Marine Bioactive Compounds against Aspergillus fumigatus: Challenges and Future Prospects
Abstract
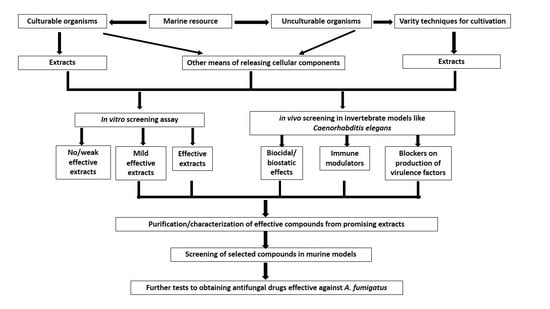
:1. Introduction
2. A. fumigatus and Aspergillosis
3. Bioactive Compounds from Marine Organisms against A. fumigatus
3.1. A. fumigatus Effective Compounds from Marine Bacteria
3.2. A. fumigatus Effective Compounds from Marine Sponges
3.3. A. fumigatus Effective Compounds from Marine Algae
3.4. A. fumigatus Effective Compounds from Sea Cucumbers
3.5. A. fumigatus Effective Compounds from Marine Fungi
4. Challenges and Future Prospects
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Latgé, J.P.; Chamilos, G. Aspergillus fumigatus and aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef] [PubMed]
- Geißel, B.; Loiko, V.; Klugherz, I.; Zhu, Z.; Wagener, N.; Kurzai, O.; van den Hondel, C.A.M.J.J.; Wagener, J. Azole-induced cell wall carbohydrate patches kill Aspergillus fumigatus. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darling, B.A.; Milder, E.A. Invasive aspergillosis. Pediatr. Rev. 2018, 39, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Linder, K.A.; McDonald, P.J.; Kauffman, C.A.; Revankar, S.G.; Chandrasekar, P.H.; Miceli, M.H. Invasive aspergillosis in patients following umbilical cord blood transplant. Bone Marrow Transpl. 2019, 54, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Ortigosa, C.; Aimanianda, C.; Muszkieta, L.; Mouyna, I.; Alsteens, D.; Pire, S.; Beau, R.; Krappmann, S.; Beauvais, A.; Dufrêne, Y.F.; et al. Chitin synthases with a myosin motor-like domain control the resistance of Aspergillus fumigatus to echinocandins. Antimicrob. Agents Chemother. 2012, 56, 6121–6131. [Google Scholar] [CrossRef] [Green Version]
- Abdolrasouli, A.; Scourfield, A.; Rhodes, J.; Shah, A.; Elborn, J.S.; Fisher, M.C.; Schelenz, S.; Armstrong-James, D. High prevalence of triazole resistance in clinical Aspergillus fumigatus isolates in a spe-cialist cardio-thoracic centre. Int. J. Antimicrob. Agents 2018, 52, 637–642. [Google Scholar] [CrossRef]
- Wirmann, L.; Ross, B.; Reimann, O.; Steinmann, J.; Rath, P.-M. Airborne Aspergillus fumigatus spore concentration during demolition of a building on a hospital area and patient risk determination for invasive aspergillosis including azole resistance. J. Hosp. Infect. 2018, 100, e91–e97. [Google Scholar] [CrossRef]
- Prigitano, A.; Esposto, M.C.; Biffi, A.; De Lorenzis, G.; Favuzzi, V.; Koncan, R.; Lo Cascio, G.; Barao Ocampo, M.; Colombo, C.; Pizzamiglio, G.; et al. Triazole resistance in Aspergillus fumigatus isolates from patients with cystic fibrosis in Italy. J. Cyst. Fibros. 2017, 16, 64–569. [Google Scholar] [CrossRef] [Green Version]
- Hagiwara, D.; Arai, T.; Takahashi, H.; Kusuya, Y.; Watanabe, A.; Kamei, K. Non-cyp51A azole-resistant Aspergillus fumigatus isolates with mutation in HMG-CoA reductase. Emerg. Infect. Dis. 2018, 24, 1889–1897. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Snelders, E.; Zwaan, B.J.; Schoustra, S.E.; Meis, J.F.; van Dijk, K.; Hagen, F.; van der Beek, M.T.; Kampinga, G.A.; Zoll, J.; et al. A novel environmental azole resistance mutation in Aspergillus fumigatus and a possible role of sexual reproduction in its emergence. mBio 2017, 8, e00791-17. [Google Scholar] [CrossRef] [Green Version]
- Prigitano, A.; Esposto, M.C.; Romanò, L.; Auxilia, F.; Tortorano, A.M. Azole-resistant Aspergillus fumigatus in the Italian environment. J. Glob. Antimicrob. Resist. 2019, 16, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Ashu, E.E.; Korfanty, G.A.; Samarasinghe, H.; Pum, N.; You, M.; Yamamura, D.; Xu, J. Widespread amphotericin B-resistant strains of Aspergillus fumigatus in Hamilton, Canada. Infect. Drug Resist. 2018, 11, 1549–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagiwara, D.; Miura, D.; Shimizu, K.; Paul, S.; Ohba, A.; Gonoi, T.; Watanabe, A.; Kamei, K.; Shintani, T.; Moye-Rowley, W.S.; et al. A novel Zn2 Cys6 transcription factor AtrR plays a key role in an azole resistance mechanism of Aspergillusfumigatus by co-regulating cyp51A and cdr1B expressions. PLoS Pathog. 2017, 13, e1006096. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Nelson-Sathi, S.; Singh, A.; Pillai, M.R.; Chowdhary, A. Genomic perspective of triazole resistance in clinical and environmental Aspergillus fumigatus isolates without cyp51A mutations. Fungal Genet. Biol. 2019, 132. [Google Scholar] [CrossRef]
- Lamoth, F.; Juvvadi, P.R.; Soderblom, E.J.; Moseley, M.A.; Asfaw, Y.G.; Steinbach, W.J. Identification of a key lysine residue in heat shock protein 90 required for azole and echinocandin resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2014, 58, 1889–1896. [Google Scholar] [CrossRef] [Green Version]
- Beer, K.D.; Farnon, E.C.; Jain, S.; Jamerson, C.; Lineberger, S.; Miller, J.; Berkow, E.L.; Lockhart, S.R.; Chiller, T.; Jackson, B.R. Multidrug-resistant Aspergillus fumigatus carrying mutations linked to environmental fungicide exposure—Three states, 2010–2017. MMWR-Morb. Mortal. Wkly. Rep. 2018, 67, 1064–1067. [Google Scholar] [CrossRef] [Green Version]
- Public Health England. English Surveillance Programme for Antimicrobial Utilization and Resistance (ESPAUR); Public Health England: London, UK, 2017; pp. 1–143.
- Reichert-Lima, F.; Lyra, L.; Pontes, L.; Moretti, M.L.; Pham, C.D.; Lockhart, S.R.; Schreiber, A.Z. Surveillance for azoles resistance in Aspergillus spp. highlights a high number of amphotericin B-resistant isolates. Mycoses 2018, 61, 360–365. [Google Scholar] [CrossRef]
- Mondol, M.A.; Shin, H.J. Antibacterial and antiyeast compounds from marine-derived bacteria. Mar. Drugs 2014, 12, 2913–2921. [Google Scholar] [CrossRef] [Green Version]
- Matobole, R.; van Zyl, L.; Parker-Nance, S.; Davies-Coleman, M.; Trindade, M. Antibacterial activities of bacteria isolated from the marine sponges Isodictya compressa and Higginsia bidentifera collected from Algoa Bay, South Africa. Mar. Drugs 2017, 15, 47. [Google Scholar] [CrossRef] [Green Version]
- Kamarudheen, N.; Rao, K.V.B. Fatty acyl compounds from marine Streptomyces griseoincarnatus strain HK12 against two major bio-film forming nosocomial pathogens; an in vitro and in silico approach. Microb. Pathog. 2019, 127, 121–130. [Google Scholar] [CrossRef]
- Mickymaray, S.; Alturaiki, W. Antifungal efficacy of marine macroalgae against fungal isolates from bronchial asthmatic cases. Molecules 2018, 23, 3032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, L.; Sun, C.; Zhang, C.; Song, S.; Sun, X.; Ju, J.; Deng, Y. Efficacy of compounds isolated from Streptomyces olivaceus against the morphogenesis and virulence of Candida albicans. Mar. Drugs 2019, 17, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivakumar, K.C.; Sajeevan, T.P.; Bright Singh, I.S. Marine derived compounds as binders of the White spot syndrome virus VP28 envelope protein: In silico insights from molecular dynamics and binding free energy calculations. Comput. Biol. Chem. 2016, 64, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Yuan, Y.; Liu, W.; Liu, L.; Qin, Q.; Yi, M. Identification of inhibitory compounds against Singapore grouper iridovirus infection by cell viability-based screening assay and droplet digital PCR. Mar. Biotechnol. 2017, 20, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Li, G.; Liu, Y.; Lu, A.; Wang, Z.; Wang, Q. Naamines and naamidines as novel agents against a plant virus and phytopathogenic fungi. Mar. Drugs 2018, 16, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melek, F.R.; Tadros, M.M.; Yousif, F.; Selim, M.A.; Hassan, M.H. Screening of marine extracts for schistosomicidal activity in vitro. Isolation of the triterpene glycosides echinosides A and B with potential activity from the sea cucumbers Actinopyga echinites and Holothuria polii. Pharm. Biol. 2012, 50, 490–496. [Google Scholar] [CrossRef]
- Tasdemir, D.; MacIntosh, A.J.J.; Stergiou, P.; Kaiser, M.; Mansour, N.R.; Bickle, Q.; Huffman, M.A. Antiprotozoal and antihelminthic properties of plants ingested by wild Japanese macaques (Macaca fuscata yakui) in Yakushima Island. J. Ethnopharmacol. 2019, 247. [Google Scholar] [CrossRef]
- Orhan, I.; Sener, B.; Kaiser, M.; Brun, R.; Tasdemir, D. Inhibitory activity of marine sponge-derived natural products against parasitic protozoa. Mar. Drugs 2010, 8, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Khanavi, M.; Toulabi, P.B.; Abai, M.R.; Sadati, N.; Hadjiakhoond, F.; Hadjiakhoondi, A.; Vatandoost, H. Larvicidal activity of marine algae, Sargassum swartzii and Chondria dasyphylla, against malaria vector Anopheles stephensi. J. Vector Dis. 2011, 48, 241–244. [Google Scholar]
- Martins, L.F.; Mesquita, J.T.; Pinto, E.G.; Costa-Silva, T.A.; Borborema, S.E.T.; Galisteo Junior, A.J.; Galisteo Junior, A.J.; Neves, B.J.; Andrade, C.H.; Shuhaib, Z.A.; et al. Analogues of marine guanidine alkaloids are in vitro effective against Trypanosoma cruzi and selectively eliminate Leishmania (L.) infantum intracellular amastigotes. J. Nat. Prod. 2016, 79, 2202–2210. [Google Scholar] [CrossRef] [Green Version]
- Peters, T.L.; Tillotson, J.; Yeomans, A.M.; Wilmore, S.C.; Lemm, E.; Jiménez-Romero, C.; Amador, L.A.; Li, L.; Amin, A.D.; Pongtornpipat, P.; et al. Target-based screening against eIF4A1 reveals the marine natural Product elatol as a novel inhibitor of translation initiation with in vivo antitumor activity. Clin. Cancer Res. 2018, 24, 4256–4270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howarth, A.; Simms, C.; Kerai, N.; Allen, O.; Mihajluk, K.; Madureira, P.A.; Sokratous, G.; Cragg, S.; Lee, S.Y.; Morley, A.D.; et al. DIVERSet JAG compounds inhibit topoisomerase II and are effective against adult and pediatric high-grade gliomas. Transla. Oncol. 2019, 12, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Rath, B.; Hochmair, M.; Plangger, A.; Hamilton, G. Anticancer activity of fascaplysin against lung cancer cell and small cell lung cancer circulating tumor cell lines. Mar. Drugs 2018, 16, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowan, J.; Shadab, M.; Nadkarni, D.H.; Kc, K.; Velu, S.E.; Yusuf, N.A. Novel marine natural product derived pyrroloiminoquinone with potent activity against skin cancer cells. Mar. Drugs 2019, 17, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Park, C.; Oh, E.; Sung, Y.; Lee, J.; Park, K.H.; Kang, H. Benzophenone compounds, from a marine-derived strain of the fungus Pestalotiopsis neglecta, inhibit proliferation of pancreatic cancer cells by targeting the MEK/ERK pathway. J. Nat. Prod. 2019, 82, 3357–3365. [Google Scholar] [CrossRef]
- Oh, S.; Son, M.; Lee, H.S.; Kim, H.S.; Jeon, Y.J.; Byun, K. Protective effect of pyrogallol-phloroglucinol-6, 6-bieckol from Ecklonia cava on monocyte-associated vascular dysfunction. Mar. Drugs 2018, 16, 441. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, K.; Antony, T. First report of spiro-compounds from marine macroalga Gracilaria salicornia: Prospective natural anti-inflammatory agents attenuate 5-lipoxygenase and cyclooxygenase-2. Nat. Prod. Res. 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Kim, M.; Son, K.-T.; Jeong, Y.; Jeon, Y.-J. Antioxidant activity of marine algal polyphenolic compounds: A mechanistic approach. J. Med. Food 2016, 19, 615–628. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, T.H.; Doan, C.T.; Tran, T.N.; Nguyen, A.D.; Kuo, Y.-H.; Wang, S.-L. Production and bioactivity-guided isolation of antioxidants with α-glucosidase inhibitory and anti-NO properties from marine chitinous materials. Molecules 2018, 23, 1124. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Lin, Y.; Cao, X.; Xiang, L.; Qi, J. Sterols from Mytilidae show anti-aging and neuroprotective effects via anti-oxidative activity. Int. J. Mol. Sci. 2014, 15, 21660–21673. [Google Scholar] [CrossRef] [Green Version]
- Prastya, M.E.; Astuti, R.I.; Batubara, I.; Takagi, H.; Wahyudi, A.T. Chemical screening identifies an extract from marine Pseudomonas sp.-PTR-08 as an anti-aging agent that promotes fission yeast longevity by modulating the Pap1-ctt1+ pathway and the cell cycle. Mol. Biol. Rep. 2019, 47, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Ianora, A. Marine organisms with anti-diabetes properties. Mar. Drugs 2016, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- de Alencar Alves, M.F.; de Almeida Barreto, F.K.; de Vasconcelos, M.A.; do Nascimento Netro, L.G.; Carneiro, R.F.; Silva, L.T.D.; Nagano, C.S.; Sampaio, A.H.; Teixeira, E.H. Antihyperglycemic and antioxidant activities of a lectin from the marine red algae, Bryothamnion seaforthii, in rats with streptozotocin-induced diabetes. Int. J. Biol. Macromol. 2010, 158, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Nasab, B.S.; Homaei, A.; Pletschke, B.I.; Salinas-Solazar, C.; Castillo-Zacarias, C.; Parra-Saldívar, R. Marine resources effective in controlling and treating diabetes and its associated complications. Process. Biochem. 2020, 92, 313–342. [Google Scholar] [CrossRef]
- Yu, J.; Proctor, R.H.; Brown, D.W.; Abe, K.; Gomi, K.; Machida, M.; Hasegawa, F.; Nierman, W.C.; Bhatnagar, D.; Cleveland, T.E. Genomics of economically significant Aspergillus and Fusarium species. In Applied Mycology and Biotechnology; Arora, D.K., Khachatourians, G.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 249–283. [Google Scholar] [CrossRef]
- Bennett, J.H. On the parasitic vegetable structures found growing in living animals. Trana. R. Soc. Edinb. 1844, 15, 277–294. [Google Scholar] [CrossRef] [Green Version]
- Stevens, D.A.; Melikian, G.L. Aspergillosis in the ‘nonimmunocompromised’ host. Immunol. Investig. 2011, 40, 751–766. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Shah, A.A.; Bashir, S.H. Craniocerebral aspergillosis of sinonasal origin in immunocompetent patients: Clinical spectrum and outcome in 25 cases. Neurosurgery 2004, 55, 602–613. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Bashir, S.H.; Ali Shah, A.; Sajjad, Z.; Ahmed, N.; Jooma, R.; Enam, S.A. Diagnostic MR imaging features of craniocerebral aspergillosis of sino-nasal origin in immunocompetent patients. Acta Neurochir. 2006, 148, 155–166. [Google Scholar] [CrossRef]
- Webb, B.J.; Vikram, H.R. Chronic invasive sinus aspergillosis in immunocompetent hosts: A geographic comparison. Mycopathologia 2010, 170, 403–410. [Google Scholar] [CrossRef]
- Mody, K.H.; Ali, M.J.; Vemuganti, G.K.; Nalamada, S.; Naik, M.N.; Honavar, S.G. Orbital aspergillosis in immunocompetent patients. Br. J. Ophthalmol. 2014, 98, 1379–1384. [Google Scholar] [CrossRef]
- Aggarwal, E.; Mulay, K.; Menon, V.; Sundar, G.; Honavar, S.G.; Sharma, M. Isolated orbital aspergillosis in immunocompetent patients: A multicenter study. Am. J. Ophthalmol. 2016, 165, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Baeesa, S.S.; Bakhaidar, M.; Ahamed, N.A.B.; Madani, T.A. Invasive orbital apex aspergillosis with mycotic aneurysm formation and subarachnoid hemorrhage in immunocompetent patients. World Neurosurg. 2017, 102, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Blaize, M.; Mayaux, J.; Nabet, C.; Lampros, A.; Marcelin, A.G.; Thellier, M.; Piarroux, R.; Demoule, A.; Fekkar, A. Fatal Invasive aspergillosis and coronavirus disease in an immunocompetent patient. Emerg. Infect. Dis. 2020, 26, 1636–1637. [Google Scholar] [CrossRef] [PubMed]
- Bora, S.; Kumar, A.; Mishra, S.; Satyarthee, G.D.; Singh, P.K.; Sawarkar, D.; Verma, S.; Borkar, S.; Sharma, R.; Chandra, S.P.; et al. Intracranial aspergillosis amongst immunocompetent patients: An experience with combined surgical and medical management of 18 patients. Clin. Neurol. Neurosurg. 2019, 186, 105511. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, W.; Ao, R.; Lan, X.; Li, Y.; Zhang, J.; Yu, S. Central nervous system aspergillosis in immunocompetent patients: Case series and literature review. Medicine 2020, 99, e22911. [Google Scholar] [CrossRef]
- Yoon, S.H.; Park, C.M.; Goo, J.M.; Lee, H.J. Pulmonary aspergillosis in immunocompetent patients without air-meniscus sign and underlying lung disease: CT findings and histopathologic features. Acta Radiol. 2011, 52, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Han, X.; Li, Y.; Zhang, C.; Xing, X. Invasive pulmonary aspergillosis in immunocompetent patients hospitalised with influenza A-related pneumonia: A multicenter retrospective study. BMC Pulm Med. 2020, 20, 239. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Wang, W.; Liu, K. Adrenal and hepatic aspergillosis in an immunocompetent patient. Infect. Dis. 2015, 47, 428–432. [Google Scholar] [CrossRef]
- Snelders, E.; Huisin’t Veld, R.A.G.; Rijs, A.J.M.M.; Kema, G.H.J.; Melchers, W.J.G.; Verweij, P.E. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl. Environ. Microb. 2009, 75, 4053–4057. [Google Scholar] [CrossRef] [Green Version]
- Fang, W.; Latgé, J.P. Microbe profile: Aspergillus fumigatus: A saprotrophic and opportunistic fungal pathogen. Microbiology 2018, 164, 1009–1011. [Google Scholar] [CrossRef]
- Vaezi, A.; Fakhim, H.; Javidnia, J.; Khodavaisy, S.; Abtahian, Z.; Vojoodi, M.; Nourbakhsh, F.; Badali, H. Pesticide behavior in paddy fields and development of azole-resistant Aspergillus fumigatus: Should we be concerned? J. Mycol. Med. 2018, 28, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, B.R.; Bapuji, M.; Sree, A. Antifungal efficacy of bacteria isolated from marine sedentary organisms. Folia Microbiol. 2002, 47, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Wahidullah, S.; Rodrigues, C.; Souza, L.D. The sponge-associated bacterium Bacillus licheniformis SAB1: A source of antimicrobial compounds. Mar. Drugs 2010, 8, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cai, K.; Yao, R. A new macrolactam derivative from the marine actinomycete HF-11225. J. Antibiot. 2018, 71, 477–479. [Google Scholar] [CrossRef]
- Santos, J.D.; Vitorino, I.; De la Cruz, M.; Díaz, C.; Cautain, B.; Annang, F.; Pérez-Moreno, G.; Gonzalez Martinez, I.; Tormo, J.R.; Martín, J.M.; et al. Bioactivities and extract dereplication of Actinomycetales isolated from marine sponges. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Subramani, R.; Sipkema, D. Marine rare actinomycetes: A promising source of structurally diverse and unique novel natural products. Mar. Drugs 2019, 17, 249. [Google Scholar] [CrossRef] [Green Version]
- Nagai, K.; Kamigiri, K.; Matsumoto, H.; Kawano, Y.; Yamaoka, M.; Shimoi, H.; Watanabe, M.; Suzuki, K. YM-202204, a new antifungal antibiotic produced by marine fungus Phoma sp. J. Antibiot. 2002, 55, 1036–1041. [Google Scholar] [CrossRef] [Green Version]
- Orwa, P.; Mugambi, G.; Wekesa, V.; Mwirichia, R. Isolation of haloalkaliphilic fungi from Lake Magadi in Kenya. Heliyon 2020, 6, e02823. [Google Scholar] [CrossRef] [Green Version]
- Al-Enazi, N.M.; Awaad, A.S.; Zain, M.E.; Alqasoumi, S.I. Antimicrobial, antioxidant and anticancer activities of Laurencia catarinensis, Laurencia majuscula and Padina pavonica extracts. Saudi Pharm. J. 2018, 26, 44–52. [Google Scholar] [CrossRef]
- Al-Enazi, N.M.; Awaad, A.S.; Alqasoumi, S.I.; Alwethairi, M.F. Biological activities of the red algae Galaxaura rugosa and Liagora hawaiiana butters. Saudi Pharm. J. 2018, 26, 25–32. [Google Scholar] [CrossRef]
- Silva, P.; Fernandes, C.; Barros, L.; Ferreira, I.C.F.R.; Pereira, L.; Gonçalves, T. The antifungal activity of extracts of Osmundea pinnatifida, an edible seaweed, indicates its usage as a safe environmental fungicide or as a food additive preventing post-harvest fungal food contamination. Food Funct. 2018, 9, 6187–6195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, K.A.; Lauritano, C.; Druka, D.; Romano, G.; Grohmann, T.; Jaspars, M.; Martín, J.; Díaz, C.; Cautain, B.; de la Cruz, M.; et al. Amphidinol 22, a new cytotoxic and antifungal amphidinol from the dinoflagellate Amphidinium carterae. Mar. Drugs 2019, 17, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshmi, V.; Mishra, S.K.; Srivastava, S.; Chaturvedi, A.; Srivastava, M.N.; Shukla, P.K. Antifungal activity of marine sponge Haliclona exigua (Krikpatrick). J. Mycol. Méd. 2010, 20, 31–35. [Google Scholar] [CrossRef]
- Xu, T.; Feng, Q.; Jacob, M.R.; Avula, B.; Mask, M.M.; Baerson, S.R.; Tripathi, S.K.; Mohammed, R.; Hamann, M.T.; Khan, I.A.; et al. The marine sponge-derived polyketide endoperoxide plakortide F acid mediates its antifungal activity by interfering with calcium homeostasis. Antimicrob. Agents Chemother. 2011, 55, 1611–1621. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Jacob, M.R.; Rao, R.R.; Wang, Y.-H.; Agarwal, A.K.; Newman, D.J. Antifungal cyclic peptides from the marine sponge Microscleroderma herdmani. Res. Rep. Med. Chem. 2010, 22, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Nazemi, M.; Alidoust Salimi, M.; Alidoust Salimi, P.; Motallebi, A.; Tamadoni Jahromi, S.; Ahmadzadeh, O. Antifungal and antibacterial activity of Haliclona sp. from the Persian Gulf, Iran. J. Mycol. Méd. 2014, 24, 220–224. [Google Scholar] [CrossRef]
- Ismail, H.; Lemriss, S.; Aoun, B.Z.; Mhadhebi, L.; Dellai, A.; Kacem, Y.; Boiron, P.; Bouraoui, A. A activity of aqueous and methanolic extracts from the Mediterranean sea cucumber, Holothuria polii. J. Mycol. Méd. 2008, 18, 23–26. [Google Scholar] [CrossRef]
- Yuan, W.-H.; Yi, Y.-H.; Tang, H.-F.; Liu, B.-S.; Wang, Z.-L.; Sun, G.-Q.; Zhang, W.; Li, L.; Sun, P. Antifungal triterpene glycosides from the sea cucumber Bohadschia marmorata. Planta Med. 2009, 75, 168–173. [Google Scholar] [CrossRef]
- Yuan, W.-H.; Yi, Y.-H.; Tan, R.-X.; Wang, Z.-L.; Sun, G.-Q.; Xue, M.; Zhang, H.-W.; Tang, H.-F. Antifungal triterpene glycosides from the sea cucumber Holothuria (Microthele) axiloga. Planta Med. 2009, 75, 647–653. [Google Scholar] [CrossRef] [Green Version]
- Barsby, T.; Kelly, M.T.; Andersen, R.J. Tupuseleiamides and basiliskamides, new acyldipeptides and antifungal polyketides produced in culture by a Bacillus laterosporus isolate obtained from a tropical marine habitat. J. Nat. Prod. 2002, 65, 1447–1451. [Google Scholar] [CrossRef]
- Lacret, R.; Oves-Costales, D.; Gómez, C.; Díaz, C.; de la Cruz, M.; Pérez-Victoria, I.; Vicente, F.; Genilloud, O.; Reyes, F. New ikarugamycin derivatives with antifungal and antibacterial properties from Streptomyces zhaozhouensis. Mar. Drugs 2015, 13, 128–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacret, R.; Pérez-Victoria, I.; Oves-Costales, D.; de la Cruz, M.; Domingo, E.; Martín, J.; Díaz, C.; Vicente, F.; Genilloud, O.; Reyes, F. MDN-0170, a new napyradiomycin from Streptomyces sp. strain CA-271078. Mar. Drugs 2016, 14, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Victoria, I.; Oves-Costales, D.; Lacret, R.; Martín, J.; Sánchez-Hidalgo, M.; Diaz, C.; Cautain, B.; Vicente, F.; Genilloud, O.; Reyes, F. Structure elucidation and biosynthetic gene cluster analysis of caniferolides A-D, new bioactive glycosylated 36-membered polyol macrolides from the marine-derived Streptomyces caniferus CA-271066. Org. Biomol. Chem. 2019, 17. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kannabiran, K. Antifungal activity of Streptomyces VITSVK5 spp. against drug resistant Aspergillus clinical isolates from pulmonary tuberculosis patients. J. Mycol. Méd. 2010, 20, 101–107. [Google Scholar] [CrossRef]
- Suthindhiran, K.; Kannabiran, K. Diversity and exploration of bioactive marine actinomycetes in the Bay of Bengal of the Puducherry coast of India. Indian J. Microbiol. 2010, 50, 76–82. [Google Scholar] [CrossRef]
- Sata, N.U.; Matsunaga, S.; Fusetani, N.; van Soest, R.W.M. Aurantosides D, E, and F: New antifungal tetramic acid glycosides from the marine sponge Siliquariaspongia japonica. J. Nat. Prod. 1999, 62, 969–971. [Google Scholar] [CrossRef]
- Edrada, R.A.; Ebel, R.; Supriyono, A.; Wray, V.; Schupp, P.; Steube, K.; van Soest, R.; Proksch, P. Swinhoeiamide A, a new highly active calyculin derivative from the marine sponge Theonella swinhoei. J. Nat. Prod. 2002, 65, 1168–1172. [Google Scholar] [CrossRef]
- Chen, Y.; McCarthy, P.J.; Harmody, D.K.; Schimoler-O’Rourke, R.; Chilson, K.; Selitrennikoff, C.; Pomponi, S.A.; Wright, A.E. New bioactive peroxides from marine sponges of the family Plakiniidae. J. Nat. Prod. 2002, 65, 1509–1512. [Google Scholar] [CrossRef]
- Sionov, E.; Roth, D.; Sandovsky-Losica, H.; Kashman, Y.; Rudi, A.; Chill, L.; Berdicevsky, I.; Segal, E. Antifungal effect and possible mode of activity of a compound from the marine sponge Dysidea herbacea. J. Infect. 2005, 50, 453–460. [Google Scholar] [CrossRef]
- Pettit, G.R.; Chicacz, Z.A.; Gao, F.; Herald, C.L.; Boyd, M.R.; Schmidt, J.M.; Hooper, J.N.A. Antineoplastic agents. 257. Isolation and structure of spongistatin 1. J. Org. Chem. 1993, 58, 1302–1304. [Google Scholar] [CrossRef]
- Pettit, R.K.; McAllister, S.C.; Pettit, G.R.; Herald, C.L.; Johnson, J.M.; Cichacz, Z.A. A broad-spectrum antifungal from the marine sponge Hyrtios erecta. Int. J. Antimicrob. Agents 1998, 9, 147–152. [Google Scholar] [CrossRef]
- Oh, K.-B.; Lee, J.H.; Chung, S.-C.; Shin, J.; Shin, H.J.; Kim, H.-K.; Lee, H.-S. Antimicrobial activities of the bromophenols from the red alga Odonthalia corymbifera and some synthetic derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Alarif, W.M.; Al-Lihaibi, S.S.; Abdel-Lateff, A.; Ayya, S.-E.N. New antifungal cholestane and aldehyde derivatives from the red alga Laurencia papillosa. Nat. Prod. Commun. 2011, 6, 1821–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Chaturvedi, A.K.; Shukla, P.K.; Lakshmi, V. Antifungal activity in triterpene glycosides from the sea cucumber Actinopyga lecanora. Bioorg. Med. Chem. Lett. 2007, 17, 4387–4391. [Google Scholar] [CrossRef]
- Han, H.; Yi, Y.-H.; Li, L.; Liu, B.-S.; La, M.-P.; Zhang, H.-W. Antifungal active triterpene glycosides from sea cucumber Holothuria scabra. Acta Pharm. Sin. 2009, 44, 620–624. [Google Scholar]
- Adibpour, N.; Nasr, F.; Nematpour, F.; Shakouri, A.; Ameri, A. Antibacterial and antifungal activity of Holothuria leucospilota isolated from Persian Gulf and Oman Sea. Jundishapur. J. Microb. 2014, 7, e8708. [Google Scholar] [CrossRef] [Green Version]
- Lakshmi, V.; Srivastava, S.; Mishra, S.K.; Shukla, P.K. Antifungal activity of bivittoside-D from Bohadschia vitiensis (Semper). Nat. Prod. Res. 2012, 26, 913–918. [Google Scholar] [CrossRef]
- Austin, B. The value of cultures to modern microbiology. Antonie Van Leeuwenhoek 2017, 110, 1247–1256. [Google Scholar] [CrossRef]
- Pulschen, A.A.; Bendia, A.G.; Fricker, A.D.; Pellizari, V.H.; Galante, D.; Rodrigues, F. Isolation of uncultured bacteria from Antarctica using long incubation periods and low nutritional media. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Bodor, A.; Bounedjoum, N.; Vincze, G.E.; Erdeiné Kis, Á.; Laczi, K.; Bende, G.; Szilágyi, Á.; Kovács, T.; Perei, K.; Rákhely, G. Challenges of unculturable bacteria: Environmental perspectives. Rev. Environ. Sci. Biotechnol. 2020, 19, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Kovalchuk, V.; Voronkina, A.; Binnewerg, B.; Schubert, M.; Muzychka, L.; Wysokowski, M.; Tsurkan, M.V.; Bechmann, N.; Petrenko, I.; Fursov, A.; et al. Naturally drug-loaded chitin: Isolation and applications. Mar. Drugs 2019, 17, 574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.A. Collagen of extracellular matrix from marine invertebrates and its medical applications. Mar. Drug 2019, 17, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breger, J.; Fuchs, B.B.; Aperis, G.; Moy, T.I.; Asbel, F.M.; Mylonakis, E. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 2007, 3, e18. [Google Scholar] [CrossRef] [PubMed]
- Moy, T.I.; Conery, A.L.; Larkins-Ford, J.; Wu, G.; Mazitschek, R.; Casadei, G.; Lewis, K.; Carpenter, A.E.; Ausubel, F.M. High-throughput screen for novel antimicrobials using a whole animal infection model. ACS Chem. Biol. 2009, 4, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Graham, C.E.; Cruz, M.R.; Garsin, D.A.; Lorenz, M.C. Enterococcus faecalis bacteriocin EntV inhibits hyphal morphogenesis, biofilm formation, and virulence of Candida albicans. Proc. Natl. Acad. Sci. USA 2017, 25, 4507–4512. [Google Scholar] [CrossRef] [Green Version]
- Manoharan, R.K.; Lee, J.H.; Kim, Y.G.; Kim, S.I.; Lee, J. Inhibitory effects of the essential oils α-longipinene and linalool on biofilm formation and hyphal growth of Candida albicans. Biofouling 2017, 33, 143–155. [Google Scholar] [CrossRef]
- Okoli, I.; Coleman, J.J.; Tempakakis, E.; An, W.F.; Holson, E.; Wagner, F.; Conery, A.L.; Larkins-Ford, J.; Wu, G.; Stern, A.; et al. Identification of antifungal compounds active against Candida albicans using an improved high-throughput Caenorhabditis elegans assay. PLoS ONE 2009, 4, e7025. [Google Scholar] [CrossRef] [Green Version]
- Peterson, N.D.; Pukkila-Worley, R. Caenorhabditis elegans in high-throughput screens for anti-infective compounds. Curr. Opin. Immunol. 2018, 54, 59–65. [Google Scholar] [CrossRef]
- Ahamefule, C.S.; Qin, Q.; Odiba, A.S.; Li, S.; Moneke, A.N.; Ogbonna, J.C.; Jin, C.; Wang, B.; Fang, W. Caenorhabditis elegans-based Aspergillus fumigatus infection model for evaluating pathogenicity and drug efficacy. Front. Cell. Infect. Microbiol. 2020, 10. [Google Scholar] [CrossRef]
- Haferburg, G.; Groth, I.; Möllmann, U.; Kothe, E.; Sattler, I. Arousing sleeping genes: Shifts in secondary metabolism of metal tolerant actinobacteria under conditions of heavy metal stress. Biometals 2009, 22, 225–234. [Google Scholar] [CrossRef]
- Morgenstern, A.; Paetz, C.; Behrend, A.; Spiteller, D. Divalent transition-metal-ion stress induces prodigiosin biosynthesis in Streptomyces coelicolor M145: Formation of coeligiosins. Chemistry 2015, 21, 6027–6032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Pan, C.; Auckloo, B.N.; Chen, X.; Chen, C.-T.A.; Wang, K.; Wu, X.; Ye, Y.; Wu, B. Stress-driven discovery of a cryptic antibiotic produced by Streptomyces sp. WU20 from Kueishantao hydrothermal vent with an integrated metabolomics strategy. Appl. Microbiol. Biotechnol. 2016, 101, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.; Shah, S.A.A.; Pan, C.; Fu, L.; Cao, X.; Shi, Y.; Wu, X.; Wang, K.; Wu, B. Production of an antibiotic enterocin from a marine actinobacteria strain H1003 by metal-stress technique with enhanced enrichment using response surface methodology. Pak. J. Pharm. Sci. 2017, 30, 313–324. [Google Scholar] [PubMed]
- Zhu, C.; Liu, X.; Chi, H.; Chen, C.; Chen, Z.; Fu, G.; Gong, H.; Huang, Y. System for the heterologous expression of NS1 protein of H9N2 avian influenza virus in the ciliate Tetrahymena thermophila. J. Vet. Med. Sci. 2018, 80, 1610–1618. [Google Scholar] [CrossRef]






| Compound | Description/Source of Compound | Compound Class | Activity (MIC μg/mL) | Reference |
|---|---|---|---|---|
| Isoikarugamycin | Ethyl acetate extract from Streptomyces zhaozhouensis CA-185989 broth | polycyclic tetramic acid macrolactams | 4–8 | [83] |
| 28-N-methylikaguramycin | Ethyl acetate extract from Streptomyces zhaozhouensis CA-185989 broth | polycyclic tetramic acid macrolactams | 4–8 | [83] |
| Ikarugamycin | Ethyl acetate extract from Streptomyces zhaozhouensis CA-185989 broth | polycyclic tetramic acid macrolactams | 4–8 | [83] |
| Caniferolides A&B | Ethyl acetate extract from Streptomyces caniferus CA-271066. | polyol macrolides | 2–4 | [85] |
| Caniferolides C&D | Ethyl acetate extract from Streptomyces caniferus CA-271066. | polyol macrolides | 4–8 | [85] |
| 4,4′-oxybis(3-phenylpropionic acid) | Ethyl acetate concentrates of methanolic extract from Bacillus licheniformis | oxyneolignan | 7–10 mm * | [65] |
| Isolate | Description | Activity (MIC μg/mL) | Reference |
|---|---|---|---|
| Streptomyces sp. VITSDK36 | Ethyl acetate extract | 1.32 | [87] |
| Streptomyces sp. VITSDK37 | Ethyl acetate extract | 0.74 | [87] |
| Streptomyces sp. VITSDK38 | Ethyl acetate extract | 1.64 | [87] |
| Streptomyces sp. VITSDK39 | Ethyl acetate extract | 1.78 | [87] |
| Streptomyces sp. VITSDK41 | Ethyl acetate extract | 2.68 | [87] |
| Streptomyces sp. VITSDK42 | Ethyl acetate extract | 1.58 | [87] |
| Actinopolyspora sp. VITSDK43 | Ethyl acetate extract | 1.58 | [87] |
| Actinopolyspora sp. VITSDK44 | Ethyl acetate extract | 2.54 | [87] |
| Micromonospora sp. VITSDK46 | Ethyl acetate extract | 1.40 | [87] |
| Sachharopolyspora sp. VITSDK47 | Ethyl acetate extract | 0.90 | [87] |
| Streptomyces VITSVK5 spp. | Ethyl acetate extract | 0.5 | [86] |
| Micrococcus sp. RRL-3 | Methanol extract | 0.5 | [64] |
| Flavobacterium sp. RRL-10 | Methanol extract | 5.5 | [64] |
| Pseudomonas sp. RRL-12 | Methanol extract | 0.9 | [64] |
| Streptomyces sp. RRL-13 | Methanol extract | 0.7 | [64] |
| Flavobacterium sp. RRL-20 | Methanol extract | 4.3 | [64] |
| Flavobacterium sp. RRL-32 | Methanol extract | 0.9 | [64] |
| Micrococcus sp. RRL-37 | Methanol extract | 0.8 | [64] |
| Bacillus sp. RRL-38 | Methanol extract | 2.0 | [64] |
| Flavobacterium sp. RRL-41 | Methanol extract | 1.3 | [64] |
| Flavobacterium sp. RRL-54 | Methanol extract | 0.5 | [64] |
| Alcaligenes sp. RRL-56 | Methanol extract | 0.3 | [64] |
| Bacillus sp. RRL-57 | Methanol extract | 2.5 | [64] |
| Streptomyces VITSVK5 spp. | Ethyl acetate extract against MDR9 | 4 * | [86] |
| Streptomyces VITSVK5 spp. | Ethyl acetate extract against MDR10 | 0.25 * | [86] |
| Streptomyces VITSVK5 spp. | Ethyl acetate extract against MDR11 | 2 * | [86] |
| Compound | Description/Source of Compound | Location of Isolation | Compound Class | Activity (MIC μg/mL) | Reference |
|---|---|---|---|---|---|
| Swinhoeiamide A | Methanol extract from Theonella swinhoei | Karkar Island, Papua New Guinea | calyculinamide-related congener | 1.0 | [89] |
| 1,2-dioxane ring peroxide acid | Ethanol extract from Plakortis halichondrioides | Long Bay, Negril, Jamaica | peroxide | 5.6 1 | [90] |
| Aurantoside A & B | Ethanol extract from Siliquariaspongia japonica | Hachijo-jima Island, Tokyo, Japan | polyene tetramic acids | 0.16 | [88] |
| Aurantoside D | Ethanol extract from Siliquariaspongia japonica | Hachijo-jima Island, Tokyo, Japan | polyene tetramic acids | 11.0 2 | [88] |
| Aurantoside E | Ethanol extract from Siliquariaspongia japonica | Hachijo-jima Island, Tokyo, Japan | polyene tetramic acids | 0.04 | [88] |
| 3,5-dibromo-2-(3,5-dibromo-2-methoxyphenoxy) phenol | Ethyl acetate-methanol (EtOAc-MeOH) extract of Dysidea herbace | Coast of Zanzibar | phenol | 7.8 | [91] |
| Microsclerodermin J | Methananol extract of Microscleroderma herdmani | Mauritius | hexapeptides | 10.0 | [77] |
| Microsclerodermin K | Methananol extract of Microscleroderma herdmani | Mauritius | hexapeptides | 10.0 | [77] |
| Microsclerodermin A | Methananol extract of Microscleroderma herdmani | Mauritius | hexapeptides | 1.3 | [77] |
| Microsclerodermin B | Methananol extract of Microscleroderma herdmani | Mauritius | hexapeptides | 0.6 | [77] |
| Araguspongin C | Methanol extract of Haliclona exigua | Tamil Nadu coast of India | heteropentacyclic compound | 50 | [75] |
| Spongistatin 1 | Extract from Hyrtios erecta | Eastern Indian Ocean | macrocyclic lactone polyether | 12.5 3 | [92,93] |
| Compound/Isolate | Description/Source of Compound | Activity (MIC μg/mL) | Reference |
|---|---|---|---|
| 2,20,3,30-tetrabromo-4,40,5,50-tetrahydroxydiphenylmethane | Compounds from Odonthalia corymbifera | 0.78 | [94] |
| 3-bromo-4-(2,3-dibromo-4,5-dihydroxybenzyl)-5-methoxymethylpyrocatechol | Compounds from Odonthalia corymbifera | 25 | [94] |
| (E)-2-{(E) tridec-2-en-2-yl} heptadec-2-enal | Chloroform/methanol extract of Laurencia papillosa | 200 | [95] |
| Extract | Ethanol extract from Laurencia catarinensis | 3.90 | [71] |
| Extract | Ethanol extract from Laurencia majuscula | 1.95 | [71] |
| Extract | Ethanol extract from Padina pavonica | 0.98 | [71] |
| Compounds | Sea Cucumber | Compound Class | Activity (MIC μg/mL) | Reference |
|---|---|---|---|---|
| Scabraside A | Holothuria scabra | Triterpene glycosides | 2.0 * | [97] |
| Echinoside A | Holothuria scabra | Triterpene glycosides | 1.0 * | [97] |
| Holothurin A1 | Holothuria scabra | Triterpene glycosides | 8.0 * | [97] |
| Holothurin B | Actinopyga lecanora | Triterpene glycosides | 3.12 | [82] |
| 3-O-b-D-xylopyranosyl-16b-acetoxyholost-7-ene | Actinopyga lecanora | Triterpene glycosides | 50.0 | [96] |
| Holothurin A | Actinopyga lecanora | Triterpene glycosides | 50.0 | [96] |
| Bivittoside-D | Bohadschia vitiensis Semper | Lanostane triterpenoid | 1.56 | [99] |
| Marmoratoside A | Bohadschia marmorata Jaeger. | Triterpene glycosides | 2.81 * | [80] |
| Marmoratoside B | Bohadschia marmorata Jaeger. | Triterpene glycosides | 44.44 * | [80] |
| 17α-hydroxy impatienside A | Bohadschia marmorata Jaeger. | Triterpene glycosides | 2.78 * | [80] |
| Impatienside A | Bohadschia marmorata Jaeger. | Triterpene glycosides | 2.81 * | [80] |
| Bivittoside D | Bohadschia marmorata Jaeger. | Triterpene glycosides | 2.80 * | [80] |
| 25-acetoxy bivittoside D | Bohadschia marmorata Jaeger. | Triterpene glycosides | 43.13 * | [80] |
| Arguside F | Holothuria (Microthele) axiloga | Holostan-type triterpene glycosides | 16.0 * | [81] |
| Impatienside B | Holothuria (Microthele) axiloga | Holostan-type triterpene glycosides | 4.0 * | [81] |
| Pervicoside D | Holothuria (Microthele) axiloga | Holostan-type triterpene glycosides | 64.0 * | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahamefule, C.S.; Ezeuduji, B.C.; Ogbonna, J.C.; Moneke, A.N.; Ike, A.C.; Wang, B.; Jin, C.; Fang, W. Marine Bioactive Compounds against Aspergillus fumigatus: Challenges and Future Prospects. Antibiotics 2020, 9, 813. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9110813
Ahamefule CS, Ezeuduji BC, Ogbonna JC, Moneke AN, Ike AC, Wang B, Jin C, Fang W. Marine Bioactive Compounds against Aspergillus fumigatus: Challenges and Future Prospects. Antibiotics. 2020; 9(11):813. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9110813
Chicago/Turabian StyleAhamefule, Chukwuemeka Samson, Blessing C. Ezeuduji, James C. Ogbonna, Anene N. Moneke, Anthony C. Ike, Bin Wang, Cheng Jin, and Wenxia Fang. 2020. "Marine Bioactive Compounds against Aspergillus fumigatus: Challenges and Future Prospects" Antibiotics 9, no. 11: 813. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9110813