Electron Paramagnetic Resonance (EPR) Spectroscopy in Studies of the Protective Effects of 24-Epibrasinoide and Selenium against Zearalenone-Stimulation of the Oxidative Stress in Germinating Grains of Wheat
Abstract
:1. Introduction
2. Results
2.1. Zearalenone Content and Antioxidant Activity
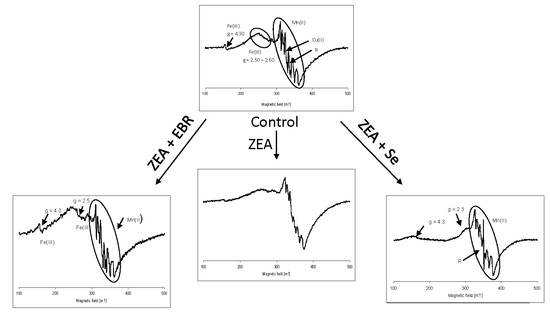
2.2. Electron Paramagnetic Resonance Spectra
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Plant Material
5.2. Chemicals
5.3. Determination of Zearalenone Concentration
5.4. Analysis of Antioxidative Enzymes
5.5. Electron Paramagnetic Resonance Spectroscopy
5.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cromey, M.G.; Shorter, S.C.; Lauren, D.R.; Sinclair, K.I. Cultivar and crop management influences on Fusarium head blight and mycotoxins in spring wheat (Triticum aestivum) in New Zealand. N. Z. J. Crop Hortic. Sci. 2002, 30, 235–247. [Google Scholar] [CrossRef]
- Manova, R.; Mladenova, R. Incidence of zearalenone and fumonisins in Bulgarian cereal production. Food Control 2009, 20, 362–365. [Google Scholar] [CrossRef]
- Maragos, C.M. Zearalenone occurrence and human exposure. World Mycotoxin J. 2010, 3, 369–383. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J.; Miguel, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Broders, K.S.; Lipps, P.E.; Paul, P.A.; Dorrance, E.A. Evaluation of Fusarium gramineaum associated with corn and soybean seeds and seedlings disease in Ohio. Plant Dis. 2007, 91, 1155–1160. [Google Scholar] [CrossRef]
- Steddom, K.; Bredehoeft, M.W.; Khan, M.; Rush, C.M. Comparison of visual and mulitspectral radiometric disease evaluations of Cercospora leaf spot of sugar beet. Plant Dis. 2005, 89, 153–158. [Google Scholar] [CrossRef]
- Beccari, G.; Prodi, A.; Tini, F.; Bonciarelli, U.; Onofri, A.; Oueslati, S.; Limayma, M.; Covarelli, L. Changes in the Fusarium head blight complex of malting barley in a tree-year field experiment in Italy. Toxins 2017, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Placinta, C.M.; D’Mello, J.P.F.; Macdonald, A.M.C. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim. Feed Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- Zhu, Y.; Hassan, Y.I.; Lepp, D.; Shao, S.; Zhou, T. Strategies and methodologies for developing microbial detoxification systems to mitigate mycotoxins. Toxins 2017, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, K.S.; Sarr, A.B.; Mayura, K.; Bailey, R.H.; Millar, D.R.; Rogers, T.D.; Corred, W.P.; Voss, K.A.; Plattner, R.D.; Kubena, L.F.; et al. Oxidative degradation and detoxification of mycotoxins using a novel source of ozone. Food Chem. Toxicol. 1997, 35, 807–820. [Google Scholar] [CrossRef]
- Abd Alla, E.S. Zearalenone: Toxigenic fungi and chemical decon-tamination in Egyptian cereals. Nahrung 1997, 41, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Łabanowska, M.; Kurdziel, M.; Filek, M. Changes of paramagnetic species in cereal grains upon short-term ozone action as a marker of oxidative stress tolerance. J. Plant Physiol. 2016, 190, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.A.D.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R.O.N. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tajrishi, M.; Madan, M.; Tuteja, N. A DESD-box helicase functions in salinity stress tolerance by improving photosynthesis and antioxidant machinery in rice (Oryza sativa L. cv. PB1). Plant Mol. Biol. 2013, 82, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Łabanowska, M.; Filek, M.; Kościelniak, J.; Kurdziel, M.; Kuliś, E.; Hartikainen, H. The effects of short-term selenium stress on Polish and Finnish wheat seedlings-EPR, enzymatic and fluorescence studies. J. Plant Physiol. 2012, 15, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Łabanowska, M.; Filek, M.; Kurdziel, M.; Bidzinska, E.; Miszalski, Z.; Hartikainen, H. EPR spectroscopy as a tool for investigation of differences in radical status in wheat plants of various tolerances to osmotic stress induced by NaCl and PEG-treatment. J. Plant Physiol. 2013, 170, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Łabanowska, M.; Kurdziel, M.; Filek, M.; Walas, S.; Tobiasz, A. The influence of the starch component on thermal radical generation in flours. Carbohydr. Polym. 2014, 101, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Golombek, A.P.; Hendrich, M.P. Quantitative analysis of dinuclear manganese(II) EPR spectra. J. Magn. Reson. 2003, 165, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Sieprawska, A.; Kornaś, A.; Filek, M. Involvement of selenium in protective mechanisms of plants under environmental stress conditions. Acta Biol. Cracoviensia 2015, 57, 9–20. [Google Scholar] [CrossRef]
- Beaman, J. Aquatic Life Ambient Water Quality Criterion for Selenium in Freshwater 2016—Fact Sheet; United States Environmental Protection Agency: Washington, DC, USA, 2016. Available online: www.epa.gov/wqc/aquatic-life-criterion-selenium (accessed on 30 June 2016).
- Hasanuzzaman, M.; Fujita, M. Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol. Trace Elem. Res. 2011, 143, 1758–1776. [Google Scholar] [CrossRef] [PubMed]
- Filek, M.; Zembala, M.; Kornaś, A.; Walas, S.; Mrowiec, H.; Hartikainen, H. The uptake and translocation of macro- and microelements in rape and wheat seedlings as affected by selenium supply level. Plant Soil 2010, 336, 303–312. [Google Scholar] [CrossRef]
- Gzyl-Malcher, B.; Filek, M.; Brezesinski, G. Influence of cadmium and selenate on the interactions between hormones and phospholipids. Langmuir 2009, 25, 13071–13076. [Google Scholar] [CrossRef] [PubMed]
- Gzyl, B.; Filek, M.; Dudek, A. Influence of phytohormones on polar and hydrophobic parts of mixed phospholipid monolayers at water/air interface. J. Colloid Interface Sci. 2004, 269, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Biesaga-Koscielniak, J.; Filek, M. Occurrence and physiology of zearalenon as a new plant hormone. In Sociology, Organic Farming, Climate Change and Soil Science; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2010; Volume 3, pp. 419–435. [Google Scholar] [CrossRef]
- Dufourc, E.J. Sterols and membrane dynamics. J. Chem. Biol. 2008, 1, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Janeczko, A.; Biesaga-Kościelniak, J.; Oklestkova, J.; Filek, M.; Dziurka, M.; Szarek-Łukaszewska, G.; Kościelniak, J. Role of 24-epibrasenoide in wheat production: Physiological effects and uptake. J. Agronomy Crop Sci. 2010, 196, 311–321. [Google Scholar]
- Łabanowska, M.; Kurdziel, M.; Filek, M.; Wesełucha-Birczyńska, A. The impact of biochemical composition and nature of paramagnetic species in grains on stress tolerance of oat cultivars. J. Plant Physiol. 2016, 199, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.F.; Brudvig, G.W. A guide to electron paramagnetic resonance spectroscopy of photosystem II membranes. Biochim. Biophys. Acta 1991, 1056, 1–18. [Google Scholar] [CrossRef]
- Baader, S.L.; Bill, E.; Trautwein, A.X.; Bruchelt, G.; Matzanke, B.F. Mobilization of iron from cellular ferritin by ascorbic acid in neuroblastoma SK-N-SH cells: An EPR study. FEBS Lett. 1996, 381, 131–134. [Google Scholar] [CrossRef]
- Masuda, T.; Goto, F.; Yoshihara, T.; Mikami, B. Crystal structure of plant ferritin reveals a novel metal binding site that functions as a transit site for metal transfer in ferritin. J. Biol. Chem. 2010, 285, 4049–4059. [Google Scholar] [CrossRef] [PubMed]
- Pfaffen, S.; Abdulqadir, R.; Le Brun, N.E.; Murphy, M.E.P. Mechanism of ferrous binding and oxidation by ferritin from a pennate diatom. J. Biol. Chem. 2013, 288, 14917–14925. [Google Scholar] [CrossRef] [PubMed]
- Malmström, B.G. Rack-induced bonding in blue-copper proteins. Eur. J. Biochem. 1994, 223, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Kurdziel, M.; Dłubacz, A.; Weselucha-Birczynska, A.; Filek, M.; Łabanowska, M. Stable radicals and biochemical compounds in embryos and endosperm of wheat grains differentiating sensitive and tolerant genotypes-EPR and Raman studies. J. Plant Physiol. 2015, 183, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Filek, M.; Łabanowska, M.; Kościelniak, J.; Biesaga-Kościelniak, J.; Kurdziel, M.; Szarejko, I.; Hartikainen, H. Characterization of barley leaf tolerance to drought stress by chlorophyll fluorescence and electron paramagnetic resonance studies. J. Agron. Crop Sci. 2015, 201, 228–240. [Google Scholar] [CrossRef]
- Polat, M.; Korkmaz, M. Detection of irradiated black tee (Camellia simensis) and rooibos tea (Aspalathus linearis) by ESR spectroscopy. Food Chem. 2008, 107, 956–961. [Google Scholar] [CrossRef]
- Reichenauer, T.G.; Goodman, B.A. Free radicals in wheat flour change during storage in air and are influenced by the presence of ozone during the growing season. Free Radic. Res. 2003, 37, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Himo, F.; Gräslund, A.; Eriksson, L.A. Density functional calculations on model tyrosyl radical. Biophys. J. 1997, 72, 1556–1567. [Google Scholar] [CrossRef]
- Schünemann, V.; Lendzian, F.; Jung, C.; Contzen, J.; Barra, A.L.; Sligar, S.G.; Trautwein, A.X. Tyrosine radical formation in the reaction of wild type and mutant cytochrome P450cam with peroxy acids. J. Biol. Chem. 2004, 279, 10919–10930. [Google Scholar] [CrossRef] [PubMed]
- Tomter, A.; Zoppellaro, G.; Bell, C.B.; Barra, A.L.; Andersen, N.H.; Solomon, E.I.; Andersson, K.K. Spectroscopic studies of the iron and manganese reconstituted tyrosyl radical in Bacillus cereus ribonucleotide reductase R2 protein. PLoS ONE 2012, 7, e33436. [Google Scholar] [CrossRef]
- Hofbauer, W.; Zouni, A.; Bittl, R.; Kern, J.; Orth, P.; Lendzian, F.; Fromme, P.; Witt, H.T.; Lubitz, W. Photosystem II single crystals studied by EPR spectroscopy at 94 GHz: The tyrosine radical. Proc. Natl. Acad. Sci. USA 2001, 98, 6623–6628. [Google Scholar] [CrossRef] [PubMed]
- Madden, K.P.; Bernhard, W.A. Thermally induced free-radical reactions in glucopyranose single crystals. An electron spin resonance–electron nuclear double resonance study. J. Phys. Chem. 1982, 86, 4033–4036. [Google Scholar] [CrossRef]
- Yamauchi, M.; Sugito, M.; Kuzuya, M. Plasma-induced free radicals of polycrystalline monocarbohydrates studied by electron spin resonance. Chem. Pharm. Bull. 1999, 47, 273–278. [Google Scholar] [CrossRef]
- Kuzuya, M.; Yamauchi, Y.; Kondo, S. Mechanolysis of glucose-based polysaccharides as studied by electron spin resonance. J. Phys. Chem. B 1999, 103, 8051–8059. [Google Scholar] [CrossRef]
- Grzesiak, M.; Filek, M.; Barbasz, A.; Kreczmer, B.; Hartikainen, H. Relationships between polyamines, ethylene, osmoprotectants and antioxidant enzymes activities in wheat seedlings after short-term PEG- and NaCl-induced stresses. Plant Growth Regul. 2013, 69, 177–189. [Google Scholar] [CrossRef]
- Gromadzka, K.; Waśkiewicz, A.; Świetlik, J.; Bocianowski, J.; Goliński, P. Possible way of zearalenone migration in the agricultural environment. Plant Soil Environ. 2015, 61, 358–363. [Google Scholar] [CrossRef]
- Luo, X.; Qi, L.; Liu, Y.; Wang, R.; Yang, D.; Li, K.; Wang, L.; Li, Y.; Zhang, Y.; Chen, Z. Effects of electron beam irradiation on zearalenone and ochratoxin a in naturally contaminated corn and corn quality parameters. Toxins 2017, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Fridovich, I. Superoxide dismutase: An enzymatic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Lück, H. Peroxidase in: Methods of Enzymatic Analysis; Bergemeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1965; p. 895. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbat especific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Spałek, T.; Pietrzyk, P.; Sojka, Z. Application of genetic algorithm joint with Powell method to non linear least squares fitting of powder EPR spectra. J. Chem. Inf. Model. 2005, 45, 18–29. [Google Scholar] [CrossRef] [PubMed]




| Genotype | ZEA (ng/g) | Genotype | ZEA (ng/g) | ||
|---|---|---|---|---|---|
| Parabola | grains | hypocotyl | Raweta | grains | hypocotyl |
| control | 60.8 ± 0.9 c | 45.2 ± 1.0 c | control | 70.4 ± 1.4 c | 52.7 ± 1.2 c |
| ZEA (30 µM) | 294.7 ± 2.3 a | 101.3 ± 2.6 a | ZEA (30 µM) | 350.0 ± 3.9 a | 155.4 ± 2.0 a |
| Se (10 µM) | 59.9 ± 1.3 c | 44.1 ± 1.1 c | Se (10 µM) | 70.3 ± 1.6 c | 53.6 ± 1.5 c |
| EBR (0.1 µM) | 59.5 ± 1.1 c | 45.0 ± 1.7 c | EBR (0.1 µM) | 70.8 ± 1.7 c | 54.0 ± 1.4 c |
| ZEA + Se | 205.6 ± 2.6 b | 81.5 ± 2.0 b | ZEA+Se | 302.1 ± 2.7 b | 128.3 ± 3.9 b |
| ZEA + EBR | 199.1 ± 3.8 b | 76.6 ± 2.2 b | ZEA + EBR | 297.0 ± 3.0 b | 126.5± 2.6 b |
| Treatment | Antioxidative Enzymes (U/mg Proteins) | |||
|---|---|---|---|---|
| SOD | CAT | POX | APX | |
| Parabola | ||||
| control | 0.29 ± 0.01 a | 0.039 ± 0.002 a | 0.37 ± 0.02 a | 0.019±0.004 a |
| ZEA (30 µM) | 0.31 ± 0.02 a | 0.042 ± 0.002 a | 0.34 ± 0.03 a | 0.018±0.002 a |
| Se (10 µM) | 0.25 ± 0.02 ab | 0.035 ± 0.002 ab | 0.36 ± 0.01 a | 0.016 ± 0.003 a |
| EBR (0.1 µM) | 0.26 ± 0.02 ab | 0.039 ± 0.001 ab | 0.39 ± 0.02 a | 0.017 ± 0.003 a |
| ZEA + Se | 0.28 ± 0.02 a | 0.040 ± 0.002 a | 0.36 ± 0.03 a | 0.016 ± 0.004 a |
| ZEA + EBR | 0.29 ± 0.01 ab | 0.037 ± 0.003 a | 0.36 ± 0.01 ab | 0.017 ± 0.003 a |
| Raweta | ||||
| control | 0.12 ± 0.02 b | 0.047 ± 0.001 b | 0.30 ± 0.01 a | 0.007 ± 0.003 a |
| ZEA (30 µM) | 0.25 ± 0.03 a | 0.053 ± 0.001 a | 0.25 ± 0.01 b | 0.010 ± 0.003 a |
| Se (10 µM) | 0.14 ± 0.01 b | 0.048 ± 0.003 b | 0.29 ± 0.02 a | 0.008 ± 0.004 a |
| EBR (0.1 µM) | 0.14 ± 0.02 b | 0.043 ± 0.004 b | 0.26 ± 0.02 ab | 0.006 ± 0.002 a |
| ZEA + Se | 0.16 ± 0.02 b | 0.045 ± 0.003 b | 0.26 ± 0.02 ab | 0.011 ± 0.004 a |
| ZEA + EBR | 0.15 ± 0.03 b | 0.047 ± 0.002 ab | 0.27 ± 0.03 ab | 0.011 ± 0.004 a |
| Signal | I | II | III | IV | V |
|---|---|---|---|---|---|
| g | g1 = 2.0058
g2 = 2.0041 g3 = 2.0013 gav = 2.0037 | 2.0023 | 2.0062 | 2.0027 | g1 = 2.0078 g2 = 2.0045 g3 = 2.0023 gav = 2.0049 |
| A (mT) | - | 1.7 | 2.0 | - | A2β = 1.7 |
| Contribution (%) | 20 | 14 | 4 | 28 | 34 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filek, M.; Łabanowska, M.; Kurdziel, M.; Sieprawska, A. Electron Paramagnetic Resonance (EPR) Spectroscopy in Studies of the Protective Effects of 24-Epibrasinoide and Selenium against Zearalenone-Stimulation of the Oxidative Stress in Germinating Grains of Wheat. Toxins 2017, 9, 178. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins9060178
Filek M, Łabanowska M, Kurdziel M, Sieprawska A. Electron Paramagnetic Resonance (EPR) Spectroscopy in Studies of the Protective Effects of 24-Epibrasinoide and Selenium against Zearalenone-Stimulation of the Oxidative Stress in Germinating Grains of Wheat. Toxins. 2017; 9(6):178. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins9060178
Chicago/Turabian StyleFilek, Maria, Maria Łabanowska, Magdalena Kurdziel, and Apolonia Sieprawska. 2017. "Electron Paramagnetic Resonance (EPR) Spectroscopy in Studies of the Protective Effects of 24-Epibrasinoide and Selenium against Zearalenone-Stimulation of the Oxidative Stress in Germinating Grains of Wheat" Toxins 9, no. 6: 178. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins9060178