Bioactive Compounds, Pharmacological Actions, and Pharmacokinetics of Wormwood (Artemisia absinthium)
Abstract
:1. Introduction
2. Method
3. Bioactive Constituents
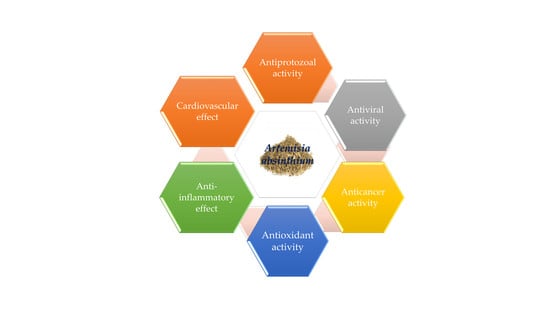
4. Pharmacological Actions
4.1. Traditional Uses of A. absinthium
4.2. Antioxidant Activity
4.3. Antioxidant Related Effects
4.3.1. Antitumor Activity
4.3.2. Neuroprotective and Antidepressant Effects
4.3.3. Immuno-modulatory and Wound Healing Activities
4.3.4. Hepatoprotective Effect
4.3.5. Renal and Hypoglycaemic Effects
4.4. Biological Activity of A. absinthium and Its Related Compounds
4.4.1. Anti-inflammatory and Antisnake Venom activity
4.4.2. Antipyretic and Analgesic Activities
4.4.3. Cardiovascular Activity
4.4.4. Growth Performance and Hormonal Effects
4.4.5. Antiulcer and Digestive Activities
4.5. Activities Related to Infectious Diseases
4.5.1. Antibacterial Activity
4.5.2. Antiviral Activity
4.5.3. Antiprotozoal Activity
4.5.4. Anti-fungal Activity
4.5.5. Anthelmintic Activity
4.5.6. Insecticidal Effect
5. Pharmacokinetics of and Stability of A. absinthium Components
6. Combination Therapy of A. absinthium and Its Related Compounds with Other Drugs
7. Side Effects and Contraindications
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Sharopov, F.S.; Sulaimonova, V.A.; Setzer, W.N. Composition of the Essential oil of Artemisia absinthium from Tajikistan. Rec. Nat. Prod. 2012, 6, 127–134. [Google Scholar]
- El Gaber, S.B.; Beshbishy, A.M.; Tayebwa, D.S.; Adeyemi, O.S.; Yokoyama, N.; Igarashi, I. Anti-piroplasmic potential of the methanolic Peganum harmala seeds and ethanolic Artemisia absinthium leaf extracts. J. Protozool. Res. 2019, 29, 8–27. [Google Scholar]
- Ahmad, W.; Hasan, A.; Abdullah, A.; Tarannum, T. Medicinal Importance of Artemisia absinthium Linn (Afsanteen) in Unani Medicine: A Review. Hippocrat. J. Unani Med. 2010, 5, 117–125. [Google Scholar]
- Nin, S.; Arfaioli, P.; Bosetto, M. Quantitative determination of some essential oil components of selected Artemisia absinthium plants. J. Essent. Oil Res. 1995, 7, 271–277. [Google Scholar] [CrossRef]
- Ahamad, J.; Mir, S.; Amin, S. A pharmacognostic review on Artemisia absinthium. Int. Res. J. Pharm. 2019, 10, 25–31. [Google Scholar] [CrossRef]
- Padosch, S.A.; Lachenmeier, D.W.; Kröner, L.U. Absinthism: A fictitious 19th century syndrome with present impact. Subst. Abuse Treat. Prev. Policy 2006, 1, 14. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Abbasi, B.H. Production of commercially important secondary metabolites and antioxidant activity in cell suspension cultures of Artemisia absinthium L. Ind. Crops Prod. 2013, 49, 400–406. [Google Scholar] [CrossRef]
- Shafi, G.; Hasan, T.N.; Syed, N.A.; Al-Hazzani, A.A.; Alshatwi, A.A.; Jyothi, A.; Munshi, A. Artemisia absinthium (AA): A novel potential complementary and alternative medicine for breast cancer. Mol. Biol. Rep. 2012, 39, 7373–7379. [Google Scholar] [CrossRef]
- Omer, B.; Krebs, S.; Omer, H.; Noor, T. Steroid-sparing effect of wormwood (Artemisia absinthium) in Crohn’s disease: A double-blind placebo-controlled study. Phytomedicine 2007, 14, 87–95. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Mavi, A.; Kilic, H.; Yildirim, A. Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkish artemisia species. J. Agric. Food Chem. 2005, 53, 1408–1416. [Google Scholar] [CrossRef]
- Ahamad, J.; Naquvi, K.; Ali, M.; Mir, S. Isoflavone glycosides from aerial parts of Artemisia absinthium. Chem. Nat. Comp. 2014, 49, 996–1000. [Google Scholar] [CrossRef]
- Rezaeinodehi, A.; Khangholi, S. Chemical composition of the essential oil of Artemisia absinthium growing wild in Iran. Pak. J. Biol. Sci. 2008, 11, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Dhen, N.; Majdoub, O.; Souguir, S.; Tayeb, W.; Laarif, A.; Chaieb, I. Chemical composition and fumigant toxicity of Artemisia absinthium essential oil against Rhyzopertha dominica and Spodoptera littoralis. Tunis. J. Plant Prot. 2014, 9, 57–61. [Google Scholar]
- Turak, A.; Shi, S.P.; Jiang, Y.; Tu, P.F. Dimeric guaianolides from Artemisia absinthium. Phytochemistry 2014, 105, 109–114. [Google Scholar] [CrossRef]
- Lou, S.N.; Lai, Y.C.; Hsu, Y.S.; Ho, C.T. Phenolic content, antioxidant activity and effective compounds of kumquat extracted by different solvents. Food Chem. 2016, 197, 1–6. [Google Scholar] [CrossRef]
- Bhat, R.R.; Rehman, M.U.; Shabir, A.; Mir, M.U.R.; Ahmad, A.; Khan, R.; Masoodi, M.H.; Madkhali, H.; Ganaie, M.A. Chemical Composition and Biological Uses of Artemisia absinthium (Wormwood). In Plant and Human Health; Springer: Berlin/Heidelberg, Germany, 2019; Volume 3, pp. 37–63. [Google Scholar]
- Ansari, S.; Shamshi, Y.; Khan, Q.A. A review of Artemisia absinthium, Linn. (afsanteen) with special reference of Unani medicine. J. Pharm. Sci. Innov. 2019, 8, 11–18. [Google Scholar] [CrossRef]
- Guarrera, P.M. Traditional phytotherapy in Central Italy (Marche, Abruzzo, and Latium). Fitoterapia 2005, 76, 1–25. [Google Scholar] [CrossRef]
- Wake, G.; Court, J.; Pickering, A.; Lewis, R.; Wilkins, R.; Perry, E. CNS acetylcholine receptor activity in European medicinal plants traditionally used to improve failing memory. J. Ethnopharm. 2000, 69, 105–114. [Google Scholar] [CrossRef]
- Gilani, A.U.H.; Janbaz, K.H. Preventive and curative effects of Artemisia absinthium on acetaminophen and CCl4-induced hepatotoxicity. Gen. Pharmacol. Vasc. Syst. Gen. Pharmacol. 1995, 26, 309–315. [Google Scholar] [CrossRef]
- Basiri, Z.; Zeraati, F.; Esna-Ashari, F.; Mohammadi, F.; Razzaghi, K.; Araghchian, M.; Moradkhani, S. Topical effects of Artemisia Absinthium ointment and liniment in comparison with piroxicam gel in patients with knee joint osteoarthritis: A randomized double-blind controlled trial. Iran. J. Med. Sci. 2017, 42, 524–531. [Google Scholar]
- Guarrera, P.M. Traditional antihelmintic, antiparasitic and repellent uses of plants in Central Italy. J. Ethnopharmacol. 1999, 68, 183–192. [Google Scholar] [CrossRef]
- Kim, S.C.; Adesogan, A.T.; Kim, J.H.; Ko, Y.D. Influence of replacing rice straw with wormwood (Artemisia montana) silage on feed intake, digestibility and ruminal fermentation characteristics of sheep. Anim. Feed Sci. Tech. 2006, 128, 1–13. [Google Scholar] [CrossRef]
- Canadanovic-Brunet, J.M.; Djilas, S.M.; Cetkovic, G.S.; Tumbas, V.T. Free-radical scavenging activity of wormwood (Artemisia absinthium L) extracts. J. Sci. Food Agric. 2005, 85, 265–272. [Google Scholar] [CrossRef]
- Basta, A.; Tzakou, O.; Couladis, M.; Pavlović, M. Chemical composition of Artemisia absinthium L. from Greece. J. Essent. Oil Res. 2007, 19, 316–318. [Google Scholar] [CrossRef]
- Hadi, A.; Hossein, N.; Shirin, P.; Najmeh, N.; Abolfazl, M. Anti-inflammatory and analgesic activities of Artemisia absinthium and chemical composition of its essential oil. Int. J. Pharm. Sci. Rev. Res. 2014, 38, 237–244. [Google Scholar]
- Jaleel, G.A.R.A.; Abdallah, H.M.I.; Gomaa, N.E.S. Pharmacological effects of ethanol extract of Egyptian Artemisia herba-alba in rats and mice. Asian Pac. J. Trop. Biomed. 2016, 6, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Nibret, E.; Wink, M. Volatile components of four Ethiopian Artemisia species extracts and their in vitro antitrypanosomal and cytotoxic activities. Phytomedicine 2010, 17, 369–374. [Google Scholar] [CrossRef]
- Manganelli, R.E.U.; Chericoni, S.; Baragatti, B. Ethnopharmacobotany in Tuscany: Plants used as antihypertensives. Fitoterapia 2000, 71, S95–S100. [Google Scholar] [CrossRef]
- Pirker, H.; Haselmair, R.; Kuhn, E.; Schunko, C.; Vogl, C.R. Transformation of traditional knowledge of medicinal plants: The case of Tyroleans (Austria) who migrated to Australia, Brazil and Peru. J. Ethnobiol. Ethnomed. 2012, 8, 44. [Google Scholar]
- Leporatti, M.L.; Ghedira, K. Comparative analysis of medicinal plants used in traditional medicine in Italy and Tunisia. J. Ethnobiol. Ethnomed. 2009, 5, 31. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimzadeh, M.; Nabavi, S.; Nabavi, S.; Pourmorad, F. Nitric oxide radical scavenging potential of some Elburz medicinal plants. Afr. J. Biotech. 2010, 9, 5212–5217. [Google Scholar]
- Qureshi, R.; Ghufran, M.; Sultana, K.; Ashraf, M.; Khan, A. Ethnomedicinal studies of medicinal plants of Gilgit District and surrounding areas. Ethnobot. Res. Appl. 2007, 5, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Pieroni, A.; Giusti, M.E.; Münz, H.; Lenzarini, C.; Turković, G.; Turković, A. Ethnobotanical knowledge of the Istro-Romanians of Žejane in Croatia. Fitoterapia 2003, 74, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Amat, N.; Upur, H.; Blažeković, B. In vivo hepatoprotective activity of the aqueous extract of Artemisia absinthium L. against chemically and immunologically induced liver injuries in mice. J. Ethnopharm. 2010, 131, 478–484. [Google Scholar] [CrossRef]
- Muto, T.; Watanabe, T.; Okamura, M.; Moto, M.; Kashida, Y.; Mitsumori, K. Thirteen-week repeated dose toxicity study of wormwood (Artemisia absinthium) extract in rats. J. Toxic. Sci. 2003, 28, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Karaman, S.; Kocabas, Y.Z. Traditional medicinal plants of K. Maras (Turkey). Sciences 2001, 1, 125–128. [Google Scholar]
- Bora, K.S.; Sharma, A. Evaluation of antioxidant and free-radical scavenging potential of Artemisia absinthium. Pharm. Biol. 2011, 49, 1216–1223. [Google Scholar] [CrossRef] [Green Version]
- Craciunescu, O.; Constantin, D.; Gaspar, A.; Toma, L.; Utoiu, E.; Moldovan, L. Evaluation of antioxidant and cytoprotective activities of Arnica montana L. and Artemisia absinthium L. ethanolic extracts. Chem. Cent. J. 2012, 6, 97. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, J.F.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef] [Green Version]
- Maobe, M.A.; Gatebe, E.; Gitu, L.; Rotich, H. Preliminary phytochemical screening of eight selected medicinal herbs used for the treatment of diabetes, malaria and pneumonia in Kisii region, southwest Kenya. Eur. J. Appl. Sci. 2013, 5, 1–6. [Google Scholar]
- Tsuchiya, T.; Suzuki, O.; Igarashi, K. Protective effects of chlorogenic acid on paraquat-induced oxidative stress in rats. Biosci. Biotech. Biochem. 1996, 60, 765–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goff, W.L.; Johnson, W.C.; Molloy, J.B.; Jorgensen, W.K.; Waldron, S.J.; Figueroa, J.V.; Matthee, O.; Adams, D.S.; McGuire, T.C.; Pino, I. Validation of a competitive enzyme-linked immunosorbent assay for detection of Babesia bigemina antibodies in cattle. Clin. Vaccine Immunol. 2008, 15, 1316–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, J.M.; Kang, H.M.; Son, K.H.; Kim, J.H.; Lee, C.W.; Kim, H.M.; Chang, S.I.; Kwon, B.M. Antitumor activity of flavones isolated from Artemisia argyi. Planta Med. 2003, 69, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Ortiz, M.P.; Wei, M.Q. Antitumor activity of artemisinin and its derivatives: From a well-known antimalarial agent to a potential anticancer drug. BioMed. Res. Int. 2011, 2012, 247597. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Z.X.; Peng, H.Q.; Sun, Z.W.; Cheng, R.L.; Ye, Z.M.; Li, W.X. Artesunate inhibits growth and induces apoptosis in human osteosarcoma HOS cell line in vitro and in vivo. J. Zhejiang Univ. Sci. B 2011, 12, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Parekh, H.S.; Liu, G.; Wei, M.Q. A new dawn for the use of traditional Chinese medicine in cancer therapy. Mol. Cancer 2009, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Bora, K.S.; Sharma, A. Neuroprotective effect of Artemisia absinthium L. on focal ischemia and reperfusion-induced cerebral injury. J. Ethnopharm. 2010, 129, 403–409. [Google Scholar] [CrossRef]
- Kharoubi, O.; Slimani, M.; Hamadouche, N.A.; Krouf, D.; Aoues, A. Protective effect of Wormwood extract on lead induced neurotoxicity and cognitive disorder. Int. J. Green Pharm. 2010, 4, 193–198. [Google Scholar] [CrossRef]
- Sansar, W.; Gamrani, H. The pharmacological effect of Artemisia absinthium extract in protecting adult rats against lead neurotoxicity. J. Neurol. Sci. 2013, 333, e598. [Google Scholar] [CrossRef]
- Hallal, N.; Kharoubi, O.; Benyettou, I.; Tair, K.; Ozaslan, M.; Aoues, A. In vivo amelioration of oxidative stress by Artemisia absinthium L. administration on mercuric chloride toxicity in brain regions. J. Biol. Sci. 2016, 16, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Zeng, K.W.; Liao, L.X.; Song, X.M.; Lv, H.N.; Song, F.J.; Yu, Q.; Dong, X.; Jiang, Y.; Tu, P.F. Caruifolin D from Artemisia absinthium L. inhibits neuroinflammation via reactive oxygen species-dependent c-jun N-terminal kinase and protein kinase c/NF-κB signaling pathways. Eur. J. Pharmacol. 2015, 767, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Ebrahimzadeh, M.; Ansaroudi, F.; Nabavi, S.; Nabavi, S. Antidepressant and antioxidant activities of Artemisia absinthium L. at flowering stage. Afr. J. Biotech. 2009, 8, 7170–7175. [Google Scholar]
- Ahangar, N.; Mirfetros, S.; Ebrahimzadeh, M. Antidepressant activity of polyphenol fraction of Artemisia absinthium L. Pharmacology 2011, 1, 825–832. [Google Scholar]
- Krebs, S.; Omer, T.N.; Omer, B. Wormwood (Artemisia absinthium) suppresses tumour necrosis factor alpha and accelerates healing in patients with Crohn’s disease–a controlled clinical trial. Phytomedicine 2010, 17, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Shahnazi, M.; Azadmehr, A.; Hajiaghaee, R.; Mosalla, S.; Latifi, R. Effects of Artemisia absinthium L. extract on the maturation and function of dendritic cells. Jundishapur J. Nat. Pharm. Prod. 2015, 10. [Google Scholar] [CrossRef]
- Danilets, M.; Bel’skiĭ, I.; Gur’Ev, A.; Belousov, M.; Bel’Skaia, N.; Trofimova, E.; Uchasova, E.; Alhmedzhanov, R.; Ligacheva, A.; Iusbov, M. Effect of plant polysaccharides on TH1-dependent immune response: Screening investigation. Eksp. Klin. Farmakol. 2010, 73, 19–22. [Google Scholar]
- Hoseinian, A.; Moslemi, H.R.; Sedaghat, R. Antioxidant properties of Artemisia absinthium accelerate healing of experimental Achilles tendon injury in rabbits. Herba Pol. 2018, 64, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Mohammadian, A.; Moradkhani, S.; Ataei, S.; Shayesteh, T.H.; Sedaghat, M.; Kheiripour, N.; Ranjbar, A. Antioxidative and hepatoprotective effects of hydroalcoholic extract of Artemisia absinthium L. in rat. J. HerbMed. Pharmacol. 2016, 5, 29–32. [Google Scholar]
- Caner, A.; Döşkaya, M.; Değirmenci, A.; Can, H.; Baykan, Ş.; Üner, A.; Başdemir, G.; Zeybek, U.; Gürüz, Y. Comparison of the effects of Artemisia vulgaris and Artemisia absinthium growing in western Anatolia against trichinellosis (Trichinella spiralis) in rats. Exp. Parasitol. 2008, 119, 173–179. [Google Scholar] [CrossRef]
- Lachenmeier, D.W. Wormwood (Artemisia absinthium L.)—A curious plant with both neurotoxic and neuroprotective properties? J. Ethnopharm. 2010, 131, 224–227. [Google Scholar] [CrossRef] [Green Version]
- Grycyk, R.A.; Kireev, I.V.; Struk, O.A.; Ivanochko, V.M. Investigation of acute toxicity and hepatoprotective effect of extracts of Artemisia vulgaris L. and Artemisia absinthium L. Herbs. Med. Clin. Chem. 2019, 4, 147–155. [Google Scholar]
- Shanmugasundaram, E.; Gopinath, K.L.; Shanmugasundaram, K.R.; Rajendran, V. Possible regeneration of the islets of Langerhans in streptozotocin-diabetic rats given Gymnema sylvestre leaf extracts. J. Ethnopharm. 1990, 30, 265–279. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; G Wasef, L.; Elewa, Y.H.; A Al-Sagan, A.; El-Hack, A.; Mohamed, E.; Taha, A.E.; M Abd-Elhakim, Y.; Prasad Devkota, H. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travlos, G.; Morris, R.; Elwell, M.; Duke, A.; Rosenblum, S.; Thompson, M. Frequency and relationships of clinical chemistry and liver and kidney histopathology findings in 13-week toxicity studies in rats. Toxicology 1996, 107, 17–29. [Google Scholar] [CrossRef]
- Hwang, D.F.; Lai, Y.S.; Chiang, M.T. Toxic effects of grass carp, snake and chicken bile juices in rats. Toxic. Lett. 1996, 85, 85–92. [Google Scholar] [CrossRef]
- Farzaneh, F.; Ebrahim, H.S.; Akbar, V. Investigating on Effect of Wormwood Extract on Reduction of Renal Toxicity in Treated Rats by Azathioprine. Biomed. Pharm. J. 2015, 8, 291–299. [Google Scholar] [CrossRef]
- Kharoubi, O.; Slimani, M.; Aoues, A.; Seddik, L. Prophylactic effects of Wormwood on lipid peroxidation in an animal model of lead intoxication. Indian J. Nephrol. 2008, 18, 51. [Google Scholar] [CrossRef]
- Daradka, H.M.; Abas, M.M.; Mohammad, M.A.; Jaffar, M.M. Antidiabetic effect of Artemisia absinthium extracts on alloxan-induced diabetic rats. Comp. Clin. Pathol. 2014, 23, 1733–1742. [Google Scholar] [CrossRef]
- Krebs, S.; Omer, B.; Omer, T.N.; Fliser, D. Wormwood (Artemisia absinthium) for poorly responsive early-stage IgA nephropathy: A pilot uncontrolled trial. Am. J. Kidney Dis. 2010, 56, 1095–1099. [Google Scholar] [CrossRef]
- Hassan, M.; Niazi, A.T.; Khan, S.; Gul, F. Antidiabetic and antihyperlipidemic effects of Artemisia absinthium L., Citrullus colocynthis (L.) Schrad. and Gymnema sylvestre (Retz.) R. Br. ex Sm. on type II diabetes hyperlipidemic patients. Indian J. Tradit. Knowl. 2018, 17, 233–239. [Google Scholar]
- Beigh, Y.A.; Ganai, A.M. Potential of wormwood (Artemisia absinthium Linn.) herb for use as additive in livestock feeding: A review. Pharm. Innov. 2017, 6, 176. [Google Scholar]
- Ahmad, F.; Khan, R.A.; Rasheed, S. Study of analgesic and anti-inflammatory activity from plant extracts of Lactuca scariola and Artemisia absinthium. J. Islamic Acad. Sci. 1992, 5, 111–114. [Google Scholar]
- Nalbantsoy, A.; Erel, Ş.B.; Köksal, Ç.; Göçmen, B.; Yıldız, M.Z.; Yavaşoğlu, N.Ü.K. Viper venom induced inflammation with Montivipera xanthina (Gray, 1849) and the anti-snake venom activities of Artemisia absinthium L. in rat. Toxicon 2013, 65, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Kim, H.; Oh, W.K.; Yu, K.A.; Choe, Y.K.; Ahn, J.S.; Kim, D.S.; Kim, S.H.; Dinarello, C.A.; Kim, K. Tetramethoxy hydroxyflavone p7F downregulates inflammatory mediators via the inhibition of nuclear factor κB. Ann. N. Y. Acad. Sci. 2004, 1030, 555–568. [Google Scholar] [CrossRef]
- Hatziieremia, S.; Gray, A.; Ferro, V.; Paul, A.; Plevin, R. The effects of cardamonin on lipopolysaccharide-induced inflammatory protein production and MAP kinase and NFκB signalling pathways in monocytes/macrophages. Br. J. Pharm. 2006, 149, 188–198. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.C.; Choi, E.J.; Oh, H.M.; Lee, S.; Lee, J.K.; Lee, M.S.; Shin, Y.I.; Choi, S.J.; Chae, J.R.; Lee, K.M. DA-9601, a standardized extract of Artemisia asiatica, blocks TNF-α-induced IL-8 and CCL20 production by inhibiting p38 kinase and NF-κB pathways in human gastric epithelial cells. World J. Gastroenterol. 2006, 12, 4850. [Google Scholar]
- Jin, H.Z.; Lee, J.H.; Lee, D.; Hong, Y.S.; Kim, Y.H.; Lee, J.J. Inhibitors of the LPS-induced NF-κB activation from Artemisia sylvatica. Phytochemistry 2004, 65, 2247–2253. [Google Scholar] [CrossRef]
- Khattak, S.G.; Gilani, S.N.; Ikram, M. Antipyretic studies on some indigenous Pakistani medicinal plants. J. Ethnopharm. 1985, 14, 45–51. [Google Scholar] [CrossRef]
- Koch, J. Assessment Report on Artemisia absinthium L., herba; EMEA/HMPC/234497/2008; European Medicines Agency: London, UK, 2009; pp. 1–26. [Google Scholar]
- Zeraati, F.; Esna-Ashari, F.; Araghchian, M.; Emam, A.H.; Rad, M.V.; Seif, S.; Razaghi, K. Evaluation of topical antinociceptive effect of Artemisia absinthium extract in mice and possible mechanisms. Afr. J. Pharm. Pharmacol. 2014, 8, 492–496. [Google Scholar]
- Hurrell, J.A.; Puentes, J.P.; Arenas, P.M. Medicinal plants with cholesterol-lowering effect marketed in the Buenos Aires-La Plata conurbation, Argentina: An Urban Ethnobotany study. Ethnobiol. Conserv. 2015, 4, 1–19. [Google Scholar]
- Daradka, H.M.; Badawneh, M.; Al-Jamal, J.; Bataineh, Y. Hypolipidemic efficacy of Artemisia absinthium extracts in rabbits. World Appl. Sci. J. 2014, 31, 1415–1421. [Google Scholar] [CrossRef]
- Khori, V.; Nayebpour, M. Effect of Artemisia absinthium on electrophysiological properties of isolated heart of rats. Physiol. Pharmacol. 2007, 10, 303–311. [Google Scholar]
- Sadoughi, S.; Rahbarian, R.; Jahani, N.; Shazdeh, S.; Hossein Zadeh Saljoughi, S.; Daee, M. Effect of aqueous extract of Artemisia absinthium L. on sex hormones, inflammatory cytokines and oxidative stress indices of ovarian tissue in polycystic ovary syndrome rat model. J. Babol Univ. Med. Sci. 2017, 19, 50–56. [Google Scholar]
- Kostadinović, L.; Lević, J.; Popović, S.; Čabarkapa, I.; Puvača, N.; Djuragic, O.; Kormanjoš, S. Dietary inclusion of Artemisia absinthium for management of growth performance, antioxidative status and quality of chicken meat. Eur. Poultry Sci. 2015, 79. [Google Scholar] [CrossRef]
- Shafi, N.; Khan, G.A.; Ghauri, E.G. Antiulcer effect of Artemisia absinthium L. in rats. Pak. J. Sci. Ind. Res. 2004, 47, 130–134. [Google Scholar]
- Sebai, H.; Jabri, M.A.; Souli, A.; Hosni, K.; Selmi, S.; Tounsi, H.; Tebourbi, O.; Boubaker, S.; El-Benna, J.; Sakly, M. Protective effect of Artemisia campestris extract against aspirin-induced gastric lesions and oxidative stress in rat. Rsc. Adv. 2014, 4, 49831–49841. [Google Scholar] [CrossRef] [Green Version]
- Azizi, K.; Moemenbellah, F.S.-H.M.D.; Fard, Q.A.; Mohammadi-Samani, S. Antiulcer activity after oral administration of the wormwood ethanol extract on lesions due to Leishmania major parasites in BALB/C mice. Asian J. Pharm. Res. Health Care 2016, 8, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, C.H.; Ko, Y. Influence of dietary addition of dried wormwood (Artemisia sp.) on the performance and carcass characteristics of Hanwoo steers and the nutrient digestibility of sheep. Asian-Australas. J. Anim. Sci. 2002, 15, 390–395. [Google Scholar] [CrossRef]
- Kreitmair, H. Artemisia absinthium L., the true wormwood. Die Pharmazie. 1951, 6, 27–28. [Google Scholar]
- Dülger, B.; Ceylan, M.; Alitsaous, M.; Uğurlu, E. Antimicrobial Activity of Artemisia absinthium L. Turk. J. Biol. 1999, 23, 377–384. [Google Scholar]
- Fiamegos, Y.C.; Kastritis, P.L.; Exarchou, V.; Han, H.; Bonvin, A.M.; Vervoort, J.; Lewis, K.; Hamblin, M.R.; Tegos, G.P. Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against gram-positive pathogenic bacteria. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengul, M.; Ercisli, S.; Yildiz, H.; Gungor, N.; Kavaz, A.; Çetin, B. Antioxidant, antimicrobial activity and total phenolic content within the aerial parts of Artemisia absinthum, Artemisia santonicum and Saponaria officinalis. Iran. J. Pharm. Res. 2011, 10, 49. [Google Scholar] [PubMed]
- Moslemi, H.R.; Hoseinzadeh, H.; Badouei, M.A.; Kafshdouzan, K.; Fard, R.M.N. Antimicrobial activity of Artemisia absinthium against surgical wounds infected by Staphylococcus aureus in a rat model. Indian J. Microbiol. 2012, 52, 601–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juteau, F.; Jerkovic, I.; Masotti, V.; Milos, M.; Mastelic, J.; Bessiere, J.M.; Viano, J. Composition and antimicrobial activity of the essential oil of Artemisia absinthium from Croatia and France. Planta Med. 2003, 69, 158–161. [Google Scholar] [CrossRef]
- Asili, J.; Emami, S.A.; Eynolghozat, R.; Noghab, Z.S.; Bazzaz, B.S.F.; Sahebkar, A. Chemical composition and in vitro efficacy of essential oil of seven Artemisia species against ESBL producing multidrug-resistant Escherichia coli. J. Essent. Oil Bear. Plants 2015, 18, 124–145. [Google Scholar] [CrossRef]
- Zanousi, M.B.P.; Nekoei, M.; Mohammadhosseini, M. Composition of the essential oils and volatile fractions of Artemisia absinthium by three different extraction methods: Hydrodistillation, solvent-free microwave extraction and headspace solid-phase microextraction combined with a novel QSRR evaluation. J. Essent. Oil Bear. Plants 2016, 19, 1561–1581. [Google Scholar] [CrossRef]
- Mihajilov-Krstev, T.; Jovanović, B.; Jović, J.; Ilić, B.; Miladinović, D.; Matejić, J.; Rajković, J.; Đorđević, L.; Cvetković, V.; Zlatković, B. Antimicrobial, antioxidative, and insect repellent effects of Artemisia absinthium essential oil. Planta Med. 2014, 80, 1698–1705. [Google Scholar] [CrossRef] [Green Version]
- Belay, G.; Tariku, Y.; Kebede, T.; Hymete, A.; Mekonnen, Y. Ethnopharmacological investigations of essential oils isolated from five Ethiopian medicinal plants against eleven pathogenic bacterial strains. Phytopharmacology 2011, 1, 133–143. [Google Scholar]
- Bartkiene, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Varinauskaite, I.; Pileckaite, G.; Paskeviciute, L.; Rutkauskaite, G.; Kanaporis, T. Plants and lactic acid bacteria combination for new antimicrobial and antioxidant properties product development in a sustainable manner. Foods 2020, 9, 433. [Google Scholar] [CrossRef] [Green Version]
- Anwar, M.; Hakim, M.; Siddiqui, M. Clinical efficacy of Artemisia absinthium Linn. viral Hepatitis with specific reference to ejection fraction of Heart. Hamdard Med. 1998, 41, 93–95. [Google Scholar]
- Ansari, S.; Siddiqui, M.A.; Malhotra, S.; Maaz, M. Antiviral efficacy of qust (Saussurea lappa) and afsanteen (Artemisia absinthium) for chronic Hepatitis B: A prospective single-arm pilot clinical trial. Pharmacogn. Res. 2018, 10, 282. [Google Scholar] [CrossRef]
- Fernández-Calienes Valdés, A.; Mendiola Martínez, J.; Scull Lizama, R.; Vermeersch, M.; Cos, P.; Maes, L. In vitro anti-microbial activity of the Cuban medicinal plants Simarouba glauca DC, Melaleuca leucadendron L and Artemisia absinthium L. Mem. Inst. Oswaldo Cruz. 2008, 103, 615–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nozari, S.; Azadmehr, A.; Jahanihashemi, H.; Nassiri, A.M.; Adine, M.; Javadi, F.; Hajiaghaee, R.; Shahnazi, M.; Saraei, M. In vitro Anti-toxoplasma effects of ethanolic extracts of Artemisia absinthium L., Carum copticum L. and Gossypium hirsutum. J. Med. Plants 2016, 2, 72–79. [Google Scholar]
- Kostadinovic, L.; Levic, J.; Galonja-Coghill, T.; Ruzicic, L. Anticoccidian effects of the Artemisia absinthium L. extracts in broiler chickens. Arch. Zootech. 2012, 15, 69. [Google Scholar]
- Habibi, H.; Firouzi, S.; Nili, H.; Razavi, M.; Asadi, S.L.; Daneshi, S. Anticoccidial effects of herbal extracts on Eimeria tenella infection in broiler chickens: In vitro and in vivo study. J. Parasitol. Dis. 2016, 40, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Tariku, Y.; Hymete, A.; Hailu, A.; Rohloff, J. In vitro evaluation of antileishmanial activity and toxicity of essential oils of Artemisia absinthium and Echinops kebericho. Chem. Biodiver. 2011, 8, 614–623. [Google Scholar] [CrossRef]
- Bailen, M.; Julio, L.F.; Diaz, C.E.; Sanz, J.; Martínez-Díaz, R.A.; Cabrera, R.; Burillo, J.; Gonzalez-Coloma, A. Chemical composition and biological effects of essential oils from Artemisia absinthium L. cultivated under different environmental conditions. Ind. Crops Prod. 2013, 49, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Díaz, R.A.; Ibáñez-Escribano, A.; Burillo, J.; Heras, L.d.l.; Prado, G.d.; Agulló-Ortuño, M.T.; Julio, L.F.; González-Coloma, A. Trypanocidal, trichomonacidal and cytotoxic components of cultivated Artemisia absinthium Linnaeus (Asteraceae) essential oil. Mem. Inst. Oswaldo Cruz. 2015, 110, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Piñón, A.; Scull, R.; Setzer, W.N. Chemistry and leishmanicidal activity of the essential oil from Artemisia absinthium from Cuba. Nat. Prod. Commun. 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Tamargo, B.; Monzote, L.; Piñón, A.; Machín, L.; García, M.; Scull, R.; Setzer, W.N. In vitro and in vivo evaluation of essential oil from Artemisia absinthium L. formulated in nanocochleates against cutaneous leishmaniasis. Medicines 2017, 4, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahrevanian, H.; Sheykhkanlooye Milan, B.; Kazemi, M.; Hajhosseini, R.; Soleymani Mashhadi, S.; Nahrevanian, S. Antimalarial effects of Iranian flora Artemisia sieberi on Plasmodium berghei In vivo in mice and phytochemistry analysis of its herbal extracts. Malar. Res. Treat. 2012, 2012, 727032. [Google Scholar]
- Zafar, M.; Hamdard, M.; Hameed, A. Screening of Artemisia absinthium for antimalarial effects on Plasmodium berghei in mice: A preliminary report. J. Ethnopharm. 1990, 30, 223–226. [Google Scholar]
- Mojab, F. Antimalarial natural products: A review. Avicenna J. Phytomed. 2012, 2, 52. [Google Scholar] [PubMed]
- Ramazani, A.; Sardari, S.; Zakeri, S.; Vaziri, B. In vitro antiplasmodial and phytochemical study of five Artemisia species from Iran and in vivo activity of two species. Parasitol. Res. 2010, 107, 593–599. [Google Scholar] [CrossRef]
- Antoine, T.; Fisher, N.; Amewu, R.; O’Neill, P.M.; Ward, S.A.; Biagini, G.A. Rapid kill of malaria parasites by artemisinin and semi-synthetic endoperoxides involves ROS-dependent depolarization of the membrane potential. J. Antimicrob. Chemother. 2014, 69, 1005–1016. [Google Scholar] [CrossRef]
- O’neill, P.M.; Barton, V.E.; Ward, S.A. The molecular mechanism of action of artemisinin—The debate continues. Molecules 2010, 15, 1705–1721. [Google Scholar] [CrossRef]
- Bhisutthibhan, J.; Pan, X.Q.; Hossler, P.A.; Walker, D.J.; Yowell, C.A.; Carlton, J.; Dame, J.B.; Meshnick, S.R. The Plasmodium falciparum translationally controlled tumor protein homolog and its reaction with the antimalarial drug artemisinin. J. Biol. Chem. 1998, 273, 16192–16198. [Google Scholar] [CrossRef] [Green Version]
- Eichhorn, T.; Winter, D.; Büchele, B.; Dirdjaja, N.; Frank, M.; Lehmann, W.D.; Mertens, R.; Krauth-Siegel, R.L.; Simmet, T.; Granzin, J. Molecular interaction of artemisinin with translationally controlled tumor protein (TCTP) of Plasmodium falciparum. Biochem. Pharm. 2013, 85, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Eckstein-Ludwig, U.; Webb, R.; Van Goethem, I.; East, J.; Lee, A.; Kimura, M.; O’neill, P.; Bray, P.; Ward, S.; Krishna, S. Artemisinins target the SERCA of Plasmodium falciparum. Nature 2003, 424, 957–961. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.J.; Chia, W.N.; Loh, C.C.; Li, Z.; Lee, Y.M.; He, Y.; Yuan, L.X.; Lim, T.K.; Liu, M. Haem-activated promiscuous targeting of artemisinin in Plasmodium falciparum. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Msaada, K.; Salem, N.; Bachrouch, O.; Bousselmi, S.; Tammar, S.; Alfaify, A.; Al Sane, K.; Ben Ammar, W.; Azeiz, S.; Haj Brahim, A. Chemical composition and antioxidant and antimicrobial activities of wormwood (Artemisia absinthium L.) essential oils and phenolics. J. Chem. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ewais, E.; Aly, M.M.; Ismail, M.; Abdel Shakour, E.; Hassanin, M. Antibacterial, antifungal, antitumor and toxicityof essential oils of Salvia officionalis, Thymus vulgaris, Eugenia caryophyllata and Artemisia absinthium. Sci. J. Flowers Ornam. Plants 2014, 1, 265–274. [Google Scholar] [CrossRef]
- Joshi, R.K. Volatile composition and antimicrobial activity of the essential oil of Artemisia absinthium growing in Western Ghats region of North West Karnataka, India. Pharm. Biol. 2013, 51, 888–892. [Google Scholar] [CrossRef] [PubMed]
- del Pilar Rodríguez-Torres, M.; Acosta-Torres, L.S.; Díaz-Torres, L.A.; Padrón, G.H.; García-Contreras, R.; Millán-Chiu, B.E. Artemisia absinthium-based silver nanoparticles antifungal evaluation against three Candida species. Mater. Res. Express 2019, 6, 085408. [Google Scholar] [CrossRef]
- Meschler, J.P.; Howlett, A.C. Thujone Exhibits Low Affinity for Cannabinoid Receptors But Fails to Evoke Cannabimimetic Responses. Pharm. Biochem. Behav. 1999, 62, 473–480. [Google Scholar] [CrossRef]
- Tariq, K.A.; Chishti, M.Z.; Ahmad, F.; Shawl, A.S. Anthelmintic activity of extracts of Artemisia absinthium against ovine nematodes. Vet. Parasitol. 2009, 160, 83–88. [Google Scholar] [CrossRef]
- Mravčáková, D.; Komáromyová, M.; Babják, M.; Urda Dolinská, M.; Königová, A.; Petrič, D.; Čobanová, K.; Ślusarczyk, S.; Cieslak, A.; Várady, M.; et al. Anthelmintic Activity of Wormwood (Artemisia absinthium L.) and Mallow (Malva sylvestris L.) against Haemonchus contortus in Sheep. Animals 2020, 10, 219. [Google Scholar] [CrossRef] [Green Version]
- Kerboeuf, D.; Riou, M.; Guegnard, F. Flavonoids and related compounds in parasitic disease control. Mini Rev. Med. Chem. 2008, 8, 116–128. [Google Scholar] [CrossRef]
- García-Rodríguez, J.J.; Andrés, M.F.; Ibañez-Escribano, A.; Julio, L.F.; Burillo, J.; Bolás-Fernández, F.; González-Coloma, A. Selective nematocidal effects of essential oils from two cultivated Artemisia absinthium populations. Z. Nat. C 2015, 70, 275–280. [Google Scholar] [CrossRef]
- Nin, S.; Bennici, A.; Roselli, G.; Mariotti, D.; Schiff, S.; Magherini, R. Agrobacterium-mediated transformation of Artemisia absinthium L.(wormwood) and production of secondary metabolites. Plant Cell Rep. 1997, 16, 725–730. [Google Scholar] [CrossRef]
- Maw, M.; Thomas, A.; Stahevitch, A. The biology of canadian weeds: 66. Artemisia absinthium L. Can. J. Plant Sci. 1985, 65, 389–400. [Google Scholar] [CrossRef]
- Chiasson, H.; Bélanger, A.; Bostanian, N.; Vincent, C.; Poliquin, A. Acaricidal properties of Artemisia absinthium and Tanacetum vulgare (Asteraceae) essential oils obtained by three methods of extraction. J. Econ. Entomol. 2001, 94, 167–171. [Google Scholar] [CrossRef]
- Kordali, S.; Aslan, I.; Çalmaşur, O.; Cakir, A. Toxicity of essential oils isolated from three Artemisia species and some of their major components to granary weevil, Sitophilus granarius (L.)(Coleoptera: Curculionidae). Ind. Crops Prod. 2006, 23, 162–170. [Google Scholar] [CrossRef]
- Jaenson, T.G.; Pålsson, K.; Borg-Karlson, A.K. Evaluation of extracts and oils of tick-repellent plants from Sweden. Med. Vet. Entomol. 2005, 19, 345–352. [Google Scholar] [CrossRef]
- Parveen, S.; Godara, R.; Katoch, R.; Yadav, A.; Verma, P.; Katoch, M.; Singh, N. In vitro evaluation of ethanolic extracts of Ageratum conyzoides and Artemisia absinthium against cattle tick, Rhipicephalus microplus. Sci. World J. 2014, 2014, 858973. [Google Scholar] [CrossRef] [PubMed]
- Ertürk, Ö.; Uslu, U. Antifeedant, growth and toxic effects of some plant extracts on Leptinotarsa decemlineata (Say.)(Coleoptera, Chrysomelidae). Fresenius Environ. Bull. 2007, 16, 602–608. [Google Scholar]
- Govindarajan, M.; Benelli, G. Artemisia absinthium-borne compounds as novel larvicides: Effectiveness against six mosquito vectors and acute toxicity on non-target aquatic organisms. Parasitol. Res. 2016, 115, 4649–4661. [Google Scholar] [CrossRef] [Green Version]
- Klonis, N.; Crespo-Ortiz, M.P.; Bottova, I.; Abu-Bakar, N.; Kenny, S.; Rosenthal, P.J.; Tilley, L. Artemisinin activity against Plasmodium falciparum requires hemoglobin uptake and digestion. Proc. Nat. Acad. Sci. USA 2011, 108, 11405–11410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodrow, C.; Haynes, R.; Krishna, S. Artemisinins. Postgrad. Med. J. 2005, 81, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Höld, K.M.; Sirisoma, N.S.; Ikeda, T.; Narahashi, T.; Casida, J.E. α-Thujone (the active component of absinthe): γ-aminobutyric acid type A receptor modulation and metabolic detoxification. Proc. Natl. Acad. Sci. USA 2000, 97, 3826–3831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; He, X.; Ortiz de Montellano, P.R. Radical intermediates in the catalytic oxidation of hydrocarbons by bacterial and human cytochrome P450 enzymes. Biochemistry 2006, 45, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Abass, K.; Reponen, P.; Mattila, S.; Pelkonen, O. Metabolism of α-thujone in human hepatic preparations in vitro. Xenobiotica 2011, 41, 101–111. [Google Scholar] [CrossRef]
- Medhi, B.; Patyar, S.; Rao, R.S.; Ds, P.B.; Prakash, A. Pharmacokinetic and toxicological profile of artemisinin compounds: An update. Pharmacology 2009, 84, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Navaratnam, V.; Mansor, S.M.; Sit, N.W.; Grace, J.; Li, Q.; Olliaro, P. Pharmacokinetics of artemisinin-type compounds. Clin. Pharmacokinet. 2000, 39, 255–270. [Google Scholar] [CrossRef]
- Morris, C.A.; Duparc, S.; Borghini-Fuhrer, I.; Jung, D.; Shin, C.S.; Fleckenstein, L. Review of the clinical pharmacokinetics of artesunate and its active metabolite dihydroartemisinin following intravenous, intramuscular, oral or rectal administration. Malar. J. 2011, 10, 263. [Google Scholar] [CrossRef] [Green Version]
- Hien, T.; Davis, T.; Chuong, L.; Ilett, K.; Sinh, D.; Phu, N.; Agus, C.; Chiswell, G.; White, N.; Farrar, J. Comparative pharmacokinetics of intramuscular artesunate and artemether in patients with severe falciparum malaria. Antimicrob. Agents Chemother. 2004, 48, 4234–4239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navaratnam, V.; Mansor, S.; Mordi, M.; Akbar, A.; Abdullah, M. Comparative pharmacokinetic study of oral and rectal formulations of artesunic acid in healthy volunteers. Eur. J. Clin. Pharmacol. 1998, 54, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Beshbishy, A.M.; Batiha, G.E.-S.; Alkazmi, L.; Nadwa, E.; Rashwan, E.; Abdeen, A.; Yokoyama, N.; Igarashi, I. Therapeutic Effects of Atranorin towards the Proliferation of Babesia and Theileria Parasites. Pathogens 2020, 9, 127. [Google Scholar] [CrossRef] [Green Version]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; Stephen Adeyemi, O.; Nadwa, E.; Rashwan, E.; Yokoyama, N.; Igarashi, I. Safety and efficacy of hydroxyurea and eflornithine against most blood parasites Babesia and Theileria. PLoS ONE 2020, 15, e0228996. [Google Scholar] [CrossRef]
- Ouji, M.; Augereau, J.M.; Paloque, L.; Benoit-Vical, F. Plasmodium falciparum resistance to artemisinin-based combination therapies: A sword of Damocles in the path toward malaria elimination. Parasite 2018, 25, 24. [Google Scholar] [CrossRef] [Green Version]
- Dohutia, C.; Chetia, D.; Gogoi, K.; Bhattacharyya, D.R.; Sarma, K. Molecular docking, synthesis and in vitro antimalarial evaluation of certain novel curcumin analogues. Braz. J. Pharm. Sci. 2017, 53, 00084. [Google Scholar] [CrossRef]
- McGuffin, M.; Hobbs, C.; Upton, R.; Goldberg, A. American Herbal Products Association Botanical Safety Handbook; CRC Press: Boston, MA, USA, 1997. [Google Scholar]
- Duke, J.B. Handbook of Medicinal Herbs, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Behrens, M.; Brockhoff, A.; Kuhn, C.; Bufe, B.; Winnig, M.; Meyerhof, W. The human taste receptor hTAS2R14 responds to a variety of different bitter compounds. Biochem. Biophys. Res. Commun. 2004, 319, 479–485. [Google Scholar] [CrossRef]
- Rivera, E.; Cid, M.P.; Zunino, P.; Baiardi, G.; Salvatierra, N.A. Central α-and β-thujone: Similar anxiogenic-like effects and differential modulation on GABAA receptors in neonatal chicks. Brain Res. 2014, 1555, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Alshibly, N.M. Effect of Artemisia absinthium L. on genotoxicity on mice bone marrow cells. World Appl. Sci. J. 2014, 30, 770–777. [Google Scholar] [CrossRef]
- Kocaoglu, C.; Ozel, A. Persistent metabolic acidosis and severe diarrhoea due to Artemisia absinthium poisoning. J. Pak. Med. Assoc. 2014, 64, 1081–1083. [Google Scholar] [PubMed]


| Taxonomy | |
|---|---|
| Kingdom | Plantae |
| Division | Magnoliophyta |
| Class | Magnoliopsida |
| Order | Asterales |
| Family | Asteraceae |
| Genus | Artemisia L- sagebrush |
| Species | absinthium |
| Compound | Class of Compound | IUPAC Name | Chemical Structure |
|---|---|---|---|
| Artemisinin | Endoperoxide-containing sesquiterpene lactone | (3R,5aS,6R,8aS,9R,12S,12aR)-Octahydro-3,6,9-trimethyl-3,12-epoxy-12H-pyrano[4,3-j]-1,2-benzodioxepin-10(3H)-one |  |
| α-Thujone | Bicyclic monoterpene ketone | (1S,4R,5R)-4-Methyl-1-(propan-2-yl)bicyclo[3.1.0]hexan-3-one |  |
| β-Thujone | Bicyclic monoterpene ketone | (1S,4S,5R)-4-Methyl-1-propan-2-ylbicyclo[3.1.0]hexan-3-one |  |
| Bornyl acetate | Acetate ester of borneol, the bicyclic monoterpene | (4,7,7-Trimethyl-3-bicyclo[2.2.1]heptanyl) acetate |  |
| 4-Terpineol | An isomer of the monoterpene alcohol, terpineol | 2-(4-Methylcyclohex-3-en-1-yl)propan-2-ol |  |
| Camphene | Bicyclic monoterpene | 2,2-Dimethyl-3-methylidenebicyclo[2.2.1]heptane |  |
| Chamazulene | A bicyclic unsaturated hydrocarbon. It is an azulene derived from sesquiterpenes | 7-Ethyl-1,4-dimethylazulene |  |
| Cadinene | Bicyclic sesquiterpenes | (1S,4aR,8aS)-4,7-Dimethyl-1-propan-2-yl-1,2,4a,5,8,8a-hexahydronaphthalene |  |
| Myrcene | Alkene natural hydrocarbon, classified as a monoterpene | 7-Methyl-3-methylene-octa-1,6-diene |  |
| trans-Sabinyl acetate | Oxygenated monoterpene | [(1R,3S)-4-methylidene-1-propan-2-yl-3-bicyclo[3.1.0]hexanyl] acetate |  |
| Guaiazulene | Bicyclic sesquiterpene, azulene derivative | 1,4-Dimethyl-7-isopropylazulene |  |
| γ-Terpinene | Monoterpene | 1-methyl-4-propan-2-ylcyclohexa-1,4-diene |  |
| Linalool | Naturally occurring acyclic monoterpene alcohol | (3R)-3,7-dimethylocta-1,6-dien-3-ol |  |
| Camphor- | Terpenoid with the chemical formula C10H16O | 1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one |  |
| Geographical Location | Traditional Use | Part Used | Ref. |
|---|---|---|---|
| Brazil | Used for the treatment of digestive discomforts | Artemisia absinthium tea | [30] |
| Italy | Used an anthelmintic, digestive, antiemetic, antiparasitic, antihypertensive, and to relieve tendonitis | Leaves and aerial parts | [18] |
| Tunisia | Antimalarial | Aerial parts | [31] |
| Iran | Antimicrobial, diuretic, anthelmintic, choleretic, digestive. | Aerial parts | [32] |
| Pakistan | Used for fever treatment and as an anthelmintic for children. | Whole herb | [33] |
| Croatia | Digestive | Aerial parts | [34] |
| France | Antibacterial, appetite stimulant, antipyretic, emmenagogue, anthelmintic. | Aerial parts | [1] |
| China | Used to treat cancers, hepatic disorders, neurodegenerative diseases, acute bacillary dysentery. | Aerial parts | [35] |
| Cuba | Antimalarial | Whole herb | [1] |
| Western Europe | Stomach medicine useful for gastric pain, a cardiac stimulant, a restorative of declining mental functions. | Aerial parts | [36] |
| Bosnia and Herzegovina | Infusion used for gastrointestinal ailments, stomachache; decoction used for stomachache. | ||
| Turkey | Used to treat stomach ache, as an appetizer, an abortive, blood depurative, diabetes, tuberculosis, antihypertensive, antimalarial, applied to wounds, antipyretic. | Aerial parts and leaves | [37] |
| Activities | Bioactive Compound | Mechanism of Action | Ref. |
|---|---|---|---|
| Antioxidant | Phenolic compounds and flavonoids | Reduction of lipid peroxidation level, decreasing TBARS level and the recovery of endogenous antioxidant (SOD, GSH) | [7] |
| Immuno-modulatory activity | Polysaccharides | Initiation of Th1 response and activation of NO synthesis | [56] |
| Wound Healing activity | β-thujone and β-pinene | Free radical scavenging activity | [24] |
| Neuroprotective | Combination of phytochemical compounds | Anticholinesterase activity | [58] |
| Antidepressant effects | Combination of phytochemical compounds | Inhibition of MAO, suppression of depression, inhibition of selective serotonin reuptake | [53] |
| Hepatoprotective Effects | Thujone | Suppression of liver microsomal drug-metabolizing enzymes, free radical scavenging activity, calcium channels blockage | [35,60] |
| Hypoglycaemic Effect | Thujyl alcohol, α- and β-thujones, azulenes, cadinene, bisabolene, sabinene, phellandrene, pinene | Stimulating AMPK, which mainly activated the translocation of insulin-stimulated GLUT4 to the cell surface | [69] |
| Anti-inflammatory | 5,6,3′,5′-tetramethoxy 7,4′-hydroxyflavone, cardomonin, caruifolin D | Suppressing the proinflammatory mediators expression (iNOS, PGE(2), NO, COX-2, NF-kB) in LPS-stimulated RAW 264.7 cells and BV2 cells | [74,75,76] |
| Antitumor activity | Chlorogenic acid, artesunate, dihydro artesunate, artemisetin | Activation of the MEK/ERK pathway, activates the mitochondrial pathway of caspase activation, stimulate cell apoptosis | [41,42,43,44] |
| Antipyretic and analgesic activities | 22-dien-3 Bat, 24-β-ethyl p-cholesta-7 | Nicotinic and muscarinic action | [79] |
| Renal Effect | α- and β-thujones | High contents of total phenolic compounds and flavonoids as well as antioxidant effect of wormwood extract | [67] |
| Antiulcer and digestive activities | Bitter substances, essential oils | Enhancing the bile production and secretion | [23] |
| Activities | Bioactive Compound | Mechanism of Action | Ref. |
|---|---|---|---|
| Antibacterial Activity | Essential oil | Suppressing the biosynthesis of proteins, RNA, DNA and polysaccharide in the bacterial cells | [98] |
| Anthelmintic Activity | α-and β-thujones | Decrease juvenile (L3) larval motility and development of egg of Ascaris suum in an in vitro model | [126] |
| Anti-fungal Activity | Essential oil | High contents of total phenolic compounds and flavonoids | [94,121] |
| Antiprotozoal Activity | Essential oil, flavonoids, artemisinin | Stimulation of both heme and mitochondrial-mediated degradation cascade, inhibit PfATP6, inhibiting LDH, SAMS, PyrK, SpdSyn, OAT enzyme activities | [116,120,140] |
| Insecticidal Effect | Essential oil | Toxicity to adults of granary weevil Sitophilus granarius L., resulting in 80–90% mortality rate of these insects | [133,134,135,136] |
| Antiviral Activity | Several bioactive compounds | Inhibiting the integrase enzyme from human immunodeficiency virus (HIV-1) from connecting the DNA from the host cell with the reversibly transcribed viral DNA | [141] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batiha, G.E.-S.; Olatunde, A.; El-Mleeh, A.; Hetta, H.F.; Al-Rejaie, S.; Alghamdi, S.; Zahoor, M.; Magdy Beshbishy, A.; Murata, T.; Zaragoza-Bastida, A.; et al. Bioactive Compounds, Pharmacological Actions, and Pharmacokinetics of Wormwood (Artemisia absinthium). Antibiotics 2020, 9, 353. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9060353
Batiha GE-S, Olatunde A, El-Mleeh A, Hetta HF, Al-Rejaie S, Alghamdi S, Zahoor M, Magdy Beshbishy A, Murata T, Zaragoza-Bastida A, et al. Bioactive Compounds, Pharmacological Actions, and Pharmacokinetics of Wormwood (Artemisia absinthium). Antibiotics. 2020; 9(6):353. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9060353
Chicago/Turabian StyleBatiha, Gaber El-Saber, Ahmed Olatunde, Amany El-Mleeh, Helal F. Hetta, Salim Al-Rejaie, Saad Alghamdi, Muhammad Zahoor, Amany Magdy Beshbishy, Toshihiro Murata, Adrian Zaragoza-Bastida, and et al. 2020. "Bioactive Compounds, Pharmacological Actions, and Pharmacokinetics of Wormwood (Artemisia absinthium)" Antibiotics 9, no. 6: 353. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9060353