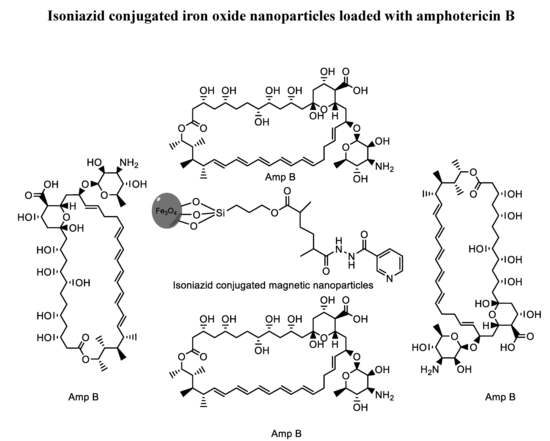
Isoniazid Conjugated Magnetic Nanoparticles Loaded with Amphotericin B as a Potent Antiamoebic Agent against Acanthamoeba castellanii
Abstract
:1. Introduction
2. Methods and Materials
2.1. Chemicals
2.2. Synthesis of N′-Methacryloylisonicotinohydrazide (MIH)
2.3. Functionalization of Magnetic Nanoparticles (MPs) with MIH
2.4. Characterization: Size, Size Distribution and Morphology
2.5. Drug Loading Studies
2.6. Drug Entrapment Efficiency Determination
- Qp: Quantity of free drug.
- Qt: Quantity of drug added.
- %EE: Entrapment efficiency of loaded drug in percent.
FTIR Spectroscopy
2.7. Hemocompatibility Study
- Rs: Absorbance of sample
- Rc: Absorbance of positive control
- % H.A: Hemolytic activity in percent
2.8. In Vitro Cytotoxicity
- At: Mean of Absorbance value of Test Sample.
- Ac: Mean of Absorbance value of Control.
2.9. Acanthamoeba Culture
2.10. Antiamoebic Assay
2.11. Anticystic Assay
3. Results and Discussion
3.1. Synthesis of N′-Methacryloylisonicotinohydrazide (MIH)
3.2. Preparation of MIH-Coated MNPs
3.3. Size, Size Distribution and Morphology
3.4. Drug Hosting Studies
3.5. FTIR Spectroscopy
3.6. Hemocompatibility
3.7. In Vitro Cytotoxicity
3.8. Isoniazid-Nanoparticles-Amphotericin B Displayed an IC50 of 45 μg/mL against A. castellanii Trophozoites
3.9. Isoniazid-Nanoparticles-Amphotericin B Displayed an IC50 of 50 μg/mL against A. castellanii Cysts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
Ethical Approval
References
- Schuster, F.L.; Visvesvara, G.S. Opportunistic amoebae: Challenges in prophylaxis and treatment. Drug Resist. Updates 2010, 7, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.M.; Khan, N.A. Tackling infection owing to brain-eating amoeba. Acta Trop. 2015, 142, 86–88. [Google Scholar] [CrossRef]
- Mungroo, M.; Anwar, A.; Khan, N.; Siddiqui, R. Brain-eating amoebae infection: Challenges and opportunities in chemotherapy. Mini Rev. Med. Chem. 2019, 19, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Hoseinzadeh, E.; Makhdoumi, P.; Taha, P.; Hossini, H.; Stelling, J.; Amjad Kamal, M. A review on nano-antimicrobials: Metal nanoparticles, methods and mechanisms. Curr. Drug Metabol. 2017, 18, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachu, R.; Chowdhury, P.; Al-Saedi, Z.; Karla, P.; Boddu, S. Ocular drug delivery barriers—role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Kalwar, K.; Shan, D. Antimicrobial effect of silver nanoparticles (AgNPs) and their mechanism–a mini review. Micro Nano Lett. 2018, 13, 277–280. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, Y.; Cui, Y.; Liu, W.; Ma, W.; Jiang, X. Small molecule-capped gold nanoparticles as potent antibacterial agents that target gram-negative bacteria. J. Am. Chem. Soc. 2010, 132, 12349–12356. [Google Scholar] [CrossRef]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and multi metal oxide nanoparticles: Synthesis, antibacterial and cytotoxic properties. J. Nanobiotechnol. 2016, 14, 73. [Google Scholar] [CrossRef] [Green Version]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Shi, X.; Huang, J.; Zhang, Y.; Wu, Z.; Xian, Y. Bacitracin-Conjugated Superparamagnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Antibacterial Activity. ChemPhysChem 2012, 13, 3388–3396. [Google Scholar] [CrossRef] [PubMed]
- Aqeel, Y.; Siddiqui, R.; Anwar, A.; Shah, M.R.; Khan, N.A. Gold nanoparticle conjugation enhances the antiacanthamoebic effects of chlorhexidine. Antimicrob. Agents Chemother. 2016, 60, 1283–1288. [Google Scholar] [CrossRef] [Green Version]
- Anwar, A.; Siddiqui, R.; Hussain, M.A.; Ahmed, D.; Shah, M.R.; Khan, N.A. Silver nanoparticle conjugation affects antiacanthamoebic activities of amphotericin B, nystatin, and fluconazole. Parasitol. Res. 2018, 117, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Gomart, G.; Denis, J.; Bourcier, T.; Dory, A.; Abou-Bacar, A.; Candolfi, E.; Sauer, A. In vitro amoebicidal activity of Titanium dioxide/UV-A combination against Acanthamoeba. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4567–4571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imran, M.; Muazzam, A.G.; Habib, A.; Matin, A. Synthesis, characterization and amoebicidal potential of locally synthesized TiO2 nanoparticles against pathogenic Acanthamoeba trophozoites in vitro. J. Photochem. Photobiol. B: Biol. 2016, 159, 125–132. [Google Scholar] [CrossRef]
- Anwar, A.; Numan, A.; Siddiqui, R.; Khalid, M.; Khan, N.A. Cobalt nanoparticles as novel nanotherapeutics against Acanthamoeba castellanii. Parasit. Vectors 2019, 12, 280. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Kolawole, G.A. Synthesis, characterization and in vitro antiprotozoal studies of iron (III) complexes of some antimalarial drugs. J. Coord. Chem. 2008, 61, 3367–3374. [Google Scholar] [CrossRef]
- Youdim, M.B.; Grünblatt, E.; Levites, Y.; Maor, G.; Mandel, S. Early and late molecular events in neurodegeneration and neuroprotection in Parkinson’s disease MPTP model as assessed by cDNA microarray; the role of iron. Neurotox. Res. 2002, 4, 679–689. [Google Scholar] [CrossRef]
- Bush, A.I. Drug development based on the metals hypothesis of Alzheimer’s disease. J. Alzheimer’s Dis. 2008, 15, 223–240. [Google Scholar] [CrossRef] [Green Version]
- Ling, D.; Hyeon, T. Chemical design of biocompatible iron oxide nanoparticles for medical applications. Small 2013, 9, 1450–1466. [Google Scholar] [CrossRef]
- Shen, M.; Cai, H.; Wang, X.; Cao, X.; Li, K.; Wang, S.H.; Guo, R.; Zheng, L.; Zhang, G.; Shi, X. Facile one-pot preparation, surface functionalization, and toxicity assay of APTS-coated iron oxide nanoparticles. Nanotechnology 2012, 23, 105601. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Meng, X.; Zhao, Y.; Li, K.; Zhao, B.; Zhu, M.; Li, Y.; Chen, X.; Wang, J. Novel water-soluble and pH-responsive anticancer drug nanocarriers: Doxorubicin–PAMAM dendrimer conjugates attached to superparamagnetic iron oxide nanoparticles (IONPs). J. Colloid Interface Sci. 2011, 363, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yang, W.; Wang, C.C.; Fu, S.K. A novel approach for preparation of thermoresponsive polymer magnetic microspheres with core–shell structure. Adv. Mater. 2003, 15, 1729–1732. [Google Scholar] [CrossRef]
- Tran, N.; Mir, A.; Mallik, D.; Sinha, A.; Nayar, S.; Webster, T.J. Bactericidal effect of iron oxide nanoparticles on Staphylococcus aureus. Int. J. Nanomed. 2010, 5, 277–283. [Google Scholar]
- Gordon, T.; Perlstein, B.; Houbara, O.; Felner, I.; Banin, E.; Margel, S. Synthesis and characterization of zinc/iron oxide composite nanoparticles and their antibacterial properties. Colloids Surfaces A: Physicochem. Eng. Asp. 2011, 374, 1–8. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Neogi, S. Gentamicin coated iron oxide nanoparticles as novel antibacterial agents. Mater. Res. Express 2017, 4, 095005. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; de Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [Green Version]
- Chertok, B.; Moffat, B.A.; David, A.E.; Yu, F.; Bergemann, C.; Ross, B.D.; Yang, V.C. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials 2008, 29, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Sonvico, F.; Mornet, S.; Vasseur, S.; Dubernet, C.; Jaillard, D.; Degrouard, J.; Hoebeke, J.; Duguet, E.; Colombo, P.; Couvreur, P. Folate-conjugated iron oxide nanoparticles for solid tumor targeting as potential specific magnetic hyperthermia mediators: Synthesis, physicochemical characterization, and in vitro experiments. Bioconj. Chem. 2005, 16, 1181–1188. [Google Scholar] [CrossRef]
- Mosimaneotsile, B.; Mathoma, A.; Chengeta, B.; Nyirenda, S.; Agizew, T.B.; Tedla, Z.; Motsamai, O.I.; Kilmarx, P.H.; Wells, C.D.; Samandari, T. Isoniazid tuberculosis preventive therapy in HIV-infected adults accessing antiretroviral therapy: A Botswana Experience, 2004–2006. JAIDS J. Acquir. Immun. Def. Syndr. 2010, 54, 71–77. [Google Scholar] [CrossRef]
- Zhang, Y.; Heym, B.; Allen, B.; Young, D.; Cole, S. The catalase—peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature 1992, 358, 591. [Google Scholar] [CrossRef] [PubMed]
- Slayden, R.A.; Lee, R.E.; Barry, C.E., 3rd. Isoniazid affects multiple components of the type II fatty acid synthase system of Mycobacterium tuberculosis. Mol. Microbiol. 2000, 38, 514–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmins, G.S.; Master, S.; Rusnak, F.; Deretic, V. Nitric oxide generated from isoniazid activation by KatG: Source of nitric oxide and activity against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2004, 48, 3006–3009. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, K.; Anwar, A.; Khan, N.A.; Siddiqui, R. Brain-eating amoebae: Silver nanoparticle conjugation enhanced efficacy of anti-amoebic drugs against Naegleria fowleri. ACS Chem. Neurosci. 2017, 8, 2626–2630. [Google Scholar] [CrossRef] [PubMed]
- Goswick, S.M.; Brenner, G.M. Activities of azithromycin and amphotericin B against Naegleria fowleri in vitro and in a mouse model of primary amebic meningoencephalitis. Antimicrob. Agents Chemother. 2003, 47, 524–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemke, A.; Kiderlen, A.F.; Petri, B.; Kayser, O. Delivery of amphotericin B nanosuspensions to the brain and determination of activity against Balamuthia mandrillaris amebas. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 597–603. [Google Scholar] [CrossRef]
- Dresco, P.A.; Zaitsev, V.S.; Gambino, R.J.; Chu, B. Preparation and properties of magnetite and polymer magnetite nanoparticles. Langmuir 1999, 15, 1945–1951. [Google Scholar] [CrossRef]
- Saif, B.; Wang, C.; Chuan, D.; Shuang, S. Synthesis and characterization of Fe3O4 coated on APTES as carriers for morin-anticancer drug. J. Biomater. Nanobiotechnol. 2015, 6, 267. [Google Scholar] [CrossRef]
- Aqeel, Y.; Siddiqui, R.; Anwar, A.; Shah, M.R.; Khoja, S.; Khan, N.A. Photochemotherapeutic strategy against Acanthamoeba infections. Antimicrob. Agents Chemother. 2015, 59, 3031–3041. [Google Scholar] [CrossRef] [Green Version]
- Anwar, A.; Mungroo, M.R.; Anwar, A.; Sullivan, W.; Khan, N.A.; Siddiqui, R. Repositioning of guanabenz in conjugation with gold and silver nanoparticles against pathogenic amoebae Acanthamoeba castellanii and Naegleria fowleri. ACS Infect. Dis. 2019, 5, 2039–2046. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Arsalani, N.; Fattahi, H.; Nazarpoor, M. Synthesis and characterization of PVP-functionalized superparamagnetic Fe3O4 nanoparticles as an MRI contrast agent. Express Polym. Lett. 2010, 4, 329–338. [Google Scholar] [CrossRef]
- Caddeo, C.; Teskač, K.; Sinico, C.; Kristl, J. Effect of resveratrol incorporated in liposomes on proliferation and UV-B protection of cells. Int. J. Pharm. 2008, 363, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Jabri, T.; Imran, M.; Shafiullah Rao, K.; Ali, I.; Arfan, M.; Shah, M.R. Fabrication of lecithin-gum tragacanth muco-adhesive hybrid nano-carrier system for in-vivo performance of Amphotericin B. Carbohydr. Polym. 2018, 194, 89–96. [Google Scholar] [CrossRef]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Imran, M.; Shah, M.R.; Ullah, F.; Ullah, S.; Elhissi, A.M.A.; Nawaz, W.; Ahmad, F.; Sadiq, A.; Ali, I. Sugar-based novel niosomal nanocarrier system for enhanced oral bioavailability of levofloxacin. Drug Deliv. 2016, 23, 3653–3664. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Simchi, A.; Imani, M.; Shokrgozar, M.A.; Milani, A.S.; Häfeli, U.O.; Stroeve, P. A new approach for the in vitro identification of the cytotoxicity of superparamagnetic iron oxide nanoparticles. Colloids Surfaces B: Biointerfac. 2010, 75, 300–309. [Google Scholar] [CrossRef]
- Khan, N.A.; Anwar, A.; Siddiqui, R. Future priorities in tackling infections due to brain-eating amoebae. ACS Chem. Neurosci. 2017, 8, 2355. [Google Scholar] [CrossRef] [Green Version]
- Ong, T.Y.; Khan, N.A.; Siddiqui, R. Brain-eating amoebae: Predilection sites in the brain and disease outcome. J. Clin. Microbiol. 2017, 55, 1989–1997. [Google Scholar] [CrossRef] [Green Version]
- Pugh, J.J.; Levy, R.A. Naegleria fowleri: Diagnosis, pathophysiology of brain inflammation, and antimicrobial treatments. ACS Chem. Neurosci. 2016, 7, 1178–1179. [Google Scholar] [CrossRef] [Green Version]
- Beskid, G.; Unowsky, J.; Behl, C.R.; Siebelist, J.A.; Tossounian, J.L.; McGarry, C.M.; Shah, N.H.; Cleeland, R. Enteral, oral, and rectal absorption of ceftriaxone using glyceride enhancers. Chemotherapy 1988, 34, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Benavides, T.; Mitjans, M.; Martínez, V.; Clapes, P.; Infante, M.R.; Clothier, R.; Vinardell, M.P. Assessment of primary eye and skin irritants by in vitro cytotoxicity and phototoxicity models: An in vitro approach of new arginine-based surfactant-induced irritation. Toxicology 2004, 197, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Lajevardi, A.; Sadr, M.H.; Yaraki, M.T.; Badiei, A.; Armaghan, M. A pH-responsive and magnetic Fe3O4@ silica@ MIL-100 (Fe)/β-CD nanocomposite as a drug nanocarrier: Loading and release study of cephalexin. New J. Chem. 2018, 42, 9690–9701. [Google Scholar] [CrossRef]
- Ghafelehbashi, R.; Yaraki, M.T.; Saremi, L.H.; Lajevardi, A.; Haratian, M.; Astinchap, B.; Rashidi, A.M.; Moradian, R. A pH-responsive citric-acid/α-cyclodextrin-functionalized Fe3O4 nanoparticles as a nanocarrier for quercetin: An experimental and DFT study. Mater. Sci. Eng. C 2020, 109, 110597. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, I.; Yaraki, M.T.; Bourbour, M.; Noorbazargan, H.; Lajevardi, A.; Shilsar, S.M.; Heidari, F.; Mousavian, S.M. Optimized doxycycline-loaded niosomal formulation for treatment of infection-associated prostate cancer: An in-vitro investigation. J. Drug Deliv. Sci. Technol. 2020, 57, 101715. [Google Scholar] [CrossRef]
- Ghafelehbashi, R.; Akbarzadeh, I.; Yaraki, M.T.; Lajevardi, A.; Fatemizadeh, M.; Saremi, L.H. Preparation, physicochemical properties, in vitro evaluation and release behavior of cephalexin-loaded niosomes. Int. J. Pharm. 2019, 569, 118580. [Google Scholar] [CrossRef]






| Nanoparticles | Ratios (Durg:NPs) | Size (nm) | PDI | Zeta Potential (mV) | Entrapment Efficiency (%) |
|---|---|---|---|---|---|
| MIH-MP | N. A. | 140.2 ± 0.45 (62%) | 0.237 ± 0.019 | −17.7 ± 0.40 | N. A. |
| MIH-MP-AmpB | 1:1 | 184 ± 2.7 | 0.265 ± 0.04 | −20.2 ± 0.41 | 76.30 ± 1.34 |
| MIH-MP-AmpB | 2:1 | 186.94 ± 1.20 (71%) | 0.346 ± 0.043 | −18.3 ± 0.92 | 64.34 ± 4.55 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, K.; Abdalla, S.A.O.; Anwar, A.; Iqbal, K.M.; Shah, M.R.; Anwar, A.; Siddiqui, R.; Khan, N.A. Isoniazid Conjugated Magnetic Nanoparticles Loaded with Amphotericin B as a Potent Antiamoebic Agent against Acanthamoeba castellanii. Antibiotics 2020, 9, 276. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9050276
Iqbal K, Abdalla SAO, Anwar A, Iqbal KM, Shah MR, Anwar A, Siddiqui R, Khan NA. Isoniazid Conjugated Magnetic Nanoparticles Loaded with Amphotericin B as a Potent Antiamoebic Agent against Acanthamoeba castellanii. Antibiotics. 2020; 9(5):276. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9050276
Chicago/Turabian StyleIqbal, Kawish, Sumayah Abdelnasir Osman Abdalla, Ayaz Anwar, Kanwal Muhammad Iqbal, Muhammad Raza Shah, Areeba Anwar, Ruqaiyyah Siddiqui, and Naveed Ahmed Khan. 2020. "Isoniazid Conjugated Magnetic Nanoparticles Loaded with Amphotericin B as a Potent Antiamoebic Agent against Acanthamoeba castellanii" Antibiotics 9, no. 5: 276. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9050276