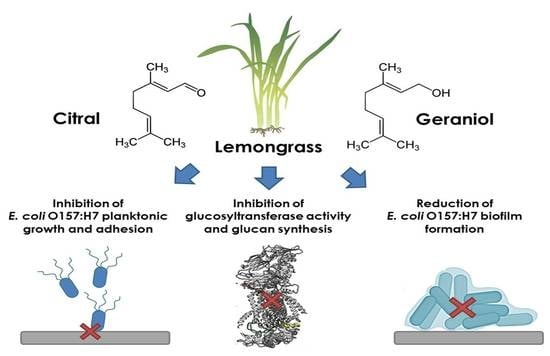
Inhibition of Glucosyltransferase Activity and Glucan Production as an Antibiofilm Mechanism of Lemongrass Essential Oil against Escherichia coli O157:H7
Abstract
:1. Introduction
2. Results
2.1. Susceptibility of Planktonic and Biofilm E. coli O157:H7 Cells to C. citratus EO, Citral, and Geraniol
2.2. Effect of C. citratus EO, Citral, and Geraniol on the Glucans Content in E. coli O157:H7 Biofilms
2.3. Inhibition of Glucosyltransferase Activity by Citral and Geraniol
3. Discussion
4. Material and Methods
4.1. Susceptibility of Planktonic and Biofilm E. coli O157:H7 Cells to C. citratus EO, Citral, and Geraniol
4.2. Effect of C. citratus EO, Citral, and Geraniol on the Glucans Content in E. coli O157:H7 Biofilms
4.3. Inhibition of Glucosyltransferase Activity by Citral and Geraniol
4.4. Molecular Docking of Glucosyltransferase with Citral and Geraniol
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gutiérrez-Pacheco, M.M.; Bernal-Mercado, A.T.; Vázquez-Armenta, F.J.; Martínez-Tellez, M.A.; González-Aguilar, G.A.; Lizardi-Mendoza, J.; Madera-Santana, T.J.; Nazzaro, F.; Ayala-Zavala, J.F. Quorum sensing interruption as a tool to control virulence of plant pathogenic bacteria. Physiol. Mol. Plant P 2019, 106, 281–291. [Google Scholar] [CrossRef]
- CDC. Reports of Selected E. coli Outbreak Investigations. Available online: https://www.cdc.gov/ecoli/outbreaks.html (accessed on 18 June 2018).
- Ryu, J.H.; Beuchat, L.R. Biofilm formation by Escherichia coli O157:H7 on stainless steel: Effect of exopolysaccharide and Curli production on its resistance to chlorine. Appl. Environ. Microbiol. 2005, 71, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Beloin, C.; Roux, A.; Ghigo, J.-M. Escherichia coli biofilms. In Bacterial Biofilms; Springer: Berlin/Heidelberg, Germany, 2008; pp. 249–289. [Google Scholar]
- Lim, E.S.; Koo, O.K.; Kim, M.-J.; Kim, J.-S. Bio-enzymes for inhibition and elimination of Escherichia coli O157: H7 biofilm and their synergistic effect with sodium hypochlorite. Sci. Rep. 2019, 9, 9920. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.L.; McNamara, J.T.; Fischer, M.; Rich, J.; Chen, H.M.; Withers, S.G.; Zimmer, J. Observing cellulose biosynthesis and membrane translocation in crystallo. Nature 2016, 531, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Zulfa, Z.; Chia, C.; Rukayadi, Y. In vitro antimicrobial activity of Cymbopogon citratus (lemongrass) extracts against selected foodborne pathogens. Int. Food Res. J. 2016, 23, 1262–1267. [Google Scholar]
- Singh, B.R.; Singh, V.; Singh, R.K.; Ebibeni, N. Antimicrobial activity of lemongrass (Cymbopogon citratus) oil against microbes of environmental, clinical and food origin. Int. Res. J. Pharm. Pharmacol. 2011, 1, 228–236. [Google Scholar]
- Ahmad, A.; Viljoen, A.M.; Chenia, H.Y. The impact of plant volatiles on bacterial Quorum sensing. Lett. Appl. Microbiol. 2015, 60, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Ramirez, L.A.; Silva-Espinoza, B.A.; Vargas-Arispuro, I.; Gonzalez-Aguilar, G.A.; Cruz-Valenzuela, M.R.; Nazzaro, F.; Ayala-Zavala, J.F. Combination of Cymbopogon citratus and Allium cepa essential oils increased antibacterial activity in leafy vegetables. J. Sci. Food Agric. 2017, 97, 2166–2173. [Google Scholar] [CrossRef] [PubMed]
- Tofiño-Rivera, A.; Ortega-Cuadros, M.; Galvis-Pareja, D.; Jiménez-Rios, H.; Merini, L.J.; Martínez-Pabón, M.C. Effect of Lippia alba and Cymbopogon citratus essential oils on biofilms of Streptococcus mutans and cytotoxicity in CHO cells. J. Ethnopharmacol. 2016, 194, 749–754. [Google Scholar] [CrossRef] [Green Version]
- Adukwu, E.C.; Allen, S.C.; Phillips, C.A. The anti-biofilm activity of lemongrass (Cymbopogon flexuosus) and grapefruit (Citrus paradisi) essential oils against five strains of Staphylococcus aureus. J. Appl. Microbiol. 2012, 113, 1217–1227. [Google Scholar] [CrossRef]
- Oliveira, M.A.C.; Borges, A.C.; Brighenti, F.L.; Salvador, M.J.; Gontijo, A.V.L.; Koga-Ito, C.Y. Cymbopogon citratus essential oil: Effect on polymicrobial caries-related biofilm with low cytotoxicity. Braz. Oral Res. 2017, 31, e89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naz, F.; Khan, F.I.; Mohammad, T.; Khan, P.; Manzoor, S.; Hasan, G.M.; Lobb, K.A.; Luqman, S.; Islam, A.; Ahmad, F.; et al. Investigation of molecular mechanism of recognition between citral and MARK4: A newer therapeutic approach to attenuate cancer cell progression. Int. J. Biol. Macromol. 2018, 107, 2580–2589. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, E.R.; Alves, D.S.; Carvalho, G.A.; de Oliveira, B.M.R.G.; Aazza, S.; Bertolucci, S.K.V. Toxicity of Cymbopogon flexuosus essential oil and citral for Spodoptera frugiperda. Ciência Agrotecnol. 2018, 42, 408–419. [Google Scholar] [CrossRef]
- Pubchem PubChem Compound Database. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 21 November 2018).
- Treesuwan, W.; Neves, M.A.; Uemura, K.; Nakajima, M.; Kobayashi, I. Preparation characteristics of monodisperse oil-in-water emulsions by microchannel emulsification using different essential oils. LWT 2017, 84, 617–625. [Google Scholar] [CrossRef]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-G.; Lee, J.-H.; Gwon, G.; Kim, S.-I.; Park, J.G.; Lee, J. Essential Oils and Eugenols Inhibit Biofilm Formation and the Virulence of Escherichia coli O157:H7. Sci. Rep. 2016, 6, 36377. [Google Scholar] [CrossRef] [Green Version]
- Bazargani, M.M.; Rohloff, J. Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control 2016, 61, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Pruthi, P.A.; Pruthi, V. Role of Exopolysaccharides in Biofilm Formation. In Introduction to Biofilm Engineering; ACS Publications: Washington, DC, USA, 2019; pp. 17–57. [Google Scholar]
- Ha, J.H.; Hauk, P.; Cho, K.; Eo, Y.; Ma, X.; Stephens, K.; Cha, S.; Jeong, M.; Suh, J.Y.; Sintim, H.O.; et al. Evidence of link between quorum sensing and sugar metabolism in Escherichia coli revealed via cocrystal structures of LsrK and HPr. Sci. Adv. 2018, 4, eaar7063. [Google Scholar] [CrossRef] [Green Version]
- Da Re, S.; Ghigo, J.M. A CsgD-independent pathway for cellulose production and biofilm formation in Escherichia coli. J. Bacteriol. 2006, 188, 3073–3087. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, N.L.; de Aguiar, S.R.M.; Falé, P.L.; Ascensão, L.; Serralheiro, M.L.M.; Lino, A.R.L. The inhibitory effect of Plectranthus barbatus and Plectranthus ecklonii leaves on the viability, glucosyltransferase activity and biofilm formation of Streptococcus sobrinus and Streptococcus mutans. Food Chem. 2010, 119, 664–668. [Google Scholar] [CrossRef]
- Koo, H.; Pearson, S.K.; Scott-Anne, K.; Abranches, J.; Cury, J.A.; Rosalen, P.L.; Park, Y.K.; Marquis, R.E.; Bowen, W.H. Effects of apigenin and tt-farnesol on glucosyltransferase activity, biofilm viability and caries development in rats. Oral Microbiol. Immunol. 2002, 17, 337–343. [Google Scholar] [CrossRef]
- Galie, S.; Garcia-Gutierrez, C.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jang, S.; Kim, H.; Kim, S. Deodorizing, antimicrobial and glucosyltransferase inhibitory activities of polyphenolics from biosource. Korean J. Chem. Eng. 2017, 34, 1400–1404. [Google Scholar] [CrossRef]
- Osawa, K.; Miyazaki, K.; Shimura, S.; Okuda, J.; Matsumoto, M.; Ooshima, T. Identification of cariostatic substances in the cacao bean husk: Their anti-glucosyltransferase and antibacterial activities. J. Dent. Res. 2001, 80, 2000–2004. [Google Scholar] [CrossRef] [PubMed]
- De-Oliveira, A.C.; Ribeiro-Pinto, L.F.; Paumgartten, F.J. In vitro inhibition of CYP2B1 monooxygenase by β-myrcene and other monoterpenoid compounds. Toxicol. Lett. 1997, 92, 39–46. [Google Scholar] [CrossRef]
- Clsi, C. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement; American National Standards Institute: Washington, DC, USA, 2014. [Google Scholar]
- Tapia-Rodriguez, M.R.; Hernandez-Mendoza, A.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Martins, C.M.; Ayala-Zavala, J.F. Carvacrol as potential quorum sensing inhibitor of Pseudomonas aeruginosa and biofilm production on stainless steel surfaces. Food Control 2017, 75, 255–261. [Google Scholar] [CrossRef]
- Gutierrez-Pacheco, M.M.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Lizardi-Mendoza, J.; Madera-Santana, T.J.; Bernal-Mercado, A.T.; Vazquez-Armenta, F.J.; Ayala-Zavala, J.F. Carvacrol inhibits biofilm formation and production of extracellular polymeric substances of Pectobacterium carotovorum subsp. carotovorum. Food Control 2018, 89, 210–218. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. Colorimetric method based on phenol sulfuric acid. Anal. Chem. 1956, 28, 356. [Google Scholar]
- Palmerini, C.A.; Datti, A.; Vanderelst, I.E.; Minuti, L.; Orlacchio, A. An approach for fluorometric determination of glycosyltransferase activities. Glycoconj. J. 1996, 13, 631–636. [Google Scholar] [CrossRef]
- Copeland, R.A. Evaluation of Enzyme Inhibitors in Drug Discovery: A Guide for Medicinal Chemists and Pharmacologists; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]







| Agent | Concentration (µM) | Km * (µM) | Vmax * (µmol UDP**-glucose min/mL) | Ki (µM) * |
|---|---|---|---|---|
| Citral | 0 | 3.42 | 714.28 | |
| 8 | 2.66 | 476.19 | 7 | |
| 10 | 2.66 | 303.03 | ||
| Geraniol | 0 | 3.42 | 714.28 | |
| 8 | 2.20 | 416.66 | 6.5 | |
| 10 | 2 | 256.41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Ramirez, L.A.; Gutiérrez-Pacheco, M.M.; Vargas-Arispuro, I.; González-Aguilar, G.A.; Martínez-Téllez, M.A.; Ayala-Zavala, J.F. Inhibition of Glucosyltransferase Activity and Glucan Production as an Antibiofilm Mechanism of Lemongrass Essential Oil against Escherichia coli O157:H7. Antibiotics 2020, 9, 102. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9030102
Ortega-Ramirez LA, Gutiérrez-Pacheco MM, Vargas-Arispuro I, González-Aguilar GA, Martínez-Téllez MA, Ayala-Zavala JF. Inhibition of Glucosyltransferase Activity and Glucan Production as an Antibiofilm Mechanism of Lemongrass Essential Oil against Escherichia coli O157:H7. Antibiotics. 2020; 9(3):102. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9030102
Chicago/Turabian StyleOrtega-Ramirez, Luis A., M. Melissa Gutiérrez-Pacheco, Irasema Vargas-Arispuro, Gustavo A. González-Aguilar, Miguel A. Martínez-Téllez, and J. Fernando Ayala-Zavala. 2020. "Inhibition of Glucosyltransferase Activity and Glucan Production as an Antibiofilm Mechanism of Lemongrass Essential Oil against Escherichia coli O157:H7" Antibiotics 9, no. 3: 102. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9030102