18, 11–17. [Google Scholar] [CrossRef]
- Malito, E.; Rappouli, R.; Burkovski, A. History of Diphthreia Vaccine Development. In Corynebacterium Diphtheriae and Related Toxigenic Species; Burkovski, A., Ed.; Springer: Amsterdam, The Netherlands, 2014; Volume 2010. [Google Scholar]
- Spaun, J.; Lyng, J. Replacement of the international standard for tetanus antitoxin and the use of the standard in the Flocculation Test. Bull World Health Organ. 1970, 42, 523–534. [Google Scholar] [PubMed]
- WHO Expert Committee on Biological Standardization. Recommendations to assure the quality, safety and efficacy of tetanus vaccines (adsorbed). WHO Tech. Rep. Ser. 2014, 980, 271–333. [Google Scholar]
- Kraner, M.E.; Müller, C.; Sonnewald, U. Comparative proteomic profiling of the choline transporter-like1 (CHER1) mutant provides insights into plasmodesmata composition of fully developed Arabidopsis thaliana leaves. Plant J. 2017, 92, 696–709. [Google Scholar] [CrossRef]
- Schäfer, W.; Eckart, R.A.; Schmid, B.; Cagköylü, H.; Hof, K.; Muller, Y.A.; Amin, B.; Lührmann, A. Nuclear trafficking of the anti-apoptotic Coxiella burnetii effector protein AnkG requires binding to p32 and Importin-α1. Cell. Microbiol. 2016, 19, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.R.; Ostasiewicz, P.; Duś, K.; Zielińska, D.F.; Gnad, F.; Mann, M. Extensive quantitative remodeling of the proteome between normal colon tissue and adenocarcinoma. Mol. Syst. Biol. 2012, 8. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Hein, M.Y.; Cox, J.; Mann, M. A “Proteomic Ruler” for protein copy number and concentration estimation without spike-in standards. Mol. Cell. Proteomics 2014, 13, 3497–3506. [Google Scholar] [CrossRef]
- Chelius, D.; Bondarenko, P.V. Quantitative profiling of proteins in complex mixtures using liquid chromatography and mass spectrometry. J. Proteome Res. 2002, 1, 317–323. [Google Scholar] [CrossRef]
- Bondarenko, P.V.; Chelius, D.; Shaler, T.A. Identification and relative quantitation of protein mixtures by enzymatic digestion followed by capillary reversed-phase liquid chromatography—Tandem mass spectrometry. Anal. Chem. 2002, 74, 4741–4749. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Rakus, D. Multi-enzyme digestion FASP and the ’Total Protein Approach’-based absolute quantification of the Escherichia coli proteome. J. Proteomics 2014, 109, 322–331. [Google Scholar] [CrossRef]
- Old, W.M.; Meyer-Arendt, K.; Aveline-Wolf, L.; Pierce, K.G.; Mendoza, A.; Sevinsky, J.R.; Resing, K.A.; Ahn, N.G. Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol. Cell. Proteomics 2005, 4, 1487–1502. [Google Scholar] [CrossRef]
- Horn, D.M.; Ueckert, T.; Fritzemeier, K.; Tham, K.; Paschke, C.; Berg, F.; Pfaff, H.; Jiang, X.; Li, S.; Lopez-Ferrer, D. New Method for Label-Free Quantification in the Proteome Discoverer Framework. Available online: https://tools.thermofisher.com/content/sfs/posters/PN-64792-Label-Free-Proteome-Discoverer-ASMS2016-PN64792-EN.pdf (accessed on 16 April 2019).
- Laird, M.R.; Melli, G.; Sahinalp, S.C.; Yu, N.Y.; Lo, R.; Dao, P.; Brinkman, F.S.L.; Wagner, J.R.; Ester, M.; Foster, L.J.; et al. PSORTb 3.0: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 2010, 26, 1608–1615. [Google Scholar] [CrossRef]
- Doytchinova, I.A.; Flower, D.R. VaxiJen: A server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinformatics 2007, 8, 1–7. [Google Scholar] [CrossRef]
- Vizcaíno, J.A.; Csordas, A.; Del-Toro, N.; Dianes, J.A.; Griss, J.; Lavidas, I.; Mayer, G.; Perez-Riverol, Y.; Reisinger, F.; Ternent, T.; et al. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 2016, 44, D447–D456. [Google Scholar] [CrossRef]
- Alam, S.I.; Bansod, S.; Singh, L. Immunization against Clostridium perfringens cells elicits protection against Clostridium tetani in mouse model: Identification of cross-reactive proteins using proteomic methodologies. BMC Microbiol. 2008, 8. [Google Scholar] [CrossRef]
- Krüger, M.; Skau, M.; Shehata, A.A.; Schrödl, W. Efficacy of Clostridium botulinum types C and D toxoid vaccination in Danish cows. Anaerobe 2013, 23, 97–101. [Google Scholar] [CrossRef] [PubMed]






| Origin | Company | Active components |
|---|---|---|
| Brazil | Butanan Institute | Tetanus toxoid ≥ 25 Lf/mL Diphtheria toxoid ≥ 2 Lf/mL |
| Bulgaria | InterVax for BB-NCIPD | Tetanus toxoid ≥ 20 Lf/mL Diphtheria toxoid ≥ 30 Lf/mL |
| Germany | GlaxoSmithKline (GSK) | Tetanus toxoid ≥ 20 I.U. Diphtheria toxoid ≥ 2 I.U. |
| India | Biological E (BE) | Tetanus toxoid ≥ 20 I.U. Diphtheria toxoid ≥ 2 I.U. |
| Russia | Microgen | Tetanus toxoid ≥ 5 BU/mL Diphtheria toxoid ≥ 5 Lf/mL |
| UniProt ID | Identifier | Annotation | Localization |
|---|---|---|---|
| P04958 | CTC_p60 | Tetanus toxin | S, M, C |
| Q890P1 | CTC_02598 | 50S ribosomal protein L2 | U |
| Q890T2 | CTC_02553 | Thioredoxin | M, C |
| Q890 × 6 | CTC_02507 | Tail-specific protease | M, C |
| Q890Z2 | CTC_02490 | ATP-dependent 6-phosphofructokinase | M, C |
| Q891M9 | CTC_02340 | Glycine betaine-binding protein | M |
| Q891P2 | CTC_02327 | V-type ATP synthase beta chain 2 | M, C |
| Q891Q6 | CTC_02312 | Conserved protein | U |
| Q891U8 | CTC_02265 | UDP-glucose 6-dehydrogenase | M, C |
| Q892B0 | CTC_02196 | Hydroxyacylglutathione hydrolase | U |
| Q892H0 | CTC_02129 | Phage protein | U |
| Q892K3 | CTC_02093 | N-acetylmuramoyl-L-alanine amidase/putative S-layer protein | M |
| Q892P7 | CTC_02047 | Dihydrolipoyl dehydrogenase | M, C |
| Q892V6 | CTC_01980 | Uncharacterized protein | M |
| Q893B5 | CTC_01913 | Uncharacterized protein | M |
| Q893R9 | CTC_01741 | Pyruvate-flavodoxin oxidoreductase | U |
| Q893T5 | CTC_01724 | Flagellar hook-associated protein 1 | S |
| Q894F4 | CTC_01593 | 3-dehydroquinate dehydratase | M |
| Q894P1 | CTC_01495 | Conserved protein | U |
| Q894P7 | CTC_01488 | Fumarate reductase flavoprotein subunit | M |
| Q894Q6 | CTC_01479 | Uncharacterized protein | M, C |
| Q894Q7 | CTC_01478 | Putative histidine decarboxylase | U |
| Q895A1 | CTC_01379 | Periplasmic transport protein, nickel or dipeptide transport | M |
| Q895E4 | CTC_01332 | Transketolase | U |
| Q895G9 | CTC_01305 | Uncharacterized protein | U |
| Q895P6 | CTC_01225 | Serine/threonine protein kinase | M |
| Q895R2 | CTC_01209 | Uncharacterized protein | U |
| Q895T9 | CTC_01178 | NADH oxidase | M, C |
| Q896G9 | CTC_01036 | Uncharacterized protein | U |
| Q896I3 | CTC_01021 | Electron transport complex subunit G | M |
| Q896J5 | CTC_01009 | Conserved protein, putative N-acetylmuramoyl-L-alanine amidase | M |
| Q896U1 | CTC_00907 | D-ribose-binding periplasmic protein | U |
| Q896W4 | CTC_00882 | Carboxyl-terminal protease | M, C |
| Q896W8 | CTC_00878 | 50S ribosomal protein L25 | U |
| Q896Y3 | CTC_00860 | D-galactose-binding periplasmic protein | U |
| Q896Y6 | CTC_00856 | Uncharacterized protein | M, C |
| Q897C9 | CTC_00811 | Fumarate reductase flavoprotein subunit | M |
| Q897E8 | CTC_00792 | Conserved protein | M |
| Q897G1 | CTC_00777 | Putative surface/cell-adhesion protein | U |
| Q897G4 | CTC_00774 | Putative surface/cell-adhesion protein | M |
| Q897G6 | CTC_00771 | Putative surface/cell-adhesion protein | M |
| Q897G7 | CTC_00770 | Putative surface/cell-adhesion protein, big2 domain | U |
| Q897G8 | CTC_00769 | Putative surface/cell-adhesion protein | M, C |
| Q897I6 | CTC_00749 | Putative surface/cell-adhesion protein, multiple big2 domain | M |
| Q897I8 | CTC_00747 | Putative surface/cell-adhesion protein, multiple big2 domain | M |
| Q897P1 | CTC_00691 | Putative S-layer protein/internalin A-like/N-acetylmuramoyl-L-alanine amidase | M |
| Q897Q0 | CTC_00681 | Conserved protein | M |
| Q897W0 | CTC_00612 | Serine protease | S |
| Q898E1 | CTC_00519 | Zink-carboxypeptidase | U |
| Q898I5 | CTC_00465 | Putative S-layer protein | M |
| Q898I7 | CTC_00462 | Putative S-layer protein/N-acetylmuramoyl-L-alanine amidase | M |
| Q898R0 | CTC_00382 | Enolase | S |
| Q898S3 | CTC_00369 | Membrane lipoprotein tmpC | M |
| Q899E7 | CTC_00234 | Putative cell wall hydrolase | S |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möller, J.; Kraner, M.E.; Burkovski, A. More than a Toxin: Protein Inventory of Clostridium tetani Toxoid Vaccines. Proteomes 2019, 7, 15. https://0-doi-org.brum.beds.ac.uk/10.3390/proteomes7020015
Möller J, Kraner ME, Burkovski A. More than a Toxin: Protein Inventory of Clostridium tetani Toxoid Vaccines. Proteomes. 2019; 7(2):15. https://0-doi-org.brum.beds.ac.uk/10.3390/proteomes7020015
Chicago/Turabian StyleMöller, Jens, Max Edmund Kraner, and Andreas Burkovski. 2019. "More than a Toxin: Protein Inventory of Clostridium tetani Toxoid Vaccines" Proteomes 7, no. 2: 15. https://0-doi-org.brum.beds.ac.uk/10.3390/proteomes7020015