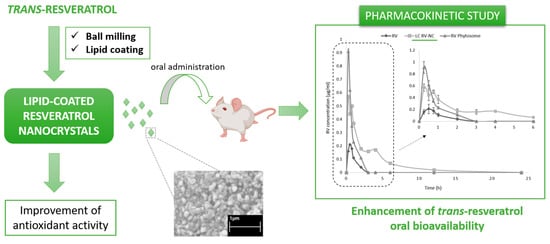
Lipid-Coated Nanocrystals as a Tool for Improving the Antioxidant Activity of Resveratrol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Trans-Resveratrol Nanocrystals
2.3. In Vitro Characterization of Trans-Resveratrol Nanocrystals
2.3.1. Physico-Chemical Parameter Determination
2.3.2. Scanning Electron Microscopy (SEM) Analysis
2.3.3. Differential Scanning Calorimetry (DSC)
2.3.4. Fourier Transform Infra-Red Spectroscopy (FTIR)
2.4. Trans-Resveratrol Quantitative Determination
2.5. In Vitro Dissolution Studies
2.6. Stability Studies
2.7. Antioxidant Activity Evaluation
2.7.1. DPPH Free Radical Scavenging Activity
2.7.2. Thiobarbituric Acid (TBA) Assay
2.8. In Vivo Pharmacokinetic Study after Oral Administration to Rats
2.9. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Francioso, A.; Mastromarino, P.; Masci, A.; d’Erme, M.; Mosca, L. Chemistry, stability and bioavailability of resveratrol. Med. Chem. 2014, 10, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors 2018, 44, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vervandier-Fasseur, D.; Latruffe, N. The Potential Use of Resveratrol for Cancer Prevention. Molecules 2019, 24, 4506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houacine, C.; Khan, I.; Yousaf, S.S. Potential Cardio-Protective Agents: A Resveratrol Review (2000–2019). Curr. Pharm Des. 2021, 27, 2943–2955. [Google Scholar] [CrossRef] [PubMed]
- Amri, A.; Chaumeil, J.C.; Sfar, S.; Charrueau, C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release 2012, 158, 182–193. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernas, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification—the correlation of in-vitro drug product dissolution and in-vivo bioavailability. Pharmaceut. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. BioFactors 2018, 44, 16–25. [Google Scholar] [CrossRef]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.S.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to Improve Oral Bioavailability and Beneficial Effects of resveratrol. Int. J. Mol. Sci. 2019, 20, 1381. [Google Scholar] [CrossRef] [Green Version]
- Peng, R.M.; Lin, G.R.; Ting, Y.; Hu, J.Y. Oral delivery system enhanced the bioavailability of stilbenes: Resveratrol and pterostilbene. Biofactors 2018, 44, 5–15. [Google Scholar] [CrossRef]
- Machado, N.D.; Fernández, M.A.; Díaz, D.D. Recent Strategies in resveratrol Delivery Systems. ChemPlusChem 2019, 84, 951–973. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.; Pereira, I.; Pereira-Silva, M.; Ferreira, L.; Caldas, M.; Collado-González, M.; Magalhães, M.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Nanotechnology-based formulations for resveratrol delivery: Effects on resveratrol in vivo bioavailability and bioactivity. Colloids Surf. B Biointerfaces 2019, 180, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Intagliata, S.; Modica, M.N.; Santagati, L.M.; Montenegro, L. Strategies to Improve resveratrol Systemic and Topical Bioavailability: An Update. Antioxidants 2019, 8, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhakar, N.K.; Matencio, A.; Caldera, F.; Argenziano, M.; Cavalli, R.; Dianzani, C.; Trotta, F. Comparative Evaluation of Solubility, Cytotoxicity and Photostability Studies of resveratrol and Oxyresveratrol Loaded Nanosponges. Pharmaceutics 2019, 11, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perris, A.; Bhattacharya, S.; Jawed, J.J.; Hoda, M. Oncotherapeutic Application of Resveratrol-based Inorganic Nanoparticles. Pharm. Nanotech. 2021, 9, 271–280. [Google Scholar] [CrossRef]
- Juère, E.; Florek, J.; Bouchoucha, M.; Jambhrunkar, S.; Wong, K.Y.; Popat, A.; Kleitz, F. In Vitro Dissolution, Cellular Membrane Permeability, and Anti-Inflammatory Response of Resveratrol-Encapsulated Mesoporous Silica Nanoparticles. Mol. Pharm. 2017, 14, 4431–4441. [Google Scholar] [CrossRef]
- Venditti, I.; Iucci, G.; Fratoddi, I.; Cipolletti, M.; Montalesi, E.; Marino, M.; Secchi, V.; Battocchio, C. Direct Conjugation of Resveratrol on Hydrophilic Gold Nanoparticles: Structural and Cytotoxic Studies for Biomedical Applications. Nanomaterials 2020, 10, 1898. [Google Scholar] [CrossRef]
- Leone, F.; Cavalli, R. Drug nanosuspensions: A ZIP tool between traditional and innovative pharmaceutical formulations. Expert Opin. Drug Deliv. 2015, 12, 1607–1625. [Google Scholar] [CrossRef]
- Sun, L.; Hu, Y.; Zhang, L. Recent Trends in Nanocrystals for Pharmaceutical Applications. Curr. Pharm. Des. 2018, 24, 2394–2402. [Google Scholar] [CrossRef]
- Müller, R.H.; Gohla, S.; Keck, C.M. State of the art of nanocrystals—special features, production, nanotoxicology aspects and intracellular delivery. Eur. J. Pharm. Biopharm. 2011, 78, 1–9. [Google Scholar] [CrossRef]
- Gigliobianco, M.R.; Casadidio, C.; Censi, R.; Di Martino, P. Nanocrystals of Poorly Soluble Drugs: Drug Bioavailability and Physicochemical Stability. Pharmaceutics 2018, 10, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halwani, A.A. Development of Pharmaceutical Nanomedicines: From the Bench to the Market. Pharmaceutics 2022, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Ančić, D.; Oršolić, N.; Odeh, D.; Tomašević, M.; Pepić, I.; Ramić, S. Resveratrol and its nanocrystals: A promising approach for cancer therapy? Toxicol. Appl. Pharmacol. 2022, 435, 115851. [Google Scholar] [CrossRef] [PubMed]
- Sinico, C.; Pireddu, R.; Pini, E.; Valenti, D.; Caddeo, C.; Fadda, A.M.; Lai, F. Enhancing topical delivery of resveratrol through a nanosizing approach. Planta Med. 2017, 83, 476–481. [Google Scholar] [CrossRef]
- Singh, S.K.; Makadia, V.; Sharma, S.; Rashid, M.; Shahi, S.; Mishra, P.R.; Wahajuddin, M.; Gayen, J.R. Preparation and in-vitro/in-vivo characterization of resveratrol nanocrystals for oral administration. Drug Deliv. Transl. Res. 2017, 7, 395–407. [Google Scholar] [CrossRef]
- Ozkan, G.; Kostka, T.; Esatbeyoglu, T.; Capanoglu, E. Effects of Lipid-Based Encapsulation on the Bioaccessibility and Bioavailability of Phenolic Compounds. Molecules 2020, 25, 5545. [Google Scholar] [CrossRef]
- Subramanian, P. Lipid-Based Nanocarrier System for the Effective Delivery of Nutraceuticals. Molecules 2021, 26, 5510. [Google Scholar] [CrossRef]
- Coimbra, M.; Isacchi, B.; van Bloois, L.; Torano, J.S.; Ket, A.; Wu, X.; Broere, F.; Metselaar, J.M.; Rijcken, C.J.; Storm, G.; et al. Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int. J. Pharm. 2011, 416, 433–442. [Google Scholar] [CrossRef]
- Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to Enhance the Bioavailability and Physiological Functions of Polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef]
- Cavalli, R.; Zara, G.P.; Caputo, O.; Bargoni, A.; Fundarò, A.; Gasco, M.R. Transmucosal transport of tobramycin incorporated in SLN after duodenal administration to rats. Part I—A pharmacokinetic study. Pharm. Res. 2000, 42, 541–545. [Google Scholar] [CrossRef]
- Singh, G. Resveratrol: Nanocarrier-based delivery systems to enhance its therapeutic potential. Nanomedicine 2020, 15, 2801–2817. [Google Scholar] [CrossRef] [PubMed]
- Shegokar, R.; Müller, R.H. Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm. 2010, 399, 129–139. [Google Scholar] [CrossRef]
- Cheng, Z.; Lian, Y.; Kamal, Z.; Ma, X.; Chen, J.; Zhou, X.; Su, J.; Qiu, M. Nanocrystals Technology for Pharmaceutical Science. Curr. Pharm. Des. 2018, 24, 2497–2507. [Google Scholar] [CrossRef] [PubMed]
- Kobierski, S.; Ofori-Kwakye, K.; Müller, R.H.; Keck, C.M. Resveratrol nanosuspensions: Interaction of preservatives with nanocrystal production. Pharmazie 2011, 66, 942–947. [Google Scholar] [PubMed]
- Dhaval, M.; Makwana, J.; Sakariya, E.; Dudhat, K. Drug Nanocrystals: A Comprehensive Review with Current Regulatory Guidelines. Curr. Drug Deliv. 2020, 17, 470–482. [Google Scholar] [CrossRef]
- Lai, F.; Schlich, M.; Pireddu, R.; Fadda, A.M.; Sinico, C. Nanocrystals as Effective Delivery Systems of Poorly Water-soluble Natural Molecules. Curr. Med. Chem. 2019, 26, 4657–4680. [Google Scholar] [CrossRef]
- Cavalli, R.; Leone, F. Nanosuspensions A versatile drug delivery platform. Chem. Today 2013, 31, 46–49. [Google Scholar]
- Kumar, B.S.; Saraswathi, R.; Kumar, K.V.; Jha, S.K.; Venkates, D.P.; Dhanaraj, S.A. Development and characterization of lecithin stabilized glibenclamide nanocrystals for enhanced solubility and drug delivery. Drug Del. 2014, 21, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, Z.; Zhang, H.; Gao, J.; Zheng, A. Progress in the development of stabilization strategies for nanocrystal preparations. Drug Del. 2021, 28, 19–36. [Google Scholar] [CrossRef]
- Yang, H.; Kim, H.; Jung, S.; Seo, H.; Nida, S.K.; Yoo, S.-Y.; Lee, J. Pharmaceutical Strategies for Stabilizing Drug Nanocrystals. Curr. Pharm. Des. 2018, 24, 2362–2374. [Google Scholar] [CrossRef]
- Tuomela, A.; Hirvonen, J.; Peltonen, L. Stabilizing Agents for Drug Nanocrystals: Effect on Bioavailability. Pharmaceutics 2016, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Hoogevest, P. Review-An update on the use of oral phospholipid excipients. Eur. J. Pharm. Sci. 2017, 108, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.S.; Sim, W.Y.; Lee, S.K.; Jeong, J.S.; Kim, J.S.; Baek, I.H.; Choi, D.H.; Park, H.; Hwang, S.J.; Kim, M.S. Preparation and Evaluation of Resveratrol-Loaded Composite Nanoparticles Using a Supercritical Fluid Technology for Enhanced Oral and Skin Delivery. Antioxidants 2019, 8, 554. [Google Scholar] [CrossRef] [Green Version]
- Ha, E.S.; Park, H.; Lee, S.K.; Sim, W.Y.; Jeong, J.S.; Baek, I.H.; Kim, M.S. Pure Trans-Resveratrol Nanoparticles Prepared by A Supercritical Antisolvent Process Using Alcohol and Dichloromethane Mixtures: Effect of Particle Size on Dissolution and Bioavailability in Rats. Antioxidants 2020, 9, 342. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.L.; John, M.; Lee, S.L.; Tyner, K.M. Development Considerations for Nanocrystal Drug Products. AAPS J. 2017, 19, 642–651. [Google Scholar] [CrossRef]
- Tian, Z.; Mai, Y.; Meng, T.; Ma, S.; Gou, G.; Yang, J. Nanocrystals for Improving Oral Bioavailability of Drugs: Intestinal Transport Mechanisms and Influencing Factors. AAPS PharmSciTech 2021, 22, 179. [Google Scholar] [CrossRef]
- Yang, Z.; Argenziano, M.; Salamone, P.; Pirro, E.; Sprio, A.E.; Di Scipio, F.; Berta, G.N. Preclinical pharmacokinetics comparison between resveratrol 2-hydroxypropyl-β-cyclodextrin complex and trans-resveratrol suspension after oral administration. J. Inc. Phenom. Macrocycl. Chem. 2016, 86, 263–271. [Google Scholar] [CrossRef]
- Vasconcelos, T.; Prezotti, F.; Araújo, F.; Lopes, C.; Loureiro, A.; Marques, S.; Sarmento, B. Third-generation solid dispersion combining Soluplus and poloxamer 407 enhances the oral bioavailability of resveratrol. Int. J. Pharm. 2021, 595, 120245. [Google Scholar] [CrossRef] [PubMed]
- Sessa, M.; Balestrieri, M.L.; Ferrari, G.; Servillo, L.; Castaldo, D.; D’Onofrio, N.; Donsì, F.; Tsao, R. Bioavailability of encapsulated resveratrol into nanoemulsion-based delivery systems. Food Chem. 2014, 147, 42–50. [Google Scholar] [CrossRef] [PubMed]






| RV-NC | LC RV-NC | |
|---|---|---|
| Average diameter ± SD (nm) | 445.8 ± 35.3 | 306.2 ± 12.5 *** |
| Polydispersity index (PDI) ± SD | 0.25 ± 0.02 | 0.22 ± 0.02 |
| Zeta potential ± SD (mV) | −2.12 ± 0.54 | −26.63 ± 3.17 |
| Parameters | RV | LC RV-NC | RV Phytosome® |
|---|---|---|---|
| AUC (h/ng/mL) | 250 ± 20 | 1570 ± 32 *** | 780 ± 26 *** |
| Tmax (min) | 30 ± 1.4 | 15 ± 0.8 | 15 ± 0.6 |
| Cmax (µg/mL) | 0.21 ± 0.03 | 0.62 ± 0.04 | 0.90 ± 0.05 |
| MRT (h) | 1.10 ± 0.02 | 3.90 ± 0.08 | 1.10 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argenziano, M.; Ansari, I.A.; Muntoni, E.; Spagnolo, R.; Scomparin, A.; Cavalli, R. Lipid-Coated Nanocrystals as a Tool for Improving the Antioxidant Activity of Resveratrol. Antioxidants 2022, 11, 1007. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11051007
Argenziano M, Ansari IA, Muntoni E, Spagnolo R, Scomparin A, Cavalli R. Lipid-Coated Nanocrystals as a Tool for Improving the Antioxidant Activity of Resveratrol. Antioxidants. 2022; 11(5):1007. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11051007
Chicago/Turabian StyleArgenziano, Monica, Irfan Aamer Ansari, Elisabetta Muntoni, Rita Spagnolo, Anna Scomparin, and Roberta Cavalli. 2022. "Lipid-Coated Nanocrystals as a Tool for Improving the Antioxidant Activity of Resveratrol" Antioxidants 11, no. 5: 1007. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11051007