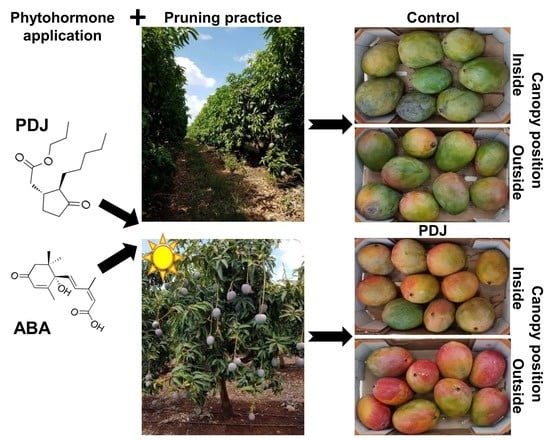
Improving the Red Color and Fruit Quality of ‘Kent’ Mango Fruit by Pruning and Preharvest Spraying of Prohydrojasmon or Abscisic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Storage Conditions
2.2. Measurements of Mango Fruit Skin Color
2.3. Mango Fruit Ripening Parameters and Fruit Decay
2.4. Statistical Analysis
3. Results
3.1. Evaluation of Mango Fruit Color Parameters
3.2. Estimation of Chlorophyll, Anthocyanin and Flavonoids in Fruit Skin
3.3. Fruit Decay
3.4. Effect of ABA on Mango Fruit Skin Color in Open Trees
3.5. Evaluation of Chlorophyll, Anthocyanin and, Flavonoids in Fruit Skin
3.6. Evaluation of Physiological Parameters and Fruit Decay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alkan, N.; Kumar, A. Post-harvest storage management of mango fruit. In Achieving Sustainable Cultivation of Mangoes; Sauco, V.G., Lu, P., Eds.; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2018; pp. 377–393. [Google Scholar]
- Medlicott, A.; Bhogal, M.; Reynolds, S. Changes in peel pigmentation during ripening of mango fruit (Mangifera indica var. Tommy Atkins). Ann. Appl. Biol. 1986, 101, 651–656. [Google Scholar] [CrossRef]
- Kuhn, D.N.; Bally, I.S.; Dillon, N.L.; Innes, D.; Groh, A.M.; Rahaman, J.; Ophir, R.; Cohen, Y.; Sherman, A. Genetic map of mango: A tool for mango breeding. Front. Plant Sci. 2017, 8, 577. [Google Scholar] [CrossRef] [PubMed]
- Muengkaew, R.; Chaiprasart, P.; Warrington, I. Changing of physiochemical properties and color development of mango fruit sprayed methyl Jasmonate. Sci. Hortic. 2016, 198, 70–77. [Google Scholar] [CrossRef]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef] [PubMed]
- Buer, C.S.; Imin, N.; Djordjevic, M.A. Flavonoids: New roles for old molecules. J. Integr. Plant Biol. 2010, 51, 98–111. [Google Scholar] [CrossRef]
- de Pascual-Teresa, S.; Moreno, D.A.; García-Viguera, C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef] [Green Version]
- Spencer, J.P. The impact of fruit flavonoids on memory and cognition. Br. J. Nutr. 2010, 104, S40–S47. [Google Scholar] [CrossRef] [Green Version]
- Berardini, N.; Fezer, R.; Conrad, J.; Beifuss, U.; Carle, R.; Schieber, A. Screening of mango (Mangifera indica L.) cultivars for their contents of flavonol O-and xanthone C-glycosides, anthocyanins, and pectin. J. Agric. Food Chem. 2005, 51, 1563–1570. [Google Scholar] [CrossRef]
- López-Cobo, A.; Verardo, V.; Diaz-de-Cerio, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Gómez-Caravaca, A.M. Use of HPLC-and GC-QTOF to determine hydrophilic and lipophilic phenols in mango fruit (Mangifera indica L.) and its by-products. Food Res. Int. 2017, 100, 423–434. [Google Scholar]
- Sudheeran, P.K.; Love, C.; Feygenberg, O.; Maurer, D.; Ovadia, R.; Oren-Shamir, M.; Alkan, N. Induction of red skin and improvement of fruit quality in ‘Kent’, ’Shelly’ and ‘Maya’ mangoes by preharvest spraying of prohydrojasmon at the orchard. Postharvest Biol. Technol. 2019, 149, 18–26. [Google Scholar] [CrossRef]
- Koshiyama, M.; Watanabe, K.; Fujisawa, H.; Mitomi, M.; Imamura, K. Development of a new plant growth regulator, prohydrojasmon. Regul. Plant Growth Dev. (Jpn.) 2006, 41, 24–33. [Google Scholar]
- Sudheeran, P.; Feygenberg, O.; Maurer, D.; Alkan, N. Improved cold tolerance of mango fruit with enhanced anthocyanin and flavonoid contents. Molecules 2018, 21, 1832. [Google Scholar] [CrossRef] [Green Version]
- Sivankalyani, V.; Feygenberg, O.; Diskin, S.; Wright, B.; Alkan, N. Increased anthocyanin and flavonoids in mango fruit peel are associated with cold and pathogen resistance. Postharvest Biol. Technol. 2016, 111, 132–139. [Google Scholar] [CrossRef]
- Sudheeran, P.K.; Ovadia, R.; Galsarker, O.; Maoz, I.; Sela, N.; Maurer, D.; Feygenberg, O.; Oren Shamir, M.; Alkan, N. Glycosylated flavonoids: Fruit’s concealed antifungal arsenal. New Phytol. 2020, 221, 1788–1798. [Google Scholar] [CrossRef]
- Cantín, C.M.; Fidelibus, M.W.; Crisosto, C.H. Application of abscisic acid (ABA) at veraison advanced red color development and maintained postharvest quality of ‘Crimson Seedless’ grapes. Postharvest Biol. Technol. 2007, 41, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, A.K.; Gray, D.J.; Lu, J.; Gu, L. Effects of exogenous abscisic acid on antioxidant capacities, anthocyanins, and flavonol contents of muscadine grape (Vitis rotundifolia) skins. Food Chem. 2011, 121, 982–988. [Google Scholar] [CrossRef]
- Kondo, S.; Uthaibutra, J.; Gemma, H. Comparison of 1-aminocyclopropane-1-carboxylic acid, abscisic acid and anthocyanin content of some apple [Malus pumila] cultivars during fruit growth and maturation. J. Jpn. Soc. Hortic. Sci. (Jpn.) 1991, 60, 505–511. [Google Scholar] [CrossRef]
- Zhang, M.; Leng, P.; Zhang, G.; Li, X. Cloning and functional analysis of 9-cis-epoxycarotenoid dioxygenase (NCED) genes encoding a key enzyme during abscisic acid biosynthesis from peach and grapefruits. J. Plant Physiol. 2009, 161, 1241–1252. [Google Scholar] [CrossRef]
- Whale, S.; Singh, Z.; Behboudian, M.; Janes, J.; Dhaliwal, S. Fruit quality in ‘Cripp’s Pink’ apple, especially colour, as affected by preharvest sprays of aminoethoxy vinylglycine and ethephon. Sci. Hortic. 2008, 111, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yin, W.; Wu, J.; Chai, L.; Yi, H. Effects of exogenous abscisic acid on the expression of citrus fruit ripening-related genes and fruit ripening. Sci. Hortic. 2016, 201, 175–183. [Google Scholar] [CrossRef]
- Ghozlen, N.B.; Cerovic, Z.G.; Germain, C.; Toutain, S.; Latouche, G. Non-destructive optical monitoring of grape maturation by proximal sensing. Sensors 2010, 11, 10040–10068. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.; Kaplunov, T.; Zutahy, Y.; Daus, A.; Lurie, S.; Lichter, A. Auto- fluorescence for analysis of ripening in Thompson Seedless and colour in Crimson Seedless table grapes. Aust. J. Grape Wine Res. 2012, 11, 353–359. [Google Scholar] [CrossRef]
- Galsurker, O.; Diskin, S.; Maurer, D.; Feygenberg, O.; Alkan, N. Fruit stem-end rot. Horticulturae 2018, 1, 50. [Google Scholar] [CrossRef] [Green Version]
- Dar, J.A.; Wani, A.A.; Ahmed, M.; Nazir, R.; Zargar, S.M.; Javaid, K. Peel color in apple (Malus domestica Borkh.): An economic quality parameter in fruit market. Sci. Hortic. 2019, 244, 50–60. [Google Scholar] [CrossRef]
- Silvestri, C.; Cirilli, M.; Zecchini, M.; Muleo, R.; Ruggieri, A. Consumer acceptance of the new red-fleshed apple variety. J. Food Prod. Mark. 2018, 24, 1–21. [Google Scholar] [CrossRef]
- Romanazzi, G.; Sanzani, S.M.; Bi, Y.; Tian, S.; Martínez, P.G.; Alkan, N. Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Biol. Technol. 2016, 122, 82–94. [Google Scholar] [CrossRef]
- Brunetti, C.; Guidi, L.; Sebastiani, F.; Tattini, M. Isoprenoids and phenylpropanoids are key components of the antioxidant defense system of plants facing severe excess light stress. Environ. Exp. Bot. 2015, 119, 54–62. [Google Scholar] [CrossRef]
- Kondo, S.; Tsukada, N.; Niimi, Y.; Seto, H. Interactions between jasmonates and abscisic acid in apple fruit, and stimulative effect of jasmonates on anthocyanin accumulation. J. Jpn. Soc. Hortic. Sci. 2001, 71, 546–552. [Google Scholar] [CrossRef]
- Singh, S.P.; Saini, M.K.; Singh, J.; Pongener, A.; Sidhu, G.S. Preharvest application of abscisic acid promotes anthocyanins accumulation in pericarp of litchi fruit without adversely affecting postharvest quality. Postharvest Biol. Technol. 2014, 96, 14–22. [Google Scholar] [CrossRef]
- Asad, M.A.U.; Zakari, S.A.; Zhao, Q.; Zhou, L.; Ye, Y.; Cheng, F. Abiotic stresses intervene with ABA signaling to induce destructive metabolic pathways leading to death: Premature leaf senescence in plants. Int. J. Mol. Sci. 2019, 21, 256. [Google Scholar] [CrossRef] [Green Version]
- Ghasemnezhad, M.; Javaherdashti, M. Effect of methyl jasmonate treatment on antioxidant capacity, internal quality and postharvest life of raspberry fruit. Casp. J. Environ. Sci. 2008, 1, 73–78. [Google Scholar]
- Osorio, G.T.; Oliveira, B.S.; Di Piero, R.M. Effect of fumigants on blue and gray molds of apple fruit. Trop. Plant Pathol. 2013, 31, 63–67. [Google Scholar]
- Furness, G.O.; Pinczewski, W.V. A comparison of the spray distribution obtained from sprayers with converging and diverging airjets with low volume air-assisted spraying on citrus and grapevines. J. Agric. Eng. Res. 1985, 31, 291–310. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.P.; Maurer, D.; Feygenberg, O.; Love, C.; Alkan, N. Improving the Red Color and Fruit Quality of ‘Kent’ Mango Fruit by Pruning and Preharvest Spraying of Prohydrojasmon or Abscisic Acid. Agronomy 2020, 10, 944. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy10070944
Kumar SP, Maurer D, Feygenberg O, Love C, Alkan N. Improving the Red Color and Fruit Quality of ‘Kent’ Mango Fruit by Pruning and Preharvest Spraying of Prohydrojasmon or Abscisic Acid. Agronomy. 2020; 10(7):944. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy10070944
Chicago/Turabian StyleKumar, Sudheeran Pradeep, Dalia Maurer, Oleg Feygenberg, Cliff Love, and Noam Alkan. 2020. "Improving the Red Color and Fruit Quality of ‘Kent’ Mango Fruit by Pruning and Preharvest Spraying of Prohydrojasmon or Abscisic Acid" Agronomy 10, no. 7: 944. https://0-doi-org.brum.beds.ac.uk/10.3390/agronomy10070944