Spermine-Mediated Tolerance to Selenium Toxicity in Wheat (Triticum aestivum L.) Depends on Endogenous Nitric Oxide Synthesis
Abstract
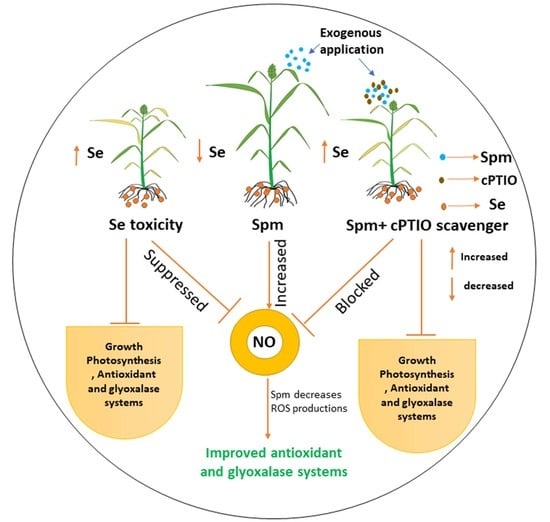
:1. Introduction
2. Materials and Methods
2.1. Wheat Variety, Plant Growth Conditions and Treatments
2.2. Selenium (Se) and Nitric Oxide (NO) Measurements
2.3. Growth Measurements
2.4. Photosynthetic Pigments and Relative Water Content (RWC)
2.5. Malondialdehyde (MDA) and Hydrogen Peroxide (H2O2) Contents and Electrolyte Leakage (EL)
2.6. Proline, Total Soluble Sugar (TSS) and Anthocyanin Contents
2.7. Antioxidant Enzyme Assays
2.8. Ascorbate (AsA) and Glutathione (GSH) Levels
2.9. Glyoxalase Systems and Lactate Dehydrogenase (LDH) Activity
2.10. Methylglyoxal (MG) and d-Lactate Contents
2.11. Gene Expression of Metal-Related Proteins
2.12. Statistical Analysis
3. Results
3.1. Se and NO Contents in Leaves and Roots of Wheat Seedlings
3.2. Spm and NO Alleviates Se Stress-Induced Growth Retardation
3.3. Spm-Induced Intrinsic NO Improves the RWC and Photosynthetic Pigments of Wheat Seedlings under Se Stress
3.4. Spm and NO Decreased the Content of MDA, H2O2 and EL of Wheat Seedlings under Se Stress
3.5. Spm and NO Decreased Proline and TSS Accumulation and Increased the Anthocyanin Content of Wheat Seedlings under Se Stress
3.6. Spm-Induced Endogenous NO Increased Antioxidant Enzyme Activities in Se-Stressed Wheat Seedlings
3.7. Spm and NO Improved the AsA-GSH Contents in Wheat Seedlings under Se Stress
3.8. Spm and NO Decreased MG Intermediates and Improved Glyoxalase Systems
3.9. Spm Induced NO-Modulated Gene Expression of Metal-Related Proteins under Se Stress
3.10. Heat Map Analysis and Principal Component Analysis (PCA) of Spm- and NO-Treated Wheat Seedlings under Se Stress
4. Discussion
4.1. Spm-Induced Endogenous NO Diminishes the Se Content in the Leaves and Roots of Se-Stressed Wheat
4.2. Spm-Induced NO Improved Growth, and Photosynthetic Performances under Se Stress
4.3. Spm-Induced NO Decreases Oxidative Damage and Improves Antioxidant Defense under Se Stress
4.4. Modulation of Gene Expression in Spm and NO-Treated Wheat Seedlings under Se Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abedi, T.; Mojiri, A. Cadmium uptake by wheat (Triticum aestivum L.): An overview. Plants 2020, 9, 500. [Google Scholar] [CrossRef] [Green Version]
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide Research Trends on Wheat and Barley: A Bibliometric Comparative Analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef] [Green Version]
- Mingorance, M.D.; Valdés, B.; Oliva, S.R. Strategies of heavy metal uptake by plants growing under industrial emissions. Environ. Int. 2007, 33, 514–520. [Google Scholar] [CrossRef]
- Hasan, M.M.; Ali, M.A.; Soliman, M.H.; Alqarawi, A.A.; Abd Allah, E.F.; Fang, X.-W. Insights into 28-homobrassinolide (HBR)-mediated redox homeostasis, AsA–GSH cycle, and methylglyoxal detoxification in soybean under drought-induced oxidative stress. J. Plant Inter. 2020, 15, 371–385. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef] [Green Version]
- Ulhassan, Z.; Huang, Q.; Gill, R.A.; Ali, S.; Mwamba, T.M.; Ali, B.; Hina, F.; Zhou, W. Protective mechanisms of melatonin against selenium toxicity in Brassica napus: Insights into physiological traits, thiol biosynthesis and antioxidant machinery. BMC Plant Biol. 2019, 19, 507. [Google Scholar] [CrossRef]
- Dai, Z.; Rizwan, M.; Gao, F.; Yuan, Y.; Huang, H.; Hossain, M.M.; Xiong, S.; Cao, M.; Liu, Y.; Tu, S. Nitric oxide alleviates selenium toxicity in rice by regulating antioxidation, selenium uptake, speciation and gene expression. Environ. Pollut. 2020, 257, 113540. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon-Smits, E.A. The fascinating facets of plant selenium accumulation biochemistry, physiology, evolution and ecology. New Phytol. 2017, 213, 1582–1596. [Google Scholar] [CrossRef] [Green Version]
- Borbély, P.; Molnár, Á.; Valyon, E.; Ördög, A.; Horváth-Boros, K.; Csupor, D.; Fehér, A.; Kolbert, Z. The effect of foliar selenium (Se) treatment on growth, photosynthesis, and oxidative-nitrosative signalling of Stevia rebaudiana leaves. Antioxidants 2021, 10, 72. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; Hasan, M.M.; Hammami, I.; Alghamdi, A.I.; Alshehri, D.; Alatawi, H.A. Green synthesized metal oxide nanoparticles mediate growth regulation and physiology of crop plants under drought stress. Plants 2021, 10, 1730. [Google Scholar] [CrossRef]
- Hasan, M.M.; Skalicky, M.; Jahan, M.S.; Hossain, M.N.; Anwar, Z.; Nie, Z.-F.; Alabdallah, N.M.; Brestic, M.; Hejnak, V.; Fang, X.-W. Spermine: Its Emerging Role in Regulating Drought Stress Responses in Plants. Cells 2021, 10, 261. [Google Scholar] [CrossRef]
- Corpas, F.J.; Hayashi, M.; Mano, S.; Nishimura, M.; Barroso, J.B. Peroxisomes are required for in vivo nitric oxide accumulation in the cytosol following salinity stress of Arabidopsis plants. Plant Physiol. 2009, 151, 2083–2094. [Google Scholar] [CrossRef] [Green Version]
- Mostofa, M.G.; Rahman, M.M.; Siddiqui, M.N.; Fujita, M.; Tran, L.P. Salicylic acid antagonizes selenium phytotoxicity in rice: Selenium homeostasis, oxidative stress metabolism and methylglyoxal detoxification. J. Hazard. Mater. 2020, 394, 122572. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Exogenous sodium nitroprusside alleviates arsenic induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 2013, 22, 584–596. [Google Scholar] [CrossRef]
- Hasan, M.M.; Alabdallah, N.M.; Alharbi, B.M.; Waseem, M.; Yao, G.; Liu, X.-D.; Abd El-Gawad, H.G.; El-Yazied, A.A.; Ibrahim, M.F.M.; Jahan, M.S.; et al. GABA: A Key Player in Drought Stress Resistance in Plants. Int. J. Mol. Sci. 2021, 22, 10136. [Google Scholar] [CrossRef]
- Hasan, M.M.; Gong, L.; Nie, Z.; Feng, X.; Ahammed, G.J.; Fang, X.W. ABA induced stomatal movements in vascular plants during dehydration versus rehydration. Environ. Exp. Bot. 2021, 186, 104436. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rahman, M.A.; Skalicky, M.; Alabdallah, N.M.; Waseem, M.; Jahan, M.S.; Ahammed, G.J.; El-Mogy, M.M.; El-Yazied, A.A.; Ibrahim, M.F.M.; et al. Ozone Induced Stomatal Regulations, MAPK and Phytohormone Signaling in Plants. Int. J. Mol. Sci. 2021, 22, 6304. [Google Scholar] [CrossRef]
- Jahan, M.S.; Wang, Y.; Shu, S.; Hasan, M.M.; El-Yazied, A.A.; Alabdallah, N.M.; Hajjar, D.; Altaf, M.A.; Sun, J.S.; Guo, S. Melatonin pretreatment confers heat tolerance and repression of heat-induced senescence in tomato through the modulation of ABA and GA mediated pathways. Front. Plant Sci. 2021, 12, 650955. [Google Scholar] [CrossRef]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small Amines with Large Effects on Plant Abiotic Stress Tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef]
- Gong, L.; Liu, X.D.; Zeng, Y.Y.; Tian, X.Q.; Li, Y.L.; Turner, N.C.; Fang, X.W. Stomatal morphology and physiology explain varied sensitivity to abscisic acid across vascular plant lineages. Plant Physiol. 2021, 186, 782–797. [Google Scholar] [CrossRef]
- Taie, H.A.; El-Yazal, M.A.S.; Ahmed, S.M.; Rady, M.M. Polyamines modulate growth, antioxidant activity, and genomic DNA in heavy metal–stressed wheat plant. Environ. Sci. Pollut. Res. 2019, 26, 22338–22350. [Google Scholar] [CrossRef]
- Groppa, M.D.; Tomaro, M.L.; Benavides, M.P. Polyamines and heavy metal stress: The antioxidant behavior of spermine in cadmium and copper-treated wheat leaves. Biometals 2017, 20, 185–195. [Google Scholar] [CrossRef]
- Agurla, S.; Gayatri, G.; Raghavendra, A.S. Polyamines increase nitric oxide and reactive oxygen species in guard cells of Arabidopsis thaliana during stomatal closure. Protoplasma 2018, 255, 153–162. [Google Scholar] [CrossRef]
- Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Freschi, L.; Corpas, F.J.; Khan, N.A. Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ. Sci. Pollut. Res. 2017, 24, 2273–2285. [Google Scholar] [CrossRef]
- Gomes, C.; Souza, R.D.; Lidia, N.; Alencar, M.; Helio, J.; Tarquinio, J.; Gomes-filho, E. Exogenous nitric oxide improves salt tolerance during establishment of Jatropha curcas seedlings by ameliorating oxidative damage and toxic ion accumulation. J. Plant Physiol. 2017, 212, 69–79. [Google Scholar]
- Arora, D.; Bhatla, S.C. Melatonin and nitric oxide regulate sunflower seedling growth under salt stress accompanying differential expression of Cu/Zn SOD and Mn SOD. Free Radic. Biol. Med. 2017, 106, 315–328. [Google Scholar] [CrossRef]
- Yao, G.; Nie, Z.F.; Turner, N.C.; Li, F.M.; Gao, T.P.; Fang, X.W.; Scoffoni, C. Combined high leaf hydraulic safety and efficiency provides drought tolerance in Caragana species adapted to low mean annual precipitation. New Phyto. 2021, 229, 230–244. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B. Lead-induced stress, which triggers the production of nitric oxide (NO) and superoxide anion (O2−) in Arabidopsis peroxisomes, affects catalase activity. Nitric Oxide. 2017, 68, 103–110. [Google Scholar] [CrossRef]
- Montilla-Bascón, G.; Rubiales, D.; Hebelstrup, K.H.; Mandon, J.; Harren, F.J.M.; Cristescu, S.M.; Mur, L.A.J.; Prats, E. Reduced nitric oxide levels during drought stress promote drought tolerance in barley and is associated with elevated polyamine biosynthesis. Sci. Rep. 2017, 7, 13311. [Google Scholar] [CrossRef]
- Signorelli, S.; Corpas, F.J.; Rodríguez-Ruiz, M.; Valderrama, R.; Barroso, J.B.; Borsani, O.; Barroso, J.B.; Monza, J. Drought stress triggers the accumulation of NO and SNOs in cortical cells of Lotus japonicus L. roots and the nitration of proteins with relevant metabolic function. Environ. Exp. Bot. 2019, 161, 228–241. [Google Scholar] [CrossRef]
- Fasani, E.; Dalcorso, G.; Varotto, C.; Li, M.; Visioli, G.; Mattarozzi, M.; Furini, A. The MTP1 promoters from Arabidopsis halleri reveal cis-regulating elements for the evolution of metal tolerance. New Phytol. 2017, 214, 1614–1630. [Google Scholar] [CrossRef] [Green Version]
- Sottile, M.L.; Nadin, S.B. Heat shock proteins and DNA repair mechanisms: An updated overview. Cell Stress Chap. 2018, 23, 303–315. [Google Scholar] [CrossRef]
- Yang, Y.J.; Bi, M.H.; Nie, Z.F.; Jiang, H.; Liu, X.D.; Fang, X.W.; Brodribb, T.J. Evolution of stomatal closure to optimize water use efficiency in response to dehydration in ferns and seed plants. New Phyto. 2021, 230, 2001–2010. [Google Scholar] [CrossRef]
- Pal, M.; Csávás, G.; Szalai, G.; Oláh, T.; Khalil, R.; Yordanova, R.; Gell, G.; Birinyi, Z.; Németh, E.; Janda, T. Polyamines may influence phytochelatin synthesis during Cd stress in rice. J. Hazard. Mater. 2017, 340, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Corpas, F.J.; Ahmad, P. Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J. Hazard. Mater. 2020, 399, 123020. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [Green Version]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts1. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teari, D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Mancinelli, A.L. Photoregulation of anthocyanin synthesis: VIII. Effect of light pretreatments. Plant Physiol. 1984, 75, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 2020, 105, 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Carlberg, I.; Mannervik, B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 1975, 250, 5475–5480. [Google Scholar] [CrossRef]
- Hossain, M.A.; Nakano, Y.; Asada, K. Monodehydroascorbate reductase in spinach chloroplast and its participation in regeneration of ascorbate for scavenging of hydrogen peroxide. Plant Cell Physiol. 1984, 25, 385–395. [Google Scholar]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hossain, M.Z.; Fujita, M. Stress induced changes of methylglyoxal level and Glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust. J. Crop Sci. 2009, 3, 53–64. [Google Scholar]
- Couldwell, D.L.; Dunford, R.; Kruger, N.J.; Lloyd, D.C.; Ratcliffe, R.G.; Smith, A.M. Response of cytoplasmic pH to anoxia in plant tissues with altered activities of fermentation enzymes: Application of methyl phosphonate as an NMR pH probe. Ann. Bot. 2009, 103, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Monošík, R.; dos Santos, V.B.; Angnes, L. A simple paper-strip colorimetric method utilizing dehydrogenase enzymes for analysis of food components. Anal. Methods 2015, 7, 8177–8184. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Benavides, M.P.; Groppa, M.D.; Recalde, L.; Verstraeten, S.V. Effects of polyamines on cadmium-and copper-mediated alterations in wheat (Triticum aestivum L.) and sunflower (Helianthus annuus L.) seedling membrane fluidity. Arch. Biochem. Biophys. 2018, 654, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Guo, S.; Sun, J.; Shu, S.; Wang, Y.; El–Yazied, A.A.; Alabdallah, N.M.; Hikal, M.; Mohamed, M.H.M.; Ibrahim, M.F.M.; et al. Melatonin–mediated photosynthetic performance of tomato seedlings under high–temperature stress. Plant Physiol. Biochem. 2021, 167, 309–320. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 2018, 255, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Alharby, H.F.; Uddin, M.N.; Ali, M.A.; Anwar, Y.; Fang, X.W.; Hakeem, K.R.; Alzahrani, Y.; Hajar, A.S. Magnetized water confers drought stress tolerance in Moringa biotype via modulation of growth, gas exchange, lipid peroxidation and antioxidant activity. Pol. J. Environ. Stud. 2020, 1, 29. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Seraj, Z.I.; Fujita, M. Exogenous sodium nitroprusside and glutathione alleviate copper toxicity by reducing copper uptake and oxidative damage in rice (Oryza sativa L.) seedlings. Protoplasma 2014, 251, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ahammed, G.J.; Zhang, X.N.; Zhang, L.; Yan, P.; Zhang, L.P.; Fu, J.Y.; Han, W.Y. Melatonin-mediated regulation of anthocyanin biosynthesis and antioxidant defense confer tolerance to arsenic stress in Camellia sinensis L. J. Hazard. Mater. 2020, 403, 123922. [Google Scholar] [CrossRef]
- del Río, L.A.; Corpas, F.J.; López-Huertas, E.; Palma, J.M. Plant superoxide dismutases: Function under abiotic stress conditions. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–26. [Google Scholar]
- Manai, J.; Kalai, T.; Gouia, H.; Corpas, F.J. Exogenous nitric oxide (NO) ameliorates salinity-induced oxidative stress in tomato (Solanum lycopersicum) plants. J. Soil Sci. Plant Nutri. 2014, 14, 433–446. [Google Scholar] [CrossRef]
- Silva, V.M.; Boleta, E.H.M.; Lanza, M.G.D.B.; Lavres, J.; Martins, J.T.; Santos, E.F. Physiological, biochemical, and ultrastructural characterization of selenium toxicity in cowpea plants. Environ. Exp. Bot. 2018, 150, 172–182. [Google Scholar] [CrossRef] [Green Version]
- González-Gordo, S.; Bautista, R.; Claros, M.G.; Cañas, A.; Palma, J.M.; Corpas, F.J. Nitric oxide-dependent regulation of sweet pepper fruit ripening. J. Exp. Bot. 2019, 70, 4557–4570. [Google Scholar] [CrossRef] [Green Version]
- Corpas, F.J.; Gupta, D.K.; Palma, J.M. Production sites of reactive oxygen species (ROS) in organelles from plant cells. In Reactive Oxygen Species and Oxidative Damage in Plants Under Stress; Gupta, D.K., Corpas, F.J., Palma, J.M., Eds.; Springer International Publishing: Newyork, NY, USA, 2015; pp. 1–22. [Google Scholar]
- Corpas, F.J.; González-Gordo, S.; Palma, J.M. Nitric oxide and hydrogen sulfide modulate the NADPH-generating enzymatic system in higher plants. J. Exp. Bot. 2021, 72, 830–847. [Google Scholar] [CrossRef]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of subcellular ROS and NO metabolism in higher plants: Multifunctional signaling molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ram, H.; Kaur, A.; Gandass, N.; Singh, S.; Deshmukh, R.; Sonah, H.; Sharma, T.R. Molecular characterization and expression dynamics of MTP genes under various spatiotemporal stages and metal stress conditions in rice. PLoS ONE 2019, 14, e0217360. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Cai, S.Y.; Ruan, X.L.; Chen, S.Y.; Mei, G.F.; Ruan, G.H.; Cao, D.D. Salicylic acid enhances sunflower seed germination under Zn2+ stress via involvement in Zn2+ metabolic balance and phytohormone interactions. Sci. Hortic. 2021, 275, 109702. [Google Scholar] [CrossRef]
- Luo, L.; Li, Z.; Tang, M.Y.; Cheng, B.Z.; Zeng, W.H.; Peng, Y.; Nie, G.; Zhang, X.Q. Metabolic regulation of polyamines and-aminobutyric acid in relation to spermidine-induced heat tolerance in white clover. Plant Biol. 2020, 22, 794–804. [Google Scholar] [CrossRef] [PubMed]
- He, J.L.; Zhuang, X.L.; Zhou, J.T.; Sun, L.Y.; Wan, H.X.; Li, H.F.; Lyu, D. Exogenous melatonin alleviates cadmium uptake and toxicity in apple rootstocks. Tree Physiol. 2020, 40, 746–761. [Google Scholar] [CrossRef]










| Treatment | Na2SeO4 (mM) | Spm (mM) | SNP (mM) | cPTIO (mM) |
|---|---|---|---|---|
| Control | 0.0 | 0.0 | 0.0 | 0.0 |
| Se | 1.0 | 0.0 | 0.0 | 0.0 |
| Se + Spm | 1.0 | 1.0 | 0.0 | 0.0 |
| Se + SNP | 1.0 | 0.0 | 0.1 | 0.0 |
| Se + Spm + SNP | 1.0 | 1.0 | 0.1 | 0.0 |
| Se + Spm + cPTIO | 1.0 | 1.0 | 0.0 | 0.1 |
| Se + SNP + cPTIO | 1.0 | 0.0 | 0.1 | 0.1 |
| Se + Spm + SNP + cPTIO | 1.0 | 1.0 | 0.1 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.M.; Alharbi, B.M.; Alhaithloul, H.A.S.; Abdulmajeed, A.M.; Alghanem, S.M.; Al-Mushhin, A.A.M.; Jahan, M.S.; Corpas, F.J.; Fang, X.-W.; Soliman, M.H. Spermine-Mediated Tolerance to Selenium Toxicity in Wheat (Triticum aestivum L.) Depends on Endogenous Nitric Oxide Synthesis. Antioxidants 2021, 10, 1835. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10111835
Hasan MM, Alharbi BM, Alhaithloul HAS, Abdulmajeed AM, Alghanem SM, Al-Mushhin AAM, Jahan MS, Corpas FJ, Fang X-W, Soliman MH. Spermine-Mediated Tolerance to Selenium Toxicity in Wheat (Triticum aestivum L.) Depends on Endogenous Nitric Oxide Synthesis. Antioxidants. 2021; 10(11):1835. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10111835
Chicago/Turabian StyleHasan, Md. Mahadi, Basmah M. Alharbi, Haifa Abdulaziz Sakit Alhaithloul, Awatif M. Abdulmajeed, Suliman Mohammed Alghanem, Amina A. M. Al-Mushhin, Mohammad Shah Jahan, Francisco J. Corpas, Xiang-Wen Fang, and Mona H. Soliman. 2021. "Spermine-Mediated Tolerance to Selenium Toxicity in Wheat (Triticum aestivum L.) Depends on Endogenous Nitric Oxide Synthesis" Antioxidants 10, no. 11: 1835. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10111835