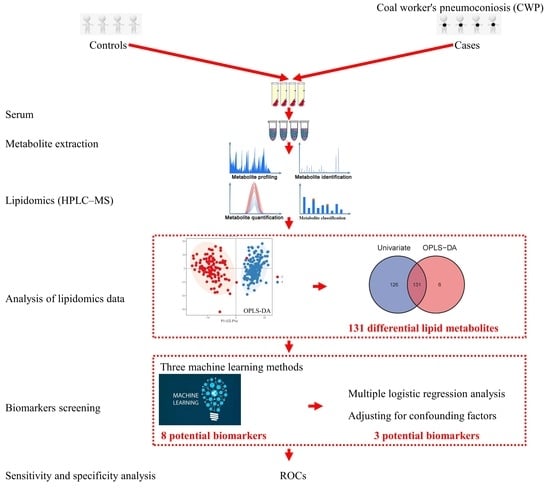
Lipidomics Profiles and Lipid Metabolite Biomarkers in Serum of Coal Workers’ Pneumoconiosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Serum Sample Collection
2.3. Serum Biochemical Lipids Measurement
2.4. Serum Sample Preparation of Lipidomics
2.5. Detection of Lipidomics in HPLC-MS
2.6. Annotation and Identification of Lipidomics Data
2.7. Analysis of Lipidomics Data
2.8. Biomarker Screening
2.9. Statistical Analysis
3. Results
3.1. The Characteristics of Subjects
3.2. Different Concentrations of Serum Biochemical Lipids between the CWP Case and Control Groups
3.3. Different Lipidomics Profiles between the CWP Case and Control Groups
3.4. Differential Lipid Metabolites between the CASE and Control Groups
3.5. Screening of Potential Biomarkers for CWP
3.6. Effect of CWP Stage on the Biomarker Screening
3.7. Sensitivity and Specificity Analysis of Potential Biomarkers for CWP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Liang, R.; Yang, M.; Ma, J.; Li, W.; Mu, M.; Xiao, Y.; Feng, X.; Dong, C.; Yu, L.; et al. Incidence and diseases burden of coal workers’ pneumoconiosis worldwide, 1990-2019: Evidence from the Global Burden of Disease Study 2019. Eur. Respir. J. 2021, 58, 2101669. [Google Scholar] [CrossRef] [PubMed]
- Lancet Improving occupational health in China. Lancet 2019, 394, 443. [CrossRef]
- Mandrioli, D.; Schlünssen, V.; Ádám, B.; Cohen, R.A.; Colosio, C.; Chen, W.; Fischer, A.; Godderis, L.; Göen, T.; Ivanov, I.D.; et al. WHO/ILO work-related burden of disease and injury: Protocol for systematic reviews of occupational exposure to dusts and/or fibres and of the effect of occupational exposure to dusts and/or fibres on pneumoconiosis. Environ. Int. 2018, 119, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J. Black lung is still a threat. Lancet Respir. Med. 2018, 6, 745–746. [Google Scholar] [CrossRef]
- Furuya, S.; Chimed-Ochir, O.; Takahashi, K.; David, A.; Takala, J. Global Asbestos Disaster. Int. J. Environ. Res. Public Health 2018, 15, 1000. [Google Scholar] [CrossRef]
- Leung, C.C.; Yu, I.T.; Chen, W. Silicosis. Lancet 2012, 379, 2008–2018. [Google Scholar] [CrossRef]
- Hou, X.; Summer, R.; Chen, Z.; Tian, Y.; Ma, J.; Cui, J.; Hao, X.; Guo, L.; Xu, H.; Wang, H.; et al. Lipid Uptake by Alveolar Macrophages Drives Fibrotic Responses to Silica Dust. Sci. Rep. 2019, 9, 399. [Google Scholar] [CrossRef]
- Castranova, V.; Vallyathan, V. Silicosis and coal workers’ pneumoconiosis. Environ. Health Perspect. 2000, 108 (Suppl. S4), 675–684. [Google Scholar] [PubMed]
- Kamp, D.W.; A Weitzman, S. The molecular basis of asbestos induced lung injury. Thorax 1999, 54, 638–652. [Google Scholar] [CrossRef]
- Liu, G.; Cheresh, P.; Kamp, D.W. Molecular Basis of Asbestos-Induced Lung Disease. Annu. Rev. Pathol. Mech. Dis. 2013, 8, 161–187. [Google Scholar] [CrossRef] [Green Version]
- Fubini, B.; Hubbard, A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic. Biol. Med. 2003, 34, 1507–1516. [Google Scholar] [CrossRef]
- Vallyathan, V.; Shi, X.; Castranova, V. Reactive oxygen species: Their relation to pneumoconiosis and carcinogenesis. Environ. Health Perspect. 1998, 106 (Suppl. S5), 1151–1155. [Google Scholar] [PubMed]
- Yang, H.-Y.; Shie, R.-H.; Chang, C.-J.; Chen, P.-C. Development of breath test for pneumoconiosis: A case-control study. Respir. Res. 2017, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Olivier, M.; Asmis, R.; Hawkins, G.A.; Howard, T.D.; Cox, L.A. The Need for Multi-Omics Biomarker Signatures in Precision Medicine. Int. J. Mol. Sci. 2019, 20, 4781. [Google Scholar] [CrossRef] [PubMed]
- Paglia, G.; Astarita, G. Metabolomics and lipidomics using traveling-wave ion mobility mass spectrometry. Nat. Protoc. 2017, 12, 797–813. [Google Scholar] [CrossRef]
- Tsugawa, H.; Ikeda, K.; Takahashi, M.; Satoh, A.; Mori, Y.; Uchino, H.; Okahashi, N.; Yamada, Y.; Tada, I.; Bonini, P.; et al. A lipidome atlas in MS-DIAL. Nat. Biotechnol. 2020, 38, 1–5. [Google Scholar] [CrossRef]
- Jang, C.; Chen, L.; Rabinowitz, J.D. Metabolomics and Isotope Tracing. Cell 2018, 173, 822–837. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Yin, P.; Xu, G. Metabolomics Toward Biomarker Discovery. In Serum/Plasma Proteomics; Humana Press: New York, NY, USA, 2017; Volume 1619, pp. 467–475. [Google Scholar] [CrossRef]
- Kumar, A.; Misra, B.B. Challenges and Opportunities in Cancer Metabolomics. Proteomics 2019, 19, e1900042. [Google Scholar] [CrossRef]
- Chen, X.; Yu, D. Metabolomics study of oral cancers. Metabolomics 2019, 15, 22. [Google Scholar] [CrossRef]
- McGarrah, R.W.; Crown, S.B.; Zhang, G.-F.; Shah, S.H.; Newgard, C.B. Cardiovascular Metabolomics. Circ. Res. 2018, 122, 1238–1258. [Google Scholar] [CrossRef] [PubMed]
- Boone, S.; Mook-Kanamori, D.; Rosendaal, F.; den Heijer, M.; Lamb, H.; de Roos, A.; le Cessie, S.; Willems van Dijk, K.; de Mutsert, R. Metabolomics: A search for biomarkers of visceral fat and liver fat content. Metabolomics 2019, 15, 139. [Google Scholar] [CrossRef] [PubMed]
- Bowerman, K.L.; Rehman, S.F.; Vaughan, A.; Lachner, N.; Budden, K.F.; Kim, R.Y.; Wood, D.L.A.; Gellatly, S.L.; Shukla, S.D.; Wood, L.G.; et al. Disease-associated gut microbiome and metabolome changes in patients with chronic obstructive pulmonary disease. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Sailani, M.R.; Contrepois, K.; Zhou, Y.; Ahadi, S.; Leopold, S.R.; Zhang, M.J.; Rao, V.; Avina, M.; Mishra, T.; et al. Longitudinal multi-omics of host–microbe dynamics in prediabetes. Nature 2019, 569, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Ristori, M.V.; Mortera, S.L.; Marzano, V.; Guerrera, S.; Vernocchi, P.; Ianiro, G.; Gardini, S.; Torre, G.; Valeri, G.; Vicari, S.; et al. Proteomics and Metabolomics Approaches towards a Functional Insight onto AUTISM Spectrum Disorders: Phenotype Stratification and Biomarker Discovery. Int. J. Mol. Sci. 2020, 21, 6274. [Google Scholar] [CrossRef]
- Morissette, M.C.; Shen, P.; Thayaparan, D.; Stämpfli, M.R. Disruption of pulmonary lipid homeostasis drives cigarette smoke-induced lung inflammation in mice. Eur. Respir. J. 2015, 46, 1451–1460. [Google Scholar] [CrossRef]
- Cao, Y.; Long, J.; Ji, Y.; Chen, G.; Shen, Y.; Gong, Y.; Li, J. Foam cell formation by particulate matter (PM) exposure: A review. Inhal. Toxicol. 2016, 28, 583–590. [Google Scholar] [CrossRef]
- Yatera, K.; Morimoto, Y.; Kim, H.-N.; Myojo, T.; Mukae, H. Foam cell formation of alveolar macrophages in Clara cell ablated mice inhaling crystalline silica. Inhal. Toxicol. 2011, 23, 736–744. [Google Scholar] [CrossRef]
- Zelnik, I.D.; Volpert, G.; Viiri, L.E.; Kauhanen, D.; Arazi, T.; Aalto-Setälä, K.; Laaksonen, R.; Futerman, A.H. Different rates of flux through the biosynthetic pathway for long-chain versus very-long-chain sphingolipids. J. Lipid Res. 2020, 61, 1341–1346. [Google Scholar] [CrossRef]
- Jensen, S.A.; Calvert, A.E.; Volpert, G.; Kouri, F.M.; Hurley, L.A.; Luciano, J.P.; Wu, Y.; Chalastanis, A.; Futerman, A.H.; Stegh, A.H. Bcl2L13 is a ceramide synthase inhibitor in glioblastoma. Proc. Natl. Acad. Sci. USA 2014, 111, 5682–5687. [Google Scholar] [CrossRef]
- Saddoughi, S.A.; Ogretmen, B. Diverse Functions of Ceramide in Cancer Cell Death and Proliferation. Adv. Cancer Res. 2013, 117, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Pratipanawatr, T.; Berria, R.; Wang, E.; DeFronzo, R.A.; Sullards, M.C.; Mandarino, L.J. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 2004, 53, 25–31. [Google Scholar] [CrossRef]
- Yu, J.; Pan, W.; Shi, R.; Yang, T.; Li, Y.; Yu, G.; Bai, Y.; Schuchman, E.H.; He, X.; Zhang, G. Ceramide Is Upregulated and Associated With Mortality in Patients With Chronic Heart Failure. Can. J. Cardiol. 2015, 31, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.R.; Xanthakis, V.; Duncan, M.S.; Gross, S.; Friedrich, N.; Völzke, H.; Felix, S.B.; Jiang, H.; Sidhu, R.; Nauck, M.; et al. Ceramide Remodeling and Risk of Cardiovascular Events and Mortality. J. Am. Heart Assoc. 2018, 7, e007931. [Google Scholar] [CrossRef]
- Grassmé, H.; Riethmüller, J.; Gulbins, E. Ceramide in cystic fibrosis. Sphingolipids Dis. 2013, 216, 265–274. [Google Scholar]
- Gardner, A.I.; Haq, I.J.; Simpson, A.J.; Becker, K.A.; Gallagher, J.; Saint-Criq, V.; Verdon, B.; Mavin, E.; Trigg, A.; Gray, M.A.; et al. Recombinant Acid Ceramidase Reduces Inflammation and Infection in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2020, 202, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Grassmé, H.; Becker, K.A.; Zhang, Y.; Gulbins, E. Ceramide in bacterial infections and cystic fibrosis. Biol. Chem. 2008, 389, 1371–1379. [Google Scholar] [CrossRef]
- Ziobro, R.; Henry, B.; Edwards, M.J.; Lentsch, A.B.; Gulbins, E. Ceramide mediates lung fibrosis in cystic fibrosis. Biochem. Biophys. Res. Commun. 2013, 434, 705–709. [Google Scholar] [CrossRef]
- Wojewodka, G.; De Sanctis, J.B.; Radzioch, D. Ceramide in Cystic Fibrosis: A Potential New Target for Therapeutic Intervention. J. Lipids 2010, 2011, 1–13. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 19, 175–191. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Tang, T.-K.; Phuah, E.-T.; Tan, C.-P.; Wang, Y.; Li, Y.; Cheong, L.-Z.; Lai, O.-M. Production, safety, health effects and applications of diacylglycerol functional oil in food systems: A review. Crit. Rev. Food Sci. Nutr. 2019, 60, 2509–2525. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Baillie, R.A.; Wiest, M.M.; Mirshahi, F.; Choudhury, J.; Cheung, O.; Sargeant, C.; Contos, M.J.; Sanyal, A.J. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 2007, 46, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Lyu, K.; Zhang, Y.; Zhang, D.; Kahn, M.; ter Horst, K.W.; Rodrigues, M.R.; Gaspar, R.C.; Hirabara, S.M.; Luukkonen, P.K.; Lee, S.; et al. A Membrane-Bound Diacylglycerol Species Induces PKCϵ-Mediated Hepatic Insulin Resistance. Cell Metab. 2020, 32, 654–664.e5. [Google Scholar] [CrossRef]
- Perry, R.J.; Samuel, V.T.; Petersen, K.F.; Shulman, G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014, 510, 84–91. [Google Scholar] [CrossRef]
- Zheng, J.-S.; Wang, L.; Lin, M.; Yang, H.; Li, D. BMI status influences the response of insulin sensitivity to diacylglycerol oil in Chinese type 2 diabetic patients. Asia Pac. J. Clin. Nutr. 2015, 24, 65–72. [Google Scholar] [PubMed]
- Johnson, A.A.; Stolzing, A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell 2019, 18, e13048. [Google Scholar] [CrossRef]
- Søgaard, D.; Baranowski, M.; Dela, F.; Helge, J.W. The Influence of Age and Cardiorespiratory Fitness on Bioactive Lipids in Muscle. J. Gerontol. Ser. A 2018, 74, 778–786. [Google Scholar] [CrossRef]










| Variables | Control Group (n = 120) | CWP Case Group (n = 150) | p Value |
|---|---|---|---|
| Age (years) | 56.63 ± 3.03 | 69.02 ± 9.07 | <0.001 * |
| Smoking n (%) | <0.001 * | ||
| Yes | 63 (52.5) | 125 (83.3) | |
| No | 57 (47.5) | 25 (16.7) | |
| Dinking n (%) | 0.934 | ||
| Yes | 69 (57.5) | 87 (58.0) | |
| No | 51 (42.5) | 63 (42.0) | |
| Chronic disease n (%) | <0.001 * | ||
| Yes | 51 (42.5) | 106 (70.7) | |
| No | 69 (57.5) | 44 (29.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Shi, J.; Zhang, Y.; Zhang, J.; Li, S.; Guan, L.; Jia, G. Lipidomics Profiles and Lipid Metabolite Biomarkers in Serum of Coal Workers’ Pneumoconiosis. Toxics 2022, 10, 496. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10090496
Chen Z, Shi J, Zhang Y, Zhang J, Li S, Guan L, Jia G. Lipidomics Profiles and Lipid Metabolite Biomarkers in Serum of Coal Workers’ Pneumoconiosis. Toxics. 2022; 10(9):496. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10090496
Chicago/Turabian StyleChen, Zhangjian, Jiaqi Shi, Yi Zhang, Jiahe Zhang, Shuqiang Li, Li Guan, and Guang Jia. 2022. "Lipidomics Profiles and Lipid Metabolite Biomarkers in Serum of Coal Workers’ Pneumoconiosis" Toxics 10, no. 9: 496. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10090496